Abstract
Interleukin-13 (IL-13) has emerged as a major cytokine mediator of fibroblast activation and pulmonary fibrosis. Normal (from noninflamed lung), Th1-type (induced by the pulmonary embolization of purified peptide derivative-coated beads in mice sensitized to purified peptide derivative), and Th2-type (induced by the pulmonary embolization of Schistosoma mansoni egg antigen-coated beads in mice sensitized with S. mansoni eggs) primary fibroblast cell lines all exhibited constitutive gene expression of two receptor chains that bind and signal IL-13-mediated cellular events: IL-4Rα and IL-13Rα1. However, all three fibroblast cell lines exhibited divergent synthetic and proliferative responses to the exogenous addition of either recombinant IL-13 or a chimeric protein comprised of IL-13 and a truncated version of Pseudomonas exotoxin (IL13-PE), which targets and kills IL-13 receptor overexpressing cells. The exogenous addition of IL-13 to Th1-type and Th2-type fibroblast cultures significantly increased the cellular expression of IL-13Rα2, which may function as an IL-13 decoy receptor. After a 24-hour exposure to IL-13, the total collagen generation and cellular proliferation by Th2-type fibroblasts were significantly higher than that observed in similar numbers of normal and Th1-type fibroblasts. In addition IL13-PE, which binds with highest affinity to IL-13Rα2, exhibited down-regulatory effects on proliferation and matrix generation expression by Th1- and Th2-type, but not normal, fibroblasts. Thus, these data demonstrate that fibroblasts derived from murine pulmonary granulomas exhibit divergent expression of functional IL-13 receptor and this expression dictates the responsiveness and susceptibility to recombinant IL-13 and IL-13 immunotoxin, respectively.
Considerable research has been directed toward understanding the cellular and molecular mechanisms through which fibroblasts are activated during pulmonary fibrosis, and recent reviews on this topic can be found elsewhere. 1-3 Transforming growth factor (TGF)-β is regarded as the premier pathological cytokine in chronic pulmonary fibrosis because of its enhanced presence in clinical idiopathic pulmonary fibrosis (IPF) 4,5 and its potent role in experimental fibrosis under a number of conditions. 6-8 Interestingly, data published recently suggested that the synthesis of TGF-β during experimental pulmonary fibrosis was regulated by interleukin (IL)-13, 8 a Th2-type cytokine whose actions were believed to be limited to that of immune cell regulation. 9 Furthermore, IL-13 is pertinent to IPF given that levels of this cytokine are markedly up-regulated in alveolar macrophages 10 and overall the cytokine pattern of this disease is more Th2-type (ie, IL-4 and IL-13) than Th1-type [ie, IL-12 and interferon (IFN)-γ]. 11-13 Detailed studies of IL-13 receptor expression by pulmonary fibroblasts during IPF have yet to appear in the literature, but isolated primary human fibroblasts from a number of normal and diseased tissues express functional IL-13 receptors. 14-16 The most common functional IL-13 receptor on nonhematopoietic cells such as fibroblasts is comprised of the IL-4Rα and IL-13Rα1 subunits, 17-19 explaining, in part, why IL-4 and IL-13 have similar effects on these cells. 15,20,21 The shared effects of IL-13 and IL-4 on fibroblasts presumably reflects the fact that both cytokines similarly activate the JAK2 tyrosine kinase-dependent signal transduction pathway. 15 However, some IL-13-dependent and IL-4-independent effects have also been observed in fibroblasts. 22 Furthermore, fibroblasts express the IL-13Rα2 subunit, 17,23 which has ∼100-fold higher affinity for IL-13 than IL-13Rα1 24,25 and possibly function as a decoy receptor because of its short cytoplasmic region.
Exploration of the therapeutic potential associated with the high-affinity IL-13Rα2 decoy receptor has garnered attention because of recent data showing that a soluble version of this receptor, sIL-13Rα2-Fc, effectively antagonized the profibrotic actions of IL-13 in the liver. 26,27 In addition, the cellular internalization of IL-13Rα2 does not elicit cell-signaling events, a feature that is optimal for receptor-directed cytokine immunotoxin therapies. 28 Along this line, we recently demonstrated that the intranasal delivery of a cytokine toxin chimera comprised of human IL-13 and a mutated form of Pseudomonas exotoxin A (IL13-PE) significantly reduced Aspergillus fumigatus-induced peribronchial 29,30 and S. mansoni-induced granulomatous 31 fibrosis in vivo. Thus, the aim of the present study was to further characterize the in vitro expression of IL-4Rα, IL-13Rα1, and IL-13Rα2 subunits by purified pulmonary fibroblast cell lines derived from noninflamed lung or from lung granulomas skewed toward a Th1-type or a Th2-type cytokine pattern. In these experimental models, the Th1-type granuloma is characterized by modest pulmonary fibrosis in contrast to the Th2-type granuloma. 32 The impact of IL-13 and IL13-PE on the synthetic and proliferative properties of these fibroblast cell lines was examined. We previously demonstrated that normal, Th1-type, and Th2-type fibroblasts exhibited distinct chemokine and cytokine synthetic characteristics that are maintained long term in culture. 33,34 The data shown herein demonstrate that IL-13 has a significant impact on the synthetic and proliferative properties of granuloma fibroblasts, but not normal fibroblasts, and the IL-13-responsiveness of granuloma fibroblasts renders them targets of IL-13-based immunotoxin therapy.
Methods and Materials
Lung Granuloma Models
Th1-Type or Type 1 Granulomas
Specific pathogen-free, female CBA/J mice (6 to 8 weeks; Jackson Laboratories, Bar Harbor, ME) were sensitized by intraperitoneal and subcutaneous injection of complete Freund’s adjuvant (Sigma Chemical Company, St. Louis, MO) diluted 1:1 with normal saline (100 μl per injection site). At day 16 after complete Freund’s adjuvant injections, groups of five mice were injected intravenously with 3000 Sepharose 4B beads covalently coupled to purified peptide derivative (PPD) antigens suspended in 500 μl of normal saline.
Th2-Type or Type 2 Granulomas
Specific pathogen-free, female CBA/J mice (6 to 8 weeks; Jackson Laboratories) were sensitized by the intraperitoneal injection of 5000 freshly isolated S. mansoni eggs suspended in 500 μl of normal saline. At day 16 after the intraperitoneal injection, groups of five mice were intravenously injected with 3000 Sepharose 4B covalently coupled to S. mansoni egg antigen suspended in 500 μl of normal saline.
Isolation and Culture of Primary Pulmonary Fibroblast Lines
Whole lungs from mice with Th1-type (PPD-induced) or Th2-type (S. mansoni-induced) granulomas were prepared as previously described in detail. 33,34 Briefly, each mouse was euthanized at day 8 after bead embolization by a sodium pentobarbital overdose, and whole lung samples were dissected free from the thoracic cavity. The whole lung samples were then finely dispersed on sterile steel mesh and the dispersed cells were then placed into 150-cm 2 cell culture flasks (Corning Inc., Corning, NY) containing Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 15% fetal bovine serum (DMEM-15). Normal fibroblasts were obtained from other groups of five noninflamed, specific pathogen-free mice according to the same protocol. All three primary lung cell lines were maintained in DMEM-15 at 37°C in a 5% CO2 incubator and were serially passaged a total of five times to yield pure populations of lung fibroblasts as previously described. 35 Our previous characterization of these primary fibroblast cell lines are described in detail elsewhere. 33,34
Preparation of RNA and cDNA from Fibroblast Cell Lines
Before each experiment, the purified fibroblast lines were added to 24-well tissue culture plates at a cell density of 1 × 10 5 cells/well. Twenty-four hours after plating, fibroblasts were exposed to fresh DMEM-15 or DMEM-15 to which 10 ng/ml of IFN-γ (R&D Systems, Minneapolis, MN) or 10 ng/ml of IL-13 (R&D Systems) had been added. After 24 hours, TRIzol Reagent (Invitrogen Life Technologies, Carlsbad, CA) was subsequently added to each well and total RNA was then prepared according to the manufacturer’s instructions. The purified RNA was subsequently reverse-transcribed into cDNA using a BRL reverse transcription kit and oligo (dT) 12–18 primers. The amplification buffer contained 50 mmol/L KCl, 10 mmol/L Tris-HCl (pH 8.3), and 2.5 mmol/L MgCl2.
