Abstract
Estrogen deficiency may contribute to the development and progression of glomerulosclerosis in postmenopausal women. The responsiveness to estrogens could be controlled by genetic traits related to those that determine the susceptibility to glomerular scarring. This study was undertaken to determine whether the intensity of the sclerotic response was modified by the estrogen status in sclerosis-prone ROP Os/+ mice. Ovariectomized ROP Os/+ mice developed more severe renal dysfunction and glomerulosclerosis than intact, ie, estrogen sufficient age-matched female mice. Ovariectomized ROP Os/+ exhibited increased accumulation of extracellular matrix, predominantly of laminin, and a marked distortion of the glomerular architecture. We found an increase in macrophage infiltration in the mesangium of ovariectomized ROP Os/+. Estrogen deficiency decreased glomerular estrogen receptor expression in ROP Os/+ mice, which we had previously found to be low in the parental ROP strain. Thus, although physiological estrogen levels in young ROP Os/+ mice could not prevent the development of glomerulosclerosis, estrogen deficiency accelerated the progression of glomerular scarring in this mouse strain. This suggests that estrogen replacement will slow but not prevent the progression of glomerulosclerosis. It underscores the importance of the genetic composition of individuals that determines the susceptibility to diseases as well as the response to treatment.
Estrogen deficiency may contribute to the development and progression of glomerulosclerosis in postmenopausal women. This is suggested by data from the United States Renal Data System, which show that the female to male ratio of patients with end-stage renal disease because of diabetes increases sharply in the postmenopausal age groups, especially of those from ethnic minorities. 1 The United States Renal Data System data as well as additional genetic and epidemiological studies also suggest that genetic factors play an important role in the susceptibility to develop glomerulosclerosis and the progression to end-stage renal disease. 2,3 The responsiveness to estrogens, which is primarily determined by the level of estrogen receptor (ER) expression, 4 could be controlled by genetic traits related to those that determine the susceptibility to glomerular scarring. We have previously shown that the renal glomerulus is a direct target tissue for estrogens. 5,6 Human and mouse mesangial cells, which play a central role in the pathogenesis of glomerulosclerosis, express both ER subtypes α and β. 5 We reported that these ERs were transcriptionally active and that estrogens positively regulated ER expression in mesangial cells. 5 We also found that estrogens provided protection from glomerulosclerosis by regulating genes involved in extracellular matrix (ECM) turnover in a manner leading to the prevention of ECM accumulation in the mesangium. 5,6 Estrogen treatment increased the expression and activity of matrix metalloproteinase (MMP)-9 in mesangial cells isolated from glomerulosclerosis-resistant mice, predominantly via ERα activation. 6 In addition, estrogens have also been shown to decrease the synthesis of type I and IV collagen in mesangial cells. 6-9 Importantly, we found that ER levels were much lower in the glomeruli and mesangial cells isolated from glomerulosclerosis-prone ROP than glomerulosclerosis-resistant C57BL6/J mice. 6
Female ROP mice develop glomerulosclerosis in response to nephron reduction or diabetes during their reproductive life span. 10-12 This suggests that their circulating estrogen levels, which are adequate to assure fertility, do not sufficiently protect the renal glomerulus against noxious stimuli, which cause glomerulosclerosis. Based on these data, we now postulate that decreased expression of ER in glomerular cells may be a critical part of the propensity of ROP mice to develop glomerulosclerosis after injury. Female ROP mice may resemble the group or subset of women who develop glomerulosclerosis during their reproductive years, ie, before menopause and who experience an acceleration and further progression of their renal disease after the onset of menopause.
