Abstract
Biochemical studies show that phosphorylated τ, like that found in paired helical filaments (PHFs), does not promote microtubule assembly leading to the view that PHF formation leads to microtubule deficiency in Alzheimer’s disease (AD). However, although this issue is one of the most important aspects to further understanding the cell biology of AD, no quantitative examination of microtubule diminution in AD and its relationship with PHFs has been performed. To examine this issue directly, we undertook a morphometric study of brain biopsy specimens from AD and control cases. Ultrastructural analysis of neurons was performed to compare the microtubule assembly state in neurons of diseased and control cases and to examine the effect of PHF accumulation. We found that both number and total length of microtubules were significantly and selectively reduced in pyramidal neurons from AD in comparison to control cases (P = 0.000004) but that this decrement in microtubule density was surprisingly unrelated to PHFs (P = 0.8). Further, we found a significant age-dependent decrease in microtubule density with aging in the control cases (P = 0.016). These findings suggest that reduction in microtubule assembly is not dependent on τ abnormalities of AD and aging.
The accumulation of paired helical filaments (PHFs) in Alzheimer’s disease (AD) is one of the most striking features of neuronal cytoskeletal pathology in AD and one noted in the earliest description of the disease. 1 The finding that PHFs derive from τ, 2 a major microtubule-stabilizing protein, and that the form of τ found in PHFs is highly phosphorylated and inactive in microtubule assembly 3 suggested that formation of PHFs may directly underlie the abnormalities in microtubule-based transport thought to occur in AD. 4,5 In this regard, the observations of increased mitochondrial components in lysosomes, 6 synaptic vesicles failing to arrive at terminals, 7,8 and vesicle accumulation in cell bodies 5 suggest that microtubule-dependent transport of organelles is hindered in AD. 4 The clinical importance of these findings is that microtubule reduction may underlie the loss of neuronal connectivity suggested as the basis of cognitive loss in AD. 9 In this study, we sought not only to quantitatively determine whether microtubules are reduced early in the course of AD but also whether the reduction is mechanistically linked to τ phosphorylation by examining neurons with and without PHFs.
Materials and Methods
Biopsy
Tissue was taken for diagnostic procedures from the frontal or parietal cortex of six patients with dementia (52 to 63 years of age), most of which had been included in other clinicopathological studies, 5,6,10 and with a definite history (duration 3 to 11 years), and clinical presentation of dementia, fulfilling the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Diseases and Related Disorders Associations (NINCDS-ADRDA working group criteria for probable AD. 11 Thirteen cases of cognitively normal individuals were examined ultrastructurally and four patients (62 to 80 years of age) were selected for this study based on the presence of normal brain tissue. Of the control cases selected, three suffered from hydrocephalus and one from a brain tumor, in which no sign of tumor infiltration or other abnormalities were evident in the tissue examined (Table 1) ▶ . Tissue was fixed in 1.5% glutaraldehyde in cacodylate buffer for 1 hour immediately on removal from the brain and postfixed in 1% osmium tetroxide for 1 hour. After dehydration in graded ethanol and propylene oxide, the tissue was embedded in Epon 812, sectioned at silver interference color, electron contrasted with uranyl acetate and lead citrate, and grids were viewed at 80 KV by using a JEOL 100CX electron microscope. Unfortunately, there is no information on whether an autopsy was performed on these cases to confirm the original diagnosis or to know the progression of pathological changes.
Table 1.
Demographic Data of Patients Used in this Study
| Case no. | Diagnosis | Age (yr) | Gender | Pyramidal neurons (n) | Nonpyramidal neurons (n) | Cortical area |
|---|---|---|---|---|---|---|
| 1 | AD | 52 | F | 1 | 1 | Parietal |
| 2 | AD | 53 | M | 0 | 2 | Prefrontal |
| 3 | AD | 55 | M | 4 | 3 | Prefrontal |
| 4 | AD | 56 | F | 2 | 1 | Frontal |
| 5 | AD | 59 | M | 2 | 0 | Prefrontal |
| 6 | AD | 64 | M | 1 | 1 | Prefrontal |
| 7 | Hydrocephalus | 62 | M | 4 | 0 | Frontal |
| 8 | Hydrocephalus | 69 | M | 4 | 0 | Frontal |
| 9 | Hydrocephalus | 74 | F | 3 | 1 | Frontal |
| 10 | Brain tumor | 80 | M | 2 | 4 | Frontal |
Abbreviations: AD, Alzheimer’s Disease; F, female; M, male.
