Abstract
Immune complex-induced tissue injury is mediated by inflammatory cell infiltration that is highly regulated by multiple adhesion molecules. To assess the relative contribution of adhesion molecules, including selectins and ICAM-1, in this pathogenetic process, the cutaneous passive Arthus reaction was examined in mice lacking E-selectin, P-selectin, or both L-selectin and ICAM-1 with anti-P- or E-selectin mAbs. Edema and hemorrhage were significantly reduced in P-selectin−/− mice compared with wild-type mice while they were not inhibited in E-selectin−/− mice. Combined E- and P-selectin blockade resulted in more significant reduction relative to L-selectin/ICAM-1−/− as well as P-selectin−/− mice. Remarkably, both E- and P-selectin blockade in L-selectin/ICAM-1−/− mice completely abrogated edema and hemorrhage. The inhibited edema and hemorrhage paralleled reduced infiltration of neutrophils and mast cells that expressed significant levels of P-selectin glycoprotein ligand-1. Similarly reduced infiltration of neutrophils and mast cells was observed in the peritoneal Arthus reaction and was associated partly with the decreased production of tumor necrosis factor-α and interleukin-6. The results of this study indicate that both endothelial selectins contribute predominantly to the Arthus reaction by regulating mast cell and neutrophil infiltration and that the full development of the Arthus reaction is mediated cooperatively by all selectins and ICAM-1.
Autoimmune diseases are frequently developed by the formation of immune complexes (ICs) and their perivascular depositions that induce acute inflammatory response with significant tissue injury, referred to as type III hypersensitivity reaction. 1 This IC injury has been implicated in the pathogenesis of vasculitis syndrome, systemic lupus erythematosus, rheumatoid arthritis, and cryoglobulinemia. 1 The classical and standard animal model for the inflammatory response in these IC-mediated diseases is the Arthus reaction. 2 In the original description by Arthus, 2 horse serum was repeatedly injected intradermally into a rabbit, resulting in edema, hemorrhage, and neutrophil infiltration in the skin. Because of its ease and reproducibility, the experimental model most commonly used is the reverse passive Arthus reaction, in which antibody (Ab) is injected at the site where the investigator wants the inflammatory response to develop and the antigen is applied intravenously immediately before or after Ab injection. 1 Analyses using gene knockout mice have revealed that activation of the complement system, especially C5a and its interaction with C5a receptor, and of Fc receptors for IgG on inflammatory cells, particularly mast cells, are both required to initiate the Arthus reaction. 1,3-6 In addition, accumulation of neutrophils and mast cells is necessary for the progression of the IC-mediated vascular tissue damage, which results in edema and hemorrhage. 3-8
Leukocyte recruitment from the circulation into a site of inflammation is a multistep process that is regulated by multiple cell-surface adhesion molecules. 9,10 Leukocytes first tether and roll on vascular endothelial cells, before they are activated to adhere firmly and subsequently emigrate into the extravascular space. The selectins primarily mediate tethering and rolling of leukocytes whereas immunoglobulin (Ig) superfamily members, including intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), and their integrin ligands, including lymphocyte function-associated antigen-1 (LFA-1, CD11a/CD18) and vary late antigen-4 (VLA-4, CD49d/CD29), are critical for the firm adhesion that follows. The selectin family consists of three cell-surface molecules expressed by leukocytes (L-selectin), vascular endothelium (E- and P-selectins), and platelets (P-selectin). 11 Although P-selectin is rapidly mobilized to the surface of activated endothelium or platelets, E-selectin expression is induced within several hours after activation with inflammatory cytokines. 11 L-selectin is constitutively expressed on most leukocytes. 11 ICAM-1 is also constitutively expressed at low levels by endothelial cells, but is rapidly up-regulated during inflammation. 12 Disrupting the expression or function of these adhesion molecules is a potential approach for intervening in the progression of IC-mediated tissue injury.
Interaction of adhesion molecules during the process of leukocyte migration into inflammatory sites is complex and highly regulated. Three selectins have partially overlapping functions for leukocyte rolling. 13,14 Specifically, E-selectin-deficient (E-selectin−/−) mice do not exhibit obvious defects of leukocyte rolling and inflammation, but blockade of P-selectin function by a monoclonal antibody (mAb) or by an induced null mutation in the P-selectin gene results in virtually eliminated leukocyte rolling and inflammation. 15-17 Comparisons among mice deficient in two selectins reveal that P-selectin cooperates with both E- and L-selectins. 14 Furthermore, interactions between integrins and their Ig superfamily ligands can mediate rolling as well as firm adhesion synergistically with selectins. 18-20 ICAM-1 functions cooperatively with L-selectin or P-selectin to mediate optimal leukocyte rolling and recruit leukocytes into inflammatory sites. 20-24 In addition, E-selectin does not only mediate rolling but also serves to promote firm adhesion. 25 This functional redundancy of adhesion molecules did not allow us to precisely predict the relative contribution of each adhesion molecule to a certain inflammatory process. In addition, the relative role of each adhesion molecule in the inflammation varies according to the tissue site and the nature of the inflammatory stimuli. For example, P-selectin expression is required for Streptococcus pneumoniae-induced inflammation in the peritoneum, but not in the lung. 23 By contrast, lung inflammation because of hemorrhage shock is mediated by P-selectin. 26 Therefore, to precisely determine the relative contribution of each adhesion molecule to a given model of inflammation, the role of each adhesion molecule should be systematically examined.
We previously showed that mice lacking L-selectin, ICAM-1, or both exhibit reduced Arthus reaction that is associated with decreased infiltration of neutrophils and mast cells. 7 However, in the absence of both L-selectin and ICAM-1, IC-induced tissue damage is not completely inhibited with ∼50% reduction in tissue injury. 7 Blocking P-selectin function by mAbs reduces the cutaneous Arthus reaction in the rat system. 27 Therefore, the remaining tissue injury induced by IC in mice lacking both L-selectin and ICAM-1 (L-selectin/ICAM-1−/− mice) may be mediated by endothelial selectins (P- and E-selectins). However, the relative contribution and interaction of selectin members and ICAM-1 to the Arthus reaction remain unknown. In this study, to clarify the role of endothelial selectins in the Arthus reaction, we first examined inflammation induced by IC in mice lacking P- or E-selectin and wild-type mice with combinations of anti-P- and/or E-selectin mAbs. Furthermore, to evaluate the role of endothelial selectins in the absence of L-selectin and ICAM-1 expression during the Arthus reaction, we investigated the IC-mediated inflammatory process in L-selectin/ICAM-1−/− mice treated with anti-P- and/or E-selectin mAbs. The results of this study indicate that both endothelial selectins contribute predominantly to the Arthus reaction by regulating the accumulation of mast cells and neutrophils, and also demonstrate that the full progression of the Arthus reaction is mediated cooperatively by all selectins and ICAM-1.
Materials and Methods
Mice
P-selectin−/− 23 and E-selectin−/− 16 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice lacking both L-selectin and ICAM-1 were generated as described elsewhere. 20 All mice were healthy, fertile, and did not display evidence of infection or disease. All mice were backcrossed between 5 and 10 generations onto the C57BL/6 genetic background. Mice used for experiments were 12 to 16 weeks old. Age-matched wild-type littermates and C57BL/6 mice (The Jackson Laboratory) were used as controls with equivalent results so all control results were pooled. All mice were housed in a pathogen-free barrier facility and screened regularly for pathogens. All studies and procedures were approved by the Committee on Animal Experimentation of Kanazawa University Graduate School of Medical Science.
Reverse Passive Arthus Reactions
For cutaneous reverse passive Arthus reactions, mice anesthetized by inhalation of diethyl ether were shaved on their dorsal skin and wiped with 70% alcohol. Rabbit IgG anti-chicken egg albumin Abs (60 μg/30 μl; Cappel, Aurora, OH) were injected intradermally with a 29-gauge needle, followed immediately by an intravenous injection of chicken egg albumin (20 mg/kg; Sigma-Aldrich, St. Louis, MO). 4 The intradermal injection of purified polyclonal rabbit IgG (60 μg/30 μl, Sigma-Aldrich) followed by intravenous installation of chicken egg albumin served as a control. The solution of chicken egg albumin contained 1% Evans blue dye (Sigma-Aldrich). For a blocking study using mAbs to E- and/or P-selectin, mAbs were injected intravenously 30 minutes before IC challenge. Abs used in this blocking study included mAbs to murine P-selectin (RB40.34, rat IgG1, 30 μg per mouse; BD PharMingen, San Diego, CA) 28 and mAbs to murine E-selectin (10E9.6, rat IgG2a, 30 μg per mouse; BD PharMingen). 28 These were the mAb concentrations required to inhibit P-selectin- and E-selectin-dependent leukocyte rolling in vivo as previously described. 24 Irrelevant isotype-matched, purified rat IgG1 mAb (R3-34) and rat IgG2a mAb (R35-95) served as controls (30 μg per mouse, BD PharMingen).
