Abstract
To investigate the molecular events that may underpin dysfunctional repair processes that characterize idiopathic pulmonary fibrosis/usual interstitial pneumonia (IPF/UIP), we analyzed the expression patterns of β-catenin on 20 IPF/UIP lung samples, together with two downstream target genes of Wnt signaling, cyclin-D1, and matrilysin. In 18 of 20 cases of IPF/UIP investigated on serial sections, nuclear β-catenin immunoreactivity and abnormal levels of cyclin-D1 and matrilysin were demonstrated in proliferative bronchiolar lesions (basal-cell hyperplasia, squamous metaplasia, bronchiolization, honeycombing). The nature of these lesions was precisely defined using specific markers (ΔN-p63, surfactant-protein-A, cytokeratin-5). Interestingly, nuclear β-catenin accumulation was also demonstrated in fibroblast foci in most (16 of 20) IPF/UIP samples, often associated with bronchiolar lesions. Similar features were not observed in normal lung and other fibrosing pulmonary diseases (diffuse alveolar damage, organizing pneumonia, nonspecific interstitial pneumonia, desquamative interstitial pneumonia). Sequence analysis performed on DNA extracted from three samples of IPF/UIP did not reveal abnormalities affecting the β-catenin gene. On the basis of these findings new models for IPF/UIP pathogenesis can be hypothesized, centered on the aberrant activation of Wnt/β-catenin signaling, with eventual triggering of divergent epithelial regeneration at bronchiolo-alveolar junctions and epithelial-mesenchymal-transitions, leading to severe and irreversible remodeling of the pulmonary tissue.
Idiopathic pulmonary fibrosis/usual interstitial pneumonia (IPF/UIP) is the most common and severe form among idiopathic interstitial pneumonias. 1-4 Many questions regarding this disease still remain unsolved in terms of etiology and natural history, and recent contrasting opinions have raised a stirring discussion regarding its pathogenesis. 5-13 The inflammatory theory of IPF/UIP has been challenged, and new models have been proposed based on the hypothesis that a dysregulated communication between mesenchymal and epithelial pulmonary components after tissue injury is key to the irreversible process of fibrosis and tissue remodeling. 7,9,10 This change in views appears particularly intriguing because it might provide the rationale for new treatment approaches, aimed at contrasting fibroblast proliferation and/or inducing fibroblast apoptosis. 7,14,15 Nevertheless, the centrality of fibroblasts/myofibroblasts in IPF/UIP still remains controversial and unproven, and little is known about the molecular mechanisms involved in the pathogenesis of this disease. Critical arguments regard the epithelial target of early injury, as well as the molecular features characterizing abnormal mesenchymal/epithelial cross-talking. In this regard, we have recently observed that bronchiolar epithelial cells and bronchiolo-alveolar junctions are a relevant target of cell injury in IPF/UIP, and that abnormal bronchiolar proliferations (including hyperplasia, metaplasia, bronchiolization, and honeycombing) may, in fact, represent substantial features of this disease. 16
Much evidence suggests that the study of the molecular pathways regulating lung development and morphogenesis may provide important information regarding the pathogenesis of pulmonary diseases. 8,16 Early phases of lung development are dependent on complex molecular networks that include a series of stimulatory and inhibitory pathways including Fgf, Egf, TGFβ/Activin, Wnt, Hedgehog, Hox, SOX, sprouty, and others. 17-22 It is reasonable to hypothesize that these molecular pathways can in part be recapitulated during postnatal life, because the complex lung architecture can reform precisely after extensive damage, as observed in various pulmonary diseases.
In this study we focused on Wnt signaling and its effector β-catenin for a number of reasons. First, this relevant signaling pathway is involved in lung development and organogenesis in mammals. 19-22 Second, the metalloproteinase matrylisin/MMP-7, which is a target of β-catenin transactivation, has recently been revealed as a key regulator of pulmonary fibrosis. 23-25 Third, the Wnt pathway has been implicated in the pathogenesis of some human fibrosing diseases. 26,27 Finally, direct evidence has been recently provided for a role of β-catenin signaling in the induction of epithelial-mesenchymal transition (EMT), an important process occurring during critical phases of embryonic development, tumor progression, and fibrotic tissue repair after injury. 28-32
It is widely accepted that either activation of the Wnt pathway or abnormalities affecting the β-catenin-transactivating functions can be demonstrated in situ by specific expression patterns of β-catenin accumulation. In particular, cytoplasmic/nuclear accumulation of β-catenin, as shown by immunohistochemistry, represents a reliable means to demonstrate posttranslational stabilization of β-catenin. 33,34 In this study we have investigated the expression patterns of β-catenin in a series of IPF/UIP samples, together with two gene products, cyclin-D1 and matrilysin/MMP-7, whose expression is under β-catenin control. 23,24,35
Materials and Methods
Study Population
The study group consisted of 20 previously untreated patients with clinical, radiographical (chest radiograph and high resolution computed tomography (HRCT)), physiological, and bronchoalveolar lavage findings consistent with the diagnosis of IPF. Histological examination of surgical lung biopsies revealed all of the major features of UIP, according to the recently defined criteria. 1-4 Five samples of normal lung (fragments of unaffected tissue from patients submitted to large excisions for lung carcinoma), two samples of fetal lung (12 and 15 weeks of gestation), 10 samples from patients with organizing pneumonia (OP/BOOP), 4 samples of nonspecific interstitial pneumonia (NSIP), 2 samples of acute interstitial pneumonia with diffuse alveolar damage (AIP/DAD), and 2 samples of desquamative interstitial pneumonia (DIP), defined according to the recent consensus criteria, 4 were analyzed as controls. All samples were fixed in buffered formalin and paraffin-embedded following standard protocols.