Therefore, we examined whether estrogen deficiency had an effect on the development and progression of glomerulosclerosis in a glomerulosclerosis-prone mouse model. In a previous study of the oligosyndactyly (Os) mutation that causes a congenital 50% reduction in nephron number, we found that young ROP Os/+ mice developed glomerular hypertrophy and glomerulosclerosis, ie, during their estrogen-sufficient period of life. 10 This was associated with an increase in both cell turnover and ECM synthesis. In contrast, sclerosis-resistant C57 Os/+ mice developed only minimal glomerular scarring despite an identical reduction in nephron number. 11 These data suggested that the development of glomerulosclerosis was primarily dependent on the genetic background. 13 Pathological stimuli such as unilateral nephrectomy or diabetes further accelerated the progression and intensity of glomerulosclerosis in young sclerosis-prone ROP Os/+ but not in sclerosis-resistant mouse strains. 11-13
In the current study, we asked whether estrogen deficiency after ovariectomy accelerated and worsened the sclerotic response in young adult female ROP Os/+ mice. In other words, we wanted to determine whether the intensity of the sclerotic response to nephron reduction was modified by the estrogen status in these mice. Ovariectomized (Ovx) ROP Os/+ mice, ie, estrogen-deficient mice, developed more severe renal dysfunction and glomerulosclerosis than intact, ie, estrogen-sufficient age-matched female mice. Ovx ROP Os/+ exhibited increased accumulation of ECM, predominantly of laminin. In addition, we found macrophage infiltration in the mesangium of Ovx ROP Os/+. Long-standing estrogen deficiency further decreased glomerular ER expression in ROP Os/+ mice, which was already significantly lower in the parental ROP strain compared to glomerulosclerosis-resistant C57BL6/J mice. Thus, although physiological estrogen levels in young ROP Os/+ mice could not prevent the development of glomerulosclerosis, estrogen deficiency accelerated the progression of glomerular scarring in this mouse strain. These data confirm that estrogens provide only a partial and relative protection in sclerosis-prone individuals. They are also in line with the results of the randomized estrogen replacement therapy trials. 14,15 This suggests that estrogen replacement, which in humans and rodents raises estrogen levels only into the lower range of what is usually seen during the menstrual/estrous cycle, will not reverse or prevent the development but may slow the rate of progression of glomerulosclerosis in susceptible individuals.
Materials and Methods
Experimental Design
Female ROP Os/+ (ROP/Le-Os Es1b/+ Es1a) mice were obtained from Jackson Laboratories (Bar Harbor, ME). At 3 to 4 months of age, six animals were ovariectomized (Ovx) using the following procedure, which was approved by the committee for animal safety at the University of Miami School of Medicine. Mice were anesthetized with ketamine/xylazine (80:5 mg/kg i.m.). A small area of both flanks was shaved and disinfected with Septisol foam (Calgon-Vestal, St. Louis, MO) and bilateral incisions were made. The ovaries and fallopian tubes were removed and the uterine horns were placed in the abdominal cavity. The incisions were closed with silk sutures. Six intact female mice served as controls.
Animal Sacrifice
Intact or Ovx animals were allowed free food and water for 10 to 11 months and were sacrificed at 14 months of age, as previously described. 11 Briefly, the left kidney was perfused with a buffer solution containing collagenase and RNase inhibitors for microdissection of glomeruli. The right kidney was perfused in situ with 6 ml of phosphate-buffered saline and 3 ml of 4% paraformaldehyde, postfixed in 4% paraformaldehyde solution for at least 12 hours, and embedded in methacrylate. Sections, which were 4-μm thick, were stained with periodic acid-Schiff. Other kidney fragments were immediately frozen in OCT.
Measurements of Urinary Albumin and Creatinine and Blood Urea Nitrogen
Urine samples were collected once a month and at sacrifice. Albumin excretion was measured by enzyme-linked immunosorbent assay (Wako International, Tokyo, Japan), corrected for creatinine concentration in the urine (kit using the Jaffe method; Stanbio, San Antonio, TX) and expressed as the urinary albumin/creatinine excretion ratio (AER). Serum was collected at the time of sacrifice and used to determine blood urea nitrogen concentrations (Urease method; Sigma Chemical Co., St. Louis, MO).
Noninvasive Measurement of Blood Pressure
Tail cuff blood pressure measurements were obtained in conscious mice using the Non-Invasive Blood Pressure system from Columbus Instruments (Columbus, OH) connected to a 133 MHz Pentium computer. Mice were acclimated to the restrainers throughout a period of several days, and on the day of measurement, were left undisturbed for 10 to 15 minutes before recording the blood pressure. An inflatable occlusion cuff was placed proximally on the tail and a sensor cuff was placed distally. The tail was warmed to a temperature between 30 to 37°C. Six measurements of systolic pressure were recorded throughout an approximate time of 10 minutes, and expressed as the mean.
