Abstract
Nitric oxide (NO) is widely known to inhibit platelet and leukocyte adhesion to endothelium through its regulatory effect on adhesion molecule expression. The objective of the present study was to investigate if NO affects the cytoadherence of Plasmodium falciparum-infected erythrocytes (IRBCs) to human microvascular endothelium (HDMECs) under flow conditions in vitro. The effect of endogenous NO was studied using the NO synthase inhibitor l-NG-nitro-arginine-methyl-ester (l-NAME). Treatment of HDMECs with 3 mmol/L of l-NAME for 4 hours significantly enhanced IRBC adhesion and the effect could be reversed by an anti-P-selectin but not an anti-VCAM-1 antibody. The effect of exogenous NO on cytoadherence was studied by using the NO donor 3-(2-hydroxy-2-nitroso-1-propylhydrazino)-1-propanamine (PPN). PPN (300 μmol/L) treatment reduced the number of adherent IRBCs on resting HDMECs by down-regulating basal ICAM-1 expression, and on tumor necrosis factor-α-stimulated HDMECs by inhibition of VCAM-1 induction and down-regulation of ICAM-1 expression. The inhibitory effect of PPN on tumor necrosis factor-α-induced VCAM-1 expression at 24 hours was evident when the NO donor was added for as short as 2 hours. These findings suggest that NO may be protective against P. falciparum infection by inhibiting cytoadherence, and underscore the therapeutic potential of NO in the treatment of severe falciparum malaria.
Severe Plasmodium falciparum malaria is a systemic disease that affects multiple vital organs. 1 Its main pathological feature is the sequestration of erythrocytes containing mature stages of the parasite (IRBCs) in the microvessels of the brain, heart, liver, lung, and kidney. Although the pathophysiology of severe falciparum malaria is still not completely understood, clinical and pathological evidence would suggest that the sequestration of IRBCs is an essential initiating event that ultimately leads to the impairment of blood flow, local tissue hypoxia, and the induction of proinflammatory cytokines. 2 Sequestration results from the cytoadherence of IRBCs to venular endothelium by a selective process involving specific parasite ligands and host endothelial receptors. We and others have shown previously that under flow conditions, IRBCs can tether and roll on ICAM-1, VCAM-1, and P-selectin, while adhesion is almost exclusively to CD36. 3-7 There is no interaction with E-selectin. In both in vitro 6 and in vivo 7 models of the human microvasculature, there is strong evidence that the interactions with the other molecules enhance subsequent adhesion to CD36, either by increasing the percentage of rolling cells that become adherent (ICAM-1 and VCAM-1), or by increasing the number of rolling and hence adherent IRBCs (P-selectin). 6 Because the molecules supporting rolling, but not CD36, can be up-regulated by cytokines such as tumor necrosis factor-α (TNF-α) and oncostatin M (OSM), the rolling component may in fact determine the degree of cytoadherence and hence disease severity in falciparum malaria.
Nitric oxide (NO) generated by endothelial cells has a vital role in maintaining vascular tone and blood flow. NO also contributes significantly to vascular homeostasis by the regulation of platelet aggregation and adhesion to endothelium thereby modulating thrombus formation, 8 and the regulation of neutrophil-endothelial cell adhesion thereby restricting the extent of inflammatory processes. 9 Inhibition of endogenous NO synthesis by the l-arginine analogs NG-monomethyl l-arginine (l-NMMA) or l-NG-nitro-arginine-methyl-ester (l-NAME) enhances leukocyte adhesion to vascular endothelial cells in animal models and in static binding assays in vitro. 10-14 The effect of endogenous NO on leukocyte adhesion appears to be mediated through the induction of P-selectin in the rat mesenteric microvasculature 11 and in e-NOS-deficient mice 14 in vivo, and on human iliac vein endothelial cells in flow chamber studies in vitro. 13 On the other hand, NO donors have been demonstrated to attenuate leukocyte-endothelial cell interaction on cytokine-activated endothelial cells through the down-regulation of the expression of ICAM-1, VCAM-1, and E-selectin. 15,16 The adhesion molecules affected appear to vary among different types of endothelial cells.
NO has been postulated to have both a pathogenic 17 and protective role 18 in falciparum malaria. In support of the latter role, a population genetics study demonstrated that a point mutation in the promoter of the nitric oxide synthase 2 (iNOS) gene, termed NOS2Lambarene (NOS2-G954C), protected heterozygous carriers against severe falciparum malaria as effectively as the sickle cell trait. 19 Peripheral blood mononuclear cells from heterozygous individuals produced seven times higher basal NO compared to wild-type cells. 20 More recently, a new single nucleotide polymorphism, −1173 C→T, in the NOS2 promoter associated with increased NO production was found to protect African children from symptomatic malaria and severe malarial anemia. 21 The protective effect of NO against P. falciparum has been attributed to the inhibition of parasite growth 18 and/or intracellular parasite killing by reactive nitrogen metabolites, 22 although the production of these metabolites by human macrophages is still unresolved. Whether NO has a protective role in falciparum malaria by regulating IRBC adhesion to vascular endothelium, in the same way that it attenuates leukocyte recruitment in inflammation, has not been determined.
In this study, we tested whether NO has an anti-adhesive effect on the rolling and adhesion of IRBCs on human microvascular endothelial cells under flow conditions in vitro through the regulation of adhesion molecule expression. Our results indicate that inhibition of endogenous NO production enhances cytoadherence through an apparent effect on P-selectin expression. In addition, exogenous NO production by NO donors reduced cytoadherence by the down-regulation of ICAM-1 and VCAM-1 expression. These findings underscore the therapeutic potential of NO against severe falciparum malaria by inhibiting cytoadherence.
Materials and Methods
Parasites
Cryopreserved parasite isolates from adult Thai patients with well-documented P. falciparum malaria were thawed and studied during the first cycle in culture as described. 4 The collection of specimens was approved by the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand. A total of 20 clinical parasite isolates were studied.
Reagents
Recombinant human TNF-α was purchased from BD Biosciences (Bedford, MA). OSM was purchased from R&D Systems, Inc. (Minneapolis, MN). The concentration of cytokines used for the stimulation of endothelial cells in 35-mm tissue culture dishes (Corning, New York, NY) were 5 to 20 ng/ml of TNF-α for 4 and 24 hours and 10 ng/ml of OSM for 24 or 48 hours. NG-nitro-l-arginine methyl ester (l-NAME) and the d-stereoisomer d-NAME were purchased from Bachem, Torrance, CA. Sodium nitroprusside and thrombin were purchased from Sigma-Aldrich Co., Oakville, Ont., Canada. 3-(2-Hydroxy-2-nitroso-1-propylhydrazino)-1-propanamine (PPN) was purchased from Cayman Chemicals, Ann Arbor, MI. The polyamine with no adducted NO was from Sigma-Aldrich. 3,3′,5,5′-Tetramethylbenzidine was purchased from DAKO Corp., Carpinteria, CA.