Gene Array Membranes
Non-Rad GEArray gene array membranes from SuperArray Inc. (Bethesda, MD) were used to analyze changes in gene profiles for various interleukin receptors, extracellular matrix- and apoptosis-associated factors. The procedures implemented were those supplied by the manufacturers. Briefly, cDNAs from all three fibroblast cell lines were created using biotin labeled 16-dUTP, and each gene array membrane was hybridized with the biotin-labeled cDNAs for ∼12 hours or overnight. Each gene array membrane was subsequently washed, blocked, and incubated with alkaline phosphatase-conjugated to streptavidin. After a subsequent incubation with CDP-star chemiluminescent substrate for 2 minutes, the gene array membranes were then exposed to X-ray film. Analysis of the spots was then completed using Scanalyse (Eisen Lab, Stanford University, Stanford, CA) and GEArray analyzer (SuperArray Inc.).
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Amplification
Specific oligonucleotide primers were added (200 ng/sample) to the buffer, along with 1 μl of reverse-transcribed cDNA sample. The following murine oligonucleotide primers (5′ to 3′ sequences) were used for RT-PCR analysis: IL-4 receptor α sense, CCGCACTTCCACGTGTGA; IL-4 receptor α antisense, CTGAAGTAACAGAACAGGC; IL-13 receptor α1 sense, GAATTTGAGCGTCTCTGTCGAA; IL-13 receptor α1 antisense, GGTTATGCCAAATGCACTTGAG. These mixtures were then first incubated for 4 minutes at 94°C and amplified using the following cycling parameters: IL-4Rα: cycled 38 times at 94°C for 45 seconds, 55°C for 45 seconds, and elongated at 72°C for 60 seconds; IL-13Rα1: cycled 38 times at 94°C for 45 seconds, 66°C for 60 seconds, and elongated at 72°C for 60 seconds. After amplification the samples were separated on a 2% agarose gel containing 0.3 μg/ml of ethidium bromide and bands visualized and photographed using a translucent UV source.
Real-Time TaqMan PCR Analysis
IL-13Rα2 gene expression was analyzed by real-time quantitative RT-PCR procedure using an Abi Prism 7700 sequence detection system (Applied Biosystems, Foster City, CA). The cDNAs from each fibroblast line at 24 hours after exposure to media, IFN-γ, or IL-13 were analyzed for IL-13Rα2 and GAPDH (an internal control). All primers and probes used were purchased from Applied Biosystems. IL-13Rα2 gene expression was normalized to GAPDH before the fold change in IL-13Rα2 expression was calculated. The fold increases in IL-13Rα2 gene expression were calculated via the comparison of gene expression in all of the fibroblast cell lines (with or without cytokine exposure) to that of untreated normal fibroblasts. IL-13Rα2 gene expression in untreated normal fibroblasts was given an arbitrary value of 1.
Immunohistochemical Analysis
Purified fibroblast lines were added to 8-well Labtek (Nunc Inc., Naperville, IL) tissue culture plates at a cell density of 1 × 10 4 cells/well. Twenty-four hours after plating, fibroblasts were exposed to fresh DMEM-15 alone or DMEM-15 to which 10 ng/ml of IFN-γ (R&D Systems) or 10 ng/ml of IL-13 (R&D Systems) had been added. After 24 hours, the fibroblasts were fixed with 4% paraformaldehyde and stained according to the procedures supplied with the Cell and Tissue Staining Kit from R&D Systems. After fixation with 4% paraformaldehyde, the fibroblasts were blocked for 15 minutes. After rinsing, cells were incubated overnight with receptor-specific primary antibodies directed toward murine IL-4Rα, IL-13Rα2, or an appropriate isotype-matched nonimmune control antibody, labeled (−Ab) on immunohistochemistry figures (R&D Systems). Fibroblasts were then washed and incubated with a biotinylated secondary antibody for 1 hour. These fibroblasts were then washed thoroughly and high sensitivity streptavidin (HSS)-horseradish peroxidase was added for a total of 30 minutes. In the final step, alveolar epithelial cell chromogen was added for 2 minutes, after which the fibroblasts were rinsed with distilled water and counterstained with hematoxylin. Aqueous mounting medium was used to hold glass coverslips in place over the stained fibroblasts and microscopic images (×400 magnification) were captured using a digital camera.
Fibroblast Proliferation Analysis: Effects of IL13-PE
Fibroblast proliferation was assessed in 24-well plates via [3H]thymidine incorporation. In the first experiment, type1, type 2, and normal fibroblasts were plated at a density of 1 × 10 5 cells/well, allowed to adhere overnight and to quadruplet wells the following was added: DMEM-15 alone, DMEM-15 plus 200 ng/ml of IL13-PE, or DMEM-15 plus 1000 ng/ml of IL13-PE. These culture plates were incubated for 24 hours at 37°C in a 5% CO2 incubator. In the second experiment, purified type1, type 2, and normal fibroblasts were plated at 1 × 10 4 cells/well and allowed to adhere overnight. The lower fibroblast density was used in these studies because all three fibroblast cell lines were exposed to 10 ng/ml of IFN-γ or IL-13 for 24 hours before the addendum of 100 μl of DMEM-15 alone or the same volume of DMEM-15 containing 200 ng/ml of IL13-PE for another 24 hours. These culture plates were incubated for the duration of the experiment at 37°C in a 5% CO2 incubator. Four hours before the conclusion of both experiments, 10 μCi of [3H]thymidine was added to each well and the plates were incubated at 37°C. Subsequently, fibroblasts were then washed three times with phosphate-buffered saline and to each well 1 ml of 1% Triton X-100 for 30 minutes. The Triton X-100-treated fibroblast samples were then added to 5-ml scintillation vials, 3 ml of scintillation fluid was added, and the vials were analyzed in a Beckman scintillation counter (model LS 5801; Beckman Instruments, Fullerton, CA).
Statistical Analysis
All results are expressed as mean ± SEM (SE). One-way analysis of variance and Tukey-Kramer multiple comparisons test were used to reveal statistical differences between the normal, type 1, and type 2 fibroblasts; P < 0.05 was considered statistically significant.
Results
Normal, Type 1, and Type 2 Fibroblasts Strongly Express the IL-13Rα1 Gene Relative to Gene Levels for Other Interleukin Receptors
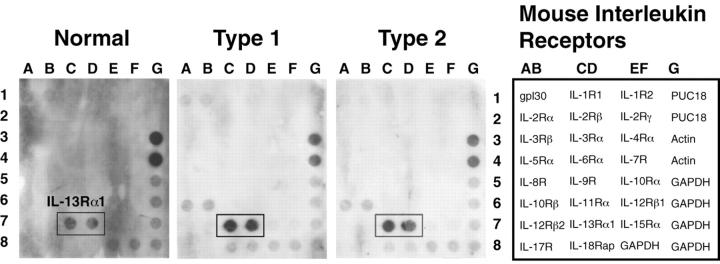
Our first experiment addressed whether cultured normal, type 1, and type 2 fibroblasts expressed unique repertoires of cytokine receptors. GEArray gene analysis of constitutive cytokine receptor expression by normal, type 1, and type 2 fibroblasts is shown in Figure 1 ▶ . Weak gene expression for gp160 (a subunit of the IL-6 receptor) was detected in all three fibroblast cell lines whereas IL-10 and IL-18 receptor gene expression were observed in type 1 and type 2 but not normal fibroblasts. Strong constitutive IL-13Rα1 gene expression was observed in normal, type 1, and type 2 fibroblasts relative to the housekeeping gene expression for β-actin and GAPDH (Figure 1) ▶ . This gene array analysis suggested that untreated normal and granuloma fibroblasts strongly express the gene for a key receptor subunit necessary for responsiveness to IL-13.
Figure 1.
GEArray analysis of cytokine receptor gene expression in tissue cultures of primary normal, type 1, and type 2 fibroblast lines. SuperArray analysis demonstrated a strong expression of the functional IL-13 receptor, IL-13Rα1, in all three fibroblast populations. The template for this SuperArray appears next to the membranes.
IL-13 Does Not Modulate the Gene Expression of IL-4Rα and IL-13Rα1 in Normal, Th1-Type, and Th2-Type Fibroblasts
RT-PCR analysis was used next to determine whether the fibroblast cell lines expressed IL-4Rα, another receptor subunit that binds IL-13, 20,36 and whether gene expression for this receptor and IL-13Rα1 were affected by exposure to 10 ng/ml of IL-13 for 24 hours. We also examined the effects of IFN-γ on the expression of both IL-13 receptor subunits in light of the evidence that this Th1 cytokine can regulate cellular responsiveness to IL-13. 37 As shown in Figure 2 ▶ , IL-13Rα1 gene expression was similar in the fibroblast cell lines under basal conditions and cytokine treatment. IL-4Rα was mostly expressed in type 1 and type 2 fibroblasts compared to normal fibroblasts. In addition, exposure to IFN-γ and IL-13 for 24 hours did not dramatically effect IL-4Rα gene expression in type 1 and type 2 fibroblasts. However, either cytokine treatment of normal fibroblasts appeared to lower IL-4Rα gene expression. These data suggested that IL-4Rα expression varied between normal and granuloma fibroblasts. Whereas, IL-13Rα1 gene expression is constitutively expressed on all fibroblast cell populations and cytokine treatment does not alter its constitutive expression.