Morphometry
A morphometric approach was used to quantify the degree of glomerulosclerosis. 11 Fifty cortical glomeruli, randomly selected from each mouse, were recorded with an Olympus BH-2 microscope and Micro Image A209RGB color video camera. Glomerular volume (μm3) and mesangial area (μm2) were measured using MetaMorph 4.5.4 Imaging System computer program (Universal Imaging Corporation, West Chester, PA). Because the glomerular lesions were too advanced to measure the mesangial areas we chose to measure the vascular spaces. The ratio vascular space/glomerular area was calculated for each mouse. The result (percent vascular space/glomerular area) was used to determine the severity of glomerulosclerosis in each animal. 16
Immunofluorescence Staining
Cryostat sections of 4-μm thickness were exposed to either rabbit anti-mouse collagen type IV (Biodesign, Saco, ME), rabbit anti-mouse laminin (Research Diagnostics, Flanders, NJ), rabbit anti-chicken tenascin (Chemicon, Temecula, CA) or rat anti-mouse CD68 (Serotec, Raleigh, NC) followed by goat anti-rabbit-conjugated fluorescein isothiocyanate. The sections were examined and graded on a scale of 0 to 4 by a renal pathologist blinded to the origin of the kidney slides.
Isolation of RNA and Quantitative Analysis of RNA Expression by Real-Time Reverse Transcriptase-Polymerase Chain Reaction
Total RNA was extracted from 100 glomeruli, microdissected from each animal, using the guanidinium thiocyanate-phenol-chloroform method as described. 17 Amplification and quantification of target RNAs was performed on an ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Foster City, CA). 6 TaqMan probes and primers for amplification of the specific transcripts were designed using the Primer Express 1.5 from Applied Biosystems. Primer sequences were identified with Tm values 10°C less than the probe. The TaqMan probes were labeled with the reporter dyes FAM, VIC, or TET at the 5′-end and with the quencher dye TAMRA at the 3′-end. The primers and probes for ERα, ERβ, MMP-2, type IV collagen, and laminin were synthesized commercially (Applied Biosystems, ABI Primer & Probes, Foster City, CA). TaqMan ribosomal RNA control reagents designed to detect 18S ribosomal RNA, which served as an endogenous control to normalize for variations in the isolated RNA amount, were purchased from Applied Biosystems.
Zymography for MMPs
A piece of kidney cortex from each mouse was lysed in buffer and protein concentration was determined using the Bradford method, as previously described. 6 Equal amounts of protein were electrophoresed on a gelatin gel and processed to determine MMP-2 and MMP-9 activity, as previously described. 6
Electron Microscopy
Kidney tissue was postfixed for 1 hour in 1.0% osmium tetroxide (in 0.2 mol/L of sodium cacodylate), prestained in 1.25% uranyl acetate for 1 hour, dehydrated through a series of graded alcohol solutions, and embedded in EPON epoxy resin.
Statistical Analysis
All values were expressed as mean ± SEM. The two-tailed unpaired Student’s t-test was used to evaluate differences between means of corresponding sets of data obtained from Ovx and intact mice.
Results
Body, Kidney, and Uterine Weight in Intact and Ovx ROP Os/+ Mice
Intact and Ovx ROP Os/+ mice were sacrificed at 14 months of age. Body and kidney weight were not different between the two groups (Table 1) ▶ . As expected, the estrogen-deficient state, which began at 3 to 4 months of age and lasted ∼10 to 11 months in the Ovx mice, resulted in a lower mean uterine weight in Ovx than in intact animals (0.103 ± 0.038 g versus 0.198 ± 0.026 g). Each group of intact and Ovx mice contained six animals. However, two of the Ovx and one of the intact mice died before they reached the age of 14 months and were not included in the analysis.
Table 1.
Comparison of Intact and Ovx Mice
| Intact (control) | Ovx | |
|---|---|---|
| n | 5 | 4 |
| Body weight (g) | 24.12 ± 1.4 | 23.5 ± 1.1 |
| Kidney weight (g) | 0.18 ± 0.035 | 0.12 ± 0.008 |
| Kidney weight/body weight ratio (g) | 0.007 ± 0.001 | 0.004 ± 0.0003 |
| Uterus weight (g) | 0.198 ± 0.026 | 0.103 ± 0.038 |
| Albumin/creatinine ratio | 0.25 ± 0.03 | 1.05 ± 0.3* |
| Serum blood urea nitrogen (n = 3) | 46.1 ± 5.3 | 67.7 ± 4.6* |
P < 0.05
Renal Function
The AER of intact and Ovx mice was determined once monthly throughout the study and at the time of sacrifice (Table 1) ▶ . AER was fourfold higher in the Ovx mice (P < 0.05). In addition, blood urea nitrogen concentrations, a measure of kidney function, also significantly increased in Ovx mice as a result of an accelerated decline in kidney function (Table 1) ▶ .
Systolic Blood Pressure in Ovx and Intact ROP Os/+ Mice
We measured systolic blood pressure in Ovx and intact mice at the age of 14 months using a noninvasive tail cuff blood pressure system. There was no difference in the systolic blood pressure between the Ovx and intact mice (120.0 ± 4.243 mmHg versus 119.3 ± 3.786 mmHg, respectively).