The peritoneal reverse passive Arthus reaction was initiated by the intravenous injection of chicken egg albumin at 20 mg/kg, followed immediately by the intraperitoneal injection of 800 μg of rabbit IgG anti-chicken egg albumin Ab or control purified rabbit polyclonal IgG in a volume of 400 μl. 4 Four or 8 hours later, the peritoneum was exposed by a middle abdominal incision, and 5 ml of ice-cold phosphate-buffered saline containing 0.1% bovine serum albumin was injected into the peritoneal cavity via a 27-gauge needle. Cells in the recovered lavage fluid were centrifuged onto glass slides and stained with Giemsa for light microscopic examination to quantify neutrophil and mast cell numbers.
Quantitation of Edema and Hemorrhage
Edema was evaluated by measuring the vascular leak 4 hours after IC challenge. 4 Mice were sacrificed and the skin containing the injection site was removed at the level of fascia above skeletal muscle and was reversed. The diameter of extravascular Evans blue dye on the fascia side of the injection site was measured directly. Evans blue dye binds to serum proteins and thereby can be used to quantify alterations in vascular permeability. The diameter of the major and minor axis of the blue spot was averaged for analysis. The amount of hemorrhage was assessed 8 hours after IC challenge by direct macroscopic measurement of the purpuric spot. The diameter of the major and minor axis of the purpuric spot was averaged for analysis.
Histological Examination
Tissues were harvested 4 or 8 hours after IC challenge using a disposable sterile 6-mm punch biopsy (Maruho, Osaka, Japan) and assessed for tissue damage and number of infiltrating neutrophils and mast cells. Tissues were cut into halves, fixed in 3.5% paraformaldehyde, and then paraffin embedded. Sections (6 μm) were stained using hematoxylin and eosin for neutrophil evaluation and toluidine blue for mast cell staining. Neutrophil and mast cell infiltration was evaluated by counting extravascular neutrophils and mast cells in the entire section and averaging the numbers present in 10 serial skin sections from the injection site. Each section was examined independently by three investigators in a blinded manner, and the mean was used for analysis.
Flow Cytometric Analysis
Isolated peritoneal lavage cells (0.5 × 106) were stained using predetermined optimal concentrations of either anti-c-kit-fluorescein isothiocyanate Ab (CD117, clone 2B8; BD PharMingen) or anti-Gr-1-fluorescein isothiocyanate Ab (clone RB6-8C5; BD PharMingen) plus either anti-P-selectin glycoprotein ligand-1 (PSGL-1)-phycoerythrin Ab (clone 2PH1, BD PharMingen) or anti-CD49d (α4 integrin, clone R1-2; BD PharMingen)-phycoerythrin Ab for 20 minutes at 4°C as described elsewhere. 29,30 Cells were washed and analyzed on a FACScan flow cytometer (BD PharMingen) by gating on c-kit-positive mast cells or Gr-1-positive granulocytes. Positive and negative populations of cells were determined using unreactive isotype-matched mAbs (Beckman Coulter) as controls for background staining.
Cytokine Enzyme-Linked Immunosorbent Assay
Levels of murine tumor necrosis factor (TNF)-α and interleukin (IL)-6 in the peritoneal lavage were determined by enzyme-linked immunosorbent assay using rat mAb pairs for murine cytokines (BD PharMingen), according to the manufacturer’s instructions. The plates were coated with cytokine-specific Abs and incubated with appropriately diluted peritoneal lavage samples. After incubation with biotinylated cytokine-specific Abs and streptavidin-horseradish peroxidase, the reaction was developed.
Statistical Analysis
The Mann-Whitney U-test was used for determining the level of significance of differences in sample means and Bonferroni’s test was used for multiple comparisons.
Results
Contribution of Endothelial Selectins to the Cutaneous Arthus Reaction
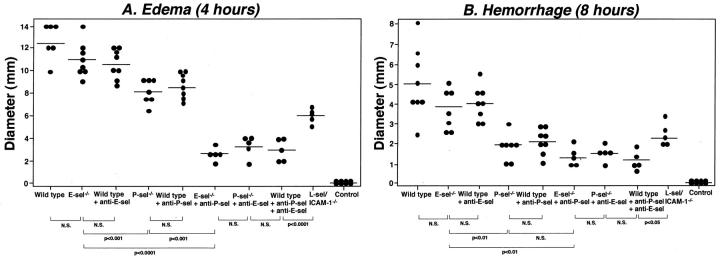
Cutaneous inflammation induced by the Arthus reaction can be separated into two distinct responses: edema, which reaches a maximum at 3 to 4 hours after IC challenge, and hemorrhage, which peaks in intensity at 8 hours. 5 To assess the role of endothelial selectins (E- and P-selectins) in the cutaneous Arthus reaction, edema and hemorrhage were evaluated 4 and 8 hours after IC challenge, respectively, in E-selectin−/− mice, P-selectin−/− mice, E-selectin−/− mice treated with anti-P-selectin mAb, P-selectin−/− mice treated with anti-E-selectin mAb, and wild-type mice treated with mAb to E- and/or P-selectins in comparison with L-selectin/ICAM-1−/− and wild-type mice. Edema that was assessed by measuring the diameter of Evans blue dye in the extravascular space was reduced in P-selectin−/− mice by 35% compared with wild-type mice (P < 0.0001; Figure 1A ▶ ). By contrast, E-selectin−/− mice developed edema that was similar to that found in wild-type mice. Similar results were obtained using wild-type mice treated with mAb to either P- or E-selectin. Blocking P-selectin by mAbs in E-selectin−/− mice inhibited edema by 77% relative to wild-type mice (P < 0.0001) and resulted in a significant further reduction of edema compared with either E-selectin−/− (P < 0.0001) or P-selectin−/− (P < 0.001) mice. Results similar to P-selectin blockade in E-selectin−/− mice were obtained when E-selectin blockade in P-selectin−/− mice or blockade of both E- and P-selectins in wild-type mice was assessed. Edema was also inhibited by 50% in L-selectin/ICAM-1−/− mice relative to wild-type mice (P < 0.0001). However, the inhibitory effect with both P- and E-selectin blockade was significantly greater than found with both L-selectin and ICAM-1 loss (P < 0.0001). Thus, P-selectin loss or blockade reduced the early response characterized by edema while blockade of both E- and P-selectins had profound inhibitory effects on edema.
Figure 1.
The effect of loss or blockade of E- and P-selectin (sel) on edema and hemorrhage in the cutaneous reverse passive Arthus reaction. Mice were injected intradermally with rabbit IgG anti-chicken egg albumin Ab, followed by systemic chicken egg albumin and 1% Evans blue dye. After 4 or 8 hours, dorsal skins were assessed from E-selectin−/− mice, P-selectin−/− mice, wild-type mice treated with mAbs to E- and/or P-selectin, E-selectin−/− mice treated with anti-P-selectin mAbs, P-selectin−/− mice treated with anti-E-selectin mAbs, L-selectin/ICAM-1−/− mice, and wild-type mice. A: Edema was evaluated as the diameter of extravasated Evans blue spot. Wild-type mice that received an intradermal injection of polyclonal rabbit IgG followed by intravenous installation of chicken egg albumin served as controls. B: Hemorrhage after 8 hours was assessed as the diameter of the purpuric spot. Edema and hemorrhage were significantly inhibited in P-selectin−/− mice, wild-type mice treated with anti-P-selectin mAb, E-selectin−/− mice treated with anti-P-selectin mAb, P-selectin−/− mice treated with anti-E-selectin mAb, wild-type mice treated with anti-P- and E-selectin mAb, and L-selectin/ICAM-1−/− mice compared with wild-type mice for both panels (P < 0.001), but not in E-selectin−/− mice and wild-type mice treated with anti-E-selectin mAb. Horizontal bars indicate mean values for each group of mice. N.S. = not significant.
Hemorrhage was macroscopically quantitated after 8 hours by measuring the size of the purpuric spot. Results comparable with edema were obtained for hemorrhage except that the inhibitory effect of P-selectin loss was greater on hemorrhage (63% decrease, P < 0.001 versus wild-type mice) than that on edema (35% decrease; Figure 1B ▶ ): the difference between P-selectin blockade alone and combined P- and E-selectin blockade was no longer observed for hemorrhage. Hemorrhage was comparable for E-selectin−/− and wild-type mice. Similar results were obtained using wild-type mice treated with mAb to either P- or E-selectin. L-selectin/ICAM-1−/− mice exhibited 54% smaller hemorrhage than that found in wild-type mice (P < 0.001), but it remained significantly larger than that found in E-selectin−/− mice with P-selectin blockade (P < 0.05). Results similar to P-selectin blockade in E-selectin−/− mice were obtained when E-selectin blockade in P-selectin−/− mice or blockade of both E- and P-selectins in wild-type mice was evaluated. Treatment with irrelevant isotype-matched mAbs instead of anti-P- or E-selectin mAbs did not affect edema or hemorrhage (data not shown). Edema and hemorrhage were not detected in mutant or wild-type mouse controls after intradermal injection of rabbit polyclonal IgG with systemic chicken egg albumin (Figure 1, A and B ▶ , and data not shown). Thus, the inhibitory effect by simultaneous blockade of E- and P-selectin on hemorrhage remained profound, with P-selectin deficiency alone inhibiting this later tissue injury more strongly than the early inflammatory response.