Morphometry
Nonbiased sampling was assured by adherence to a protocol established before microscopy. Micrographs of all neurons containing the nucleolus were made from a single ultrathin section analyzed for each case. Neurons of the third cortical layer identified by the dominance of pyramidal neurons were studied. Micrographs of section planes containing the nucleolus at magnifications of ×5000 were used for identification, and micrographs of ×20,000 were also taken so that a coded montage including the entire cytoplasm could be made to obtain measurements of microtubule number and total length. Four neuropathologists independently assigned cellular identity and only those cells with unanimous agreement of identity were analyzed. Between two to seven neurons met these criteria and were analyzed for each case. Micrographs were examined with a stereomicroscope at ×10 to ×20 (Carl Zeiss Inc., Thornwood, NY) and perikaryal microtubules were identified as 25-nm electron dense tubules with cross-sectional lumen diameter of 10 nm 12,13 and counted for each neuron. Both the number of microtubules and total length per unit area of microtubules were determined with a micrometer (Bausch & Lomb, Rochester, NY), making necessary conversions according to magnification. For length determination, each microtubule cross-sectional length was included in the data as 25 nm (diameter), while the microtubule density measurement consisted of both number of longitudinal and cross-sectioned microtubules. In no case did the micrographs show a definitive axon attached to the neuronal cell body, therefore, these processes were not analyzed. The cytoplasmic area (excluding the nucleus and regions of PHF) of each neuron was measured by using NIH Image J program, version 1.06a (http://rsp.info.nih.gov/ij).
The sample population was classified as either pyramidal or nonpyramidal neurons. The individual morphometric data for both length and density were analyzed and means ± SEM calculated. The nonparametric Student’s t-test, hierarchical/nested analysis of variance and regression analysis were used for statistics. Observations of P < 0.05 were considered significant. We took into account the number of neurons and from which subject they came from when we performed the statistical analysis. The number of neurons (n = 36) studied provided sufficient statistical power to render significance (eg, P = 0.000004) for our findings.
We quantified vesicles present at, or near, the presynaptic side in the cases examined. Synapses were visually identified as disk-shaped thickenings of the membrane having at least three synaptic vesicles of 40 nm in diameter adjacent to them as previously described. 14,15 The number of vesicles 0 to 500 nm from the synaptic cleft was determined.
Paraffin-embedded biopsy tissue was available for two control cases, which were immunostained with 4G8, a monoclonal antibody to amyloid β. For immunocytochemistry, after deparaffinization with xylene, sections were hydrated through graded ethanol. Endogenous peroxidase activity in the tissue was eliminated by a 30-minute incubation with 3% H2O2 in methanol, and nonspecific binding sites were blocked in a 30-minute incubation with 10% normal goat serum in Tris-buffered saline (50 mmol/L Tris-HCl, 150 mmol/L NaCl, pH 7.6). The immunostaining was as previously described 16 for the peroxidase-anti-peroxidase procedure by using 3,3′-diaminobenzidine as co-substrate. 17 The sections were dehydrated through ethanol and xylene solutions and then mounted in Permount (Fisher, Pittsburgh, PA).
To measure the levels of amyloid deposition, sections immunostained for amyloid (4G8) were analyzed by using an Axiocam digital camera and KS300 image analysis software (Carl Zeiss, Inc.). The percent area covered by amyloid, by the immunocytochemical method, was measured in five fields totaling 1 mm2 in each case. The values for each case were averaged and expressed as amyloid density or amyloid burden.