Histology
Although there was some degree of mesangial sclerosis in the intact animals, Ovx ROP Os/+ mice showed more advanced sclerosis with marked decrease of the vascular lumen (Figure 1) ▶ . Approximately 10% of the glomeruli were close to complete obsolescence with only occasional loops recognizable. The remaining peripheral basement membrane appeared markedly thickened. The lesions were diffuse and relatively homogeneous. The interstitial tissue contained occasional inflammatory cells and proteinaceous casts in the tubules in both the cortex and medullary regions (Figure 1, A and C) ▶ . Some tubular basement membranes were severely thickened. In contrast, the intact animals had a minor reduction in the vascular lumen with moderate thickening of the basement membrane. There was no apparent increase in cellularity in the glomeruli. Occasional vessels showed intimal thickening.
Figure 1.
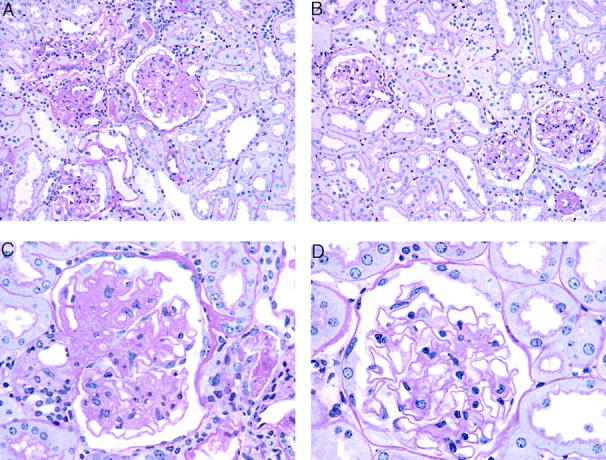
Light microscopy. Methacrylate-embedded sections were stained with periodic acid-Schiff silver methanamine. Sections from a ROP Os/+ mouse 10 months after ovariectomy (A, C). Sections from an intact ROP Os/+ mouse (B, D). Original magnifications: ×200 (A, B); ×400 (C, D).
Morphometry of the Glomeruli of Ovx and Intact ROP Os/+ Mice
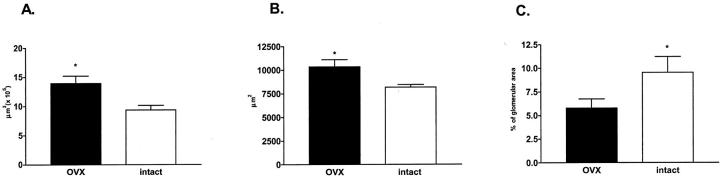
There was a conspicuous increase in the size of glomeruli in Ovx ROP Os/+ mice (Figure 2) ▶ . Specifically, glomerular volume (13.9 ± 1.28 × 105 μm3 versus 9.40300 ± 1.5 × 105 μm3) and glomerular area (10,330 ± 761 μm2 versus 8169 ± 272 μm2) were increased (P < 0.05), whereas the percent ratio vascular space/glomerular area was decreased (Figure 2C ▶ , P < 0.05) in Ovx mice.
Figure 2.
Morphometric analysis. Glomerular volume (A) and glomerular area (B) were increased and percent vascular space (C) was decreased in Ovx (black bars) compared to intact ROP Os/+ mice (open bars) (*, P < 0.05).
Immunofluorescence Microscopy
There were large amounts of type IV collagen, laminin, and tenascin in the mesangial areas of both Ovx and intact ROP Os/+ mice. However, there was a marked increase in laminin deposition in the glomeruli of Ovx ROP Os/+ mice (Figure 3) ▶ . In addition, staining with CD68, a specific marker of macrophages, revealed an approximately threefold increase in the number of macrophages/glomerulus in Ovx animals (Figure 4) ▶ .
Figure 3.
Immunofluorescence staining of laminin. A: ROP Os/+ mouse 10 months after ovariectomy. B: Intact ROP Os/+ mouse. Original magnifications ×400.
Figure 4.

Immunofluorescence staining of macrophages. A: Representative staining of ROP Os/+ mice 10 months after ovariectomy with CD68 anti-macrophage antibody. B: Intact ROP Os/+ mouse. Original magnifications ×630.