Contribution of Endothelial Selectins to the Cutaneous Arthus Reaction in L-Selectin/ICAM-1−/− Mice
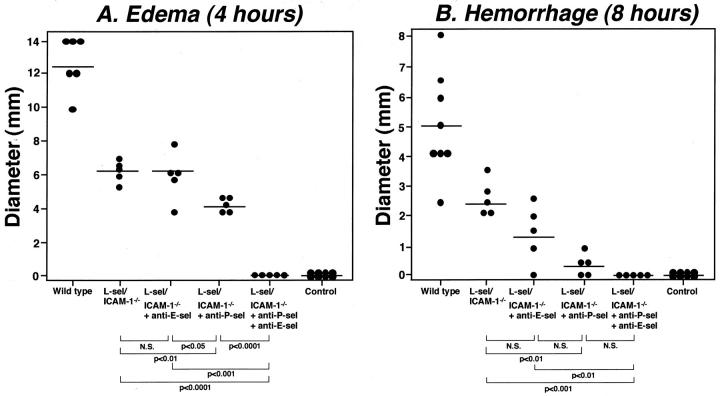
Edema and hemorrhage were not completely inhibited in L-selectin/ICAM-1−/− mice, with ∼50% inhibition of the responses (Figure 1) ▶ . Because blockade of both E- and P-selectins significantly inhibited IC-mediated inflammation (Figure 1) ▶ , we assessed the contribution of E- and P-selectins to the remaining inflammatory response observed in L-selectin/ICAM-1−/− mice. Anti-E-selectin mAb treatment in L-selectin/ICAM-1−/− mice did not affect edema and hemorrhage. By contrast, anti-P-selectin mAb treatment in L-selectin/ICAM-1−/− mice significantly inhibited both edema and hemorrhage compared with L-selectin/ICAM-1−/− mice (P < 0.01; Figure 2 ▶ ). Remarkably, the combination of P- and E-selectin blockade in L-selectin/ICAM-1−/− mice completely eliminated both edema (P < 0.0001 versus L-selectin/ICAM-1−/− mice) and hemorrhage (P < 0.001). The inhibitory effect of P-selectin loss in L-selectin/ICAM-1−/− mice on hemorrhage (94% decrease relative to wild-type mice) was greater than that on edema (66%) as in wild-type mice (Figure 1) ▶ : the combined blockade of both E- and P-selectins in L-selectin/ICAM-1−/− mice significantly inhibited edema relative to P-selectin blockade alone in these mice (P < 0.0001), but such difference was no longer observed for hemorrhage. Thus, the remaining inflammatory responses in L-selectin/ICAM-1−/− mice were dependent on E- and P-selectin function.
Figure 2.
The effect of loss or blockade of E- and P-selectin (sel) on edema and hemorrhage in L-selectin/ICAM-1−/− mice during the cutaneous reverse passive Arthus reaction. Edema (A) and hemorrhage (B) were induced and evaluated in L-selectin/ICAM-1−/− mice, L-selectin/ICAM-1−/− mice treated with anti-E-selectin mAbs, L-selectin/ICAM-1−/− mice treated with anti-P-selectin mAbs, L-selectin/ICAM-1−/− mice treated with mAbs to both E- and P-selectins, as described in Figure 1 ▶ . Edema and hemorrhage were significantly inhibited in L-selectin/ICAM-1−/− mice, L-selectin/ICAM-1−/− mice treated with anti-E-selectin mAbs, L-selectin/ICAM-1−/− mice treated with anti-P-selectin mAbs, L-selectin/ICAM-1−/− mice treated with mAbs to both E- and P-selectins compared with wild-type mice for both panels (P < 0.001). Horizontal bars indicate mean values for each group of mice. N.S. = not significant.
Leukocyte Infiltration in the Cutaneous Arthus Reaction
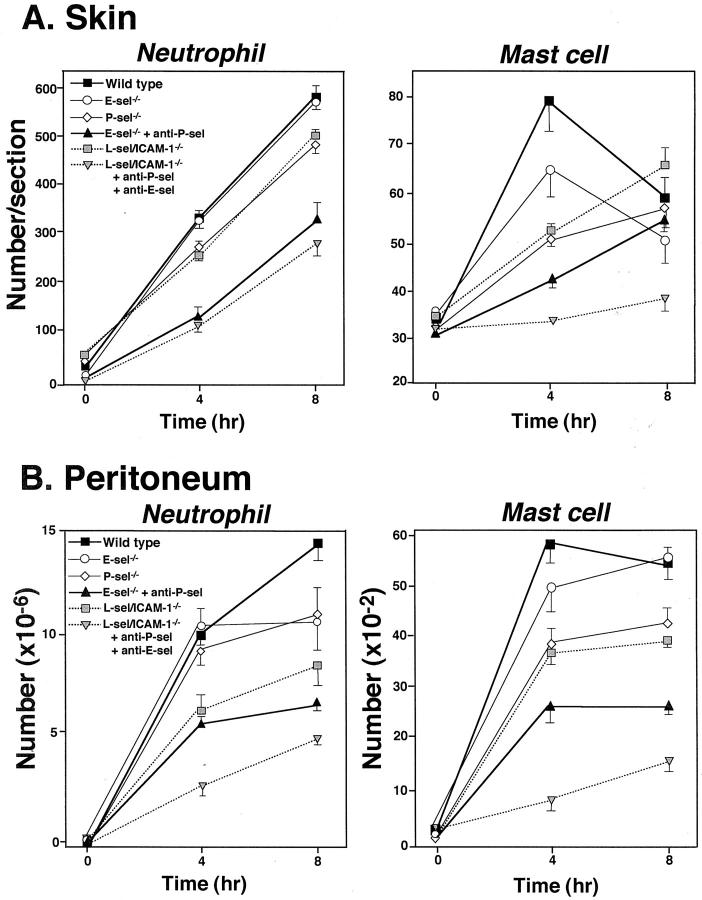
Extravascular neutrophils were assessed in skin tissue sections after 4 and 8 hours of IC formation in E-selectin−/− mice, P-selectin−/− mice, E-selectin−/− mice treated with anti-P-selectin mAb, L-selectin/ICAM-1−/− mice, and L-selectin/ICAM-1−/− mice treated with mAbs to both E- and P-selectins in comparison with wild-type mice (Figures 3A and 4) ▶ . Previous studies have shown that blood neutrophil numbers in E-selectin−/− mice are normal while they exhibit 2.4-fold and 5.8-fold increase in P-selectin−/− and L-selectin/ICAM-1−/− mice relative to wild-type mice, respectively. 15,16,20,31 However, before IC challenge, there were no significant differences in cutaneous neutrophil numbers between mutant and wild-type mice. After 4 hours of IC challenge, neutrophil numbers were significantly reduced in P-selectin−/− mice (28%, P < 0.05), L-selectin/ICAM-1−/− mice (32%, P < 0.05), E-selectin−/− mice with P-selectin blockade (69%, P < 0.005), and L-selectin/ICAM-1−/− mice with blockade of both E- and P-selectin (70%, P < 0.005) compared with wild-type mice while they were not affected by E-selectin loss alone. P-selectin blockade in E-selectin−/− mice exhibited a reduction in neutrophil accumulation that was significantly lower than that found in L-selectin/ICAM-1−/− as well as P-selectin−/− mice (P < 0.01). The combined blockade of E- and P-selectins in L-selectin/ICAM-1−/− mice significantly inhibited neutrophil numbers relative to L-selectin/ICAM-1−/− mice (P < 0.01), but did not result in a further reduction of neutrophil numbers compared with those found in E-selectin−/− mice with P-selectin blockade. Similar results were obtained after 8 hours of IC formation.
Figure 3.
Arthus reaction-induced recruitment of neutrophils and mast cells in the skin (A) and the peritoneum (B) from E-selectin (E-sel)−/− mice, P-selectin−/− mice, E-selectin−/− mice treated with anti-P-selectin mAbs, L-selectin/ICAM-1−/− mice, L-selectin/ICAM-1−/− mice treated with mAbs to both E- and P-selectins, and wild-type mice at 4 and 8 hours after IC challenge. Numbers of neutrophils and mast cells per skin section were determined by counting in H&E- and toluidine blue-stained skin sections, respectively. The peritoneal reverse passive Arthus reaction was induced by the intravenous injection of chicken egg albumin, followed immediately by the intraperitoneal injection of rabbit IgG anti-chicken egg albumin Ab. Cells in the recovered lavage fluid were then centrifuged onto glass slides and stained with Giemsa to quantify neutrophil and mast cell numbers. All values represent the mean ± SEM of results obtained from 5 to 10 mice in each group. Statistical analysis is provided in the Results.