Immunohistochemical Staining and Antibodies
All cases were immunostained with a pan-β-catenin monoclonal antibody (clone 15B8; Sigma Chemical Co., St. Louis, MO). Heat-induced antigen retrieval was performed using a microwave oven and 0.01 mol/L of citrate buffer, pH 6.0, for 30 minutes. All samples were processed using a sensitive avidin-streptavidin-peroxidase technique (Biogenex, San Ramon, CA) in a automated cell staining system (GenoMx i6000, BioGenex).
To better define the nature and differentiation level of the epithelial and mesenchymal lesions, we used antibodies recognizing low-molecular weight cytokeratin 8/18/19 (clone 5D3, Biogenex); cytokeratin-5 (CK5, clone XM26; Novocastra, Newcastle, UK) expressed in bronchiolar basal cells; urine protein 1, a rabbit antibody (DAKO, Glostrup, Denmark) recognizing CC10 antigen in Clara cells; SP-A monoclonal antibody (clone PE-10, DAKO) recognizing surfactant protein-A; 1A4 monoclonal antibody (DAKO) recognizing α-smooth muscle actin (no antigen retrieval); TN2 monoclonal antibody (DAKO) recognizing tenascin. In addition, a panel of antibodies was selected to thoroughly investigate the molecular network characterizing lesions expressing nuclear β-catenin: mAb clone 4A4 (Santa Cruz Biotechnology, Santa Cruz, CA), reacting broadly with all known variants of human p63; p40 antibody (Oncogene Research Products, Boston, MA), a polyclonal rabbit antiserum specifically recognizing the truncated ΔN-p63 isoforms lacking the transactivating domain; 36 p53-specific monoclonal antibody (clone DO-1, DAKO); monoclonal antibody recognizing p21WAF1 (clone SX118, DAKO). Details regarding these molecules in normal lung and IPF/UIP samples have been previously described. 16,37 Finally, to better evaluate the function of Wnt/β-catenin pathway activation in IPF/UIP samples we immunohistochemically investigated on serial sections the expression of cyclin-D1 (clone DCS-6; Progen Biot., Heidelberg, Germany) and matrylisin/MMP-7 (clone 141-7B2; Chemicon, Temecula, CA).
Evaluation of Immunostaining
Normal pulmonary tissue structures were used, when present, as internal controls for immunostaining with β-catenin, and only preparations in which normal bronchial/bronchiolar segments were present showing clear-cut membrane-bound β-catenin expression (linear pattern), were considered as suitable for interpretation. Nuclear staining was defined as negative when void nuclei were evident, together with clear-cut membrane immunostaining, and as positive when nuclei were immunoreactive. The nuclear pattern was better evaluated on lightly hematoxylin-counterstained preparations, in which positive nuclei changed their color from blue to brown.
Epithelial lesions were defined as bronchiolar or bronchiolo-alveolar-junctional when the cell phenotype included CK5 and ΔN-p63 expression. 16 Only nuclear staining was interpreted as positive for p63, ΔN-p63, p53, p21WAF1.
Molecular Analysis: β-Catenin Gene Sequencing
Three representative samples of IPF/UIP were used for molecular analysis. DNA was extracted from 10-μm paraffin sections with the DNeasy Tissue kit (Qiagen, Chatsworth, CA). For sequence analysis, Exon 3 of the β-catenin gene was amplified by polymerase chain reaction using primers synthesized with an Applied Biosystem synthesizer (Foster City, CA). Primer sequences (ATTTGATGGAGTTGGACATGGC and CCAGCTACTTGTTCTTGAG TGAAGG) were as previously described. 38 Polymerase chain reaction was performed in a 10-μl standard reaction mixture containing 50 ng of DNA, 5 pmol of each primer, 2 μmol/L dNTPs, 1.5 mmol/L MgCl2, 0.5 U of TaqDNA polymerase (Promega, Madison, WI). For sequence analysis a 50-μl polymerase chain reaction was gel-purified with the QIAEX gel extraction kit (Qiagen). Sequence reactions were performed using the Applied Biosystem Dye Terminator Cycle-Sequencing kit (Perkin Elmer, Foster City, CA) and analyzed on a Applied Biosystem model 373 automated DNA sequencer (Perkin Elmer). Four pilomatrixoma samples harboring β-catenin mutations were included as positive controls. 39
Results
β-Catenin Intracellular Expression Pattern in Normal Adult and Fetal Lung
In the fetal lung β-catenin nuclear immunostaining was demonstrated in budding alveolar structures as previously described (Figure 1a) ▶ . 21 In the normal adult lung, β-catenin expression was strictly confined to cell membranes in all endothelial and epithelial cells, as shown by a clear-cut linear pattern of immunostaining (Figure 1b) ▶ . Nuclear accumulation was evident in a small proportion of cuboidal alveolar cells, recognized as type II pneumocytes by morphology and immunophenotype, characterized by expression of CK8/18/19 and SP-A, and lack of CK5 and ΔN-p63 (not shown).
Figure 1.
