Abstract
The pathogeneses of toxic epidermal necrolysis (TEN) and Stevens-Johnson syndrome (SJS), both severe blistering diseases usually associated with drug intake, are not fully elucidated. Histologically, both TEN and SJS are characterized by extensive keratinocyte apoptosis. Previous studies have shown that keratinocyte apoptosis in TEN and SJS was induced by a suicidal interaction between Fas and Fas ligand (FasL), which are both expressed by keratinocytes. However, our preliminary examinations demonstrated that FasL is hardly detected on keratinocytes. We hypothesized that soluble FasL (sFasL) is secreted by peripheral blood mononuclear cells (PBMCs), and this interacts with the Fas expressed on keratinocytes in TEN and SJS. To justify this hypothesis, we investigated whether sFasL secreted by PBMCs could induce the keratinocyte apoptosis in TEN and SJS. Enzyme-linked immunosorbent assay analysis demonstrated that there was no significant sFasL increase in any samples of healthy controls (<40 pg/ml, n = 14) and patients with an ordinary erythema multiforme-type drug eruption (41.5 ± 3.1 pg/ml, n = 14), whereas high concentrations are detected in all samples of TEN and SJS patients (TEN: 131.5 ± 57.4 pg/ml, n = 8; SJS: 119.1 ± 41.0 pg/ml, n = 14) (P < 0.0001). In vitro analysis using cultured keratinocytes revealed that the sera of TEN and SJS patients induced abundant keratinocyte apoptosis compared to erythema multiforme-type drug eruption sera. Furthermore, on stimulation with the causal drug, PBMCs obtained from TEN and SJS patients secreted high levels of sFasL. Taken together, these results indicate that sFasL secreted by PBMCs, not keratinocytes, plays a crucial role in the apoptosis and pathomechanism of TEN and SJS, and that the serum sFasL level may be a good indicator for the early diagnosis of TEN and SJS.
Toxic epidermal necrolysis (TEN) and Stevens-Johnson syndrome (SJS) are severe blistering diseases mostly caused by drug intake with no effective treatment. 1,2 Histologically, TEN and SJS are characterized by marked keratinocyte apoptosis in the epidermis with dermo-epidermal separation, resulting in bullae. 3 The pathophysiology of these diseases is not well known, although immune mechanisms 4 and altered drug metabolism 5 have been postulated.
Recent data suggested that the activation of Fas through FasL is an initial important step leading to diffuse apoptotic cell death of epidermal cells in TEN. 6 FasL mediates apoptotic cell death by binding to Fas, inducing the activation of caspases. 7 Fas is expressed almost ubiquitously in a variety of cells, including keratinocytes, 8 whereas FasL is mainly expressed in activated T cells and natural killer cells. 9 Although Viard and colleagues 6 reported that FasL is also expressed in keratinocytes of TEN lesions, it remains controversial whether keratinocytes express biofunctional forms of FasL. 10 Soluble FasL (sFasL), which is cleaved from a membrane-bound FasL by a matrix metalloproteinase-like enzyme, 11,12 also has the potential to mediate apoptosis. 13
In this study, we investigated whether significant amounts of sFasL are secreted by peripheral blood mononuclear cells (PBMCs) in TEN and SJS, and if so, whether the sFasL could induce apoptosis in keratinocytes in TEN and SJS lesions. We demonstrated increased serum sFasL concentrations in all samples of TEN and SJS patients, whereas there was no significant increase of sFasL in any samples from patients with ordinary erythema multiforme-type drug eruptions (EMDE) and healthy controls. Secondly, Fas, but not FasL expression was detected on keratinocytes from TEN patients as well as normal skin. Thirdly, the sera of TEN and SJS patients mediated keratinocyte apoptosis in vitro, and lastly, sFasL secretion and FasL PBMC mRNA levels were up-regulated by the causal drug.
In conclusion, sFasL predominantly produced by PBMCs in TEN and SJS plays a pivotal role in the pathomechanism of TEN, and serum sFasL levels may be a good indicator for the early diagnosis in TEN and SJS.
Materials and Methods
Reagents
Anti-FasL, Fas monoclonal antibody (mAb), and fluorescein isothiocyanate-conjugated rabbit anti-mouse IgG were purchased from PharMingen (San Diego, CA). Soluble FasL was purchased from PeproTech (London, UK). The reagents for the terminal dUTP nick-end labeling (TUNEL) assay were purchased from WAKO (Tokyo, Japan). All other chemicals were of reagent grade.