Figure 2.
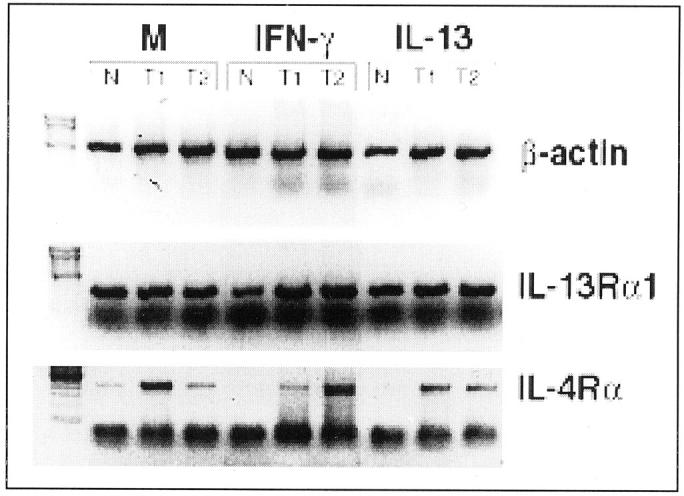
RT-PCR analysis of β-actin, IL-13Rα1, and IL-4Rα gene expression in tissue cultures of primary normal (N), Th1-type (T1), and Th2-type (T2) fibroblast lines. Gene expression of IL-13Rα1 was constitutively expressed on all three fibroblast populations whereas IL-4Rα expression varied between normal, type 1, and type 2 fibroblast populations.
IL-13 Modulates the Gene Expression of IL-13Rα2 in Th1-Type and Th2-Type Fibroblasts
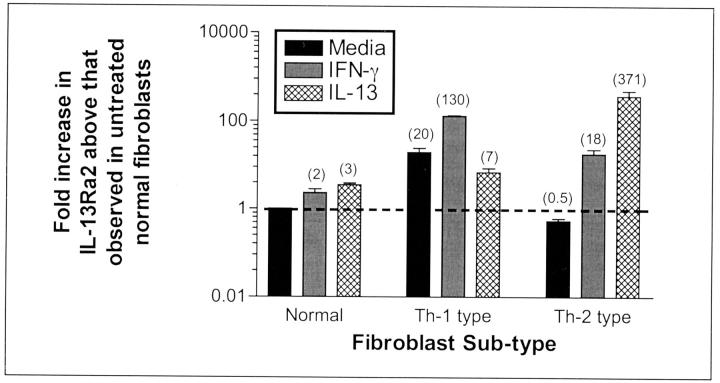
IL-13Rα2 is a nonsignaling decoy receptor 25 that has been previously described in cultured human synovial fibroblasts. 23 IL-13Rα2 acts as a potent inhibitor of IL-13 binding to its cell surface receptor leading to the initial suggestion 24 and later demonstration 26,27 that it modulates the effects of IL-13 in vivo. The results from a quantitative analysis of IL-13Rα2 gene expression using TaqMan real-time PCR are summarized in Figure 3 ▶ . The IL-13Rα2 gene expression in untreated normal fibroblasts was assigned an arbitrary value of 1 and the fold increases in IL-13Rα2 gene greater than this baseline are shown for each fibroblast population and its corresponding treatment. In normal fibroblasts, the exogenous addition of either IFN-γ or IL-13 (both at 10 ng/ml) for 24 hours modestly increased IL-13Rα2 gene expression between twofold and threefold. In Th1-type fibroblasts, the expression of IL-13Rα2 was increased 20-fold more than that measured in normal fibroblasts under similar baseline conditions. The exogenous addition of IFN-γ increased whereas exogenous IL-13 decreased the gene expression of IL-13Rα2 in type 1 fibroblasts. Untreated type 2 fibroblasts expressed 50% less IL-13Rα2 gene expression compared with untreated normal fibroblasts. Interestingly, the exogenous addition of either IFN-γ or IL-13 markedly increased IL-13Rα2 gene expression 18- and 371-fold, respectively, more than that detected in untreated normal fibroblasts. Taken together, these data showed that IL-13 decoy receptor gene expression differed markedly between the normal, type 1, and type 2 fibroblasts, and these cells exhibited divergent expression of this receptor in response to IFN-γ and IL-13.
Figure 3.
Real-time TaqMan PCR in tissue cultures of primary normal, Th1-type, and Th2-type fibroblast lines. Numbers in parentheses indicate fold increases in IL-13Rα2 gene expression in normal, Th1-type, and Th2-type fibroblasts with or without cytokine treatment above IL-13Rα2 gene expression in untreated normal fibroblasts. IFN-γ or IL-13 treatments had little effect on the gene expression of IL-13Rα2 in normal fibroblasts, but both cytokines markedly altered the expression of this IL-13 receptor subunit in Th1- and Th2-type fibroblasts.
Immunohistochemical Localization of IL-4Rα and IL-13Rα2 on Normal, Th1-Type, and Th2-Type Fibroblasts
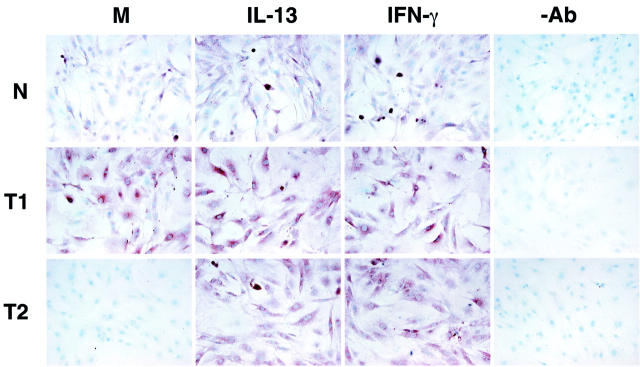
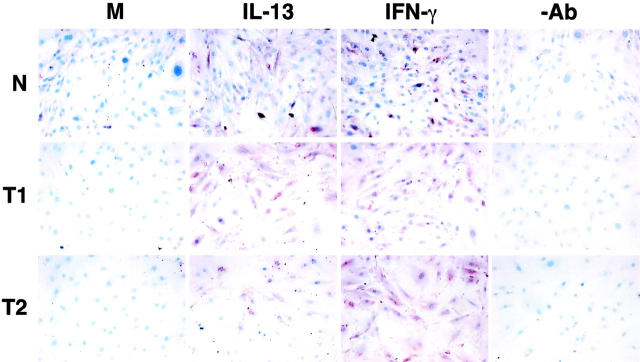
Immunohistochemical analysis of IL-4Rα and IL-13Rα2 protein expression confirmed that normal, type 1, and type 2 fibroblasts expressed both receptor subunits of the IL-13 receptor. Because of the lack of a specific antibody directed against mouse IL-13Rα1, we were unable to examine the expression of this receptor subunit in the fibroblast cell lines. Coinciding with the RT-PCR analysis, Th1-type (T1) fibroblasts strongly expressed IL-4Rα under baseline (M) conditions unlike the normal (N) and type 2 (T2) fibroblast lines (Figure 4) ▶ . IL-4Rα protein expression did not seem to be altered in type 1 fibroblasts after a 24-hour exposure to either IFN-γ or IL-13. In contrast, the IFN-γ and IL-13 treatments markedly enhanced the expression of IL-4Rα protein in normal fibroblasts, and both cytokines strongly up-regulated this receptor in type 2 fibroblasts (Figure 4) ▶ . As summarized in Figure 5 ▶ , no constitutive fibroblast-associated IL-13Rα2 protein expression was noted in normal (N), type 1 (T1), or type 2 (T2) fibroblasts. Exposure of normal fibroblasts to either 10 ng/ml of IFN-γ or IL-13 for 24 hours induced IL-13Rα2 protein expression on all fibroblast populations. This immunohistochemical analysis confirmed that normal, type 1, and type 2 fibroblasts exhibited cell-associated expression of IL-4Rα and IL-13Rα2 and the expression of both subunits in these cells were altered by the exogenous addition of either IFN-γ or IL-13.
Figure 4.
Immunohistochemical analysis of IL-4Rα in tissue cultures of primary normal (N), Th1-type (T1), and Th2-type (T2) fibroblast lines. T1 fibroblasts strongly expressed IL-4Rα under basal conditions (M) compared to N and T2 fibroblasts. Cytokine treatments enhanced IL-4Rα protein expression on all three fibroblast populations. −Ab is an abbreviation representing the control antibody used.
Figure 5.