Mast cell numbers were also assessed in skin tissue sections stained with toluidine blue (Figures 3B and 5) ▶ . Before IC challenge, skin mast cell numbers did not significantly differ between mutant and wild-type mice. By contrast, 4 hours after IC challenge, mast cell numbers were significantly reduced in P-selectin−/− mice (35%, P < 0.01), L-selectin/ICAM-1−/− mice (34%, P < 0.01), E-selectin−/− mice with P-selectin blockade (47%, P < 0.001), and L-selectin/ICAM-1−/− mice with both E- and P-selectin blockade (58%, P < 0.0001) compared with wild-type mice whereas they were similar for E-selectin−/− and wild-type mice. However, 8 hours after IC challenge, only L-selectin/ICAM-1−/− mice with the combined blockade of E- and P-selectin exhibited significantly decreased mast cell numbers by 37% relative to wild-type mice (P < 0.01). Indeed, mast cell numbers did not significantly increase after IC challenge in L-selectin/ICAM-1−/− mice treated with anti-E- and P-selectin Abs. These mice exhibited significantly reduced mast cell numbers compared with E-selectin−/− mice with P-selectin blockade (P < 0.05) as well as L-selectin/ICAM-1−/− mice (P < 0.01) after both 4 and 8 hours. P-selectin blockade in E-selectin−/− mice resulted in a further reduction of mast cell numbers relative to P-selectin−/− mice after 4 hours (P < 0.05), but not after 8 hours. Regarding both neutrophil and mast cell numbers, results similar to E-selectin−/− and P-selectin−/− mice were obtained using wild-type mice treated with anti-E-selectin mAb and those treated with anti-P-selectin mAb, respectively (data not shown). Furthermore, results comparable with P-selectin blockade in E-selectin−/− mice were observed when E-selectin blockade in P-selectin−/− mice or blockade of both E- and P-selectins in wild-type mice was assessed (data not shown). Treatment with irrelevant isotype-matched mAbs instead of anti-P- or E-selectin mAbs did not affect neutrophil and mast cell recruitment (data not shown). Any neutrophil and mast cell influx was not detected in mutant or wild-type mouse controls after intradermal injection of rabbit polyclonal IgG with systemic chicken egg albumin (data not shown). Thus, edema and hemorrhage inhibited by loss or blockade of adhesion molecules generally paralleled the reduced accumulation of neutrophils and mast cells; however, the accumulation of mast cells, but not neutrophils, was significantly decreased in mice with blockade of all four receptors relative to mice with blockade of both E- and P-selectins.
Leukocyte Infiltration in the Peritoneal Arthus Reaction
The intraperitoneal injection of Ab with the intravenous injection of Ag elicits a reverse passive Arthus reaction characterized by leukocyte influx into the peritoneal cavity. 1 After 4 hours of IC challenge, neutrophil numbers in the peritoneal cavity were significantly reduced in L-selectin/ICAM-1−/− mice (40%, P < 0.01), E-selectin−/− mice with P-selectin blockade (44%, P < 0.005), and L-selectin/ICAM-1−/− mice with both E- and P-selectin blockade (74%, P < 0.0001) compared with wild-type mice while they were not affected by either E- or P-selectin loss alone (Figure 3B) ▶ . The major differences from the cutaneous Arthus reaction are as follows: firstly, L-selectin/ICAM-1−/− mice exhibited a reduction in neutrophil numbers similar to that found in E-selectin−/− mice with P selectin blockade; secondly, L-selectin/ICAM-1−/− mice with blockade of both E- and P-selectins exhibited significantly diminished neutrophil numbers relative to E-selectin−/− mice with P-selectin blockade (P < 0.01). Similar results were obtained after 8 hours of IC formation.
Mast cell recruitment was also significantly reduced in P-selectin−/− mice (34%, P < 0.01), L-selectin/ICAM-1−/− mice (36%, P < 0.01), E-selectin−/− mice with P-selectin blockade (60%, P < 0.001), and L-selectin/ICAM-1−/− mice with both E- and P-selectin blockade (84%, P < 0.0001) after 4 hours of IC challenge compared with wild-type mice whereas they were similar for E-selectin−/− and wild-type mice (Figure 3B) ▶ . In general, results obtained for mast cell accumulation in the peritoneum after 4 hours were comparable with those in the skin (Figure 3A) ▶ . By contrast, after 8 hours, the difference observed after 4 hours between groups of mice remained in the peritoneal Arthus reaction while such difference was lost in the cutaneous Arthus reaction except for the significant difference between wild-type mice and L-selectin/ICAM-1−/− mice with blockade of both E- and P-selectins. In addition, unlike the cutaneous Arthus reaction, mast cell recruitment was significantly increased after 8 hours (P < 0.01), but not after 4 hours after IC challenge in L-selectin/ICAM-1−/− mice with blockade of both E- and P-selectin. With regard to both neutrophil and mast cell numbers, results similar to E-selectin−/− and P-selectin−/− mice were obtained using wild-type mice treated with anti-E-selectin mAb and those treated with anti-P-selectin mAb, respectively (data not shown). Furthermore, results comparable with P-selectin blockade in E-selectin−/− mice were observed when E-selectin blockade in P-selectin−/− mice or blockade of both E- and P-selectins in wild-type mice was evaluated (data not shown). There was no neutrophil and mast cell influx in mutant or wild-type mouse controls after intraperitoneal injection of rabbit polyclonal IgG with systemic chicken egg albumin (data not shown). In addition, treatment with irrelevant isotype-matched mAbs instead of anti-P- or E-selectin mAbs did not affect neutrophil and mast cell recruitment (data not shown). Collectively, the effect of the loss or blockade of each adhesion molecule on leukocyte recruitment in the peritoneal Arthus reaction was similar to that observed in the cutaneous Arthus reaction.
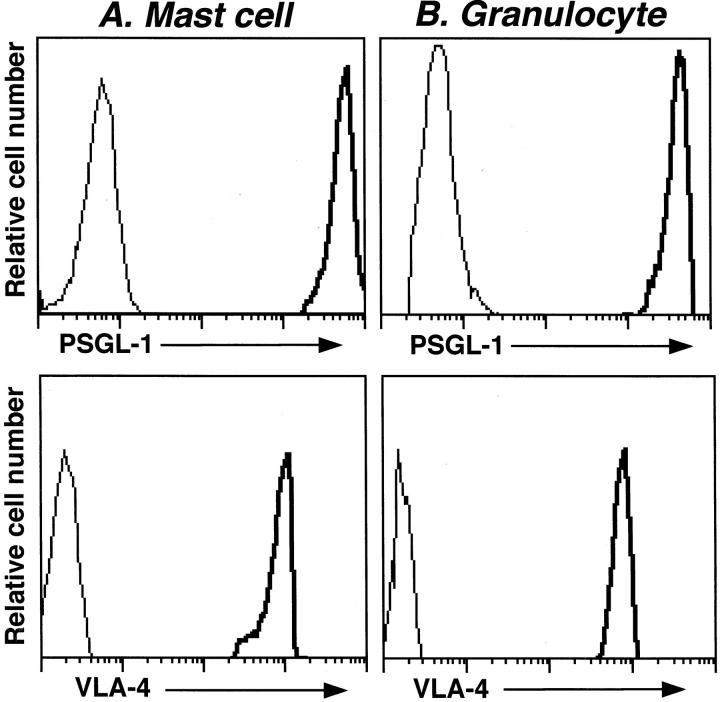
Expression of PSGL-1 and CD49d on Peritoneal Mast Cells
Reduced Arthus reaction-induced mast cell accumulation in the absence or blockade of adhesion molecules suggests a role for P- and E-selectins in mast cell recruitment. PSGL-1 is a dominant physiological ligand for P-selectin, 32,33 but also binds E-selectin. 34 In addition, the slight increase in neutrophil and mast cell numbers was observed in the absence of all selectins and ICAM-1 after 8 hours of IC challenge (Figure 3) ▶ , suggesting that VLA-4 (CD49d/CD29) may be involved in the remaining leukocyte infiltration. Therefore, murine peritoneal mast cells expressing c-kit 30,35 and granulocytes expressing Gr-1 were analyzed for cell surface PSGL-1 and CD49d expression by flow cytometry. PSGL-1 was significantly expressed on the surface of mast cells and granulocytes from wild-type mice compared with staining using an unreactive isotype-matched mAb (Figure 6) ▶ . Similarly, significant CD49d expression on the surface of mast cells and granulocytes from wild-type mice was detected. Deficiency of P- or E-selectin did not influence PSGL-1 or CD49d expression by mast cells or granulocytes (data not shown). Thus, PSGL-1 and CD49d were highly expressed on the surface of mouse peritoneal mast cells.