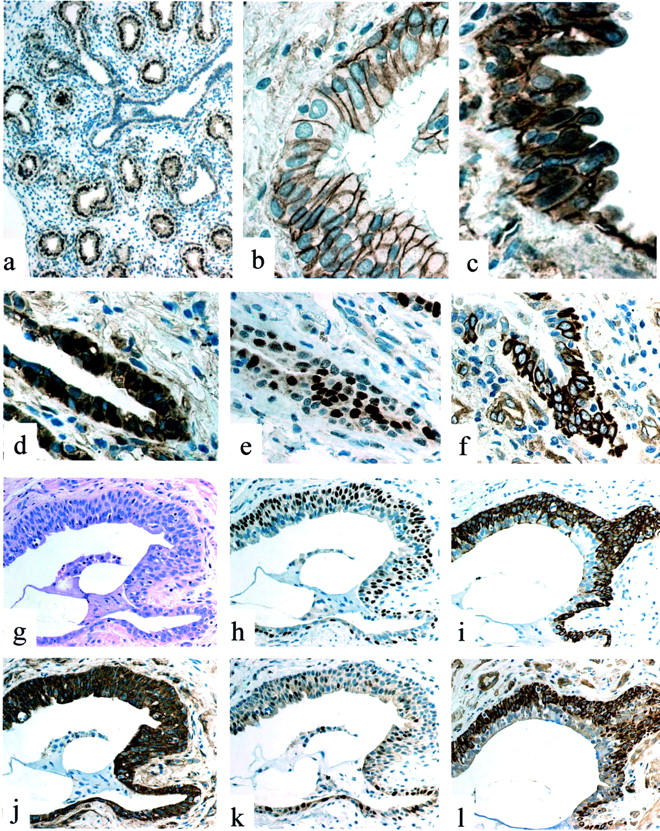
a: Fetal lung (12 weeks): nuclear expression of β-catenin is evident in alveolar buds, but not in airway cells. b: Normal lung: discrete membrane immunoreactivity of β-catenin in basal and ciliated cells in a bronchiole. c: IPF/UIP: aberrant nuclear accumulation of β-catenin in a proliferative bronchiolar lesion. d: IPF/UIP: nuclear expression of β-catenin in basal cells of an abnormal bronchiole. e: IPF/UIP (serial section to d): cyclin-D1-expressing cells. f: IPF/UIP (serial section to d): matrilysin/MMP-7 abnormal expression. g: IPF/UIP: H&E appearance of a small honeycombing bronchiolar lesion. h: IPF/UIP (serial section to g): basal cell hyperplasia as evidenced by ΔN-p63 nuclear expression. i: IPF/UIP (serial section to g): basal cell hyperplasia as evidenced by CK5 expression. j: IPF/UIP (serial section to g): abnormal intracellular expression of β-catenin in both basal and luminal epithelial cells. k: IPF/UIP (serial section to g): increased expression of cyclin-D1. l: IPF/UIP (serial section to g): aberrant expression of matrilysin/MMP-7 in basal cells.
β-Catenin Intracellular Expression Pattern in IPF/UIP Samples
In IPF/UIP patients the number of cells expressing nuclear β-catenin was highly increased, especially in areas where abnormal remodeling of lung architecture was evident.
Bronchiolar Lesions
A striking number of epithelial cells expressing β-catenin nuclear accumulation were demonstrated in proliferative bronchiolar lesions in most (18 of 20) samples (Figure 1; c, d, and j) ▶ . Nuclear β-catenin accumulation was heterogeneously distributed in these abnormal structures, mainly occurring in clusters of hyperplastic basal cells. The presence of nuclear β-catenin was particularly evident in bronchioles exhibiting honeycomb modifications (Figure 1, g and l) ▶ and/or bronchiolization (a process of migrating bronchiolar cells progressively colonizing alveolar spaces). Interestingly, at the same sites nuclear overexpression of p53 and p21waf1 could be demonstrated as previously described. 16 The bronchiolar nature of all these lesions was confirmed on serial sections by the use of antibodies recognizing ΔN-p63 and high-molecular weight cytokeratin CK5 (Figure 1, h and i) ▶ , and by the absence of both surfactant-A and CC10 antigens as previously demonstrated. 16
Alveolar Structures
Cells expressing nuclear β-catenin were found lining damaged alveolar structures, recognized as cuboidal type II pneumocytes by morphology and immunophenotype on serial sections (surfactant-A-positive and ΔN-p63-negative). The number of positive cuboidal pneumocytes progressively increased from normal to severely affected alveoli (Figure 2, a and b) ▶ . β-catenin nuclear expression was observed in all enlarged and/or atypical cuboidal cells.
Figure 2.
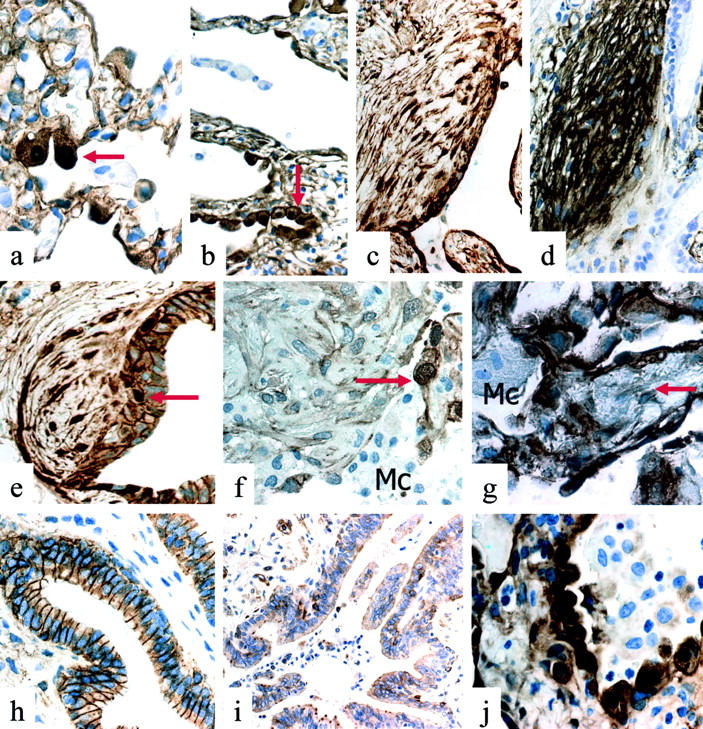
a: IPF/UIP: nuclear accumulation of β-catenin in cuboidal type II pneumocytes (arrow) in scarcely affected alveolar structures. b: IPF/UIP: nuclear accumulation of β-catenin in cuboidal type II pneumocytes (arrow) in severely affected alveolar structures. c: IPF/UIP: nuclear accumulation of β-catenin in spindled fibroblasts in subepithelial fibroblast foci. d: IPF/UIP (serial section to c): strong tenascin expression in spindled fibroblasts in subepithelial fibroblast foci. e: IPF/UIP: nuclear accumulation of β-catenin in spindled cells of subepithelial fibroblast foci. Note nuclear expression of β-catenin also in bronchiolar basal cells (arrow). f: OP/BOOP: nuclear β-catenin expression is not observed in spindled cells of an intraluminal Masson’s body and in macrophages (Mc). Note intense nuclear immunostaining in hyperplastic type II pneumocytes (arrow). g: DAD: nuclear β-catenin expression is evident in alveolar pneumocytes. Interstitial fibroblasts (arrow) and macrophages (Mc) lack evident immunoreactivity. h: NSIP: membrane β-catenin expression in bronchiolar cells. i: NSIP: scarce expression of matrilysin/MMP-7 in bronchiolar cells. j: NSIP: nuclear β-catenin expression in many alveolar pneumocytes.