Immunohistochemical analysis of IL-13Rα2 in tissue cultures of primary normal (N), Th1-type (T1), and Th2-type (T2) fibroblast lines. Protein expression of IL-13αR2 under basal conditions (M) was not present in any of the fibroblast populations. Cytokine treatments induced IL-13Rα2 protein expression on all three fibroblast populations. −Ab is an abbreviation representing the control antibody used.
Contrasting Effects of IL-13 and IL13-PE on the Proliferation of Normal, Th1-Type, and Th2-Type Fibroblasts
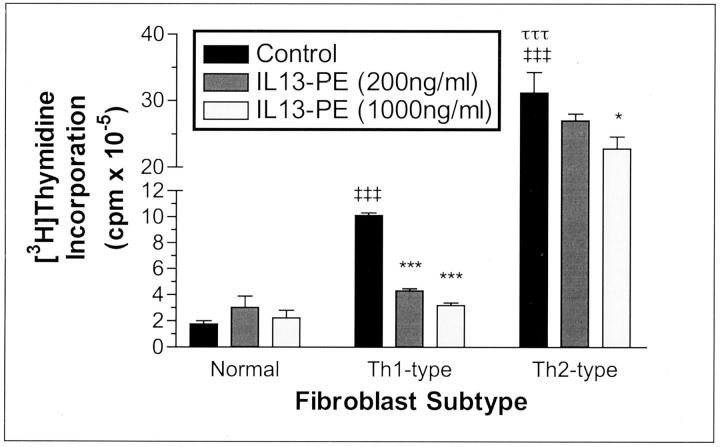
Exaggerated, uncontrolled fibroblast proliferation is a prominent feature of experimental Th2-type granulomatous responses and IPF for which no adequate therapies currently exist. 38 In light of the paucity of therapeutics that effectively regulate fibroblast proliferation, we examined in the present study whether lung fibroblast proliferation could be modulated using an IL-13-based immunotoxin approach. 39 IL13-PE has been previously used to target a number of cancerous cell types in vitro 40 and in vivo. 41,42 More recently we have observed that IL13-PE significantly reduces peribronchial 29 and granulomatous 31 fibrosis in vivo. In the present study, we observed that IL13-PE treatment had a dose-dependent inhibitory effect on the proliferation of type 1 and 2, but not normal, fibroblasts (Figure 6) ▶ . IL13-PE had the greater inhibitory effect on Th1-type fibroblasts as evidenced by the 57% decrease in type 1 fibroblast proliferation after exposure to 200 ng/ml of IL13-PE and a larger 68% decrease in the proliferation of these fibroblasts after exposure to 1000 ng/ml of IL13-PE. Taking into consideration that the [3H]thymidine incorporation by type 2 fibroblasts exposed to media alone was ∼189-fold and fourfold higher than that observed in similarly exposed normal and type 1 fibroblasts, respectively, the reduction in type 2 fibroblast proliferation was ∼13% at the lower dose of IL13-PE and 26% at the higher dose of IL13-PE. These data highlighted that even in the context of aggressive fibroblast proliferation as observed in type 2 fibroblasts, IL13-PE significantly inhibited fibroblast growth.
Figure 6.
Dose-dependent effect of IL13-PE on cellular proliferation in tissue cultures of primary normal, Th1-type, and Th2-type fibroblast lines. Fibroblasts were incubated for 24 hours with DMEM-15 alone (control), DMEM-15 plus 200 ng/ml of IL13-PE, or DMEM-15 plus 1000 ng/ml of IL13-PE. Four hours before the conclusion of the experiment, 10 μCi of [3H] thymidine was added to each well. IL13-PE treatment significantly inhibited, in a dose-dependent manner, the proliferation of Th1- and Th2-type but not normal fibroblasts. ‡‡‡, P ≤ 0.001 compared with untreated (media alone) normal fibroblasts. *, P ≤ 0.05; ***, P ≤ 0.001 compared with untreated Th1- or Th2-type fibroblasts.
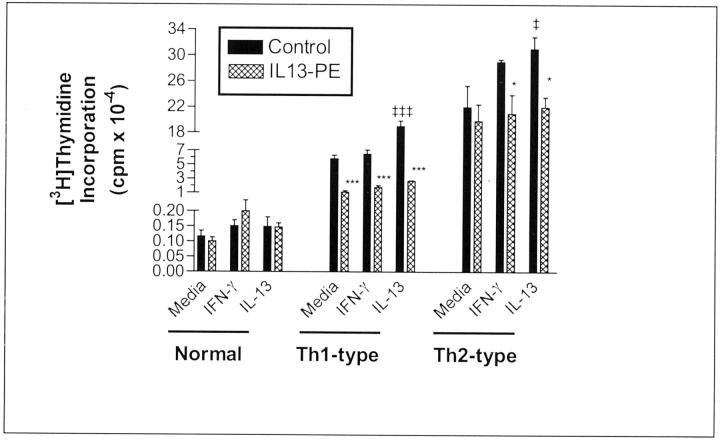
Further examination of IL13-PE’s inhibitory effect on fibroblast proliferation is summarized in Figure 7 ▶ . In these experiments, normal, type 1, and type 2 fibroblasts were exposed to media alone or media plus IFN-γ, media plus IL-13 (both cytokines at 10 ng/ml) for 24 hours before the addition of 200 ng/ml of IL13-PE for an additional 24 hours. Regardless of the culture conditions, IL13-PE did not affect [3H]thymidine incorporation by normal fibroblasts. Exposure of type 1 fibroblasts to IL-13, but not IFN-γ, significantly increased the proliferation of these cells. The inclusion of IL13-PE into cultures of type 1 fibroblasts reduced [3H]thymidine incorporation by 80% (media alone), 71% (IFN-γ treatment), and 85% (IL-13 treatment). In cultures of type 2 fibroblasts, only IL-13, not IFN-γ, treatment significantly increased the proliferation of these cells above the baseline levels. After IFN-γ or IL-13 treatment, IL13-PE reduced fibroblast proliferation by 27% and 28%, respectively. The inclusion of IL13-PE into cultures of Th2-type fibroblasts exposed to media alone reduced their proliferation by 10%. Thus, these data confirmed the inhibitory effect of IL13-PE on the proliferation of type 1 and type 2 fibroblasts.
Figure 7.
Effect of 200 ng/ml of IL13-PE on cellular proliferation in tissue cultures of primary normal, Th1-type, and Th2-type fibroblast lines. Fibroblasts were incubated for 24 hours with 10 ng/ml of IFN-γ or IL-13 for 24 hours before the addition of 100 μl of DMEM-15 alone (control) or the same volume of DMEM-15 containing 200 ng/ml of IL13-PE for another 24 hours. Four hours before the conclusion of the experiment, 10 μCi of [3H] thymidine was added to each well. The proliferation of Th1- and Th2-type but not normal fibroblasts was inhibited after exposure to IL13-PE. ‡‡‡, P ≤ 0.001 compared with Th1-type fibroblasts exposed to media alone. ‡, P ≤ 0.05 compared with Th2-type fibroblasts exposed to media alone. ***, P ≤ 0.001 compared with Th1-type fibroblasts exposed to the same cytokine in the absence of IL13-PE treatment (control). *, P ≤ 0.05 compared with Th2-type fibroblasts exposed to the same cytokine in the absence of IL13-PE treatment (control).
IL13-PE Treatment Significantly Altered Extracellular Matrix-Associated Gene Expression in Normal, Type 1, and Type 2 Fibroblasts
We next examined whether IL13-PE treatment modulated the expression of genes associated with extracellular matrix deposition and degradation using a specific GEArray, and these data are summarized in Table 1 ▶ . Fibrotic lung disease appears to result, in part, from an increase in matrix deposition and a concomitant imbalance in the expression and/or activity of proteases that regulate this process. 43 When compared to normal fibroblasts, procollagen 1 gene expression was increased more in the type 1 and type 2 fibroblasts (Table 1) ▶ . Interestingly, Th1-type fibroblasts exhibited a greater increase in a number of factors that regulate extracellular matrix deposition including thrombospondin-1, urokinase (uPA), tissue inhibitor of metalloproteinase (TIMP-1), and hyaluronidase gene expression compared with normal fibroblasts. In contrast, only hyaluronidase gene expression was increased in Th2-type fibroblasts compared with normal fibroblasts. These data suggested that Th1-type, but not Th2-type, fibroblasts expressed a number of factors critical for modulating the deposition of extracellular matrix. This observation is in keeping with in vivo observations showing that Th1-type granulomas exhibit little fibrosis or fibroblast activation. 34 On examining total soluble collagen levels in cultured normal, type 1, and type 2 fibroblasts, type 2 fibroblasts contained significantly greater quantities of collagen under basal conditions compared with normal and type 1 fibroblasts (data not shown). Furthermore, IL-13 cytokine treatment significantly increased collagen secretion in type 2 fibroblasts compared to the cytokine-treated normal and type 1 fibroblasts.