Cell Culture
Human dermal microvascular endothelial cells (HDMECs) were harvested from discarded neonatal human foreskins using 0.5 mg/ml of type IA collagenase (Boehringer Mannheim Biochemicals, Indianapolis, IN) as described previously. 6 The protocol was approved by the Conjoint Ethics Review Board of the University of Calgary. The cells were maintained in endothelial basal medium (EBM) (BioWhittaker Inc., Walkerville, MD) with supplements provided by the manufacturer. Experiments were performed with cells from passage one to five on which adhesion molecule expression was shown to be stable. 6 We and others have shown previously that HDMECs express CD36 and ICAM-1 constitutively. 6,23,24 Stimulation with TNF-α for 4 hours up-regulates ICAM-1 expression, and at 24 hours, ICAM-1 expression is further augmented while VCAM-1 expression is induced. 6,24 Viability of HDMECs after treatment with pharmacological agents was verified by trypan blue exclusion and a CellTiter 96 Aqueous One Solution Assay (MTS test) from Promega (Madison, WI) according to the instructions from the manufacturer.
Antibodies
The monoclonal antibodies (mAbs) used were known to specifically inhibit the binding of IRBCs to the respective adhesion molecules. 6 OKM5 (anti-CD36) was a kind gift of Ortho Diagnostics System (Raritan, NJ); 84H10 (anti-ICAM-1) was purchased from R&D Systems, Inc.; 4B9 (anti-VCAM-1) was a kind gift of Dr. R. Lobb, Biogen Inc., Boston, MA. The anti-P-selectin mAb TS10-6-6, raised in our laboratory, has been characterized extensively. 5 The noninhibitory anti-P-selectin mAb S12 was a kind gift of Dr. R. P. McEver, University of Oklahoma, Oklahoma City, OK. A commercial affinity-purified polyclonal anti-P-selectin Ab was purchased from Pharmingen (San Diego, CA), a polyclonal anti-von Willebrand Factor (vWF) Ab from DAKO Corp., and a mAb against vWF from ICN Pharmaceuticals Inc., Irvine, CA. Peroxidase-labeled goat anti-mouse and goat anti-rabbit IgG secondary Abs were from Jackson ImmunoResearch Laboratories, West Grove, PA.
Measurement of vWF Release by HDMECs
Confluent monolayers grown in 35-mm2 tissue culture dishes were washed twice with PBS before fresh medium was added. Cells were treated with 3 mmol/L of l-NAME for 3, 4, and 5 hours, or thrombin 1 U/ml for 10 and 20 minutes. The supernatants were aspirated, centrifuged, and stored at −70°C. The concentration of vWF in supernatants was measured by an enzyme-linked immunosorbent assay (ELISA) using 96-well plates coated with 2.5 μg/ml of a polyclonal rabbit anti-human vWF. vWF was detected with 10 μg/ml of an anti-human vWF mAb.
Adhesion Molecule Expression
Cell surface expression of CD36, ICAM-1, VCAM-1, and P-selectin on resting and stimulated HDMECs was determined by flow cytometry 6 and ELISA. 16 Quantitation of total cellular P-selectin from HDMEC monolayers treated with 3 mmol/L of l-NAME for 4 hours by ELISA was performed on cell lysates as described. 25 Monolayers stimulated with OSM were included as a positive control.
Real-Time Polymerase Chain Reaction (PCR) for P-Selectin mRNA
Total RNA from resting and l-NAME- or OSM-stimulated HDMECs was extracted using the RNeasy Mini Kit (Qiagen Inc., Missisauga, Ont., Canada) according to the manufacturer’s instruction. Reverse transcription was performed using Superscript II RT (Invitrogen, Burlington, Ont., Canada). Each 20 μl of reaction mixture contained 2 μg of RNA, 5× first strand buffer (250 μmol/L Tris-Cl, 375 μmol/L KCl, 15 μmol/L MgCl2), 10 μmol/L of dNTP mix, Superscript II RT, RNA guard, RNase inhibitor (Amersham Pharmacia Biotech Inc, Piscataway, NJ), and 25 μmol/L random hexamer (Amersham Pharmacia). Real-time PCR was performed by using an ABI Prism 7000 sequence detection system (Applied Byosystem Inc., Foster City, CA) in a 5′ nuclease assay. P-selectin primers and TaqMan probes were designed using the Primer Express software (ABI). The P-selectin sense primer sequence was AGACTCCCCACCAATGTGTGA and the P-selectin antisense primer was CCACGAGTGTCAGAACAATCCA. The P-selectin TaqMan probe sequence was FAM-CCATCAAGTGCCCAGAACTCTTTGCC-TAMRA. The TaqMan predeveloped GAPDH assay (PDAR) was used for normalization of gene expression. Each 25 μl of reaction mixture contained 1 μl of sample from the RT reaction, universal master mix (ABI), P-selectin forward and reverse primers at a final concentration of 900 nmol/L, and P-selectin TaqMan probe at a final concentration of 200 nmol/L. A standard curve was set up by serial 10-fold dilution of an OSM-treated sample. Thermocycling was performed as follows: 50°C for 2 minutes for activating AmpErase UNG, 95°C for 10 minutes to activate the Amplitaq gold polymerase. Each PCR cycle consisted of 95°C for 15 seconds and 60°C for 1 minute for combined annealing and elongation. The cycles were performed for a total of 40 times to ensure that all reactions have proceeded to completion. The increase in fluorescence at each cycle was measured by the ABI 7000 and plotted against the cycle number. A threshold was established and the relative gene expression from any given sample was related to the cycle number at which amplification crossed the threshold (Ct). The relative quantitation of the samples was determined using the ddCt method according to the manufacturer’s instruction.
Adhesive Interactions under Flow Condition
IRBC-endothelial cell interactions at fluid shear stresses approximating those in the microvasculature were studied using a parallel plate flow chamber as described. 6 A rolling IRBC was defined as one that displayed a typical end-on-end rolling motion at a velocity of <150 μm/second, compared to a centerline red blood cell flow rate of >1000 μm/second, and a velocity of >150 μm/second for noninteracting cells in close proximity to the endothelial monolayer. The flux of rolling IRBCs was determined as the number that rolled past a fixed line on the monitor screen for the duration of the experiment, and expressed as the number of rolling IRBCs/minute/mm2. An IRBC was considered adherent if it remained stationary for >10 seconds, and the results were expressed as the number of adherent IRBCs per mm2 of surface area. In inhibition studies, HDMEC monolayers were preincubated with the monoclonal antibodies at 10 μg/ml of Hanks’ balanced salt solution at 37°C for 30 minutes before being used in flow chamber experiments.
Statistical Analysis
All data are presented as mean ± SEM or mean percentage change ± SEM from control. Raw data between two groups were compared by Student’s t-test for paired samples. Raw data from greater than two groups were compared by analysis of variance for paired samples, using post hoc analysis with Bonferoni’s correction for multiple comparisons. Probabilities of 0.05 or less were considered statistically significant.
Results
Endogenous NO Enhanced Cytoadherence of IRBCs to HDMECs
As we reported previously, 6 IRBCs from clinical P. falciparum isolates rolled and adhered to HDMECs optimally at 1 dyne/cm2. The mean rolling flux was 28 ± 5 IRBCs/minute/mm2 and adhesion was 170 ± 28 IRBCs/mm2 for the 20 parasite isolates that were studied. Although some IRBCs bypassed the rolling step and adhered immediately after tethering, >90% of the adherent cells rolled for various distances before becoming adherent. On the other hand, the proportion of rolling cells that became arrested differed for the different clinical parasite isolates.