Fibroblast Foci
Nuclear expression of β-catenin was observed in spindle cells forming fibroblast foci present in 16 of 20 samples in which these lesions could be clearly identified and immunohistochemically analyzed on serial sections (Figure 2, c and e) ▶ . These foci, characterized as myofibroblastic by intense α-smooth muscle actin and tenascin immunoreactivity on serial sections (Figure 2d) ▶ , were frequently intramural and located under abnormal bronchiolar segments, as forming strictly related lesions (Figure 2e) ▶ . This pattern was different from that observed in intra-alveolar inflammatory polyps (Masson’s bodies) present in OP/BOOP (Figure 2f) ▶ and interstitial fibroblasts of AIP/DAD (Figure 2g) ▶ samples used as control, in which only a minority (less than 10%) of spindle cells expressed nuclear β-catenin.
Expression of Cyclin-D1 and Matrilysin
A high number of cells expressing both cyclin-D1 and matrilysin could be demonstrated in IPF/UIP samples by immunohistochemical analysis on serial sections (Figure 1; e, f, k, and l) ▶ . Matrilysin immunoreactivity was evident in all types of proliferative bronchiolar lesions, with particular intensity in hyperplastic and atypical basal cells (Figure 1, f and l) ▶ . This pattern was decidedly different from normal and pathological control samples in which expression of matrilysin appeared as inconsistent (Figure 2i) ▶ .
Both cyclin D1 and matrilysin were clearly located at sites corresponding to nuclear overexpression of β-catenin, although the distribution of the three molecules was not identical on serial sections of proliferative epithelial lesions. In fact, β-catenin nuclear and/or cytoplasmic overexpression was evident in the vast majority of cells (Figure 1, d and j) ▶ , whereas matrilysin and cyclin-D1 were only expressed in a proportion of cells (Figure 1; e, f, k, and l) ▶ . The reason for this finding is not clear, but could be ascribed to undefined differentiation signals. In line with this view is the observation that both matrilysin and ΔN-p63 stop to be expressed in more superficially located cells of bronchiolar lesions (Figure 1, h and l) ▶ .
β-Catenin Intracellular Expression Pattern in Other Interstitial Pneumonias
In all cases of OP/BOOP, AIP/DAD, NSIP, and DIP analyzed, bronchiolar changes were extremely rare and no abnormal expression of β-catenin could be demonstrated by immunohistochemical analysis (Figure 2h) ▶ . Accordingly, matrilysin overexpression was not observed in bronchiolar epithelium (Figure 2i) ▶ . On the other hand, the proportion of alveolar pneumocytes expressing nuclear β-catenin was prominent in samples obtained from patients with pulmonary diseases in which extensive alveolar damage and repair take place, such as AIP/DAD, OP/BOOP, and NSIP (Figure 2j) ▶ .
Sequence Analysis of β-Catenin Gene
Evidence of mutations could not be found in the three samples of IPF/UIP after sequencing of the amplified region. All four pilomatricoma samples analyzed as known positive controls in the same experiments carried missense mutations in the third exon of the β-catenin gene.
Discussion
In this work, we provide evidence of a previously unrecognized involvement of the Wnt/β-catenin signaling pathway in IPF/UIP, as evidenced by extensive nuclear accumulation of β-catenin at different involved sites. We used the immunohistochemical approach to reveal subcellular localization of β-catenin, from the membrane location toward the nucleus. This morphological approach is able to detect intracellular redistribution and nuclear accumulation of β-catenin with high sensitivity, and has been widely used to demonstrate the activation status of the Wnt pathway in human development and pathology. 21,33,34,40-42 To further support the functional significance of β-catenin nuclear immunoreactivity we investigated in situ the expression of two target genes of the Wnt/β-catenin pathway, cyclin-D1 and matrilysin, demonstrating significant overexpression of both molecules at the same involved sites overexpressing nuclear β-catenin.
Finally, we searched for mutations affecting the β-catenin gene, showing that β-catenin gene abnormalities are probably not implicated in the observed activation of the Wnt pathway, although our technique might miss mutations occurring in a small percentage of cells. Further studies are warranted to investigate in IPF/UIP samples other genetic and/or expression abnormalities affecting the complex array of molecules involved in the Wnt/β-catenin cascade, which include APC, Axin, and GSK3β, as well as Wnt ligands and frizzled receptors. 43 In fact, on the basis of our data it is not possible to define whether Wnt/β-catenin activation is causative or secondary to the disease. Nevertheless, the absence of similar features in all other interstitial lung diseases investigated in this study strongly suggests the pathogenic relevance of Wnt/β-catenin aberrant activation in IPF/UIP.