Table 1.
Extracellular Matrix-Associated Genes Up-Regulated in Th1 (T1)- and Th2 (T2)-Type Fibroblasts Compared with Untreated Normal (N) Fibroblasts
| Gene name | T1 versus N | T2 versus N | N (PE versus media) | T1 (PE versus media) | T2 (PE versus media) |
|---|---|---|---|---|---|
| Collagen 1a1 | 1.6 | 2.3 | 2.6 | 2.8 | 1.5 |
| Fibronectin | 0.6 | 0.9 | 3.0 | 2.2 | 1.3 |
| Collagenase-like A | Down | Down | 0.0 | 0.0 | Up |
| MMP-13 | 0.0 | 0.0 | Up | Up | Up |
| Thrombospondin 1 | 1.7 | 0.3 | 6.5 | 0.0 | 0.1 |
| uPA | Up | 0.0 | 1.0 | 1.9 | Up |
| PAI-1 | 0.6 | Down | 1.2 | 1.0 | 45.6 |
| tPA | 0.7 | 0.2 | 1.0 | 1.5 | 3.2 |
| MMP-3 (stromlysin) | 0.0 | 0.0 | Up | 0.0 | Up |
| MMP-7 (matrilysin) | 0.0 | 0.1 | 0.5 | Up | 56.3 |
| TIMP-1 | 2.3 | 0.5 | 1.2 | 1.3 | 2.0 |
| TIMP-2 | 1.3 | 0.2 | 1.7 | 0.6 | 5.5 |
| Hyaluronidases | Up | Up | Up | 7.8 | Down |
All three fibroblast cell lines were also analyzed in the presence and absence of IL13-PE (PE versus media) treatment. Data shown indicate fold induction in gene expression compared with normal fibroblasts (column 1 and 2) or compared with untreated fibroblasts cell lines (columns 3 to 5).
The treatment of all three fibroblast cell lines for 24 hours with 200 ng/ml of IL13-PE had a profound effect on the expression of a number of extracellular matrix-associated genes (Table 1) ▶ . IL13-PE treatment stimulated increase for matrix metalloproteinase-13 (MMP-13 or interstitial collagenase) gene expression in all three fibroblast cell lines. In addition, collagenase-like A, MMP-3 (also known as stromelysin) and MMP-7 (also known as matrilysin) were markedly increased in type 2 fibroblasts after IL13-PE treatment. Interestingly, the increases in the major endogenous regulators of MMPs, TIMP-1 and TIMP-2 were, by comparison to changes in MMPs, modest. Thrombospondin-1 is a major activator of latent TGF-β, 44 and IL13-PE treatment of type 1 and type 2 fibroblasts down-regulated or inhibited the gene expression of this profibrotic factor. Other major regulators of the pulmonary fibrotic process such as uPA, and tissue plasminogen activator (tPA) and plasminogen activator inhibitor-1 (PAI-1) were selectively up-regulated by IL13-PE in type 2 fibroblasts (Table 1) ▶ . Together, this gene array analysis suggested that IL13-PE strongly up-regulated the gene expression of proteases necessary for the degradation of tissue matrix in all three lung fibroblast lines.
IL13-PE Treatment Significantly Increased Apoptosis-Related Gene Expression in Type 1 and Type 2 Fibroblasts
Recent studies by Kawakami and colleagues 45 showed that IL13-PE treatment induced apoptotic cell death in head and neck cancer cell lines. In the present study, we assessed whether IL13-PE had a similar effect in the three primary lung fibroblast cell lines. Data from specific GEArray apoptosis gene arrays is summarized in Table 2 ▶ . The analysis of changes in gene expression between normal fibroblasts and the type 1 and type 2 fibroblasts revealed some interesting differences between these cell lines. First, it was apparent that both granuloma fibroblasts lines expressed markedly higher gene levels of the proapoptotic factor Bax, whereas gene expression for the anti-apoptotic Bcl-2 was markedly decreased in both lines compared with untreated fibroblasts. Second, the gene expression of the proapoptotic effector caspase-3 was greater than 200-fold higher in type 2 fibroblasts compared with normal fibroblasts. Third, the gene expression for the receptor for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) was increased greater in both granuloma fibroblast lines compared with the normal fibroblast line. Finally, type 1, but not type 2, fibroblasts exhibited ∼60-fold higher gene expression for Fas-ligand (Fas-L) compared with normal fibroblasts. These data suggested that both granuloma fibroblast lines expressed higher gene levels of proapoptotic factors that presumably reflects the augmented activation levels of these cells more than that in normal fibroblasts.
Table 2.
Apoptosis-Associated Genes Up-Regulated in Th1 (T1)- and Th2 (T2)-Type Fibroblasts Compared with Untreated Normal (N) Fibroblasts
| Gene name | T1 versus N | T2 versus N | N (PE versus media) | T1 (PE versus media) | T2 (PE versus media) |
|---|---|---|---|---|---|
| Apaf1 | 0.8 | 2.2 | 0.01 | 1.4 | 8.4 |
| APRIL | 0.04 | 0.03 | 4.5 | 2.5 | 107.9 |
| Bad | 0.1 | 0.2 | 0.0 | 1.7 | 5.4 |
| Bax | Up | Up | 3.8 | 1.8 | 6.2 |
| Bcl2 | 0.04 | 0.2 | 0.4 | 0.2 | 1.2 |
| Bid | Down | Down | 3.2 | Up | Up |
| Casp1 | 0.0 | 0.05 | 0.01 | 64.8 | 132.3 |
| Casp3 | 0.4 | 247.5 | Up | 1.5 | Down |
| Casp8 | 0.2 | 0.7 | 3.8 | 2.4 | 5.6 |
| Casp9 | Down | Down | 3.4 | 1.5 | Up |
| TRAIL-R/DR5 | Up | Up | 3.8 | 1.6 | 13.7 |
| TRAIL | Down | 0.9 | 4.4 | 1.5 | 6.2 |
| Tramp/Apo-3/DDR3 | 0.1 | 1.1 | 2.8 | 1.1 | 0.3 |
| Tnfsf12 (Apo-3L) | 0.01 | Down | 0.04 | 0.5 | Up |
| CD40 | 0.06 | Down | 11.4 | Down | Up |
| Fas antigen | Down | 0.4 | Up | 1.5 | Up |
| Fas ligand | 60 | 0.5 | 3.8 | Up | Up |
| p53 | 0.4 | 0.7 | 1.1 | 1.0 | 8.1 |
All three fibroblast cell lines were also analyzed in the presence and absence of IL13-PE (PE versus media) treatment. Data shown indicate fold induction in gene expression compared with normal fibroblasts (column 1 and 2) or compared with untreated fibroblasts cell lines (columns 3 to 5).
The effects of IL13-PE on gene expression for pro- and anti-apoptotic genes are also summarized in Table 2 ▶ . IL13-PE treatment for 24 hours altered gene expression in all three fibroblast lines, but the greatest changes were observed in the type 2 fibroblast cells. For example, several of the genes involved in the Fas/tumor necrosis factor proapoptotic pathway were up-regulated in normal, type 1, and type 2 fibroblasts exposed to IL13-PE. Although, two anti-apoptotic factors including APRIL (a proliferation-inducing ligand) and CD40 were also markedly increased in type 2 fibroblasts after IL13-PE treatment, Bcl-2 gene expression was modestly affected in these cells after the same treatment. However, Bcl-2 gene expression was decreased in normal and type 1 fibroblasts after IL13-PE treatment compared with the appropriate untreated fibroblast line (Table 2) ▶ . Thus, it was apparent that the gene expression for proapoptotic factors was increased in type 1 and type 2 fibroblasts compared with control fibroblasts, and the IL13-PE treatment further increased the expression of a number of proapoptotic factors in all three fibroblast lines, but this increase was much more dramatic in the Th2-type fibroblasts. However, IL13-PE-treated Th2-type fibroblasts also exhibited marked gene expression for two potentially important anti-apoptotic factors.