Glomerular mRNA Expression
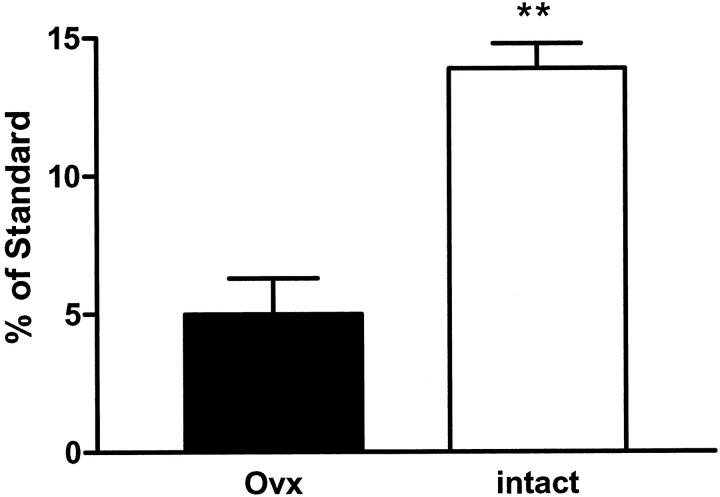
Using real-time reverse transcriptase-polymerase chain reaction, we amplified and measured the expression of ERα, ERβ, MMP-2, type IV collagen, and laminin mRNA. There was a profound decrease in mRNA expression of both ER subtypes (Figure 5) ▶ . ERβ was barely detectable in both the intact and Ovx mice confirming our previous data that ERα is the predominant subtype in the glomerulus. Expression of MMP-2, collagen type IV, and laminin mRNA was not different between the two groups, although there was a trend of higher type IV collagen in the Ovx group (data not shown) that did not reach statistical significance.
Figure 5.
Expression of ERα mRNA. ERα mRNA expression (normalized to 18S mRNA) was measured by real-time polymerase chain reaction, in isolated glomeruli from Ovx (black bars) and intact (open bars) ROP Os/+ mice. The graph represents the mean ± SEM of three animals per group (**, P < 0.05).
MMP Activity
MMP-2 and -9 activity were assessed in the cortex tissue of intact and Ovx mice. There was no difference between the groups (data not shown). This correlated with the expression of the MMP-2 mRNA found in extracted glomeruli, suggesting that MMP-2 transcription did not increase despite increased mesangial matrix in the presence of estrogen deficiency.
Electron Microscopy of Glomerular Lesions
The glomerular architecture of the Ovx mice was markedly distorted because of glomerular capillary collapse and mesangial sclerosis. The podocyte processes showed segmental effacement by ultrastructural examination (Figure 6) ▶ .
Figure 6.

Electron microscopy. A: Low power of a glomerulus from an Ovx ROP Os/+ mouse demonstrates extensive sclerosis. B: Glomerulus with preserved architecture and minimal sclerosis (intact ROP Os/+). Original magnifications ×4250.
Discussion
The purpose of this study was to determine whether the intensity of the sclerotic response to nephron reduction was modified by the estrogen status in sclerosis-prone ROP Os/+ mice. In general, mice start to have regular estrous cycles around 3 to 4 months of age and go into persistent vaginal cornification, a period to some extent comparable to the perimenopausal state in humans, between the ages of 11 to 16 months. 18 To determine the effects of estrogen deficiency and to minimize the influence of aging per se on the scarring process, we studied young, female ROP Os/+ mice during their reproductive life span. Therefore, mice were Ovx at 3 months and were 12 to 14 months old at the time of sacrifice while intact animals of the same age served as control. At the time of sacrifice, serum estradiol levels did not differ between the two groups. However, uterine weight, which is often used as a surrogate measure of biologically active estrogens, was decreased in OVX compared to intact mice suggesting that estradiol levels were chronically lower in the OVX mice.