Figure 6.
Expression of PSGL-1 and CD49d on peritoneal mast cells and granulocytes. Peritoneal cells from untreated wild-type mice were stained with either anti-c-kit-fluorescein isothiocyanate Ab or anti-Gr-1-fluorescein isothiocyanate Ab plus either anti-PSGL-1-phycoerythrin Ab or anti-CD49d-phycoerythrin Ab. Flow cytometric analysis was performed by gating on c-kit-positive mast cells or Gr-1-positive granulocytes. Representative histograms are shown for PSGL-1 or CD49d expression on mast cells (A) or granulocytes (B). The thin lines represent control staining using an unreactive isotype-matched mAb. These results represent those obtained with five wild-type mice.
Cytokine Levels in the Peritoneal Arthus Reaction
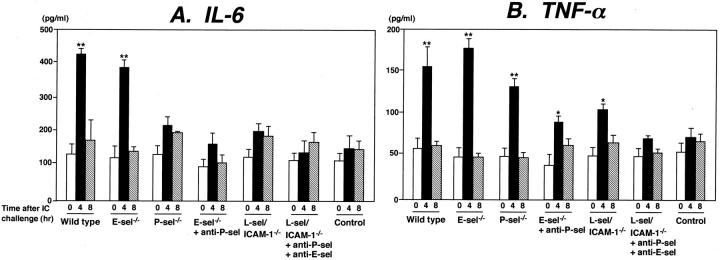
IC-induced inflammation in the peritoneum is associated with the production and release of proinflammatory cytokines, including TNF-α and IL-6, by infiltrating leukocytes. 4,36,37 To assess the relative roles of adhesion molecules in the release of IL-6 and TNF-α during the peritoneal Arthus reaction, IL-6 and TNF-α levels were measured in peritoneal lavage samples after 4 and 8 hours of IC formation. IL-6 levels were increased by 3.4-fold after 4 hours compared with the levels before IC challenge in wild-type mice (P < 0.01), but returned to the baseline levels by 8 hours (Figure 7A) ▶ . A similar increase in IL-6 levels was detected in E-selectin−/− mice. By contrast, IL-6 levels were not significantly elevated after 4 hours in P-selectin−/− mice, E-selectin−/− mice with P-selectin blockade, L-selectin/ICAM-1−/− mice, and L-selectin/ICAM-1−/− mice with both E- and P-selectin blockade: The IL-6 levels in these mice were ∼50 to 70% lower than those found in wild-type mice (P < 0.01).
Figure 7.
Arthus reaction-induced IL-6 (A) and TNF-α (B) production and release in the peritoneal lavage fluid from E-selectin (E-sel)−/− mice, P-selectin−/− mice, E-selectin−/− mice treated with anti-P-selectin mAbs, L-selectin/ICAM-1−/− mice, L-selectin/ICAM-1−/− mice treated with mAbs to both E- and P-selectins, and wild-type mice before and at 4 and 8 hours after IC challenge. IL-6 and TNF-α levels in the peritoneal lavage samples were determined by enzyme-linked immunosorbent assay. Wild-type mice that received an intravenous injection of chicken egg albumin followed by an intraperitoneal injection of polyclonal rabbit IgG served as controls. All values represent the mean ± SEM of results obtained from five to eight mice in each group. *, P < 0.05 and **, P < 0.01 versus levels before IC challenge in each group of mice.
TNF-α levels were increased 2.9-fold after 4 hours relative to the levels before IC challenge in wild-type mice (P < 0.01), but returned to the baseline levels by 8 hours (Figure 7B) ▶ . A similar increase in TNF-α levels was observed in P-selectin−/− as well as E-selectin−/− mice. Furthermore, TNF-α levels were significantly elevated in E-selectin−/− mice with P-selectin blockade and L-selectin/ICAM-1−/− mice after 4 hours (P < 0.05) relative to the baseline levels, but were ∼20 to 40% lower than those found in wild-type mice at the same time point (P < 0.05). By contrast, TNF-α levels in L-selectin/ICAM-1−/− mice with both E- and P-selectin blockade did not significantly increase after 4 hours: the TNF-α levels in these mice were reduced by 54% compared with those of wild-type mice (P < 0.01). There was no increase in IL-6 and TNF-α levels in mutant or wild-type mouse control after intraperitoneal injection of rabbit polyclonal IgG with systemic chicken egg albumin (Figure 7 ▶ and data not shown). Thus, the reduced peritoneal inflammatory responses by loss or blockade of adhesion molecules were generally associated with the reduced TNF-α release, but partly with the decreased IL-6 release.
Discussion
In the present study, IC-induced edema and hemorrhage were significantly inhibited in P-selectin−/− mice compared with wild-type mice, whereas they were not inhibited in E-selectin−/− mice (Figure 1) ▶ . Similar results were obtained using wild-type mice treated with mAbs to either E- or P-selectin. The combined blockade of P- and E-selectins profoundly inhibited the Arthus reaction that resulted in a further reduction compared with loss or blockade of E- or P-selectin alone, indicating the overlapping function of E- and P-selectins. Consistently, the cutaneous contact hypersensitivity response and TNF-α-induced leukocyte rolling are almost eliminated by loss of both E- and P-selectins whereas loss of each molecule alone results in a normal or modest inhibition. 16,17,38 We previously showed that the combined loss of L-selectin and ICAM-1 results in a greater inhibition of the Arthus reaction than the loss of either L-selectin or ICAM-1 alone. 7 However, the inhibitory effect of P- and E-selectin blockade on the Arthus reaction (∼75% inhibition) was significantly greater than that found with deficiency of both L-selectin and ICAM-1 (∼50%, Figure 1 ▶ ). Remarkably, blockade of E- and P-selectins in L-selectin/ICAM-1−/− mice completely eliminated edema and hemorrhage after IC challenge (Figure 2) ▶ , indicating that L-selectin and ICAM-1-independent pathways are dependent on E- and P-selectins. Furthermore, the reduced Arthus reaction by loss or blockade of adhesion molecules correlated with neutrophil and mast cell accumulation in the skin and peritoneum and was associated partly with reduced levels of IL-6 and TNF-α in the peritoneum (Figures 3, 4, 5, and 7) ▶ . Taken together, the results of this study indicate that the endothelial selectins predominantly contribute to the Arthus reaction by regulating the accumulation of mast cells and neutrophils, and also indicate that the full development of the Arthus reaction is mediated cooperatively by all selectin members and ICAM-1.
L-selectin deficiency alone has a significant effect on the inflammatory response in both skin and peritoneum because L-selectin−/− mice have decreased contact hypersensitivity responses, delayed rejection of allogeneic skin transplants, and decreased leukocyte recruitment into an inflamed peritoneum. 21,39,40 By contrast, E-selectin−/− mice generate normal inflammatory responses to peritonitis or contact hypersensitivity responses. 15,38 Consistently, both cutaneous and peritoneal Arthus reaction are inhibited in L-selectin−/− mice 7 but were not reduced in E-selectin−/− mice (Figures 1 and 3) ▶ . P-selectin plays an important role in the peritoneal inflammation because P-selectin−/− mice exhibited reduced leukocyte influx during the peritoneal Arthus reaction (Figure 3) ▶ as well as thioglycollate-induced peritonitis. 31 However, it has been shown that P-selectin deficiency alone does not significantly affect cutaneous inflammation: P-selectin−/− mice show normal or modestly inhibited contact hypersensitivity reactions and normal rejection of allogeneic skin transplants. 38,39,41 In contrast, the current study reveals that P-selectin loss alone significantly inhibited the cutaneous Arthus reaction (Figure 1) ▶ . Previous studies have shown that P-selectin−/− mice have a complete absence of trauma-induced rolling within 1 hour while rolling is not reduced in the venules of P-selectin−/− mice treated with TNF-α for 2 hours, indicating that P-selectin contributes significantly to leukocyte rolling during the early phase of inflammatory responses, but is not strictly required during inflammation at later times. 31,42 However, during the cutaneous Arthus reaction, P-selectin deficiency or blockade inhibited hemorrhage after 8 hours more strongly than edema after 4 hours (Figure 1) ▶ . Thus, the results indicate that P-selectin contributes to prolonged tissue injury as well as early inflammatory response during the cutaneous Arthus reaction, and also suggest that the requirement of P-selectin is influenced by the inflammatory stimuli and tissue site.