To determine whether endogenous NO has an effect on the adhesive interactions between IRBCs and microvascular endothelium, endothelial monolayers were treated with the nonspecific NOS inhibitor l-NAME in doses ranging from 10−6 to 10−2 mol/L (Figure 1) ▶ for 30 minutes, and 2, 4, 8, and 24 hours (Figure 2) ▶ . The viability and cytotoxicity of treated cells were monitored by trypan blue dye exclusion and the MTS assay. No significant toxic effect was detected until a dose of 10 mmol/L was reached (100 ± 0% for control versus 59 ± 11% for treated cells by MTS assay, P < 0.05; n = 3). The results show that 3 mmol/L of l-NAME treatment for 4 hours led to a significant enhancement of IRBC adhesion without an increase in the rolling flux (Figure 1) ▶ . However, the percentage of rolling IRBCs that became adherent rose from 43 ± 10% on untreated to 60 ± 12% on l-NAME-treated endothelial cells (n = 9, P < 0.05). d-NAME, a stereoisomer of l-NAME, did not have any effect on IRBC rolling or adhesion (data not shown). The increase in IRBC adhesion induced by l-NAME could be reversed by an anti-P-selectin mAb TS10-6-6, but not an anti-VCAM-1 mAb 4B9 that inhibits IRBC adhesion to soluble VCAM-1 by ∼60% 26 (Figure 3) ▶ . mAb TS10-6-6 had no effect on IRBC rolling or adhesion on untreated HDMECs or on CD36 transfectants (data not shown). In parallel experiments, l-NAME-treated monolayers also supported the rolling and adhesion of leukocytes infused as a 1:10 dilution of fresh whole blood 27 (Table 1) ▶ . The adhesive interactions were inhibited by mAb TS10-6-6. Cell surface expression of CD36, ICAM-1, VCAM-1, and P-selectin determined by ELISA and flow cytometry was not increased on l-NAME-treated endothelial cells (data not shown).
Figure 1.
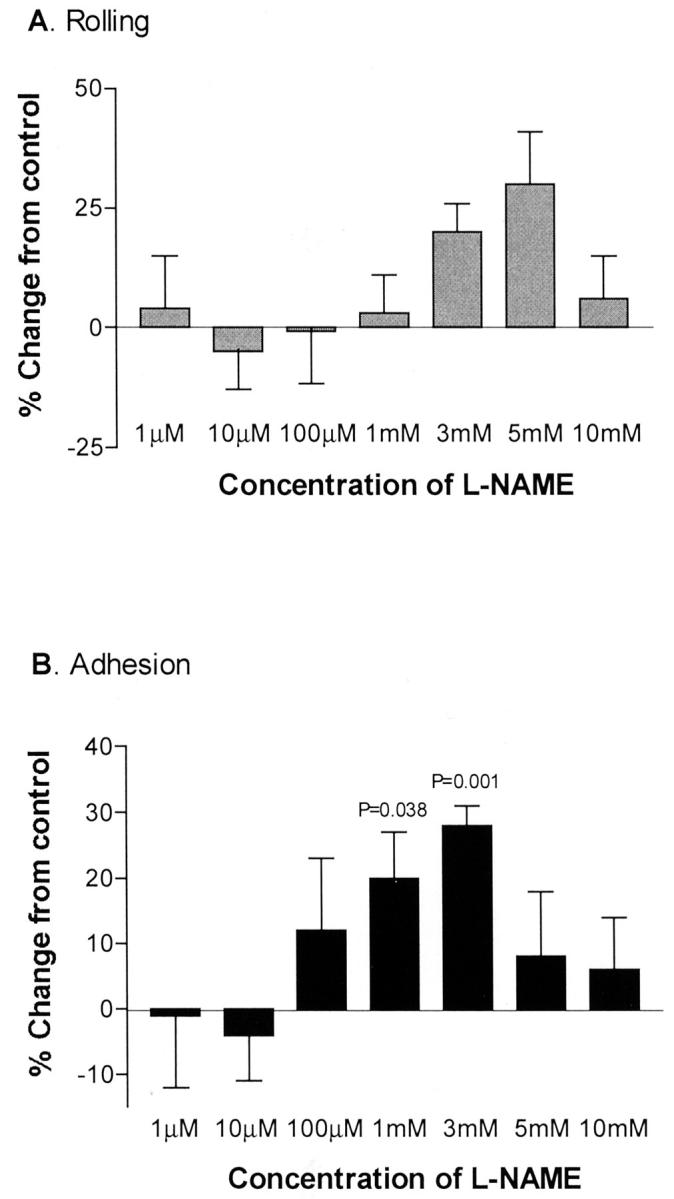
IRBC rolling and adhesion on HDMECs treated with 10−6 to 10−2 mol/L of l-NAME for 4 hours. Increased adhesion was seen on monolayers treated with 1 mmol/L and 3 mmol/L of l-NAME for 4 hours (n = 9). P value relative to untreated HDMECs.
Figure 2.

IRBC rolling and adhesion on HDMECs treated with 3 mmol/L of l-NAME for 30 minutes to 24 hours. Increased adhesion was seen on monolayers treated for 4 hours (n = 4). P value relative to untreated HDMECs.
Figure 3.
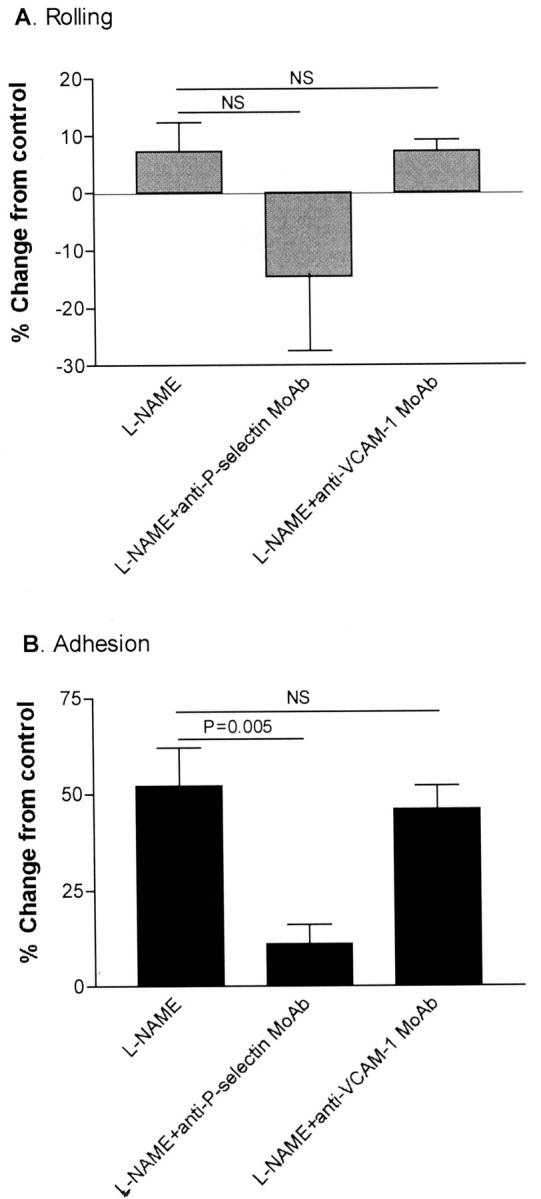
An anti-P-selectin mAb (TS10-6-6), but not an anti-VCAM-1 mAb (4B9), inhibited the effect of l-NAME on IRBC adhesion on HDMECs (n = 7).