Although Ovx ROP Os/+ mice had more microalbuminuria and higher blood urea nitrogen levels than age-matched intact females, there was no difference in blood pressure between both groups. Thus, the development and progression of renal dysfunction was not because of hypertension in the ROP Os/+ mice. In addition, estrogen deficiency did not cause a rise in the blood pressure of this sclerosis-prone mouse model as it had been recently described in mice, which lack ERβ expression. 19
Although both intact and Ovx ROP Os/+ mice developed glomerular hypertrophy and glomerulosclerosis, these abnormalities were more pronounced and severe in the Ovx animals. This was associated with a marked distortion of the glomerular architecture because of basement membrane collapse as well as segmental effacement of the podocyte processes in the Ovx mice. There were large amounts of type IV collagen, laminin, and tenascin in the mesangial area of intact and Ovx ROP Os/+ mice. This confirmed previous studies of our laboratory showing that the glomeruli of ROP Os/+ mice contained twofold to threefold more type IV collagen, laminin, and tenascin mRNA than those of a sclerosis-resistant mouse strain carrying the Os/+ mutation (C57/Os/+). 11 However, whereas the staining for α1-collagen IV and tenascin were similar in both groups, there was a marked increase in laminin deposition in the glomerulus of Ovx ROP Os/+ mice. Interestingly, we found no difference in the glomerular mRNA expression of the ECM-digesting MMP-2 as well as of collagen type IV, tenascin, and laminin B1 in intact and Ovx ROP Os/+ mice. Similarly, we did not find any change in MMP-2 activity in cortical extracts, which may not reveal changes in glomeruli. This suggests that increased laminin deposition is because of mechanisms other than transcriptional regulation of this gene by estrogens. Interestingly, estrogens have been shown to stimulate the synthesis of glycoproteins, such as laminin B1, by stimulating the glycosylation apparatus without increasing protein synthesis. 20 Thus, ovariectomy accelerated the progression of glomerulosclerosis, even though in the intact mice physiological estrogen levels did not prevent glomerular lesions.
In addition, the number of macrophages was increased in the mesangium of Ovx ROP Os/+ mice. Infiltrating macrophages have been suggested to play a pivotal role in the progression to glomerulosclerosis because treatment of mesangial cells with macrophage-conditioned medium up-regulated the production of ECM components including laminin in a dose- and time-dependent manner, independently of cell proliferation. 21 Our data support the hypothesis that macrophage infiltration accelerates the disease process because renal dysfunction and glomerulosclerosis were much more severe in Ovx ROP Os/+ mice. In addition, there exists a link between increased macrophage infiltration and estrogen deficiency. In murine macrophages and human coronary artery endothelial cells, 17β-estradiol suppressed the expression of monocyte chemoattractant protein-1, a chemokine that mainly attracts blood monocytes. 22,23 Estrogen deficiency and/or unresponsiveness may, therefore, promote macrophage recruitment to the glomerulus. On the other hand, suppressing macrophage activation and/or recruitment may represent a potential mechanism by which estrogens slow the glomerular scarring process in sclerosis-prone individuals. Taken together, macrophage infiltration induced by estrogen deficiency is associated with acceleration but not initiation of glomerulosclerosis.
We confirmed that female ROP Os/+ mice, which develop glomerulosclerosis during their estrogen-sufficient period of life, express lower glomerular ER levels than sclerosis-resistant mouse strains. This is in accordance with a previous study, in which we found that expression of ERα and ERβ was significantly lower in the glomerulus of the parental ROP strain than of glomerulosclerosis-resistant C57BL6/J mice. 6 Studies of ER have shown that physiological ER levels limit the estrogen-mediated transcriptional activity well below the cellular capacity to respond to estrogens. 4 In addition, the linear relationship, which exists between the amount of steroid hormone receptors and the level of transcriptional activation of target genes after steroid treatment, suggests that the glomerular responsiveness to estrogens is decreased in female ROP Os/+ mice. In other words, physiological estrogen levels cannot exert the same degree of ER-mediated responses in the glomerulus of female ROP Os/+ compared to C57BL6/J mice. This genetically determined difference in ER expression may contribute to the susceptibility to glomerulosclerosis as part of the sclerosis-prone or -resistant phenotype.
Long-standing estrogen deficiency decreased glomerular ER expression in female ROP Os/+ mice. Since we had previously found that estrogens positively regulated ER expression in mesangial cells, 5 we postulated that lack of estrogens would lower glomerular ER expression. In fact, this study provides the first in vivo evidence that chronic estrogen deficiency is associated with a decrease in glomerular ER expression. Thus, we propose that relative estrogen unresponsiveness because of lower ER levels contributes to the development of glomerulosclerosis in intact ROP Os/+ mice and that absolute estrogen deficiency, ie, ovariectomy, accelerates this process.
In several recent randomized trials studying estrogen replacement therapy for the primary or secondary prevention of coronary heart disease, estrogen replacement therapy failed to prevent cardiovascular disease in postmenopausal women. 14,15 For instance, estrogen replacement therapy did not change the thickness of the coronary artery diameter in women participating in the Estrogen Replacement in Atherosclerosis trial. This suggests that these women were resistant to the potential beneficial effects of estrogens, at least, in this specific vascular bed. 14 It is tempting to speculate that the decreased estrogen responsiveness in the coronary arteries of women developing atherosclerosis is part of a genetic susceptibility comparable to that leading to glomerular disease in female ROP Os/+ mice.