Patients
Under a protocol approved by the institutional review board, sera were obtained from TEN patients (three men and five women; average age, 42 ± 20 years) and SJS patients (six men and eight women; average age, 37 ± 15 years) with actively progressing reactions meeting the criteria for TEN and SJS as previously defined; 14 SJS refers to cases with less than 10% of body surface involvement and TEN to those with more than 10% involvement. In all cases, medication was suspected of causing the eruption. The extent of detachment (erosion, blisters, and areas with positive Nikolsky sign) was measured and expressed as the percentage of body surface area that was detached. Sera were also obtained early at the start of the disease course. Skin biopsies were obtained from three patients. Sera of patients with EMDE (eight men and six women; average age, 40 ± 10 years) and normal healthy volunteers (eight men and six women; average age, 43 ± 17 years) were also obtained. Discarded normal skin material from skin surgery served as controls.
Tissue Preparation
The biopsied skin samples were immediately placed in embedding medium (TissueTek; Miles Scientific, Naperville, IL), frozen on dry ice and stored at −80°C until use.
Enzyme-Linked Immunosorbent Assay (ELISA) for Soluble FasL
The concentrations of sFasL were determined by sFasL ELISA kit (MBL, Nagoya, Japan). The developed reaction was quantified by reading at 490 nm. The limit of detection was 40 pg/ml. Each individual sample was analyzed in triplicate.
Cell Culture
Cultured normal human keratinocytes (second passage) were obtained from Kurabo (Osaka, Japan). The cultures were fed on eight-well slide plates (Corning-Costar, Cambridge, UK) in a medium of serum-free modified MCDB 153 supplemented with hydrocortisone (0.5 μg/ml), insulin (5 μg/ml), epidermal growth factor (10 ng/ml), bovine pituitary extracts (150 μg/ml), and calcium (0.15 mmol/L). The keratinocytes were grown in a humidified atmosphere under 5% CO2 at 37°C. The tertiary cultured cells were harvested at 70 to 80% confluence for assay
Immunohistochemistry
The skin sections were incubated with anti-FasL mAb or anti-Fas mAb at room temperature for 1 hour, followed by incubation with fluorescein isothiocyanate-conjugated rabbit anti-mouse IgG at room temperature for 1 hour. Then, the sections were examined using a confocal laser microscope (Laser Scanning Confocal Imaging System MRC 1024; Bio-Rad, Richmond, CA) equipped with a fluorescence microscope (Zeiss, Oberkochen, Germany).
Nick-End Labeling of DNA from Apoptotic Keratinocytes
Keratinocytes were plated into culture plates at a density of 1 × 106 cells/ml, and cultured until just before confluence. Then, the cells were incubated at 37°C for 24 hours with medium containing healthy control sera, EMDE patients sera, TEN patients sera, or TEN patients sera plus an inhibitory anti-FasL mAb (NOK-2) or the negative control mAb. Apoptotic cells were identified by histochemical techniques by staining for double-stranded DNA breaks. TUNEL was performed as previously described. 15 Briefly, TUNEL reaction mixture was added to the samples, and followed by a 60-minute incubation at 37°C. Incorporated fluorescein was detected by anti-fluorescein mAb Fab fragments from sheep, conjugated with alkaline phosphatase.
Propidium Iodide Staining from Apoptotic Keratinocytes
Alternatively, cell apoptosis was measured by flow cytometry after propidium iodide staining. Detached cells and trypsinized adherent cells were collected, fixed in 70% ethanol for 1 hour on ice, washed with phosphate-buffered saline, and treated for 15 minutes with RNase (200 μg/ml) at room temperature. Cells were stained with propidium iodide (5 μg/ml) and analyzed on a BD PharMingen cell sorter.
In Vitro Lymphocyte Stimulation
Freshly isolated PBMCs from a TEN patient (65-year-old man; causal drug, carbamazepine) was used for the in vitro lymphocyte stimulation assay. PBMCs (1 × 106/ml) were cultured with carbamazepine (10 or 100 μg/ml) for 24 hours. The supernatants were subsequently analyzed for sFasL. PBMCs were collected to analyze FasL mRNA expression.
Reverse Transcriptase-Polymerase Chain Reaction Analysis of FasL mRNA
FasL mRNA expression was determined by reverse transcriptase-polymerase chain reaction. Total RNA was isolated from cells using TRIzol (Life Technologies, Inc., Rockville, MD) according to the manufacturer’s instructions. The sequences of the primer pairs in this experiment were as follows: human glyceraldehyde-3-phosphate dehydrogenase (G3PDH, used as an internal control), 5′-GCT CAG ACA CCA TGG GGA AGG T-3′, 5′-GTG GTG CAG GAG GCA TTG CTG A-3′; human FasL, 5′-GTG CCC AGA AGG CCT GGT CAA AGG-3′, 5′-TTG CAA GAT TGA CCC CGG AAG TAT-3′. Amplifications were performed in a thermocycler (GeneAmp PCR system 9600; Perkin-Elmer, Norwalk, CT) as follows: 94°C for 3 minutes, followed by 21 (G3PDH) or 35 (FasL) cycles (94°C for 1 minute; 57°C for 1.5 minutes; 72°C for 2 minutes). Aliquots of each amplification were analyzed by electrophoresis in 5% acrylamide-Tris-borate gels. The lanes were normalized to G3PDH mRNA levels as described.