In this study nuclear β-catenin accumulation could be demonstrated at three different involved sites in IPF/UIP samples: 1) bronchiolar proliferative lesions, 2) damaged alveolar structures, and 3) fibroblast foci. Bronchiolar proliferative lesions, including basal-cell hyperplasia, squamous metaplasia, honeycombing, and bronchiolization, are common in IPF/UIP, and represent well-recognized peculiar features of this disease. 1-4,16 According to our study, the aberrant activation of the Wnt pathway was particularly evident and extended in these lesions, involving in most cases the basal cell compartment. This observation is intriguing, because basal cells are considered the renewal component of bronchial and bronchiolar structures. In addition, these findings are unique, because bronchioles in normal lung samples and in various interstitial diseases other than IPF/UIP herein investigated with the same methods showed only membranous β-catenin immunoreactivity. On the other hand, β-catenin nuclear expression observed in cuboidal hyperplastic pneumocytes can be considered as part of a physiological response to alveolar damage, because type II pneumocytes exhibit this pattern in a variety of conditions in which alveolar proliferation/regeneration takes place, including fetal development, 21 and a variety of pulmonary diseases such as AIP/DAD and OP/BOOP, as shown in this study.
Nuclear β-catenin localization was also observed in spindle cells forming intramural fibroblast foci (fibroproliferative plaques) occurring in IPF/UIP, at variance with intra-alveolar Masson’s bodies in cases of organizing pneumonia and interstitial myofibroblasts in AIP/DAD. This finding is relevant because abnormal activation of the Wnt pathway could in fact provide autocrine survival signals necessary to induce the peculiar resistance to apoptosis characterizing intramural fibroblast foci of IPF/UIP. 7,44-46 In this regard, it is worth noting that nuclear accumulation of β-catenin because of gene mutations is a central feature in the pathogenesis of aggressive fibromatosis (desmoid tumor), a class of mesenchymal lesions that share with fibroblastic foci of IPF/UIP some morphological and phenotypic features, including aberrant activation of the Wnt pathway, as shown in this study. 47,48 Further support to our hypothesis has been recently provided by an experimental study in which β-catenin stabilization is able to dysregulate mesenchymal cell proliferation, motility, and invasiveness causing proliferative fibroblastic lesions in transgenic mice. 49
As described above, the distribution of β-catenin in a pattern of abnormal nuclear accumulation involves adjacent compartments, focused on bronchiolo-alveolar junctions. This peculiar zonal distribution could explain, in our view, some typical histological features of UIP such as the temporal and spatial heterogeneity, and honeycomb architectural derangement. 1 In our view, these data are consistent with a pathogenic model in which the variegated appearance, from early lesions to extensive fibrosis and remodeling characterizing IPF/UIP lesions, is produced by the progressive interference on physiological tissue repair mechanisms because of abnormal β-catenin activation, starting from foci of ongoing injury and repair processes, as following a gradient of Wnt signal concentration. Deregulated expression of Wnt target genes could exert divergent effects on different airway components (namely bronchiolar and alveolar), eventually leading to alveolar loss on one hand, and bronchiolar proliferation on the other. Accordingly, in different systems and cell types, the activation of the Wnt pathway is able to either trigger or inhibit survival and death by modulating the availability of cyclin-D1 and c-myc, two proteins that play roles in both cellular proliferation and apoptosis. 50,51 In this model, alveolar cells could be particularly vulnerable to deranged Wnt signaling, because diverse differentiation and death inducing signals, including p53, p21waf1, and transactivating isoforms of p63 are simultaneously expressed in repairing alveoli after injury. 16,52-55 On the other hand, bronchiolar basal cells could be protected from apoptosis by the constitutive expression of truncated dominant-negative ΔN-p63 isoforms exerting potent anti-apoptotic signals. 16,56
The pathogenic role of Wnt pathway activation in IPF/UIP is further supported by the in situ demonstration of abnormal expression of the metalloproteinase matrilysin (MMP-7), another important downstream target gene of β-catenin/LEF-1 signaling. An abnormal increase of matrilysin expression in IPF/UIP has also been demonstrated in a recent microarray gene expression analysis. 25 Further support is also provided by experimental studies, because matrilysin knockout mice are protected from bleomycin-induced pulmonary fibrosis. 25 The role of matrilysin in the development of bronchiolar proliferative lesions and lung remodeling can be ascribed to the peculiar multifunctional roles of this metalloproteinase, including the induction of epithelial cell migration, apoptosis, and metaplastic conversion. 57,58
A final relevant topic to be discussed in this context is the possible involvement of EMTs in the pathogenesis of IPF/UIP. These intriguing phenomena, known to occur in embryogenesis and carcinoma progression, allow cells to dissociate from the epithelial tissue from which they originate and to migrate freely. 29 In addition, definitive experimental evidence has been provided that fibroblasts can directly derive from epithelial cells in tissue fibrosis by epithelial to mesenchymal transition. 30,32 Interestingly, β-catenin-signaling plays a relevant role in inducing a mesenchymal phenotype in epithelial cells, as shown in experimental EMT, 28,31 thus it is possible to argue that the aberrant nuclearization of β-catenin observed in bronchiolar lesions of IPF/UIP can be involved in a EMT-related process increasing basal-cell motility (eg, by altering the expression of metalloproteinases), thus promoting bronchiolization and tissue remodeling. Another interesting possibility in this model, is that part of the abnormal fibroblasts in IPF/UIP could directly derive from epithelial basal cell precursors at sites of ongoing injury/repair processes, forming the peculiar lesions in which fibroblast-foci and abnormal bronchiolar segments are strictly associated (as shown in Figure 2, c and e ▶ ). Interestingly, EMT can be experimentally induced by cytokines (transforming growth factor-β1, fibroblast-growth-factor-2, insulin-like growth-factor, interleukin-8), whose expression can be tuned by complex regulatory loops with β-catenin signaling and which are potentially involved in the pathogenesis of pulmonary fibrosis. 28,59-64
In conclusion, although the precise molecular mechanisms leading to abnormal activation of the Wnt pathway observed in IPF/UIP could not be defined in this study, our findings can contribute to decipher the molecular mechanisms involved in the pathogenesis of this disease, and might also help in the search for new pharmacological strategies to counteract irreversible lung remodeling. In fact, intense investigation is currently focused on molecules exerting modulatory and inhibitory actions on the Wnt pathway. 65-67 These molecules could represent potential candidates for new treatment strategies for cancers in which the Wnt pathway is deranged, as well as in IPF/UIP, a deadly disease in which conventional treatments have proved to be unsatisfactory.