Table 1.
Effect of l-NAME on Leukocyte Rolling and Adhesion on HDMECs (n = 3)
| Control | l-NAME | l-NAME + anti-P-selectin |
|---|---|---|
| Rolling* | ||
| 3 | 12 | <1 |
| 0 | 8 | <1 |
| 3 | 8 | 2 |
| Adhesion† | ||
| 7 | 36 | 9 |
| 0 | 27 | 2 |
| 31 | 92 | 14 |
*Number of rolling IRBC/mm2/minute.
†Number of adherent IRBC/mm2 in 10 minutes.
l-NAME Treatment Did Not Increase P-Selectin Translocation or Synthesis
The above findings suggested that the enhancement of IRBC adhesion to HDMECs when endogenous NO production was inhibited was mediated by P-selectin. To determine the mechanism of induction of P-selectin expression on HDMECs, P-selectin mobilization from Weibel-Pallade bodies was studied indirectly by measuring vWF release 28 by ELISA. No increase in the release of vWF was observed on l-NAME-treated compared to untreated HDMECs (45 ± 10 versus 43 ± 9 ng/ml, n = 3). In contrast, stimulation with the established agonist thrombin resulted in a 2.2 ± 0.2-fold increase in vWF release (n = 2). In addition, real-time PCR showed that l-NAME did not augment the expression (0.9-fold to 1.4-fold) of steady state P-selectin mRNA expression in HDMECs compared to a 4- to 35-fold increase in OSM-stimulated cells (n = 3). There was also no significant increase in total P-selectin protein in cell lysates as measured by ELISA (882 ± 464 μg/106 cells versus 1362 ± 493 μg/106 cells, n = 6, P > 0.05). The lack of effect of l-NAME on total P-selectin protein in cell lysates was seen with both primary and passaged HDMECs.
Effect of NO Donor on IRBC Adhesion to Resting HDMECs
We next investigated the effect of exogenous NO on IRBC cytoadherence by using the NO donor PPN. Treatment with PPN at 300 μmol/L for 24 hours resulted in a 32% decrease in IRBC adhesion (Figure 4B) ▶ , which appeared to be mediated by an inhibitory effect of PPN on the basal (constitutive) ICAM-1 expression (Figure 5A) ▶ . No cytotoxicity or loss of viability of PPN-treated cells was detected by both the MTS assay and trypan blue exclusion.
Figure 4.
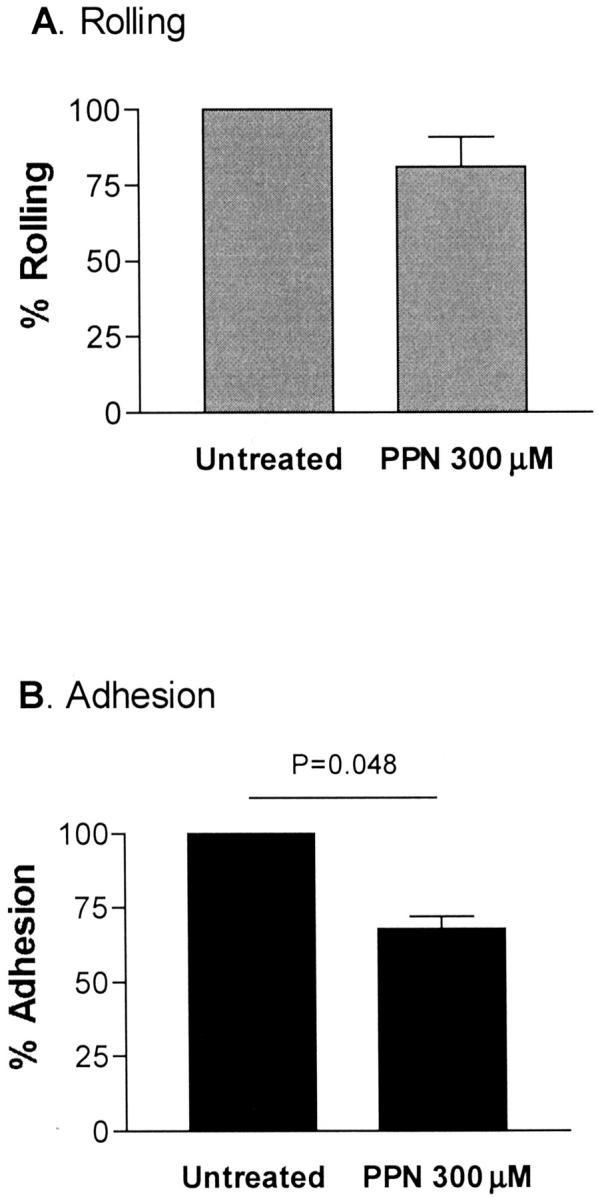
PPN (300 μmol/L for 24 hours) inhibited adhesion but not rolling on IRBCs on resting HDMECs (n = 6). P value relative to untreated HDMECs.
Figure 5.
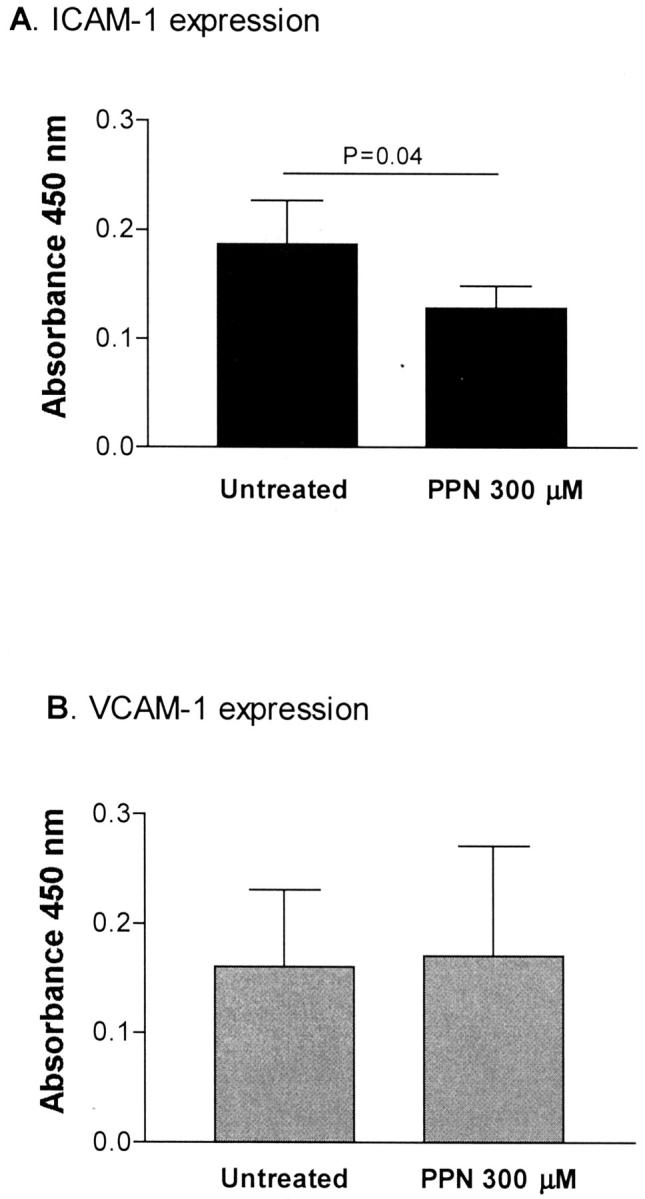
PPN (300 μmol/L for 24 hours) inhibited constitutive ICAM-1 expression on resting HDMECs (n = 5). P value relative to untreated HDMECs.