Statistical Analysis
Results are shown as mean ± SD. A paired Student’s t-test was used for comparison of paired conditions.
Results
Serum sFasL Level Was Increased in TEN and SJS Patients
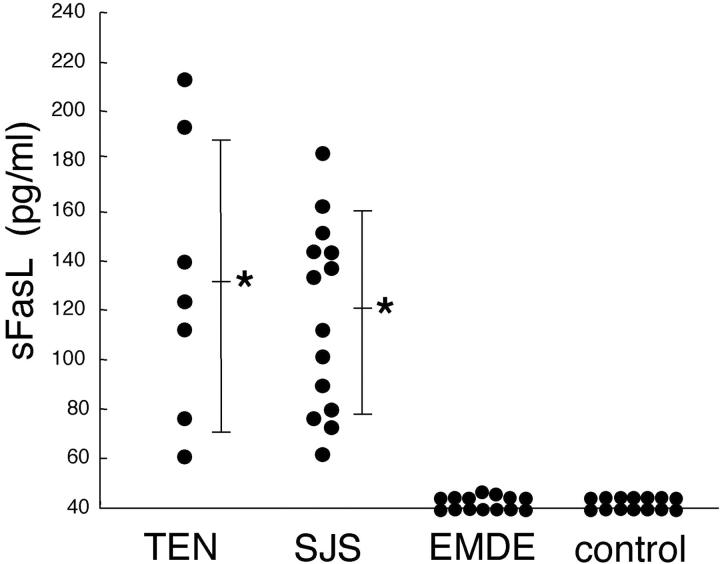
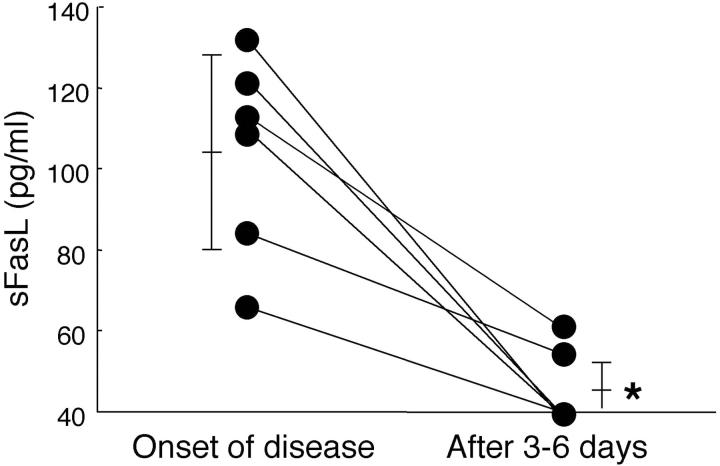
The serum levels of sFasL were examined in patients with TEN (n = 8), SJS (n = 14), EMDE (n = 14), and healthy controls (n = 14). Serum sFasL concentrations increased in all samples from TEN and SJS patients (131.5 ± 57.4 pg/ml and 119.1 ± 41.0 pg/ml, respectively; mean ± SD), whereas there were no significant increases in sFasL from any samples of patients with ordinary EMDE (42.1 ± 3.5 pg/ml) (P < 0.0001) or healthy controls (not detected in any samples) (P < 0.0001) (Figure 1) ▶ . There was no significant difference between serum sFasL levels in TEN and SJS patients (P = 0.26). Time course analysis of serum sFasL level in six TEN and SJS patients demonstrated significant sFasL reduction within 4 to 6 days after initial analysis (105.5 ± 25.4 pg/ml to 46.7 ± 10.4 pg/ml, n = 6; P < 0.001) (Figure 2) ▶ . In contrast to serum sFasL levels, the percentage area of skin detachment did not change or worsen compared to that of the disease onset in these patients (data not shown). All these patients were treated with high-dose corticosteroids (1 mg/kg/day).
Figure 1.
High levels of sFasL were detected in serum samples from patients only with TEN and SJS, but not with EMDE or healthy controls. Serum sFasL was examined by sFasL ELISA described in the Materials and Methods. Serum sFasL concentrations increased in all samples of TEN (n = 8) and SJS (n = 14) patients, whereas there was no significant increase in sFasL in any samples of patients with EMDE (n = 14) or healthy controls (n = 14) (the limit of detection was 40 pg/ml). The bar indicates average values and an asterisk indicates statistical significance as compared with EMDE or controls (*, P < 0.0001).