Footnotes
Address reprint requests to Marco Chilosi M.D., Dipartimento di Patologia, Università di Verona, Policlinico G.B.Rossi, P.le L.Scuro, 37134 Verona, Italy. E-mail: marco.chilosi@univr.it.
Supported in part by the Associazione Italiana per la Ricerca sul Cancro (Milan) and the Fondazione Cassa di Risparmio di Verona, Italy.
References
- 1.Katzenstein AL, Myers JL: Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med 1998, 157:1301-1315 [DOI] [PubMed] [Google Scholar]
- 2.: American Thoracic Society: Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 2000, 161:646-664 [DOI] [PubMed] [Google Scholar]
- 3.Poletti V, Kitaichi M: Facts and controversies in the classification of idiopathic interstitial pneumonias. Sarcoidosis Vasc Diffuse Lung Dis 2001, 17:229-238 [PubMed] [Google Scholar]
- 4.: American Thoracic Society/European Respiratory Society: International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med 2002, 165:277-304 [DOI] [PubMed] [Google Scholar]
- 5.Ward PA, Hunninghake GW: Lung inflammation and fibrosis. Am J Respir Crit Care Med 1998, 157:S123-S129 [DOI] [PubMed] [Google Scholar]
- 6.Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J: Transient expression of IL-1 beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest 2001, 107:1529-1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selman M, King TE, Jr, Pardo A: Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 2001, 134:136-151 [DOI] [PubMed] [Google Scholar]
- 8.Gross TJ, Hunninghake GW: Idiopathic pulmonary fibrosis. N Engl J Med 2001, 345:517-525 [DOI] [PubMed] [Google Scholar]
- 9.Sheppard D: Pulmonary fibrosis: a cellular overreaction or a failure of communication? J Clin Invest 2001, 107:1501-1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gauldie J, Kolb M, Sime PJ: A new direction in the pathogenesis of idiopathic pulmonary fibrosis? Respir Res 2002, 3:1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crystal RG, Bitterman PB, Mossman B, Schwarz MI, Sheppard D, Almasy L, Chapman HA, Friedman SL, King TE, Jr, Leinwand LA, Liotta L, Martin GR, Schwartz DA, Schultz GS, Wagner CR, Musson RA: Future research directions in idiopathic pulmonary fibrosis: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med 2002, 166:236-246 [DOI] [PubMed] [Google Scholar]
- 12.Gauldie J: Pro: inflammatory mechanisms are a minor component of the pathogenesis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2002, 165:1205-1206 [DOI] [PubMed] [Google Scholar]
- 13.Strieter RM: Con: inflammatory mechanisms are not a minor component of the pathogenesis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2002, 165:1206-1207 [DOI] [PubMed] [Google Scholar]
- 14.Ziesche R, Hofbauer E, Wittmann K, Petkov V, Block LH: A preliminary study of long-term treatment with interferon gamma-1b and low-dose prednisolone in patients with idiopathic pulmonary fibrosis. N Engl J Med 1999, 341:1264-1269 [DOI] [PubMed] [Google Scholar]
- 15.Selman M: From anti-inflammatory drugs through antifibrotic agents to lung transplantation: a long road of research, clinical attempts, and failures in the treatment of idiopathic pulmonary fibrosis. Chest 2002, 122:759-761 [DOI] [PubMed] [Google Scholar]
- 16.Chilosi M, Poletti V, Murer B, Lestani M, Cancellieri A, Montagna L, Piccoli P, Cangi G, Semenzato G, Doglioni C: Abnormal re-epithelialization and lung remodeling in idiopathic pulmonary fibrosis: the role of ΔN-p63. Lab Invest 2002, 82:1335-1345 [DOI] [PubMed] [Google Scholar]
- 17.Warburton D, Tefft D, Mailleux A, Bellusci S, Thiery JP, Zhao J, Buckley S, Shi W, Driscoll B: Do lung remodeling, repair, and regeneration recapitulate respiratory ontogeny? Am J Respir Crit Care Med 2001, 164:S59-S62 [DOI] [PubMed] [Google Scholar]
- 18.Takash W, Canizares J, Bonneaud N, Poulat F, Mattei MG, Jay P, Berta P: SOX7 transcription factor: sequence, chromosomal localisation, expression, transactivation and interference with Wnt signalling. Nucleic Acids Res 2001, 29:4274-4283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weidenfeld J, Shu W, Zhang L, Millar SE, Morrisey EE: The WNT7b promoter is regulated by TTF-1, GATA6, and Foxa2 in lung epithelium. J Biol Chem 2002, 277:21061-21070 [DOI] [PubMed] [Google Scholar]
- 20.Tebar M, Destree O, de Vree WJ, Ten Have-Opbroek AA: Expression of Tcf/Lef and sFrp and localization of beta-catenin in the developing mouse lung. Mech Dev 2001, 109:437-440 [DOI] [PubMed] [Google Scholar]
- 21.Eberhart CG, Argani P: Wnt signaling in human development: beta-catenin nuclear translocation in fetal lung, kidney, placenta, capillaries, adrenal, and cartilage. Pediatr Dev Pathol 2001, 4:351-357 [DOI] [PubMed] [Google Scholar]
- 22.Lako M, Strachan T, Bullen P, Wilson DI, Robson SC, Lindsay S: Isolation, characterisation and embryonic expression of WNT11, a gene which maps to 11q13.5 and has possible roles in the development of skeleton, kidney and lung. Gene 1998, 219:101-110 [DOI] [PubMed] [Google Scholar]