NO Donors Inhibited IRBC Adhesion on TNF-α-Stimulated HDMECs
We have previously shown that both ICAM-1 and VCAM-1 contributed to the increased IRBC adhesion seen on TNF-α-stimulated monolayers. 6 In this study, treatment with 300 μmol/L of PPN reduced the number of adherent cells on TNF-α-stimulated HDMECs by 24% (Figure 6) ▶ . VCAM-1 expression was completely inhibited by PPN, whereas inducible ICAM-1 expression was reduced (Figure 7) ▶ . The control polyamine (ie, without adducted NO) had no effect on IRBC adhesion or adhesion molecule expression (data not shown). Similar results were obtained with a structurally unrelated NO donor sodium nitroprusside that releases NO when it enters the cell with a half-life of 30 minutes at 37°C (data not shown). The inhibitory effect of PPN on TNF-α-induced VCAM-1 expression was evident when the monolayers were treated for as short as 30 minutes, and the maximum effect was reached at 2 hours (Figure 8) ▶ . Neither NO donor had any effect on CD36 expression on resting or TNF-α-stimulated HDMECs (data not shown).
Figure 6.

PPN (300 μmol/L) inhibited IRBC adhesion on HDMECs stimulated by TNF-α (10 ng/ml) for 24 hours. There was no significant inhibition of IRBC rolling (n = 6). P value relative to TNF-α-stimulated HDMECs.
Figure 7.
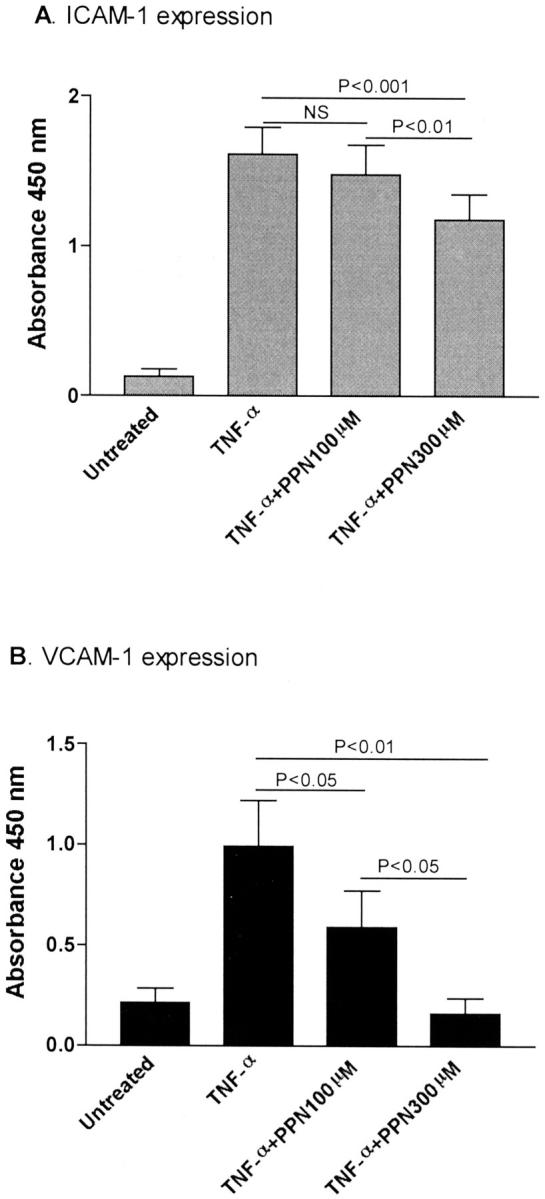
Inhibition of ICAM-1 and VCAM-1 expression on TNF-α-stimulated HDMECs by 300 μmol/L of PPN.
Figure 8.
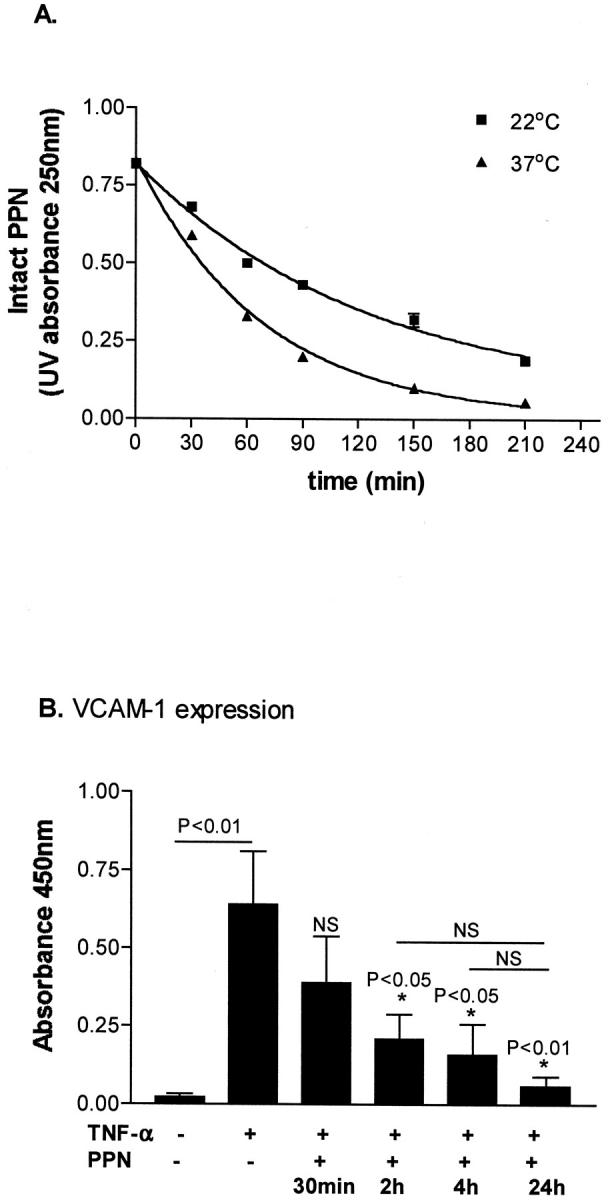
A: Rate of PPN decomposition at 22°C and 37°C. The half-life of PPN is 85.4 minutes at 22°C and 46.9 minutes at 37°C (n = 2). B: Incubation of PPN for 2 hours was sufficient for optimal inhibition of VCAM-1 expression at 24 hours induced by TNF-α (n = 3).
NO Donor Did Not Attenuate Effect of l-NAME on IRBC Adhesion
To determine whether exogenous NO could reverse the enhancing effect of l-NAME on IRBC adhesion, HDMECs were treated with 300 μmol/L of PPN for 30 minutes at 37°C before the addition of 3 mmol/L of l-NAME. Flow chamber studies were performed after 4 hours. The results with three parasite isolates showed that exogenous NO had no significant effect on the increase in IRBC adhesion induced by l-NAME treatment (25 ± 4% for l-NAME alone versus 23 ± 3% for PPN plus l-NAME, P > 0.05, n = 3).