Discussion
IL-13 has recently joined the ranks of immunomodulatory cytokines that exert major profibrotic effects in the lung 8,29,46-48 and liver. 26,27 The profibrotic effect of IL-13 in the lung is postulated to involve irreversible fibroblast activation, triggered either directly 14,49 or indirectly through TGF-β. 8,50 Regardless of its mode of action, a recently published Affymetrix gene array analysis of human lung fibroblasts demonstrated that IL-13 has major stimulatory effects on a multitude of genes that contribute to lung remodeling or fibrotic events. 51 Considering that our previous studies showed that primary fibroblast cell lines derived from normal mouse lung, a Th1-type (type 1) pulmonary granulomatous response and a Th2-type (type 2) pulmonary granulomatous response differentially respond to the exogenous addition of IL-4, 33,34 in the present study we primarily examined the impact of IL-13 on the synthetic and proliferative properties on these cell lines. The data provided herein show that receptor subunits that recognize and bind IL-13, namely IL-4Rα and IL-13Rα2, are expressed at varying levels in all three lung fibroblast cell lines, whereas IL-13Rα1 is constitutively expressed in all three cell lines. The expression of these receptor subunits was demonstrated at the gene and protein level. Unlike normal fibroblasts, both type 1 and type 2 fibroblast cell lines generated significantly greater amounts of collagen and proliferated much more aggressively in response to IL-13 (data not shown). Among the fibroblast lines examined, type 2 fibroblasts exhibited the greatest baseline collagen generation and cell proliferation rate, presumably reflecting the highly fibrotic nature of the S. mansoni-induced granulomatous response. 26,27 Most importantly, the present study showed that IL13-PE, an IL-13 immunotoxin, effectively inhibited the exuberant synthetic and proliferative properties of type 1 and type 2 but not normal fibroblasts. Thus, the present study demonstrated that major functions of lung fibroblasts are altered by IL-13, particularly after these cells have participated in inflammatory events characterized by little (ie, type 1) or copious (ie, type 2) lung fibrosis.
The high-affinity IL-13Rα2, in its monomeric form, appears to be a silent or decoy IL-13 receptor 23 and these properties have been exploited therapeutically to inhibit hepatic fibrosis because of S. mansoni eggs. 26 In the present study, we observed that IL-13Rα2 protein was not constitutively expressed on the cell surface of any of the fibroblast cell lines, despite the increased gene expression for this receptor subunit in type 1 compared with untreated normal fibroblasts. We also observed a discrepancy between our TaqMan PCR analysis showing that IL-13Rα2 gene expression was strongly induced by IL-13 compared to IFN-γ in type 2 fibroblasts and the immunohistochemical analysis showed the opposite IL-13Rα2 protein profile. One explanation for these discrepancies may relate to the propensity of mouse IL-13Rα2, but not human IL-13Rα2, to be released in a soluble form from the cells that express it. 25 Accordingly, untreated type 2 cells may release soluble IL-13Rα2 on gene translation. It is also conceivable that the release of IL-13Rα2 by the untreated or cytokine-stimulated cells may in turn lead to their dramatic up-regulation in IL-13Rα2 gene expression. Previous studies have shown that IL-13Rα2 protein is rapidly mobilized from intracellular stores to the cell surface after cellular exposure to IFN-γ. 37 This possibility is the focus of additional studies addressing the regulation of IL-13Rα2 protein expression and release by lung fibroblasts.
Previous studies have documented that fibroblasts from various tissue sources express functional IL-13 receptor(s) that promote the induction of cytokines (ie, IL-6, granulocyte colony stimulating factor, granulocyte macrophage colony stimulating factor, and TGF-β) and adhesion molecules (ie, β1 integrin and VCAM-1). 14,16,22 IL-13 has also been shown to induce gene expression for procollagen 3α1, 49 and to inhibit cytokine-induced MMP-1 and MMP-3 production while it concomitantly enhanced TIMP-1 generation. 49 Using GEArray gene chip analysis, we observed that all three fibroblast cell lines differed in their expression of extracellular matrix-associated genes both before and after IL13-PE treatment. Recent studies have documented that IL-13 is a potent inducer of collagen production in vivo in the lung 8 and in vitro in skin and keloid fibroblasts. 49 Previous in vitro studies have also shown that IL-13 inhibits proteases that degrade extracellular matrix (ie, MMP-1 and MMP-3) while enhancing specific tissue inhibitors of these proteases (ie, TIMP-1 and TIMP-2). 49 This imbalance in protease regulation by the pulmonary fibroblast is thought to favor the excess accumulation of extracellular matrix during pulmonary fibrotic events. 52 In the present study it was apparent that IL13-PE treatment markedly enhanced the gene expression of a number of extracellular matrix-specific proteases including collagenases, matrix metalloproteinases, and hyaluronidases, but this fusion protein also up-regulated a number of tissue inhibitors or regulators of these factors such as TIMP-1 and TIMP-2 in type 2 fibroblasts. IL13-PE treatment of both type 1 and type 2 fibroblasts down-regulated the gene expression of the TGF-β-activating factor thrombospondin-1. Thrombospondin-1 is prominently expressed in the lungs of IPF patients. 53 The significance of the concomitant up-regulation of pro- and anti-fibrotic factors after IL13-PE treatment is not appreciated at present but it is possible that the increased activity of metalloproteinases would prevent or reverse the increased deposition of extracellular matrix, particularly that associated with Th2-type lung diseases. 32 A balance of pro- and anti-fibrotic proteases is critical because excess protease 54 or anti-protease 55 activity has been associated with excessive pulmonary fibrosis induced by the anti-neoplastic drug bleomycin. Furthermore, the in vivo profibrotic effects of IL-13 observed in IL-13 transgenic mice was dependent on the activity of extracellular matrix proteases. 8 Several genes associated with the plasminogen activation system were also examined in the present study. This system is comprised of a number of factors including uPA, tPA, and PAI, and it is well established that the activation of these factors is necessary to maintain the fibrinolytic system in the lung, and any disruption to the normal activity of the fibrinolytic system seems to exacerbate experimental fibrosis. 56,57 Interestingly, uPA gene expression was up-regulated in type 1 fibroblasts compared with normal and type 2 fibroblasts, but the treatment of type 2 fibroblasts with IL13-PE induced uPA gene expression from that observed in untreated Th2-type fibroblast. Thus, the predominate effect of IL13-PE on the cultured fibroblast cell lines, particularly the type 2 line, was the induction of potent regulators/modulators of extracellular matrix deposition suggesting that this immunotoxin may provide clear beneficial effects in the context of pathological fibrotic events in the lung.
A number of previous studies suggest that the dysregulation of apoptotic pathways may explain, in part, the pathogenesis of a number of chronic lung diseases including IPF. 58 Our interest in the effect of IL13-PE on the induction of genes associated with apoptotic pathways in lung fibroblasts stemmed from a previous study in which it was shown that IL-13 immunotoxin induced cytotoxicity in tumor cell lines via its ability to up-regulate proapoptotic and down-regulate anti-apoptotic factors in these cells. 45 In this previous study, it was observed that Bax (a proapoptotic factor) was up-regulated while Bcl-2 (a suppressor of cell death) was down-regulated in these tumor lines after their exposure to IL13-PE. 45 The GEArray analysis of untreated normal, type 1, and type 2 fibroblasts revealed some intriguing features of the granuloma fibroblasts. Compared with normal fibroblasts, Th1- and Th2-type fibroblasts expressed markedly greater levels of genes encoding for Bax and TRAIL receptor. The TRAIL receptor binds the tumor necrosis factor-related proapoptotic ligand TRAIL, a potent inducer of fibroblast apoptosis. 59 Also of note was the marked level of FasL gene expression in Th1-type, but not Th2-type, fibroblasts compared with normal fibroblasts. In the lung, it is thought that cells up-regulate FasL to monitor and regulate local immune activity. 58 The inclusion of IL13-PE into cultures of lung fibroblasts markedly up-regulated the expression of a number of proapoptotic factors, but the most pronounced changes were observed in cultures of type 2 fibroblasts. Examples of important proapoptotic factors in fibroblasts include Apaf-1 60 and its co-factor caspase 9, 61 and p53, 61 and all three genes that were increased in type 2 cells, but not in the other two fibroblast lines after IL13-PE treatment. Overall, it appeared that IL13-PE was a potent inducer of apoptosis-related genes in Th2-type fibroblasts, but it was apparent from the proliferation studies that these cells were much less susceptible to the inhibitory effects of the fusion cytotoxin compared with the Th1-type fibroblasts. Further investigation is underway to explore this resistance in greater detail, and we will be focusing on two anti-apoptotic factors that are strongly or selectively induced by IL13-PE in Th2-type fibroblasts: APRIL 62 and CD40. 63 APRIL confers protection from TRAIL-induced apoptosis whereas CD40 confers protection from tumor necrosis factor-α- and Fas-induced apoptosis. 63,64 Our previous studies of untreated Th2-type fibroblasts have revealed that the surface expression of CD40 is 100-fold higher on these cells compared with that expressed on normal and Th1-type fibroblasts (unpublished data).