The most important finding in this study is the complete inhibition of cutaneous Arthus reaction by loss or blockade of all four adhesion receptors, E-, P-, and L-selectins and ICAM-1 (Figure 2) ▶ . Consistent with this finding, mast cell accumulation in the skin was completely abrogated after IC challenge in these mice (Figures 3A and 5) ▶ ; however, neutrophil numbers in the skin and peritoneum and mast cell numbers in the peritoneum were significantly increased after IC challenge (Figures 3 and 4) ▶ . This raised the possibility that an adhesion pathway independent of all selectin members and ICAM-1 exits. Selectins are bypassed by VCAM-1, the ligand for VLA-4 that also supports leukocyte rolling and adhesion in vitro. 18,19 Furthermore, intravital microscopy studies have shown that a selectin-independent pathway is VLA-4-dependent in the cremasteric microvasculature from mice challenged with TNF-α. 43 A recent study also has shown that VLA-4 is the predominant E- and P-selectin-independent mechanism for leukocyte migration to skin in response to various inflammatory stimuli. 44 Thus, the remaining neutrophil and mast cell accumulation after IC challenge in L-selectin/ICAM-1−/− mice with blockade of both E- and P-selectins may be mediated by VLA-4. Consistently, peritoneal neutrophi1s and mast cells expressed significant levels of CD49d (Figure 6) ▶ . Nonetheless, VLA-4 plays a minor, if any, role in the Arthus reaction because loss or blockade of all selectins and ICAM-1 completely eliminated proinflammatory cytokine release in the peritoneal lavage during the peritoneal Arthus reaction (Figure 7) ▶ , as well as edema and hemorrhage in the skin (Figure 2) ▶ .
Mast cell recruitment into tissues is thought to occur by release of immature mast cell precursors from the bone marrow into the peripheral blood, followed by migration of these precursors into tissues and their subsequent differentiation into mature mast cells. 45 Increased numbers of mast cells are noted at sites of inflammation. 46 Several studies have shown that rolling of immature bone marrow-derived mast cell precursors is mediated by the P-selectin/PSGL-1 pair. 47,48 In the current study, IC challenge induced rapid mature mast cell recruitment that was significantly inhibited by blockade of P-selectin, of both E- and P-selectins, of both L-selectin and ICAM-1, or of all selectins and ICAM-1 (Figures 3 and 5) ▶ . Peritoneal murine mature mast cells express significant levels of PSGL-1 (Figure 6) ▶ , L-selectin, 7 and CD18 (β2 integrin), a ligand for ICAM-1. 7,35 Similar rapid mast cell migration is also observed into the central nervous system from the blood within 1 to 2 hours in response to altered physiological conditions. 49 Additionally, in a chronic contact hypersensitivity model with repeated hapten sensitization for 24 days at 2-day intervals, mast cell number was increased 30 minutes after elicitation on day 24 (Y Shimada and S Sato, submitted). These results suggest that mature mast cells are rapidly recruited to inflammatory sites using multiple adhesion molecules possibly through circulation. Furthermore, our finding that complete inhibition of cutaneous edema and hemorrhage by blockade of all selectins and ICAM-1 was associated with elimination of the rapid recruitment of mast cells, but not neutrophils (Figures 2 to 5) ▶ , indicates that mast cells play a critical role in the progression of the cutaneous Arthus reaction.
Proinflammatory cytokines, including TNF-α and IL-6, are produced and released by mast cells, neutrophils, and monocytes. 50,51 In this study, TNF-α levels after 4 hours in the peritoneal lavage generally correlated with numbers of neutrophils and mast cells (Figures 3B and 7B) ▶ . On the other hand, IL-6 levels increased in wild-type and E-selectin−/− mice after 4 hours whereas they did not increase in P-selectin−/− mice, L-selectin/ICAM-1−/− mice, and E-selectin−/− mice with P-selectin blockade despite the significant infiltration of neutrophils and mast cells into the peritoneum (Figures 3B and 7A) ▶ . Deficiency of adhesion molecules influences not only leukocyte trafficking, but also survival and the state of activation by their role in outside-in signaling. 52 Therefore, it is possible that infiltrating neutrophils and mast cells may be functionally impaired for IL-6 release in the absence of adhesion molecules. Alternatively, although we could not detect significantly increased peritoneal macrophage numbers even after 8 hours (data not shown), functional alteration of residual macrophages may be related to lack of the IL-6 elevation. Remarkably, both TNF-α and IL-6 release was completely eliminated in L-selectin/ICAM-1−/− mice with both E- and P-selectin blockade. The results suggest that multiple adhesion molecules cooperatively control neutrophil and mast cell accumulation or possibly their function after IC challenge and thereby influence the release of proinflammatory cytokines from these leukocytes, which results in the regulation of the Arthus reaction.
Because the passive Arthus reaction does not require antigen sensitization, we can exclude the possible role of ICAM-1 and L-selectin on the sensitization phase because ICAM-1 functions as one of the co-stimulatory molecules on the surface of antigen-presenting cells 53 while L-selectin mediates naive T cells migrating into the draining lymph nodes. 54,55 Therefore, the Arthus reaction is a useful, simplified model to evaluate direct roles of adhesion molecules in effector phase of inflammation. The current study together with the results from our previous study demonstrates that edema and hemorrhage are inhibited by 30 to 40% with L-selectin deficiency, by ∼45% with ICAM-1 deficiency, by 40 to 60% with P-selectin deficiency or blockade, by ∼50% with both L-selectin and ICAM-1 deficiency, by ∼75% with both E- and P-selectin blockade, and by 100% with loss or blockade of all four receptors (Figures 1 and 2) ▶ . 7 This study is the first to reveal the relative contribution and interaction of adhesion molecules to the Arthus reaction. The results of this study indicate that cell adhesion molecules play critical roles with different relative contributions in the progression of the Arthus reaction. Furthermore, our results may provide important basic information for the therapy of IC-mediated human diseases by combined blockade of multiple adhesion molecules.
Figure 4.
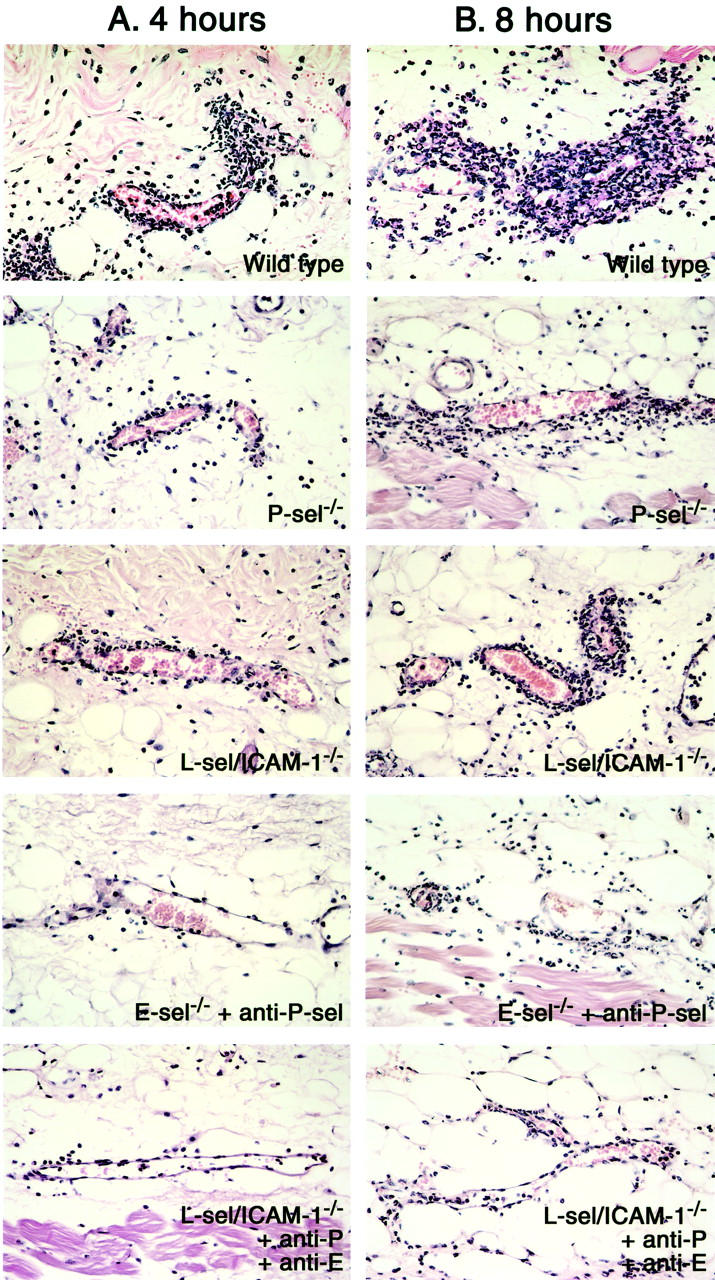
Histological tissue sections showing neutrophil infiltration in the skin of P-selectin (P-sel)−/− mice, E-selectin−/− mice treated with anti-P-selectin mAbs, L-selectin/ICAM-1−/− mice, L-selectin/ICAM-1−/− mice treated with mAbs to both E- and P-selectins, and wild-type mice at 4 (A) and 8 hours (B) after IC challenge. Neutrophils were revealed by H&E staining. Original magnifications, ×100.