Discussion
Many cell-associated or soluble inflammatory mediators have been implicated in the complex pathogenesis of severe P. falciparum malaria. Unfortunately, few functional studies have been performed to define their effects. The controversial role of NO is no exception. Conclusions regarding its pathogenic or protective roles have been based mainly on the correlation of clinical severity with total blood NO metabolite levels. 29-35 It is well known that plasma nitrite and nitrate levels can be affected by a number of confounding factors, including food intake and renal clearance. Furthermore, it has been shown that plasma nitrite but not nitrate levels can best reflect regional NO production. 36
The research described in this report is the first study to address whether NO has an anti-adhesive effect on the cytoadherence of IRBCs to endothelial cells. We found that both endogenous and exogenous NO attenuated cytoadherence on resting and TNF-α-stimulated HDMECs, but appeared to do so by different mechanisms. Results from inhibition experiments with receptor-specific mAb in a functional assay suggest that the increase in the number of adherent IRBCs induced by l-NAME was mediated by P-selectin. The involvement of P-selectin in mediating the effect of l-NAME on cell recruitment is corroborated by both in vivo and in vitro studies on other types of endothelial cells. 11,13,14 However, we were unable to demonstrate an increase in cell surface P-selectin expression, an increase in P-selectin mRNA, or increased translocation of stored protein from Weibel-Pallade bodies. A well-recognized difficulty with demonstrating P-selectin on endothelial cells is the low number of molecules (10 to 50 molecules/μm2) that are expressed on agonist- or cytokine-stimulated endothelium. 28,37,38 This is in contrast to 250 to 350 molecules/μm2 for adhesion molecules such as E-selectin and ICAM-1 (H Setiadi, personal communication). Nonetheless, this density of P-selectin has been shown to be sufficient for mediating leukocyte adhesion under flow conditions as a result of selectin and/or ligand dimerization, 39 or the clustering of the molecules around clathrin-coated pits. 38
A further mechanism that may be at play is the effect of l-NAME on the endocytosis of P-selectin. Under basal conditions, some P-selectin protein is recycled between the plasma membrane and endosomes, resulting in low levels of constitutive P-selectin protein on the surface of human endothelial cells. 37 Extremely low P-selectin expression is also believed to contribute to the basal leukocyte rolling on unstimulated endothelial cells in postcapillary venules of the cremaster muscle of wild-type mice that is absent in P-selectin knockouts. 40 Modifications to the recycling pathway by inhibiting reinternalization of the surface P-selectin without changing the amount of total P-selectin has been implicated in the increased recruitment of neutrophils on H2O2-treated human umbilical vein endothelial cells. 41 In support of this postulate, it has been shown that different rates of endocytosis of E-selectin, and not synthesis, are responsible for the differential expression of the adhesion molecule on HDMECs and human umbilical vein endothelial cells. 42 There is some evidence that NO may accelerate surface receptor internalization. NO generated from sodium nitroprusside can induce internalization of muscarinic acetylcholine receptors in transfected Chinese hamster ovary cells. 43 It is thus possible that inhibition of endogenous NO synthesis will impede surface receptor internalization resulting in an accumulation of cell surface-expressed molecules. These interesting mechanistic possibilities are beyond the scope of the present report, but certainly warrant further investigations.
A low level of P-selectin expression induced by l-NAME would be in keeping with the observation that whereas P-selectin induction by OSM resulted in an increase of both the number of rolling and adherent IRBCs, 6 l-NAME produced a modest increase in the number of adherent IRBCs by increasing the percentage of rolling cells that became arrested. This observation illustrates an important difference between leukocyte and IRBC recruitment that we have previously demonstrated. Whereas leukocyte recruitment occurs in a defined progression from selectins to immunoglobulin superfamily, IRBCs can tether and roll on a number of different molecules before adhesion to CD36. In fact, CD36 by itself can mediate all three components of the adhesive interactions. 4 If the cumulative affinity of all interactions is sufficiently high, the IRBCs will adhere. Otherwise, they will continue rolling.
The adhesion molecules involved in the action of NO donors on cytoadherence were more readily defined. The results indicate that PPN could attenuate the adhesion of IRBCs on resting HDMECs through a reduction in constitutive ICAM-1 expression. Basal ICAM-1 expression was not high, and the reduction was small although significant, compatible with a previous report that the NO donor SIN-1 could reduce constitutive ICAM-1 expression on human umbilical vein endothelial cells. 44 These observations suggest that a critical level of ICAM-1 expression was necessary to support IRBC tethering and rolling, and that a drop in the expression below this critical level could lead to a reduction in the strength of the adhesive interactions between IRBCs and the substratum. On TNF-α-stimulated HDMECs, treatment with PPN reduced IRBC adhesion. The inhibitory effect was mediated by a reduction in the expression of VCAM-1 and ICAM-1. These findings are in agreement with previous reports that NO donors inhibit TNF-α-induced VCAM-1 transcription by suppressing promoter activity through the inhibition of nuclear factor-κB activation and nuclear translocation. 16
The data present in this study are from the direct effect of NO on endothelial cells in vitro, and may not completely represent the activity of the molecule on cytoadherence in vivo. It has been demonstrated that the basal NO release in cultured endothelial cells is substantially lower than those in freshly isolated cells. 45 Furthermore, at least in animal models, the anti-adhesive effect of endogenous NO on leukocyte recruitment in vivo involves the stabilization of perivascular mast cells via the scavenging of O2, consequently preventing the release of proinflammatory cytokines from these cells. 46,47 These mechanisms would obviously not have been operative in our flow chamber assay. To address these issues, we have recently established a human/SCID mouse chimeric model that allows for the direct visualization of IRBC interactions with grafted human microvasculature in vivo by intravital microscopy. 7 Human mast cells are retained in the skin graft. 48 Preliminary experiments using this model show that l-NAME administration to the SCID mice for 4 hours led to an increase in leukocyte rolling and adhesion that could be inhibited by anti-P- and E-selectin antibodies (P Kubes, unpublished data). These results indicate that expression of selectins in the human microvessels can be induced by l-NAME in vivo, supporting the potential of this model for studying the role of endogenous NO on cytoadherence in vivo.
The effect of NO on cytoadherence demonstrated in this study was less pronounced than its effect on leukocyte recruitment. This is likely because of the dominant role of CD36 in mediating cytoadherence to the microvasculature, 6,7 and the fact that IRBCs can tether, roll, and adhere directly on CD36, the expression of which was not affected by NO. It remains to be determined whether the reduction on IRBC cytoadherence by NO would be greater in organs expressing predominantly ICAM-1, such as the brain, 49 and whether the reduction will be sufficient to be clinically beneficial. The effect of inhaled NO on the outcome of severe falciparum malaria has not been studied to any extent. In one study, inhaled NO was administered to patients with severe falciparum malaria who developed the adult respiratory distress syndrome. 50 All three patients died. The results may reflect the poor prognosis of malaria-associated adult respiratory distress syndrome (∼80% mortality), or that giving NO in the late stages of the infection when multiple organ failure has already occurred may not improve the outcome of the disease. Much more experimental and clinical data are necessary to clarify these important issues.