- 23.Brabletz T, Jung A, Dag S, Hlubek F, Kirchner T: Beta-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol 1999, 155:1033-1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crawford HC, Fingleton BM, Rudolph-Owen LA, Goss KJ, Rubinfeld B, Polakis P, Matrisian LM: The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene 1999, 18:2883-2891 [DOI] [PubMed] [Google Scholar]
- 25.Zuo F, Kaminski N, Eugui E, Allard J, Yakhini Z, Ben-Dor A, Lollini L, Morris D, Kim Y, DeLustro B, Sheppard D, Pardo A, Selman M, Heller RA: Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci USA 2002, 99:6292-6297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Surendran K, McCaul SP, Simon TC: A role for Wnt-4 in renal fibrosis. Am J Physiol 2002, 282:F431-F441 [DOI] [PubMed] [Google Scholar]
- 27.Shackel NA, McGuinness PH, Abbott CA, Gorrell MD, McCaughan GW: Insights into the pathobiology of hepatitis C virus-associated cirrhosis: analysis of intrahepatic differential gene expression. Am J Pathol 2002, 160:641-654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morali OG, Delmas V, Moore R, Jeanney C, Thiery JP, Larue L: IGF-II induces rapid beta-catenin relocation to the nucleus during epithelium to mesenchyme transition. Oncogene 2001, 20:4942-4950 [DOI] [PubMed] [Google Scholar]
- 29.Thiery JP: Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002, 2:442-454 [DOI] [PubMed] [Google Scholar]
- 30.Yang J, Liu Y: Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol 2001, 159:1465-1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim K, Lu Z, Hay ED: Direct evidence for a role of beta-catenin/LEF-1 signaling pathway in induction of EMT. Cell Biol Int 2002, 26:463-476 [DOI] [PubMed] [Google Scholar]
- 32.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG: Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 2002, 110:341-350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abraham SC, Klimstra DS, Wilentz RE, Yeo CJ, Conlon K, Brennan M, Cameron JL, Wu TT, Hruban RH: Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor beta-catenin mutations. Am J Pathol 2002, 160:1361-1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brabletz T, Herrmann K, Jung A, Faller G, Kirchner T: Expression of nuclear beta-catenin and c-myc is correlated with tumor size but not with proliferative activity of colorectal adenomas. Am J Pathol 2000, 156:865-870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestell R, Ben-Ze’ev A: The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA 1999, 96:5522-5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hibi K, Trink B, Patturajan M, Westra WH, Caballero OL, Hill DE, Ratovitski EA, Jen J, Sidransky D: AIS is an oncogene amplified in squamous cell carcinoma. Proc Natl Acad Sci USA 2000, 97:5462-5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chilosi M, Doglioni C: Constitutive p63 expression in airway basal cells. A molecular target in diffuse lung diseases. Sarcoidosis Vasc Diffuse Lung Dis 2001, 18:23-26 [PubMed] [Google Scholar]
- 38.Kitaeva MN, Grogan L, Williams JP, Dimond E, Nakahara K, Hausner P, DeNobile JW, Soballe PW, Kirsch IR: Mutations in beta-catenin are uncommon in colorectal cancer occurring in occasional replication error-positive tumors. Cancer Res 1997, 57:4478-4481 [PubMed] [Google Scholar]
- 39.Chan EF, Gat U, McNiff JM, Fuchs E: A common human skin tumour is caused by activating mutations in beta-catenin. Nat Genet 1999, 21:410-413 [DOI] [PubMed] [Google Scholar]
- 40.Polakis P: Wnt signaling and cancer. Genes Dev 2000, 14:1837-1851 [PubMed] [Google Scholar]
- 41.Wang HL, Wang J, Xiao SY, Haydon R, Stoiber D, He TC, Bissonnette M, Hart J: Elevated protein expression of cyclin D1 and Fra-1 but decreased expression of c-Myc in human colorectal adenocarcinomas overexpressing beta-catenin. Int J Cancer 2002, 101:301-310 [DOI] [PubMed] [Google Scholar]
- 42.Kirchner T, Brabletz T: Patterning and nuclear beta-catenin expression in the colonic adenoma-carcinoma sequence. Analogies with embryonic gastrulation. Am J Pathol 2000, 157:1113-1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wodarz A, Nusse R: Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol 1998, 14:59-88 [DOI] [PubMed] [Google Scholar]
- 44.Lappi-Blanco E, Soini Y, Paakko P: Apoptotic activity is increased in the newly formed fibromyxoid connective tissue in bronchiolitis obliterans organizing pneumonia. Lung 1999, 177:367-376 [DOI] [PubMed] [Google Scholar]
- 45.Ueda Y, Hijikata M, Takagi S, Takada R, Takada S, Chiba T, Shimotohno K: Wnt/beta-catenin signaling suppresses apoptosis in low serum medium and induces morphologic change in rodent fibroblasts. Int J Cancer 2002, 99:681-688 [DOI] [PubMed] [Google Scholar]
- 46.King TE, Jr, Tooze JA, Schwarz MI, Brown KR, Cherniack RM: Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med 2001, 164:1171-1181 [DOI] [PubMed] [Google Scholar]