In conclusion, these findings demonstrate the impact of IL-13 on the synthetic and proliferative properties of granuloma fibroblasts. Despite the activation of these cells in very different cytokine milieus in the lung, IL-13 affected their generation of collagen, proliferation and increased the receptor gene expression for the high-affinity IL-13 receptor subunit, IL13Rα2. The responsiveness of these cells to IL-13 also meant that these cells could be targeted directly with an IL-13 immunotoxin. Given the encouraging clinical findings from the use of cytokine immunotoxins in the treatments of cancer, 39 it is conceivable that IL13-PE may also be a relevant therapy in the treatment of debilitating lung fibrotic diseases such as IPF.
Footnotes
Address reprint requests to Dr. Cory M. Hogaboam, Ph.D., Department of Pathology, University of Michigan Medical School, 1301 Catherine Rd., Ann Arbor MI 48109-0602. E-mail: hogaboam@med.umich.edu.
Supported by the National Institutes of Health and the Biomedical Research Council of the University of Michigan Medical School.
References
- 1.Lasky JA, Brody AR: Interstitial fibrosis and growth factors. Environ Health Perspect 2000, 108(Suppl 4):751-762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen JT, Spiteri MA: Growth factors in idiopathic pulmonary fibrosis: relative roles. Respir Res 2002, 3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coker RK, Laurent GJ: Pulmonary fibrosis: cytokines in the balance. Eur Respir J 1998, 11:1218-1221 [DOI] [PubMed] [Google Scholar]
- 4.Broekelmann TJ, Limper AH, Colby TV, McDonald JA: Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci USA 1991, 88:6642-6646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coker RK, Laurent GJ, Shahzeidi S, Lympany PA, du Bois RM, Jeffery PK, McAnulty RJ: Transforming growth factors-beta 1, -beta 2, and -beta 3 stimulate fibroblast procollagen production in vitro but are differentially expressed during bleomycin-induced lung fibrosis. Am J Pathol 1997, 150:981-991 [PMC free article] [PubMed] [Google Scholar]
- 6.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J: Adenovector-mediated gene transfer of active transforming growth factor beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest 1997, 100:768-776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao J, Shi W, Wang YL, Chen H, Bringas P, Jr, Datto MB, Frederick JP, Wang XF, Warburton D: Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Physiol 2002, 282:L585-L593 [DOI] [PubMed] [Google Scholar]
- 8.Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, Senior RM, Elias JA: Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1). J Exp Med 2001, 194:809-821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKenzie GJ, Bancroft A, Grencis RK, McKenzie AN: A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr Biol 1998, 8:339-342 [DOI] [PubMed] [Google Scholar]
- 10.Hancock A, Armstrong L, Gama R, Millar A: Production of interleukin 13 by alveolar macrophages from normal and fibrotic lung. Am J Respir Cell Mol Biol 1998, 18:60-65 [DOI] [PubMed] [Google Scholar]
- 11.Wallace WA, Ramage EA, Lamb D, Howie SE: A type 2 (Th2-like) pattern of immune response predominates in the pulmonary interstitium of patients with cryptogenic fibrosing alveolitis (CFA). Clin Exp Immunol 1995, 101:436-441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace WA, Howie SE: Immunoreactive interleukin 4 and interferon-gamma expression by type II alveolar epithelial cells in interstitial lung disease. J Pathol 1999, 187:475-480 [DOI] [PubMed] [Google Scholar]
- 13.Lukacs NW, Hogaboam C, Chensue SW, Blease K, Kunkel SL: Type 1/type 2 cytokine paradigm and the progression of pulmonary fibrosis. Chest 2001, 120:5S-8S [DOI] [PubMed] [Google Scholar]
- 14.Doucet C, Brouty-Boye D, Pottin-Clemenceau C, Jasmin C, Canonica GW, Azzarone B: IL-4 and IL-13 specifically increase adhesion molecule and inflammatory cytokine expression in human lung fibroblasts. Int Immunol 1998, 10:1421-1433 [DOI] [PubMed] [Google Scholar]
- 15.Murata T, Husain SR, Mohri H, Puri RK: Two different IL-13 receptor chains are expressed in normal human skin fibroblasts, and IL-4 and IL-13 mediate signal transduction through a common pathway. Int Immunol 1998, 10:1103-1110 [DOI] [PubMed] [Google Scholar]
- 16.Doucet C, Brouty-Boye D, Pottin-Clemenceau C, Canonica GW, Jasmin C, Azzarone B: Interleukin (IL) 4 and IL-13 act on human lung fibroblasts. Implication in asthma. J Clin Invest 1998, 101:2129-2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lutz RA, Feng N, Moser R: Binding of interleukin-13 and interleukin-4 to the interleukin (IL)-4/IL-13 receptor of human synovial fibroblasts. J Recept Signal Transduct Res 1999, 19:181-190 [DOI] [PubMed] [Google Scholar]
- 18.Murata T, Taguchi J, Puri RK, Mohri H: Sharing of receptor subunits and signal transduction pathway between the IL-4 and IL-13 receptor system. Int J Hematol 1999, 69:13-20 [PubMed] [Google Scholar]
- 19.Murata T, Obiri NI, Debinski W, Puri RK: Structure of IL-13 receptor: analysis of subunit composition in cancer and immune cells. Biochem Biophys Res Commun 1997, 238:90-94 [DOI] [PubMed] [Google Scholar]
- 20.Obiri NI, Debinski W, Leonard WJ, Puri RK: Receptor for interleukin 13. Interaction with interleukin 4 by a mechanism that does not involve the common gamma chain shared by receptors for interleukins 2, 4, 7, 9, and 15. J Biol Chem 1995, 270:8797-8804 [DOI] [PubMed] [Google Scholar]
- 21.Chomarat P, Banchereau J: Interleukin-4 and interleukin-13: their similarities and discrepancies. Int Rev Immunol 1998, 17:1-52 [DOI] [PubMed] [Google Scholar]
- 22.Schlaak JF, Schwarting A, Knolle P, Meyer zum Buschenfelde KH, Mayet W: Effects of Th1 and Th2 cytokines on cytokine production and ICAM-1 expression on synovial fibroblasts. Ann Rheum Dis 1995, 54:560-565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng N, Lugli SM, Schnyder B, Gauchat JF, Graber P, Schlagenhauf E, Schnarr B, Wiederkehr-Adam M, Duschl A, Heim MH, Lutz RA, Moser R: The interleukin-4/interleukin-13 receptor of human synovial fibroblasts: overexpression of the nonsignaling interleukin-13 receptor alpha2. Lab Invest 1998, 78:591-602 [PubMed] [Google Scholar]
- 24.Zhang JG, Hilton DJ, Willson TA, McFarlane C, Roberts BA, Moritz RL, Simpson RJ, Alexander WS, Metcalf D, Nicola NA: Identification, purification, and characterization of a soluble interleukin (IL)-13-binding protein. Evidence that it is distinct from the cloned IL-13 receptor and IL-4 receptor alpha-chains. J Biol Chem 1997, 272:9474-9480 [DOI] [PubMed] [Google Scholar]
- 25.Donaldson DD, Whitters MJ, Fitz LJ, Neben TY, Finnerty H, Henderson SL, O’Hara RM, Jr, Beier DR, Turner KJ, Wood CR, Collins M: The murine IL-13 receptor alpha 2: molecular cloning, characterization, and comparison with murine IL-13 receptor alpha 1. J Immunol 1998, 161:2317-2324 [PubMed] [Google Scholar]
- 26.Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA: An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest 1999, 104:777-785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiaramonte MG, Cheever AW, Malley JD, Donaldson DD, Wynn TA: Studies of murine schistosomiasis reveal interleukin-13 blockade as a treatment for established and progressive liver fibrosis. Hepatology 2001, 34:273-282 [DOI] [PubMed] [Google Scholar]
- 28.Kawakami K, Taguchi J, Murata T, Puri RK: The interleukin-13 receptor alpha2 chain: an essential component for binding and internalization but not for interleukin-13-induced signal transduction through the STAT6 pathway. Blood 2001, 97:2673-2679 [DOI] [PubMed] [Google Scholar]
- 29.Blease K, Jakubzick C, Schuh JM, Joshi BH, Puri RK, Hogaboam CM: IL-13 fusion cytotoxin ameliorates chronic fungal-induced allergic airway disease in mice. J Immunol 2001, 167:6583-6592 [DOI] [PubMed] [Google Scholar]