In addition to causing estrogen deficiency, ovariectomy also lowers circulating blood progesterone levels as well as serum concentrations of other nonsteroidal hormones such as inhibin and activin. Progesterone action is mediated via progesterone receptors (PR), which are expressed in arterial and venous smooth muscle cells. 24 However, we previously found that PR expression in mesangial cells is near the limit of detection by reverse transcriptase-polymerase chain reaction (unpublished observation, M. Potier) suggesting that progesterone action and effects are very limited in this vascular bed. Thus, progesterone deficiency may not play an important role in the progression of glomerulosclerosis.
In summary, we found that the potential estrogen-mediated protection against the development and progression of glomerulosclerosis depended primarily on the genetic background. In female ROP Os/+, estrogens provided only a relative and partial protection against glomerulosclerosis because of a low glomerular ER expression, which appears to be genetically determined. Because estrogen responsiveness is decreased in glomerular cells, estrogens cannot prevent the development or progression of glomerulosclerosis due to stimuli such as nephron reduction and diabetes in young glomerulosclerosis-prone mice. The glomerular estrogenunresponsiveness exists even during the reproductive life of sclerosis-prone mice, therefore, at a time when estrogen levels, despite their cyclic variations, are highest. On the other hand, ovariectomy accelerated the progression of glomerulosclerosis, which indicates that estrogens provide minimal protection against glomerulosclerosis. One can conclude from these data that estrogen replacement, which in humans and rodents raises estrogen levels only into the lower range of what is usually seen during the menstrual/estrous cycle, will not reverse or prevent the development or progression of glomerulosclerosis in susceptible individuals. At best, estrogen replacement will only slow the progression of glomerulosclerosis, which is accelerated by estrogen deficiency. These findings underscore the importance of the genetic background in determining both the susceptibility to diseases as well as the response to treatment.
Acknowledgments
We thank Alton Thomas and Milton Arana for helping to perform the ovariectomies and care of the mice, and Dr. Y. Zang and Judith Pignac-Kobinger for helping with the microdissections and polymerase chain reaction.
Footnotes
Address reprint requests to Michael Karl, M.D. Vascular Biology Institute, University of Miami School of Medicine, 1600 N.W. 10th Ave., RMSB, Rm 1043-R104, Miami, FL 33136. E-mail: mkarl@miami.edu.
Supported by the National Institutes of Health (NIA R01, AG17170-01 to S. J. E.; NIA R01 AG19366-01 to G. E. S.; and NIA R01, AG17170-01 to L. J. S.), the American Heart Association (grant-in-aid AHA 0051513B bx;1to S. J. E.; and post doctoral fellowship AHA 0020544B to M. P.), and the Florida Department of Health (grant BM041 to S. J. E.).
M. K. is the recipient of a Career Development Award of the American Diabetes Association.
S. J. E. and M. K. contributed equally to this article.
References
- 1.: U. S. Renal Data System: USRDS 2000 Annual Data Report. 2000. The National Institutes of Health, NIDDK Bethesda MD
- 2.Butkus DE: Familial clustering of end-stage renal disease in Mississippi. J Miss State Med Assoc 2002, 43:71-77 [PubMed] [Google Scholar]
- 3.Lenz O, Zheng F, Vilar J, Doublier S, Lupia E, Schwedler S, Striker LJ, Striker GE: The inheritance of glomerulosclerosis in mice is controlled by multiple quantitative trait loci. Nephrol Dial Transplant 1998, 13:3074-3078 [DOI] [PubMed] [Google Scholar]
- 4.Webb P, Lopez GN, Greene GL, Baxter JD, Kushner PJ: The limits of the cellular capacity to mediate an estrogen response. Mol Endocrinol 1992, 6:157-167 [DOI] [PubMed] [Google Scholar]
- 5.Potier M, Elliot SJ, Tack I, Lenz O, Striker GE, Striker LJ, Karl M: Expression and regulation of estrogen receptors in mesangial cells: influence on matrix metalloproteinase-9. J Am Soc Nephrol 2001, 12:241-251 [DOI] [PubMed] [Google Scholar]