Figure 5.
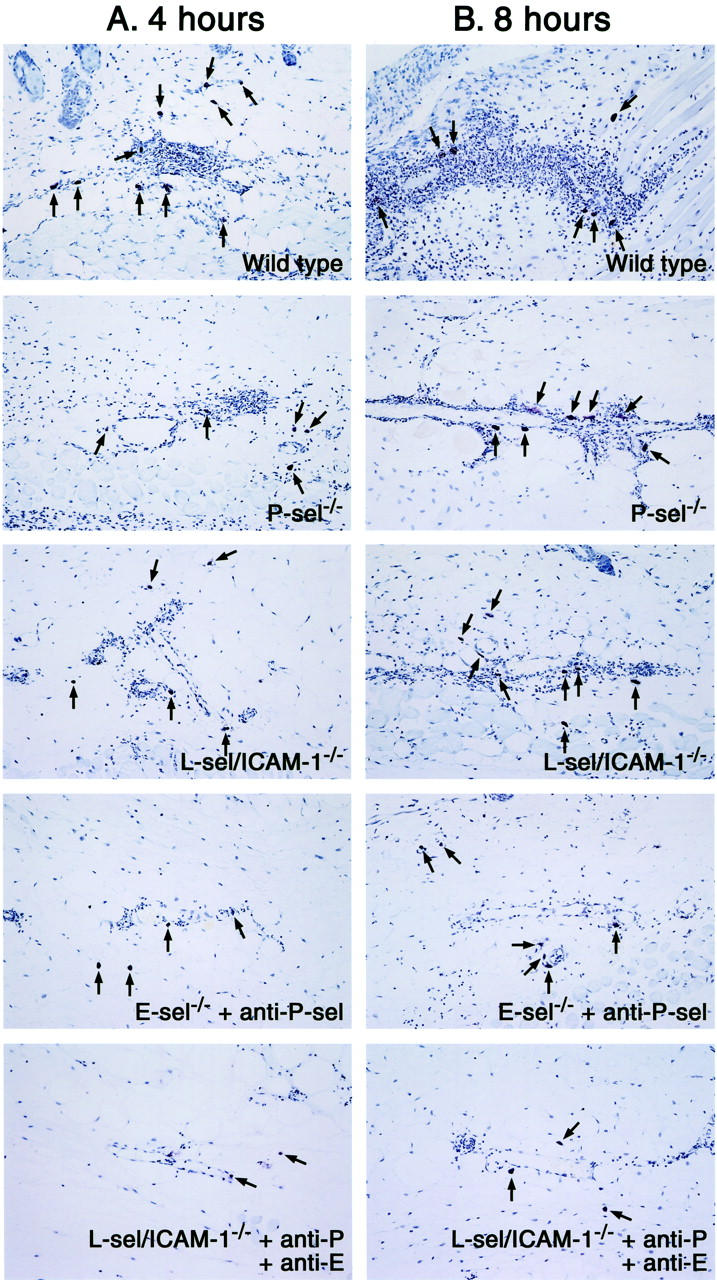
Histological tissue sections showing mast cell accumulation in the skin of P-selectin (P-sel)−/− mice, E-selectin−/− mice treated with anti-P-selectin mAbs, L-selectin/ICAM-1−/− mice, L-selectin/ICAM-1−/− mice treated with mAbs to both E- and P-selectins, and wild-type mice at 4 (A) and 8 hours (B) after IC challenge. Mast cells (arrows) were detected as cells with metachromatic staining of granules in toluidine blue-stained sections. Original magnifications, ×50.
Footnotes
Address reprint requests to Shinichi Sato, M.D., Ph.D., Department of Dermatology, Kanazawa University Graduate School of Medical Science, 13-1 Takaramachi, Kanazawa, Ishikawa 920-8641, Japan. E-mail: s-sato@med.kanazawa-u.ac.jp.
Supported by a grant-in-aid from the Ministry of Education, Science, and Culture of Japan (to S. S.) and the National Institutes of Health (grants CA54464 and CA81776 to T. F. T.).
References
- 1.Kohl J, Gessner JE: On the role of complement and Fc γ-receptors in the Arthus reaction. Mol Immunol 1999, 36:893-903 [DOI] [PubMed] [Google Scholar]
- 2.Arthus M: Injections répetées de serum de cheval chez le lapin. V R Soc Biol 1903, 55:817-820 [Google Scholar]
- 3.Baumann U, Kohl J, Tschernig T, Schwerter-Strumpf K, Verbeek JS, Schmidt RE, Gessner JE: A codominant role of FcγRI/III and C5aR in the reverse Arthus reaction. J Immunol 2000, 164:1065-1070 [DOI] [PubMed] [Google Scholar]
- 4.Hopken UE, Lu B, Gerard NP, Gerard C: Impaired inflammatory responses in the reverse Arthus reaction through genetic deletion of the C5a receptor. J Exp Med 1997, 186:749-756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sylvestre DL, Ravetch JV: Fc receptors initiate the Arthus reaction: redefining the inflammatory cascade. Science 1994, 265:1095-1098 [DOI] [PubMed] [Google Scholar]
- 6.Baumann U, Chouchakova N, Gewecke B, Kohl J, Carroll MC, Schmidt RE, Gessner JE: Distinct tissue site-specific requirements of mast cells and complement components C3/C5a receptor in IgG immune complex-induced injury of skin and lung. J Immunol 2001, 167:1022-1027 [DOI] [PubMed] [Google Scholar]
- 7.Kaburagi Y, Hasegawa M, Nagaoka T, Shimada Y, Hamaguchi Y, Komura K, Saito E, Yanaba K, Takehara K, Kadono T, Steeber DA, Tedder TF, Sato S: The cutaneous reverse Arthus reaction requires intercellular adhesion molecule 1 and L-selectin expression. J Immunol 2002, 168:2970-2978 [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Ramos BF, Jakschik BA: Augmentation of reverse Arthus reaction by mast cells in mice. J Clin Invest 1991, 88:841-846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Springer TA: Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol 1995, 57:827-872 [DOI] [PubMed] [Google Scholar]
- 10.Butcher EC: Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell 1991, 67:1033-1036 [DOI] [PubMed] [Google Scholar]
- 11.Tedder TF, Li X, Steeber DA: The selectins and their ligands: adhesion molecules of the vasculature. Adv Mol Cell Biol 1999, 28:65-111 [Google Scholar]
- 12.Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA: Induction by IL-1 and interferon-γ: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol 1986, 137:245-253 [PubMed] [Google Scholar]
- 13.Ley K, Tedder TF: Leukocyte interactions with vascular endothelium: new insights into selectin-mediated attachment and rolling. J Immunol 1995, 155:525-528 [PubMed] [Google Scholar]
- 14.Robinson SD, Frenette PS, Rayburn H, Cummiskey M, Ullman-Cullere M, Wagner DD, Hynes RO: Multiple, targeted deficiencies in selectins reveal a predominant role for P-selectin in leukocyte recruitment. Proc Natl Acad Sci USA 1999, 96:11452-11457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labow MA, Norton CR, Rumberger JM, Lombard-Gillooly KM, Shuster DJ, Hubbard J, Bertko R, Knaack PA, Terry RW, Harbison ML, Kontgen F, Stewart CL, McIntyre KW, Will PC, Burns DK, Wolitzky BA: Characterization of E-selectin-deficient mice: demonstration of overlapping function of the endothelial selectins. Immunity 1994, 1:709-720 [DOI] [PubMed] [Google Scholar]
- 16.Frenette PS, Mayadas TN, Rayburn H, Hynes RO, Wagner DD: Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectins. Cell 1996, 84:563-574 [DOI] [PubMed] [Google Scholar]
- 17.Bullard DC, Kunkel EJ, Kubo H, Hicks MJ, Lorenzo I, Doyle NA, Koerschuk CM, Ley K, Beaudet AL: Infectious susceptibility and severe deficiency of leukocyte rolling and recruitment in E-selectin and P-selectin double mutant mice. J Exp Med 1996, 183:2329-2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alon R, Kassner PD, Carr MC, Finger EB, Hemler ME, Springer TA: The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J Cell Biol 1995, 128:1243-1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berlin C, Bargatze RF, Campbell JJ, von Andrian UH, Szabo MC, Hasslen SR, Nelson RD, Berg EL, Erlandsen SL, Butcher EC: α4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell 1995, 80:413-422 [DOI] [PubMed] [Google Scholar]
- 20.Steeber DA, Campbell MA, Basit A, Ley K, Tedder TF: Optimal selectin-mediated rolling of leukocytes during inflammation in vivo requires intercellular adhesion molecule-1 expression. Proc Natl Acad Sci USA 1998, 95:7562-7567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steeber DA, Tang MLK, Green NE, Zhang X-Q, Sloane JE, Tedder TF: Leukocyte entry into sites of inflammation requires overlapping interaction between the L-selectin and ICAM-1 pathways. J Immunol 1999, 163:2176-2186 [PubMed] [Google Scholar]
- 22.Nagaoka T, Kaburagi Y, Hamaguchi Y, Hasegawa M, Takehara K, Steeber DA, Tedder TF, Sato S: Delayed wound healing in the absence of intercellular adhesion molecule-1 or L-selectin expression. Am J Pathol 2000, 157:237-247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bullard DC, Qin L, Lorenzo I, Quinlin WM, Doyle NA, Bosse R, Vestweber D, Doerschuk CM, Beaudet AL: P-selectin/ICAM-1 double mutant mice: acute emigration of neutrophils into the peritoneum is completely absent but is normal into pulmonary alveoli. J Clin Invest 1995, 95:1782-1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunkel EJ, Jung U, Bullard DC, Norman KE, Wolitzky BA, Vestweber D, Beaudet AL, Ley K: Absence of trauma-induced leukocyte rolling in mice deficient in both P-selectin and intercellular adhesion molecule-1 (ICAM-1). J Exp Med 1996, 183:57-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ley K, Allietta M, Bullard DC, Morgan S: Importance of E-selectin for firm leukocyte adhesion in vivo. Circ Res 1998, 83:287-294 [DOI] [PubMed] [Google Scholar]