Acknowledgments
We thank Zosia Williams for technical assistance with the ELISA for measurement of vWF release; Eko W. Raharjo and Tara McCrae for real time PCR; Dr. Hendra Setiadi, University of Oklahoma for advice on P-selectin protein detection and helpful discussions; and Dr. Caroline Lane, Valley View Family Practice Clinic, Calgary for providing the skin specimens. K. Chotivanich is supported by the Wellcome Trust, UK and P. Kubes is a scientist of the Canadian Institutes of Health Research.
Footnotes
Address reprint requests to Dr. May Ho, Department of Microbiology and Infectious Diseases, 3330 Hospital Dr. NW, Calgary, Alberta, Canada T2N 4N1. E-mail: mho@ucalgary.ca.
Supported by a Canadian Institutes of Health Research grant, an Anemia Institute of Research and Education grant, and an Alberta Heritage Foundation for Medical Research Senior Scholar award (to M. H.).
References
- 1.White NJ, Ho M: The pathophysiology of malaria. Adv Parasitol 1992, 31:83-173 [DOI] [PubMed] [Google Scholar]
- 2.MacPherson GG, Warrell MJ, White NJ, Looareesuwan S, Warrell DA: Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol 1985, 119:385-401 [PMC free article] [PubMed] [Google Scholar]
- 3.Cooke BM, Brendt AR, Craig AG, MacGregor J, Newbold CI, Nash GB: Rolling and stationary cytoadhesion of red blood cells parasitised by Plasmodium falciparum: separate roles for ICAM-1, CD36 and thrombospondin. Br J Haematol 1994, 87:162-170 [DOI] [PubMed] [Google Scholar]
- 4.Udomsangpetch R, Reinhardt PH, Schollaardt T, Elliott JF, Kubes P, Ho M: Promiscuity of clinical Plasmodium falciparum isolates for multiple adhesion molecules under flow conditions. J Immunol 1997, 158:4358-4364 [PubMed] [Google Scholar]
- 5.Ho M, Schollaardt T, Niu X, Looareesuwan S, Patel KD, Kubes P: Characterization of Plasmodium falciparum-infected erythrocytes and P-selectin interaction under flow conditions. Blood 1998, 91:4803-4809 [PubMed] [Google Scholar]
- 6.Yipp BG, Anand S, Schollaardt T, Patel KD, Looareesuwan S, Ho M: Synergism of multiple adhesion molecules in mediating cytoadherence of Plasmodium falciparum-infected erythrocytes to microvascular endothelial cells under flow. Blood 2000, 96:2292-2298 [PubMed] [Google Scholar]
- 7.Ho M, Hickey MJ, Murray AG, Andonegui G, Kubes P: Visualization of Plasmodium falciparum-endothelium interactions in human microvasculature: mimicry of leukocyte recruitment. J Exp Med 2000, 192:1205-1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radomski MW, Palmer RM, Moncada S: Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet 1987, 2:1057-1058 [DOI] [PubMed] [Google Scholar]
- 9.Hickey MJ, Kubes P: Role of nitric oxide in regulation of leucocyte-endothelial cell interactions. Exp Physiol 1997, 82:339-348 [DOI] [PubMed] [Google Scholar]
- 10.Kubes P, Suzuki M, Granger DN: Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA 1991, 88:4651-4655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davenpeck KL, Gauthier TW, Lefer AM: Inhibition of endothelial-derived nitric oxide promotes P-selectin expression and action in the rat microcirculation. Gastroenterology 1994, 107:1050-1058 [DOI] [PubMed] [Google Scholar]
- 12.Niu XF, Smith CW, Kubes P: Intracellular oxidative stress induced by nitric oxide synthesis inhibition increases endothelial cell adhesion to neutrophils. Circ Res 1994, 74:1133-1140 [DOI] [PubMed] [Google Scholar]
- 13.Armstead VE, Minchenko AG, Schuhl RA, Hayward R, Nossuli TO, Lefer AM: Regulation of P-selectin expression in human endothelial cells by nitric oxide. Am J Physiol 1997, 272:H740-H746 [DOI] [PubMed] [Google Scholar]
- 14.Lefer DJ, Jones SP, Girod WG, Baines A, Grisham MB, Cockrell AS, Huang PL, Scalia R: Leukocyte-endothelial cell interactions in nitric oxide synthase-deficient mice. Am J Physiol 1999, 276:1943-1950 [DOI] [PubMed] [Google Scholar]
- 15.De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Jr, Shin WS, Liao JK: Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest 1995, 96:60-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan BV, Harrison DG, Olbrych MT, Alexander RW, Medford RM: Nitric oxide regulates vascular cell adhesion molecule 1 gene expression and redox-sensitive transcriptional events in human vascular endothelial cells. Proc Natl Acad Sci USA 1996, 93:9114-9119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark IA, Cowden WB: Why is the pathology of falciparum worse than that of vivax malaria? Parasitol Today 1999, 15:458-461 [DOI] [PubMed] [Google Scholar]
- 18.Rockett KA, Awburn MM, Cowden WB, Clark IA: Killing of Plasmodium falciparum in vitro by nitric oxide derivatives. Infect Immun 1991, 59:3280-3283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kun JF, Mordmuller B, Lell B, Lehman LG, Luckner D, Kremsner PG: Polymorphism in promoter region of inducible nitric oxide synthase gene and protection against malaria. Lancet 1998, 351:265-266 [DOI] [PubMed] [Google Scholar]
- 20.Kun JF, Mordmuller B, Perkins DJ, May J, Mercereau-Puijalon O, Alpers M, Weinberg JB, Kremsner PG: Nitric oxide synthase 2 (Lambarene) (G-954C), increased nitric oxide production, and protection against malaria. J Infect Dis 2001, 184:330-336 [DOI] [PubMed] [Google Scholar]
- 21.Hobbs MR, Udhayakumar V, Levesque MC, Booth J, Roberts JM, Tkachuk AN, Pole A, Coon H, Kariuki S, Nahlen BL, Mwaikambo ED, Lal AL, Granger DL, Anstey NM, Weinberg JB: A new NOS2 promoter polymorphism associated with increased nitric oxide production and protection from severe malaria in Tanzanian and Kenyan children. Lancet 2002, 260:1468-1475 [DOI] [PubMed] [Google Scholar]
- 22.Balmer P, Phillips HM, Maestre AE, McMonagle FA, Phillips RS: The effect of nitric oxide on the growth of Plasmodium falciparum, P chabaudi and P berghei in vitro. Parasite Immunol 2000, 22:97-106 [DOI] [PubMed] [Google Scholar]
- 23.Petzelbauer P, Bender JR, Wilson J, Pober JS: Heterogeneity of dermal microvascular endothelial cell antigen expression and cytokine responsiveness in situ and in cell culture. J Immunol 1993, 151:5062-5072 [PubMed] [Google Scholar]
- 24.McCormick CJ, Craig A, Roberts D, Newbold CI, Berendt AR: Intercellular adhesion molecule-1 and CD36 synergize to mediate adherence of Plasmodium falciparum-infected erythrocytes to cultured human dermal microvascular endothelium. J Clin Invest 1997, 100:2521-2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia L, Pan J, Yao L, McEver RP: A proteasome inhibitor, an antioxidant, or a salicylate, but not a glucocorticoid, blocks constitutive and cytokine-inducible expression of P-selectin in human endothelial cells. Blood 1998, 91:1625-1632 [PubMed] [Google Scholar]