Figure 2.
Serum sFasL in patients with TEN and SJS decreased significantly between 3 to 6 days after the start of the disease course. Sera from six patients with TEN and SJS were obtained at the beginning of the disease and after 3 to 6 days. Levels of sFasL significantly declined from 105.5 ± 25.4 pg/ml to 46.7 ± 10.4 pg/ml during the 3 to 6 days after the disease onset (n = 6; *, P < 0.01).
Fas and FasL Expression and Apoptosis in Skin Lesions of TEN
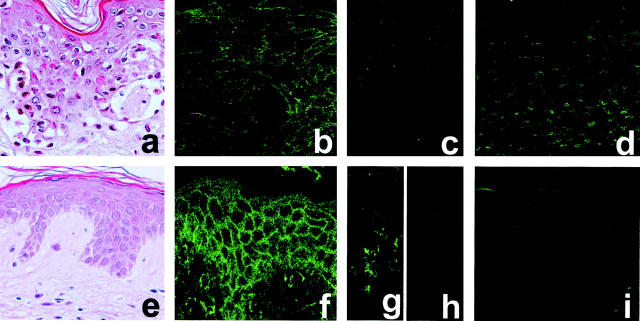
To assess the possibility that the interaction of Fas and FasL induces keratinocyte cell death leading to TEN and SJS, we analyzed Fas and FasL expression in skin samples from individuals with TEN (n = 3) and healthy controls (n = 3). Immunohistochemical analysis of adjacent frozen skin sections revealed that keratinocytes in both TEN patients and healthy controls similarly expressed Fas molecules on the cell surface (Figure 3, b and f) ▶ . Although previous reports showed that keratinocytes in TEN patients expressed high levels of FasL, 6 we could not detect FasL expression on keratinocytes in TEN patients or in control samples (Figure 3, c and h) ▶ . However, inflammatory cells in a lesion of contact dermatitis expressed FasL as previously reported (Figure 3g) ▶ . 16 The TUNEL assay demonstrated that skin sections from individuals with TEN showed numerous keratinocyte apoptotic figures, whereas no apoptotic keratinocytes were detected in normal control epidermis (Figure 3, d and i) ▶ . These observations are consistent with a previous report. 3
Figure 3.
Fas is expressed on keratinocytes in TEN skin lesions, however FasL expression is not detected. Skin sections obtained from TEN patients (a–d) or healthy controls (e, f, h, i) were subjected to H&E staining (a, e), immunohistochemical staining with anti-Fas (b, f) or Fas L mAb (c, h), or TUNEL analysis (d, i). Histological examinations showed numerous apoptotic keratinocytes (H&E) (a). Fas and FasL expression in skin were analyzed as described in the Materials and Methods. Fas expression was observed in keratinocytes of TEN skin lesions as well as normal controls (b, f), whereas no apparent FasL expression was observed in keratinocytes of both samples (c, h). FasL expression was detected in lesion of contact dermatitis (g). In the TUNEL assay, apoptotic cells were identified in situ by histochemical techniques with staining of double-stranded DNA breaks as described in the Materials and Methods. Skin sections from individuals with TEN showed numerous keratinocyte apoptotic figures (d), whereas no apoptoses were detected in normal skin (i).
Keratinocyte Apoptosis Is Induced by the Sera from TEN Patients
To assess the possibility that the sFasL in patients’ sera was the critical mediator of TEN and SJS, we investigated whether sera of TEN patients could induce apoptosis in cultured keratinocytes. The TUNEL assay and the propidium iodide staining with Sub-G0 DNA content demonstrated that the addition of TEN patient sera to the medium significantly induced keratinocyte apoptosis (Figure 4, a and c) ▶ , whereas keratinocytes cultured with medium containing EMDE patient sera showed significantly less apoptotic cell death (Figure 4, b and d) ▶ . In addition, keratinocyte apoptosis was induced by TEN serum in a dose-dependent manner (Figure 4e) ▶ .
Figure 4.