- 30.Blease K, Schuh J, Jakubzick C, Lukacs NW, Kunkel SL, Joshi BH, Puri RK, Kaplan MH, Hogaboam CM: Stat6-deficient mice develop airway hyperresponsiveness and peribronchial fibrosis during chronic fungal asthma. Am J Pathol 2002, 160:481-490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jakubzick C, Kunkel SL, Lukacs NW, Joshi BH, Puri RK, Hogaboam CM: Interleukin-13 fusion cytotoxin arrests Schistosoma mansoni egg-induced pulmonary granuloma formation in mice. Am J Pathol 2002, 161:1283-1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunkel SL, Lukacs NW, Strieter RM, Chensue SW: Th1 and Th2 responses regulate experimental lung granuloma development. Sarcoidosis Vasc Diffuse Lung Dis 1996, 13:120-128 [PubMed] [Google Scholar]
- 33.Hogaboam CM, Bone-Larson CL, Lipinski S, Lukacs NW, Chensue SW, Strieter RM, Kunkel SL: Differential monocyte chemoattractant protein-1 and chemokine receptor 2 expression by murine lung fibroblasts derived from Th1- and Th2-type pulmonary granuloma models. J Immunol 1999, 163:2193-2201 [PubMed] [Google Scholar]
- 34.Hogaboam CM, Gallinat CS, Bone-Larson C, Chensue SW, Lukacs NW, Strieter RM, Kunkel SL: Collagen deposition in a non-fibrotic lung granuloma model after nitric oxide inhibition. Am J Pathol 1998, 153:1861-1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith RE, Hogaboam CM, Strieter RM, Lukacs NW, Kunkel SL: Cell-to-cell and cell-to-matrix interactions mediate chemokine expression: an important component of the inflammatory lesion. J Leukoc Biol 1997, 62:612-619 [DOI] [PubMed] [Google Scholar]
- 36.Murata T, Noguchi PD, Puri RK: Receptors for interleukin (IL)-4 do not associate with the common gamma chain, and IL-4 induces the phosphorylation of JAK2 tyrosine kinase in human colon carcinoma cells. J Biol Chem 1995, 270:30829-30836 [DOI] [PubMed] [Google Scholar]
- 37.Daines MO, Hershey GK: A novel mechanism by which interferon-gamma can regulate interleukin (IL)-13 responses. Evidence for intracellular stores of IL-13 receptor alpha-2 and their rapid mobilization by interferon-gamma. J Biol Chem 2002, 277:10387-10393 [DOI] [PubMed] [Google Scholar]
- 38.Kuwano K, Hagimoto N, Hara N: Molecular mechanisms of pulmonary fibrosis and current treatment. Curr Mol Med 2001, 1:551-573 [DOI] [PubMed] [Google Scholar]
- 39.Puri RK: Cytotoxins directed at interleukin-4 receptors as therapy for human brain tumors. Methods Mol Biol 2001, 166:155-176 [DOI] [PubMed] [Google Scholar]
- 40.Debinski W, Obiri NI, Powers SK, Pastan I, Puri RK: Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and pseudomonas exotoxin. Clin Cancer Res 1995, 1:1253-1258 [PubMed] [Google Scholar]
- 41.Husain SR, Puri RK: Interleukin-13 fusion cytotoxin as a potent targeted agent for AIDS-Kaposi’s sarcoma xenograft. Blood 2000, 95:3506-3513 [PubMed] [Google Scholar]
- 42.Kawakami K, Joshi BH, Puri RK: Sensitization of cancer cells to interleukin 13-pseudomonas exotoxin-induced cell death by gene transfer of interleukin 13 receptor alpha chain. Hum Gene Ther 2000, 11:1829-1835 [DOI] [PubMed] [Google Scholar]
- 43.Swiderski RE, Dencoff JE, Floerchinger CS, Shapiro SD, Hunninghake GW: Differential expression of extracellular matrix remodeling genes in a murine model of bleomycin-induced pulmonary fibrosis. Am J Pathol 1998, 152:821-828 [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy-Ullrich JE, Poczatek M: Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev 2000, 11:59-69 [DOI] [PubMed] [Google Scholar]
- 45.Kawakami M, Kawakami K, Puri RK: Apoptotic pathways of cell death induced by an interleukin-13 receptor-targeted recombinant cytotoxin in head and neck cancer cells. Cancer Immunol Immunother 2002, 50:691-700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA: Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest 1999, 103:779-788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu Z, Ma B, Zheng T, Homer RJ, Lee CG, Charo IF, Noble P, Elias JA: IL-13-induced chemokine responses in the lung: role of CCR2 in the pathogenesis of IL-13-induced inflammation and remodeling. J Immunol 2002, 168:2953-2962 [DOI] [PubMed] [Google Scholar]
- 48.Blease K, Jakubzick C, Westwick J, Lukacs N, Kunkel SL, Hogaboam CM: Therapeutic effect of IL-13 immunoneutralization during chronic experimental fungal asthma. J Immunol 2001, 166:5219-5224 [DOI] [PubMed] [Google Scholar]
- 49.Oriente A, Fedarko NS, Pacocha SE, Huang SK, Lichtenstein LM, Essayan DM: Interleukin-13 modulates collagen homeostasis in human skin and keloid fibroblasts. J Pharmacol Exp Ther 2000, 292:988-994 [PubMed] [Google Scholar]
- 50.Richter A, Puddicombe SM, Lordan JL, Bucchieri F, Wilson SJ, Djukanovic R, Dent G, Holgate ST, Davies DE: The contribution of interleukin (IL)-4 and IL-13 to the epithelial-mesenchymal trophic unit in asthma. Am J Respir Cell Mol Biol 2001, 25:385-391 [DOI] [PubMed] [Google Scholar]
- 51.Lee JH, Kaminski N, Dolganov G, Grunig G, Koth L, Solomon C, Erle DJ, Sheppard D: Interleukin-13 induces dramatically different transcriptional programs in three human airway cell types. Am J Respir Cell Mol Biol 2001, 25:474-485 [DOI] [PubMed] [Google Scholar]
- 52.Sempowski GD, Derdak S, Phipps RP: Interleukin-4 and interferon-gamma discordantly regulate collagen biosynthesis by functionally distinct lung fibroblast subsets. J Cell Physiol 1996, 167:290-296 [DOI] [PubMed] [Google Scholar]
- 53.Kuhn C, Mason RJ: Immunolocalization of SPARC, tenascin, and thrombospondin in pulmonary fibrosis. Am J Pathol 1995, 147:1759-1769 [PMC free article] [PubMed] [Google Scholar]
- 54.Zuo F, Kaminski N, Eugui E, Allard J, Yakhini Z, Ben-Dor A, Lollini L, Morris D, Kim Y, DeLustro B, Sheppard D, Pardo A, Selman M, Heller RA: Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci USA 2002, 99:6292-6297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Madtes DK, Elston AL, Kaback LA, Clark JG: Selective induction of tissue inhibitor of metalloproteinase-1 in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 2001, 24:599-607 [DOI] [PubMed] [Google Scholar]
- 56.Swaisgood CM, French EL, Noga C, Simon RH, Ploplis VA: The development of bleomycin-induced pulmonary fibrosis in mice deficient for components of the fibrinolytic system. Am J Pathol 2000, 157:177-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hattori N, Degen JL, Sisson TH, Liu H, Moore BB, Pandrangi RG, Simon RH, Drew AF: Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. J Clin Invest 2000, 106:1341-1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fine A, Janssen-Heininger Y, Soultanakis RP, Swisher SG, Uhal BD: Apoptosis in lung pathophysiology. Am J Physiol 2000, 279:L423-L427 [DOI] [PubMed] [Google Scholar]
- 59.Esser JM, Esser P: Apoptosis mediating ligand-receptor systems in human tenon fibroblasts. Graefes Arch Clin Exp Ophthalmol 2001, 239:320-321 [DOI] [PubMed] [Google Scholar]
- 60.Susin SA, Daugas E, Ravagnan L, Samejima K, Zamzami N, Loeffler M, Costantini P, Ferri KF, Irinopoulou T, Prevost MC, Brothers G, Mak TW, Penninger J, Earnshaw WC, Kroemer G: Two distinct pathways leading to nuclear apoptosis. J Exp Med 2000, 192:571-580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soengas MS, Alarcon RM, Yoshida H, Giaccia AJ, Hakem R, Mak TW, Lowe SW: Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition. Science 1999, 284:156-159 [DOI] [PubMed] [Google Scholar]
- 62.Roth W, Wagenknecht B, Klumpp A, Naumann U, Hahne M, Tschopp J, Weller M: APRIL, a new member of the tumor necrosis factor family, modulates death ligand-induced apoptosis. Cell Death Differ 2001, 8:403-410 [DOI] [PubMed] [Google Scholar]
- 63.Hess S, Gottfried E, Smola H, Grunwald U, Schuchmann M, Engelmann H: CD40 induces resistance to TNF-mediated apoptosis in a fibroblast cell line. Eur J Immunol 1998, 28:3594-3604 [DOI] [PubMed] [Google Scholar]
- 64.Bjorck P, Banchereau J, Flores-Romo L: CD40 ligation counteracts Fas-induced apoptosis of human dendritic cells. Int Immunol 1997, 9:365-372 [DOI] [PubMed] [Google Scholar]