- 26.Ockenhouse CF, Tegoshi T, Maeno Y, Benjamin C, Ho M, Kan KE, Thway Y, Win K, Aikawa M, Lobb RR: Human vascular endothelial cell adhesion receptors for Plasmodium falciparum-infected erythrocytes: roles for endothelial leukocyte adhesion molecule 1 and vascular cell adhesion molecule 1. J Exp Med 1992, 176:1183-1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerfoot SM, Raharjo E, Ho M, Kaur J, Serirom S, McCafferty DM, Burns AR, Patel KD, Kubes P: Exclusive neutrophil recruitment with oncostatin M in a human system. Am J Pathol 2001, 159:1531-1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hattori R, Hamilton KK, Fugate RD, McEver RP, Sims PJ: Stimulated secretion of endothelial von Willebrand factor is accompanied by rapid redistribution to the cell surface of the intracellular granule membrane protein GMP-140. J Biol Chem 1989, 264:7768-7771 [PubMed] [Google Scholar]
- 29.Nussler AK, Eling W, Kremsler PG: Patients with Plasmodium falciparum malaria and Plasmodium vivax malaria show increased nitrite and nitrate plasma levels. J Infect Dis 1994, 169:1418-1419 [DOI] [PubMed] [Google Scholar]
- 30.al Yaman FM, Mokela D, Genton B, Rockett KA, Alpers MP, Clark IA: Association between serum levels of reactive nitrogen intermediates and coma in children with cerebral malaria in Papua New Guinea. Trans R Soc Trop Med Hyg 1996, 90:270-273 [DOI] [PubMed] [Google Scholar]
- 31.Cot S, Ringwald P, Mulder B, Miailhes P, Yap-Yap J, Nussler AK, Eling WM: Nitric oxide in cerebral malaria. J Infect Dis 1994, 169:147-1418 [DOI] [PubMed] [Google Scholar]
- 32.Kremsner PG, Winkler S, Wildling E, Prada J, Bienzle U, Graninger W, Nussler AK: High plasma levels of nitrogen oxides are associated with severe disease and correlate with rapid parasitological and clinical cure in Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg 1996, 90:44-47 [DOI] [PubMed] [Google Scholar]
- 33.Anstey NM, Weinberg JB, Hassanali MY, Mwaikambo ED, Manyenga D, Misukonis MA, Arnelle DR, Hollis D, McDonald MI, Granger DL: Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J Exp Med 1996, 184:557-567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agbenyega T, Angus B, Bedu-Addo G, Baffoe-Bonnie B, Griffin G, Vallance P, Krishna S: Plasma nitrogen oxides and blood lactate concentrations in Gambian children with malaria. Trans R Soc Trop Med Hyg 1997, 91:298-302 [DOI] [PubMed] [Google Scholar]
- 35.Dondorp AM, Planche T, de Bel EE, Angus BJ, Chotivanich KT, Silamut K, Romign JA, Ruangveerayuth R, Hoek FJ, Kager PA, Vreeken J, White NJ: Nitric oxides in plasma, urine, and cerebrospinal fluid in patients with severe falciparum malaria. Am J Trop Med Hyg 1998, 59:497-502 [DOI] [PubMed] [Google Scholar]
- 36.Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelish M, Kelm M: Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci USA 2001, 98:12814-12819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao L, Pan J, Setiadi H, Patel KD, McEver RP: Interleukin 4 or oncostatin M induces a prolonged increase in P-selectin mRNA and protein in human endothelial cells. J Exp Med 1996, 184:81-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramachandran V, Yago T, Epperson TK, Kobzdij MM, Nollert MU, Cumming RD, Zhu C, McEver RP: Dimerization of a selectin and its ligand stabilizes cell rolling and enhances tether strength in shear flow. Proc Natl Acad Sci USA 2001, 98:10166-10171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Setiadi H, Sedgewick G, Erlandsen SL, McEver RP: Interactions of the cytoplasmic domain of P-selectin with clathrin-coated pits enhance leukocyte adhesion under flow. J Cell Biol 1998, 142:859-871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanwar S, Smith CW, Kubes P: An absolute requirement for P-selectin expression in ischemia-reperfusion-induced leukocyte recruitment in cremaster muscle. Microcirculation 1998, 5:281-287 [PubMed] [Google Scholar]
- 41.Patel KD, Zimmerman GA, Prescott SM, McEver RP, McIntyre TM: Oxygen radicals induce human endothelial cells to express GMP-140 and bind neutrophils. J Cell Biol 1991, 112:749-759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kluger MS, Shiao SL, Bothwell AL, Pober JS: Cutting edge: internalization of transduced E-selectin by cultured human endothelial cells: comparison of dermal microvascular and umbilical vein cells and identification of a phosphoserine-type di-leucine motif. J Immunol 2002, 168:2091-2095 [DOI] [PubMed] [Google Scholar]
- 43.Maggio R, Barbier P, Toso A, Barletta D, Corsini G U: Sodium nitroprusside induces internalization of muscarinic receptors stably expressed in Chinese hamster ovary cell lines. J Neurochem 1995, 65:943-946 [DOI] [PubMed] [Google Scholar]
- 44.Biffl WL, Moore EE, Moore FA, Barnett C: Nitric oxide reduces endothelial expression of intercellular adhesion molecule (ICAM-1). J Surg 1996, 63:328-332 [DOI] [PubMed] [Google Scholar]
- 45.Matsushita H, Chang E, Glassford AJ, Cooke JP, Chiu CP, Tsao PS: eNOS activity is reduced in senescent human endothelial cells: preservation by hTERT immortalization. Circ Res 2001, 26:793-798 [DOI] [PubMed] [Google Scholar]
- 46.Kubes P, Kanwar S, Niu XF, Gaboury JP: Nitric oxide synthesis inhibition induces leukocyte adhesion via superoxide and mast cells. EMBO J 1993, 7:1293-1299 [DOI] [PubMed] [Google Scholar]
- 47.Niu XF, Ibbotson G, Kubes P: A balance between nitric oxide and oxidants regulates mast cell-dependent neutrophil-endothelial cell interactions. Circ Res 1996, 79:992-999 [DOI] [PubMed] [Google Scholar]
- 48.Christofidou-Solomidou M, Murphy GF, Albelda SM: Induction of E-selectin-dependent leukocyte recruitment by mast cell degranulation in human skin grafts transplanted on SCID mice. Am J Pathol 1996, 148:177-188 [PMC free article] [PubMed] [Google Scholar]
- 49.Turner GD, Morrison H, Jones M, Davis TM, Looareesuwan S, Buley ID, Gratter KC, Newbold CI, Pukritayakamee S, Nagachinta B: An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule 1 in cerebral sequestration. Am J Pathol 1994, 145:1057-1069 [PMC free article] [PubMed] [Google Scholar]
- 50.Losert H, Schmid K, Wilfing A, Winkler S, Staudinger T, Kletzmayr J, Burgmann H: Experiences with severe P. falciparum malaria in the intensive care unit. Intensive Care Med 2000, 26:195-201 [DOI] [PubMed] [Google Scholar]