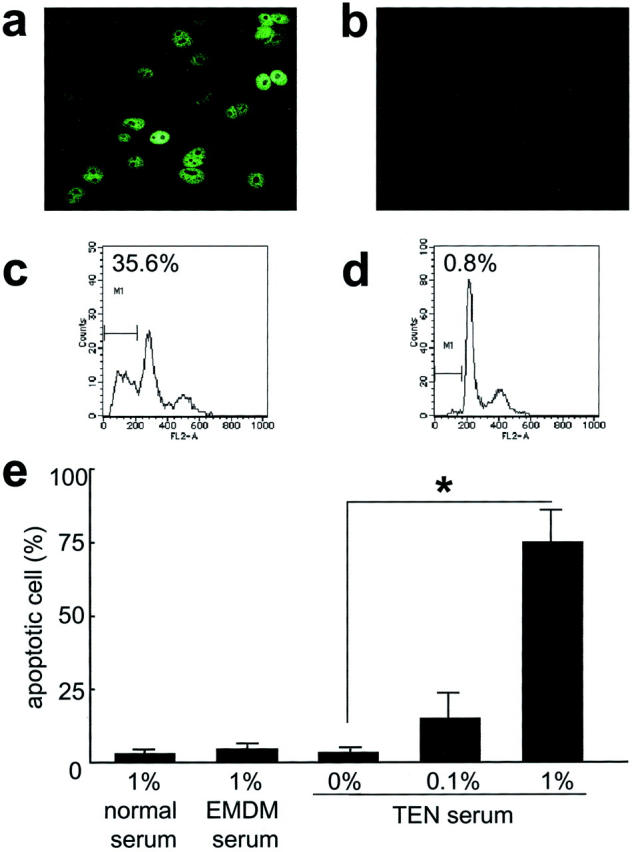
Keratinocyte apoptosis is induced by sera of TEN and SJS patients. Human keratinocytes were incubated at 37°C for 24 hours with medium containing 1% serum healthy control sera or 0.1% and 1% TEN patients’ sera. Apoptotic cells were identified by the TUNEL assay (a, b) or propidium iodide staining using FACS analysis (c, d). Sera of TEN patients induced a greater number of apoptotic keratinocytes (a, c), whereas keratinocytes cultured in medium containing normal serum showed significantly less apoptotic cell death (b, d). Apoptotic cells identified by the TUNEL assay were measured (e). *, P < 0.001.
Keratinocyte Apoptosis Induced by Sera of TEN Patients Is Mediated through Fas-FasL Interaction
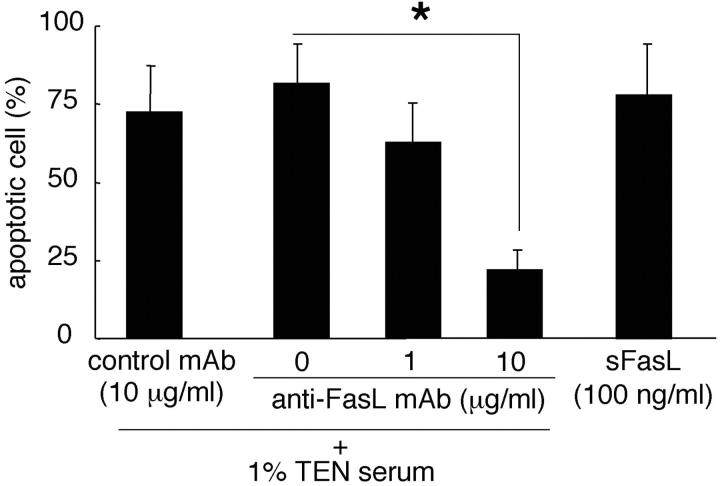
To prove that apoptosis is mediated Fas-FasL interaction we performed the in vitro keratinocyte apoptosis assay using various TEN serum concentrations, the neutralizing anti-FasL mAb, the negative control mAb, and sFasL. Culture with an inhibitory anti-FasL mAb reduced the number of apoptotic cells in a dose-dependent manner, whereas the negative control mAb failed to mediate apoptosis (Figure 5) ▶ . Soluble FasL alone was confirmed to trigger keratinocyte apoptosis. These data strongly support the idea that TEN patients’ sera mediates keratinocyte apoptotic death involving Fas and sFasL interaction.
Figure 5.
Keratinocyte apoptosis induced by sera of TEN and SJS patients was mediated through Fas-FasL interaction. Human keratinocytes were incubated at 37°C for 24 hours with medium containing 1% TEN patients’ sera plus an inhibitory anti-FasL mAb (1 and 10 μg/ml) or sFasL (100 ng/ml). Apoptotic cells were identified by the TUNEL assay. Culture with an inhibitory anti-FasL mAb (10 μg/ml) significantly reduced the number of apoptotic cells in a dose-dependent manner. Furthermore, sFasL mediated keratinocyte apoptosis. *, P < 0.001.