- 26.Scalia R, Armstead VE, Minchenko AG, Lefer AM: Essential role of P-selectin in the initiation of the inflammatory response induced by hemorrhage and reinfusion. J Exp Med 1999, 189:931-938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohnishi M, Koike H, Kawamura N, Tojo SJ, Hayashi M, Morooka S: Role of P-selectin in the early stage of the Arthus reaction. Immunopharmacology 1996, 34:161-170 [DOI] [PubMed] [Google Scholar]
- 28.Bosse R, Vestweber D: Only simultaneous blocking of the L- and P-selectin completely inhibits neutrophil migration into mouse peritoneum. Eur J Immunol 1994, 24:3019-3024 [DOI] [PubMed] [Google Scholar]
- 29.Sato S, Miller AS, Inaoki M, Bock CB, Jansen PJ, Tang MLK, Tedder TF: CD22 is both a positive and negative regulator of B lymphocyte antigen receptor signal transduction: altered signaling in CD22-deficient mice. Immunity 1996, 5:551-562 [DOI] [PubMed] [Google Scholar]
- 30.Gommerman JL, Oh DY, Zhou X, Tedder TF, Maurer M, Galli SJ, Carroll MC: A role for CD21/CD35 and CD19 in responses to acute septic peritonitis: a potential mechanism for mast cell activation. J Immunol 2000, 165:6915-6921 [DOI] [PubMed] [Google Scholar]
- 31.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD: Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell 1993, 74:541-554 [DOI] [PubMed] [Google Scholar]
- 32.Moore KL, Patel KD, Breuhl RE, Fugang L, Johnson DA, Lichenstein HS, Cummings RD, Bainton DF, McEver RP: P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J Cell Biol 1995, 128:661-671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norman KE, Moore KL, McEver RP, Ley K: Leukocyte rolling in vivo is mediated by P-selectin glycoprotein ligand-1. Blood 1996, 86:4417-4421 [PubMed] [Google Scholar]
- 34.Asa D, Raycroft L, Ma L, Aeed PA, Kaytes PS, Elhammer AP, Geng JG: The P-selectin glycoprotein ligand functions as a common human leukocyte ligand for P- and E-selectins. J Biol Chem 1995, 270:11662-11670 [DOI] [PubMed] [Google Scholar]
- 35.Rosenkranz AR, Coxon A, Maurer M, Gurish MF, Austen KF, Friend DS, Galli SJ, Mayadas TN: Impaired mast cell development and innate immunity in Mac-1 (CD11b/CD18, CR3)-deficient mice J Immunol 1998, 161:6463-6467 [PubMed] [Google Scholar]
- 36.Heller T, Gessner JE, Schmidt RE, Klos A, Bautsch W, Kohl J: Fc receptor type I for IgG on macrophages and complement mediate the inflammatory response in immune complex peritonitis. J Immunol 1999, 162:5657-5661 [PubMed] [Google Scholar]
- 37.Zhang Y, Ramos BF, Jakschik BA: Neutrophil recruitment by tumor necrosis factor from mast cells in immune complex peritonitis. Science 1992, 258:1957-1959 [DOI] [PubMed] [Google Scholar]
- 38.Staite ND, Justen JM, Sly LM, Beaudet AL, Bullard DC: Inhibition of delayed-type contact hypersensitivity in mice deficient in both E-selectin and P-selectin. Blood 1996, 88:2973-2979 [PubMed] [Google Scholar]
- 39.Tang MLK, Hale LP, Steeber DA, Tedder TF: L-selectin is involved in lymphocyte migration to sites of inflammation in the skin: delayed rejection of allografts in L-selectin-deficient mice. J Immunol 1997, 158:5191-5199 [PubMed] [Google Scholar]
- 40.Tedder TF, Steeber DA, Pizcueta P: L-selectin deficient mice have impaired leukocyte recruitment into inflammatory sites. J Exp Med 1995, 181:2259-2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Subramaniam M, Saffaripour S, Watson SR, Mayadas TN, Hynes RO, Wagner DD: Reduced recruitment of inflammatory cells in a contact hypersensitivity response in P-selectin-deficient mice. J Exp Med 1995, 181:2277-2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ley KE, Bullard D, Arbones ML, Bosse R, Vestweber D, Tedder TF, Beaudet AL: Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J Exp Med 1995, 181:669-675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCafferty DM, Kanwar S, Granger DN, Kubes P: E/P-selectin-deficient mice: an optimal mutation for abrogating antigen but not tumor necrosis factor-α-induced immune responses. Eur J Immunol 2000, 30:2362-2371 [DOI] [PubMed] [Google Scholar]
- 44.Issekutz AC, Issekutz TB: The role of E-selectin, P-selectin, and very late activation antigen-4 in T lymphocyte migration to dermal inflammation. J Immunol 2002, 168:1934-1939 [DOI] [PubMed] [Google Scholar]
- 45.Kirshenbaum AS, Kessler SW, Goff JP, Metcalfe DD: Demonstration of the origin of human mast cells from CD34+ bone marrow progenitor cells. J Immunol 1991, 146:1410-1415 [PubMed] [Google Scholar]
- 46.Ishizaka T, Ishizaka K: Biology of immunoglobulin E. Molecular basis of reaginic hypersensitivity. Prog Allergy 1975, 19:60-121 [PubMed] [Google Scholar]
- 47.Sriramarao P, Anderson W, Wolitzky BA, Broide DH: Mouse bone marrow-derived mast cells roll on P-selectin under conditions of flow in vivo. Lab Invest 1996, 74:634-643 [PubMed] [Google Scholar]
- 48.Steegmaier M, Blanks JE, Borges E, Vestweber D: P-selectin glycoprotein ligand-1 mediates rolling of mouse bone marrow-derived mast cells on P-selectin but not efficiently on E-selectin. Eur J Immunol 1997, 27:1339-1345 [DOI] [PubMed] [Google Scholar]
- 49.Silverman AJ, Sutherland AK, Wilhelm M, Silver R: Mast cells migrate from blood to brain. J Neurosci 2000, 20:401-408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vassalli P: The pathophysiology of tumor necrosis factors. Annu Rev Immunol 1992, 10:411-452 [DOI] [PubMed] [Google Scholar]
- 51.Akira S, Taga T, Kishimoto T: Interleukin-6 in biology and medicine. Adv Immunol 1993, 54:1-78 [DOI] [PubMed] [Google Scholar]
- 52.Longhurst CM, Jennings LK: Integrin-mediated signal transduction. Cell Mol Life Sci 1998, 54:514-526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Seventer GA, Shimizu Y, Horgan KJ, Shaw S: The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol 1990, 144:4579-4586 [PubMed] [Google Scholar]
- 54.Catalina MD, Carroll MC, Arizpe H, Takashima A, Estess P, Siegelman MH: The route of antigen entry determines the requirement for L-selectin during immune responses. J Exp Med 1996, 184:2341-2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu J, Grewal IS, Geba GP, Flavell RA: Impaired primary T cell responses in L-selectin-deficient mice. J Exp Med 1996, 183:589-598 [DOI] [PMC free article] [PubMed] [Google Scholar]