- 6.Potier M, Karl M, Zheng F, Elliot SJ, Striker GE, Striker LJ: Estrogen-related abnormalities in glomerulosclerosis-prone mice: reduced mesangial cell estrogen receptor expression and prosclerotic response to estrogens. Am J Pathol 2002, 160:1877-1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lei J, Silbiger S, Ziyadeh FN, Neugarten J: Serum-stimulated alpha 1 type IV collagen gene transcription is mediated by TGF-beta and inhibited by estradiol. Am J Physiol 1998, 274:F252-F258 [DOI] [PubMed] [Google Scholar]
- 8.Neugarten J, Medve I, Lei J, Silbiger SR: Estradiol suppresses mesangial cell type I collagen synthesis via activation of the MAP kinase cascade. Am J Physiol 1999, 277:F875-F881 [DOI] [PubMed] [Google Scholar]
- 9.Silbiger S, Lei J, Neugarten J: Estradiol suppresses type I collagen synthesis in mesangial cells via activation of activator protein-1. Kidney Int 1999, 55:1268-1276 [DOI] [PubMed] [Google Scholar]
- 10.He C, Zalups RK, Henderson DA, Striker GE, Striker LJ: Molecular analysis of spontaneous glomerulosclerosis in Os/+ mice, a model with reduced nephron mass. Am J Physiol 1995, 269:F266-F273 [DOI] [PubMed] [Google Scholar]
- 11.He CJ, Esposito C, Phillips C, Zalups RK, Henderson DA, Striker GE, Striker LJ: Dissociation of glomerular hypertrophy, cell proliferation and glomerulosclerosis in mouse strains heterozygous for a mutation (Os) which induces a 50% reduction in nephron number. J Clin Invest 1996, 97:1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esposito C, He CJ, Striker GE, Zalups RK, Striker LJ: Nature and severity of the glomerular response to nephron reduction is strain-dependent in mice. Am J Pathol 1999, 154:891-897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng F, Striker G, Esposito C, Lupia E, Striker L: Strain differences rather than hyperglycemia determine the severity of glomerulosclerosis in mice. Kidney Int 1998, 54:1999-2007 [DOI] [PubMed] [Google Scholar]
- 14.Herrington DM, Reboussin DM, Brosnihan KB, Sharp PC, Shumaker SA, Snyder TE, Furberg CD, Kowalchuk GJ, Stuckey TD, Rogers WJ, Givens DH, Waters D: Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N Engl J Med 2000, 343:522-529 [DOI] [PubMed] [Google Scholar]
- 15.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E: Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA 1998, 280:605-613 [DOI] [PubMed] [Google Scholar]
- 16.Pesce CM, Striker LJ, Peten E, Elliot SJ, Striker GE: Glomerulosclerosis at both early and late stages is associated with increased cell turnover in mice transgenic for growth hormone. Lab Invest 1991, 65:601-605 [PubMed] [Google Scholar]
- 17.Peten EP, Garcia-Perez A, Terada Y, Woodrow D, Martin BM, Striker GE, Striker LJ: Age-related changes in alpha 1- and alpha 2-chain type IV collagen mRNAs in adult mouse glomeruli: competitive PCR. Am J Physiol 1992, 263:F951-F957 [DOI] [PubMed] [Google Scholar]
- 18.vom Saal FS, Finch CE, Nelson JF: Natural history and mechanisms of reproductive aging in humans, laboratory rodents, and other selected vertebrates. Knobil E Neill JD eds. The Physiology of Reproduction. 1994:pp 1213-1314 Raven Press New York
- 19.Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME: Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science 2002, 295:505-508 [DOI] [PubMed] [Google Scholar]
- 20.Carson DD, Farrar JD, Laidlaw J, Wright DA: Selective activation of the N-glycosylation apparatus in uteri by estrogen. J Biol Chem 1990, 265:2947-2955 [PubMed] [Google Scholar]
- 21.Pawluczyk IZ, Harris KP: Macrophages promote prosclerotic responses in cultured rat mesangial cells: a mechanism for the initiation of glomerulosclerosis. J Am Soc Nephrol 1997, 8:1525-1536 [DOI] [PubMed] [Google Scholar]
- 22.Frazier-Jessen MR, Mott FJ, Witte PL, Kovacs EJ: Estrogen suppression of connective tissue deposition in a murine model of peritoneal adhesion formation. J Immunol 1996, 156:3036-3042 [PubMed] [Google Scholar]
- 23.Seli E, Pehlivan T, Selam B, Garcia-Velasco JA, Arici A: Estradiol down-regulates MCP-1 expression in human coronary artery endothelial cells. Fertil Steril 2002, 77:542-547 [DOI] [PubMed] [Google Scholar]
- 24.Hodges YK, Richer JK, Horwitz KB, Horwitz LD: Variant estrogen and progesterone receptor messages in human vascular smooth muscle. Circulation 1999, 99:2688-2693 [DOI] [PubMed] [Google Scholar]