PBMCs of TEN Patients Produce sFasL after the Stimulation with the Causal Drug
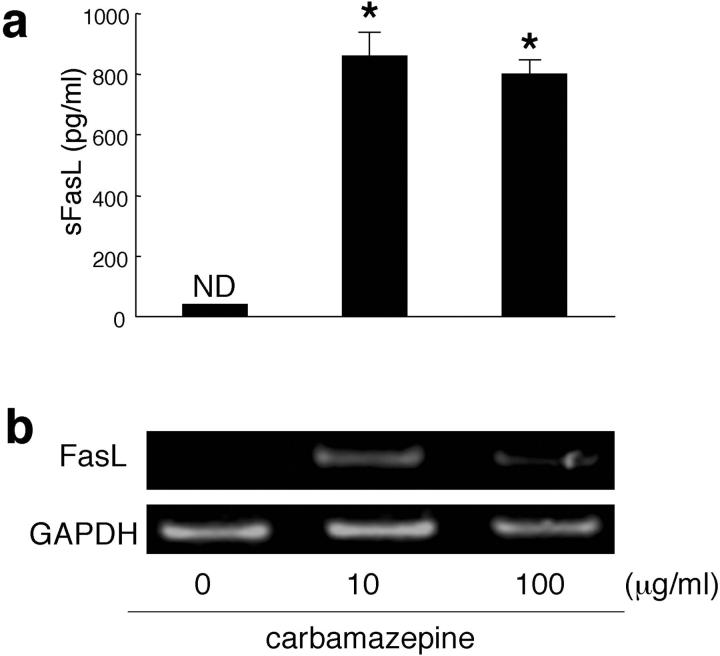
To analyze the origin of sFasL detected in TEN and SJS sera, we assessed whether sFasL is produced by PBMCs after stimulation with the causal drug. PBMCs freshly isolated from a TEN patient (65-year-old man; causal drug, carbamazepine) were cultured with the causal drug, carbamazepine (10 or 100 μg/ml), for 24 hours and culture supernatants were examined for sFasL. High amounts of sFasL were detected in the sample with carbamazepine (10 μg/ml) (Figure 6a) ▶ . In contrast, no sFasL was found in supernatants without the causal drug. FasL mRNA was also detected from PBMC samples only with the causal drug stimulation (such as carbamazepine, 10 μg/ml) for 24 hours (Figure 6b) ▶ .
Figure 6.
PBMCs of a TEN patient produced sFasL and expressed FasL mRNA after stimulation with a causal drug. PBMCs freshly isolated from a TEN patient (1 × 106/ml) were cultured with the causal drug, carbamazepine (10 and 100 μg/ml) for 24 hours. The amounts of sFasL in supernatants were measured by the sFasL ELISA kit. FasL mRNA of PBMCs was examined by reverse transcriptase-polymerase chain reaction as described in the Materials and Methods. Large amounts of sFasL were detected after the addition of carbamazepine (a). In contrast, no sFasL were found without carbamazepine (*, P < 0.001, compared with 10 or 100 μg/ml of carbamazepine). Similarly, FasL mRNA was detected only with stimulation by 10 and 100 μg/ml of carbamazepine (b).
Discussion
TEN and SJS are severe blistering diseases usually associated with drug intake in which apoptotic keratinocyte cell death results in the separation of large areas of skin at the dermo-epidermal junction, producing the appearance of scalded skin. 3 The pathophysiology of these diseases is not well known, although immune mechanisms 4 and altered metabolism of drugs 5 have been postulated.
TEN and SJS are usually a pauci-inflammatory process. Indeed, TEN and SJS lesions contain relatively few inflammatory cells. Our initial hypothesis was that apoptosis is not mediated by lesional infiltrating cells, but by circulating soluble factors.
The present study demonstrates that PBMCs from TEN and SJS patients secrete sFasL on stimulation with the causal drug. In addition, patients sera induced apoptosis in cultured keratinocytes, indicating that sFasL produced by PMBCs may contribute to the pathogenesis of TEN and SJS.
The precise mechanisms responsible for enhanced keratinocyte-specific apoptosis in TEN and SJS remain unclear. It remains controversial that keratinocyte apoptosis is mediated by soluble factors such as tumor necrosis factor-α, nitric oxide, 17 or peripheral blood cells, particularly cytotoxic T cells. For example, Nassif and colleagues 18 reported that T lymphocytes present within the lesions of TEN patients might exhibit, without any restimulation, a drug-specific cytotoxicity against autologous cells. On the other hand, proinflammatory cytokines including tumor necrosis factor-α, which mediates apoptosis, 10 are likely to be involved in TEN. 3 Although several therapeutic approaches for TEN targeting tumor necrosis factor-α were assessed, almost all trials failed to decrease mortality. 19 Viard and colleagues 6 reported that Fas-FasL interaction is directly involved in the epidermal necrolysis of TEN. They showed that keratinocytes of TEN patients express lytically active FasL. However, the exposure of Fas-sensitive target cells to cryostat sections of skin that express FasL does not result in target cell apoptosis. 6 The reason why keratinocyte FasL is nonfunctional within the keratinocytes remains unclear at present, but one possibility may be because of its cellular localization. Another possibility may be because of other control mechanisms known to regulate the lytic potential of FasL, such as metalloproteinase-mediated surface cleavage and inactivation. 10
Unexpectedly, our data showed a lack of correlation between sFasL levels and the degree of skin detachment in both TEN and SJS. We expected that the degree of detachment would be parallel to FasL levels. TEN and SJS are very rare diseases, for example, the incidence of TEN is 0.4 to 1.2 cases per million. We therefore suggest that the patient background may be a strong contributing factor, for example HLA typing, indicate heterogeneous disease severity. In addition, we suggest the trigger levels may be different between individuals, so severity and sFasL levels do not show correlation.