- 47.Alman BA, Li C, Pajerski ME, Diaz-Cano S, Wolfe HJ: Increased beta-catenin protein and somatic APC mutations in sporadic aggressive fibromatoses (desmoid tumors). Am J Pathol 1997, 151:329-334 [PMC free article] [PubMed] [Google Scholar]
- 48.Saito T, Oda Y, Tanaka K, Matsuda S, Tamiya S, Iwamoto Y, Tsuneyoshi M: Beta-catenin nuclear expression correlates with cyclin D1 overexpression in sporadic desmoid tumours. J Pathol 2001, 195:222-228 [DOI] [PubMed] [Google Scholar]
- 49.Cheon SS, Cheah AY, Turley S, Nadesan P, Poon R, Clevers H, Alman BA: Beta-catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc Natl Acad Sci USA 2002, 99:6973-6978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Packham G, Cleveland JL: c-Myc and apoptosis. Biochim Biophys Acta 1995, 1242:11-28 [DOI] [PubMed] [Google Scholar]
- 51.Kim K, Pang KM, Evans M, Hay ED: Overexpression of beta-catenin induces apoptosis independent of its transactivation function with LEF-1 or the involvement of major G1 cell cycle regulators. Mol Biol Cell 2000, 11:3509-3523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.You Z, Saims D, Chen S, Zhang Z, Guttridge DC, Guan KL, MacDougald OA, Brown AM, Evan G, Kitajewski J, Wang CY: Wnt signaling promotes oncogenic transformation by inhibiting c-Myc-induced apoptosis. J Cell Biol 2002, 157:429-440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Staversky RJ, Watkins RH, Wright TW, Hernady E, LoMonaco MB, D’Angio CT, Williams JP, Maniscalco WM, O’Reilly MA: Normal remodeling of the oxygen-injured lung requires the cyclin-dependent kinase inhibitor p21(Cip1/WAF1/Sdi1). Am J Pathol 2002, 161:1383-1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adamson A, Perkins S, Brambilla E, Tripp S, Holden J, Travis W, Guinee D, Jr: Proliferation, c-myc, and cyclin D1 expression in diffuse alveolar damage: potential roles in pathogenesis and implications for prognosis. Hum Pathol 1999, 30:1050-1057 [DOI] [PubMed] [Google Scholar]
- 55.Guinee D, Jr, Fleming M, Hayashi T, Woodward M, Zhang J, Walls J, Koss M, Ferrans V, Travis W: Association of p53 and WAF1 expression with apoptosis in diffuse alveolar damage. Am J Pathol 1996, 149:531-538 [PMC free article] [PubMed] [Google Scholar]
- 56.Yang A, McKeon F: p63 and p73: p53 mimics, menaces and more. Nat Rev Mol Cell Biol 2000, 1:199-207 [DOI] [PubMed] [Google Scholar]
- 57.Seiki M: The cell surface: the stage for matrix metalloproteinase regulation of migration. Curr Opin Cell Biol 2002, 14:624-632 [DOI] [PubMed] [Google Scholar]
- 58.Crawford HC, Scoggins CR, Washington MK, Matrisian LM, Leach SD: Matrix metalloproteinase-7 is expressed by pancreatic cancer precursors and regulates acinar-to-ductal metaplasia in exocrine pancreas. J Clin Invest 2002, 109:1437-1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levy L, Neuveut C, Renard CA, Charneau P, Branchereau S, Gauthier F, Tran Van Nhieu J, Cherqui D, Petit-Bertron AF, Mathieu D, Buendia MA: Transcriptional activation of interleukin-8 by {beta}-catenin-TCF4. J Biol Chem 2002, 277:42386-42393 [DOI] [PubMed] [Google Scholar]
- 60.Lynch JP, III, Standiford TJ, Rolfe MW, Kunkel SL, Strieter RM: Neutrophilic alveolitis in idiopathic pulmonary fibrosis. The role of interleukin-8. Am Rev Respir Dis 1992, 145:1433-1439 [DOI] [PubMed] [Google Scholar]
- 61.Liu JY, Sime PJ, Wu T, Warshamana GS, Pociask D, Tsai SY, Brody AR: Transforming growth factor-beta(1) overexpression in tumor necrosis factor-alpha receptor knockout mice induces fibroproliferative lung disease. Am J Respir Cell Mol Biol 2001, 25:3-7 [DOI] [PubMed] [Google Scholar]
- 62.Khalil N, O’Connor RN, Flanders KC, Unruh H: TGF-beta 1, but not TGF-beta 2 or TGF-beta 3, is differentially present in epithelial cells of advanced pulmonary fibrosis: an immunohistochemical study. Am J Respir Cell Mol Biol 1996, 14:131-138 [DOI] [PubMed] [Google Scholar]
- 63.Strutz F, Zeisberg M, Ziyadeh FN, Yang CQ, Kalluri R, Muller GA, Neilson EG: Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int 2002, 61:1714-1728 [DOI] [PubMed] [Google Scholar]
- 64.El-Hariry I, Pignatelli M, Lemoine NR: FGF-1 and FGF-2 regulate the expression of E-cadherin and catenins in pancreatic adenocarcinoma. Int J Cancer 2001, 94:652-661 [DOI] [PubMed] [Google Scholar]
- 65.Bordonaro M, Lazarova DL, Augenlicht LH, Sartorelli AC: Cell type- and promoter-dependent modulation of the Wnt signaling pathway by sodium butyrate. Int J Cancer 2002, 97:42-51 [DOI] [PubMed] [Google Scholar]
- 66.Joe AK, Liu H, Suzui M, Vural ME, Xiao D, Weinstein IB: Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin Cancer Res 2002, 8:893-903 [PubMed] [Google Scholar]
- 67.Hanai J, Gloy J, Karumanchi SA, Kale S, Tang J, Hu G, Chan B, Ramchandran R, Jha V, Sukhatme VP, Sokol S: Endostatin is a potential inhibitor of Wnt signaling. J Cell Biol 2002, 158:529-539 [DOI] [PMC free article] [PubMed] [Google Scholar]