TEN is associated with a mortality rate of ∼30%, most frequently as a result of sulfonamide, anticonvulsant, or nonsteroidal anti-inflammatory drug use. 20,21 Particularly, before the bullae stage is reached, impending TEN and SJS is difficult to distinguish from other drug eruptions. As these disorders progress so rapidly, a gold standard marker for diagnosis is urgently required. Our study revealed that high levels of serum sFasL were detected in TEN and SJS patients, especially in the early stages, suggesting the possibility of using this as a diagnostic marker of TEN and SJS.
There is no specific treatment for TEN at present. Some retrospective studies have claimed a benefit in the use of corticosteroids, 22 whereas other reports showed no benefit or even increased morbidity and mortality. 23,24 Other trials that included cyclosporin and monoclonal chimeric IgG anti-tumor necrosis factor-α antibodies, have been used in isolated cases and in short uncontrolled series, 25,26 allowing no conclusions on their efficacy. The extent of epidermal detachment is the main prognostic factor in TEN patients, therapies with a potential to stop this process of epithelial apoptosis might be highly useful during the initial phase of the disease. As demonstrated in our study, sFasL secreted by PBMCs may mediate keratinocyte apoptosis, which might significantly contribute to the pathogenesis of TEN and SJS. Therefore, a reduction in serum sFasL might be a new therapeutic approach in the treatment of TEN and SJS.
The knowledge of sFasL is pivotal in understanding the pathogenesis in TEN and SJS, and opens a future for more focused diagnostic and therapeutic applications.
Acknowledgments
We thank Dr. Kikuo Tsuchiya for providing serum samples of patients, Ms. Ayumi Honda for her excellent technical assistance, and Dr. James R. McMillan for his manuscript proofreading.
Footnotes
Address reprint requests to Riichiro Abe, M.D., Ph.D., Department of Dermatology, Hokkaido University Graduate School of Medicine, N 15 W 7, Kita-ku, Sapporo 060-8638, Japan. E-mail: aberi@med.hokudai.ac.jp.
References
- 1.Becker DS: Toxic epidermal necrolysis. Lancet 1998, 351:1417-1420 [DOI] [PubMed] [Google Scholar]
- 2.Roujeau JC: The spectrum of Stevens-Johnson syndrome and toxic epidermal necrolysis: a clinical classification. J Invest Dermatol 1994, 102:28-30 [DOI] [PubMed] [Google Scholar]
- 3.Wolkenstein PC, Adle H, Wechsler J, Garchon HJ, Revuz J, Roujeau JC: Apoptosis as a mechanism of keratinocyte death in toxic epidermal necrolysis. Br J Dermatol 1996, 134:710-714 [DOI] [PubMed] [Google Scholar]
- 4.Correia O, Delgado L, Ramos JP, Resende C, Torrinha JA: Cutaneous T-cell recruitment in toxic epidermal necrolysis. Further evidence of CD8+ lymphocyte involvement. Arch Dermatol 1993, 129:466-468 [PubMed] [Google Scholar]
- 5.Wolkenstein P, Charue D, Laurent P, Revuz J, Roujeau JC, Bagot M: Metabolic predisposition to cutaneous adverse drug reactions. Role in toxic epidermal necrolysis caused by sulfonamides and anticonvulsants. Arch Dermatol 1995, 131:544-551 [PubMed] [Google Scholar]
- 6.Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, Hunziker T, Saurat JH, Tschopp J, French LE: Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science 1998, 282:490-493 [DOI] [PubMed] [Google Scholar]
- 7.Nagata S: Apoptosis by death factor. Cell 1997, 88:355-365 [DOI] [PubMed] [Google Scholar]
- 8.Sayama K, Yonehara S, Watanabe Y, Miki Y: Expression of Fas antigen on keratinocytes in vivo and induction of apoptosis in cultured keratinocytes. J Invest Dermatol 1994, 103:330-334 [DOI] [PubMed] [Google Scholar]
- 9.Iwai K, Miyawaki T, Takizawa T, Konno A, Ohta K, Yachie A, Seki H, Taniguchi N: Differential expression of bcl-2 and susceptibility to anti-Fas-mediated cell death in peripheral blood lymphocytes, monocytes, and neutrophils. Blood 1994, 84:1201-1208 [PubMed] [Google Scholar]
- 10.Wehrli P, Viard I, Bullani R, Tschopp J, French LE: Death receptors in cutaneous biology and disease. J Invest Dermatol 2000, 115:141-148 [DOI] [PubMed] [Google Scholar]
- 11.Kayagaki N, Kawasaki A, Ebata T, Ohmoto H, Ikeda S, Inoue S, Yoshino K, Okumura K, Yagita H: Metalloproteinase-mediated release of human Fas ligand. J Exp Med 1995, 182:1777-1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka M, Suda T, Takahashi T, Nagata S: Expression of the functional soluble form of human Fas ligand in activated lymphocytes. EMBO J 1995, 14:1129-1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka M, Suda T, Haze K, Nakamura N, Sato K, Kimura F, Motoyoshi K, Mizuki M, Tagawa S, Ohga S, Hatake K, Drummond AH, Nagata S: Fas ligand in human serum. Nat Med 1996, 2:317-322 [DOI] [PubMed] [Google Scholar]
- 14.Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC: Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol 1993, 129:92-96 [PubMed] [Google Scholar]
- 15.Gavrieli Y, Sherman Y, BenSasson SA: Identification of programmed cell death by in situ specific labeling of nuclear DNA fragmentation. J Cell Biol 1992, 119:493-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trautmann A, Akdis M, Kleemann D, Altznauer F, Simon HU, Graeve T, Noll M, Brocker EB, Blaser K, Akdis CA: T cell-mediated Fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J Clin Invest 2000, 106:25-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lerner LH, Qureshi AA, Reddy BV, Lerner EA: Nitric oxide synthase in toxic epidermal necrolysis and Stevens-Johnson syndrome. J Invest Dermatol 2000, 114:196-199 [DOI] [PubMed] [Google Scholar]
- 18.Nassif A, Bensussan A, Dorothée G, Mami-Chouaib F, Bachot N, Bagot M, Boumsell L, Roujeau JC: Drug specific cytotoxic T-cells in the skin lesions of a patient with toxic epidermal necrolysis. J Invest Dermatol 2002, 118:728-733 [DOI] [PubMed] [Google Scholar]
- 19.Wolkenstein P, Latarjet J, Roujeau JC, Duguet C, Boudeau S, Vaillant L, Maignan M, Schuhmacher MH, Milpied B, Pilorget A, Bocquet H, Brun-Buisson C, Revuz J: Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet 1998, 352:1586-1589 [DOI] [PubMed] [Google Scholar]
- 20.Roujeau JC, Stern RS: Severe adverse cutaneous reactions to drugs. N Engl J Med 1994, 331:1272-1285 [DOI] [PubMed] [Google Scholar]
- 21.Rzany B, Mockenhaupt M, Baur S, Schroder W, Stocker U, Mueller J, Hollander N, Bruppacher R, Schopf E: Epidemiology of erythema exsudativum multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis in Germany (1990–1992): structure and results of a population-based registry. J Clin Epidemiol 1996, 9:769-773 [DOI] [PubMed] [Google Scholar]
- 22.Sherertz EF, Jegasothy BV, Lazarus GS: Phenytoin hypersensitivity reaction presenting with toxic epidermal necrolysis and severe hepatitis. Report of a patient treated with corticosteroid “pulse therapy.” J Am Acad Dermatol 1985, 12:178-181 [DOI] [PubMed] [Google Scholar]
- 23.Halebian PH, Corder VJ, Madden MR, Finklestein JL, Shires GT: Improved burn center survival of patients with toxic epidermal necrolysis managed without corticosteroids. Ann Surg 1986, 204:503-512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelemen JJ, Cioffi WG, McManus WF, Mason AD, Pruitt BA: Burn center care for patients with toxic epidermal necrolysis. J Am Coll Surg 1995, 180:273-278 [PubMed] [Google Scholar]
- 25.Hewitt J, Ormerod AD: Toxic epidermal necrolysis treated with cyclosporin. Clin Exp Dermatol 1992, 17:264-265 [DOI] [PubMed] [Google Scholar]
- 26.Fischer M, Fiedler E, Marsch WC, Wohlrab J: Antitumour necrosis factor-antibodies (infliximab) in the treatment of a patient with toxic epidermal necrolysis. Br J Dermatol 2002, 146:707-708 [DOI] [PubMed] [Google Scholar]