Results
Electron micrographs showed neurofibrillary tangles and amyloid β deposits for AD cases, consistent with the pathological assessment at the time of biopsy. Some of the neurons (33.3%) in the AD cases had neurofibrillary tangles with 16.4 ± 6.5% of the cytoplasm infiltrated with PHFs. Quantitative measurement of one control case immunostained with 4G8 showed no amyloid β and the other revealed a few sparse deposits (amyloid burden = 0.79%) consistent with normal aging. 17
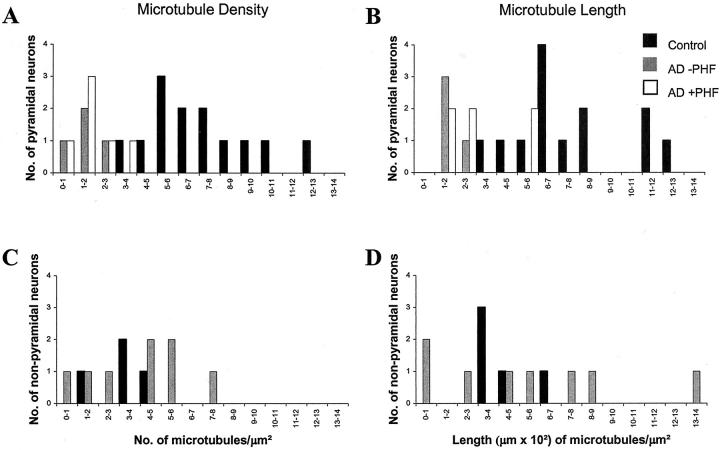
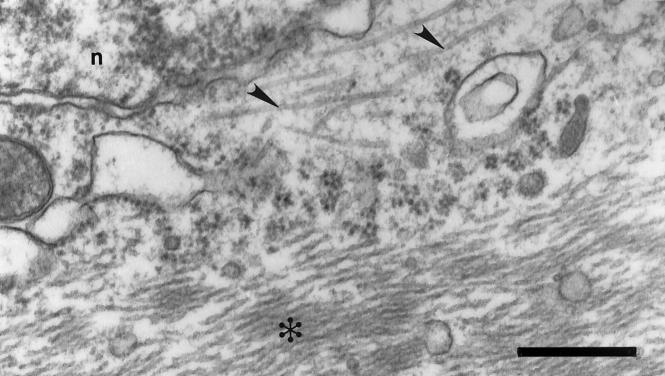
A comparison of the microtubule density of pyramidal and nonpyramidal neurons was performed. The two groups were distinguished in the micrographs by the observations of four independent experienced observers based on morphological criteria. Two-thirds of the neurons in the experimental population were pyramidal, while the remaining one-third were nonpyramidal. Within the pyramidal classification (total, n = 23; AD, n = 13; control, n = 10), AD neurons showed a significant reduction in microtubule density (1.2 ± 0.8 microtubules/μm2) when compared to controls (7.1 ± 2.6 microtubules/μm2) (P = 0.000004) (Figure 1A) ▶ . The total length of microtubules for individual AD neurons was also reduced (170.8 ± 54.4 μm of microtubules/μm2) when compared to controls (768.9 ± 77.2 μm of microtubules/μm2) (P = 0.00004) (Figure 1B) ▶ . Within the nonpyramidal classification (total, n = 13; AD, n = 8; control, n = 5), there was no difference for microtubule density (P = 0.90) (Figure 1C) ▶ or length (P = 0.55) (Figure 1D) ▶ . The greater change in microtubule density for pyramidal neurons versus nonpyramidal neurons acts as an internal control for alterations brought about by tissue handling and fixation. Although one might think the pyramidal-specific decrement in microtubules was related to PHF formation, surprisingly, neither microtubule density (P = 0.80) nor length (P = 0.15) was related to PHFs, and microtubules were often seen in close juxtaposition to PHFs (Figure 2) ▶ .
Figure 1.
Microtubule density (A, C) and length (B, D) both show striking reductions for pyramidal neurons in AD when compared to controls (P = 0.000004, density; P = 0.00004, length), whereas no reduction was seen for adjacent nonpyramidal neurons (P = 0.90, density; P = 0.55, length) in the same section. Surprisingly, there was no difference of microtubule density (P = 0.80) or length (P = 0.15) dependent on the presence of PHFs.
Figure 2.
Abundant microtubules were often seen in close juxtaposition to PHFs. Electron microscopic examinations of microtubules for both AD and control cases were performed in specimens preserved from biopsy fixed in 1.5% glutaraldehyde and 1% osmium tetroxide. Microtubules (arrowhead) could often be seen in close juxtaposition to PHFs (asterisk) in AD. n, nucleus. Scale bar, 250 nm.
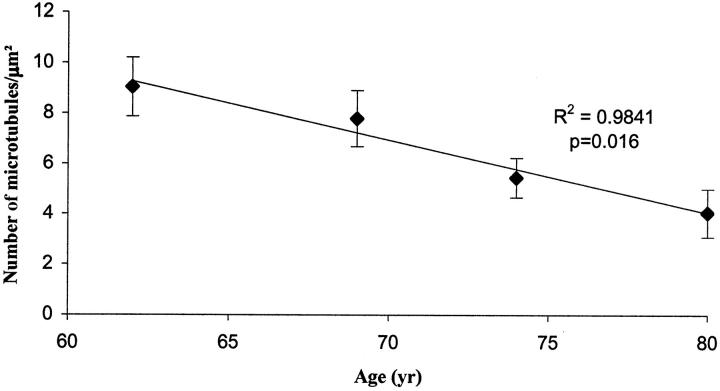
When we further analyzed the microtubule density in the control cases, we found an age-dependent decrease in microtubule density among pyramidal neurons in the control individuals (P = 0.016; regression analysis, r2 = 0.9841) (Figure 3) ▶ . Analysis of covariance was performed comparing neurons from patients with AD versus controls and by using age as a covariate. The mean age was 56.1 years (n = 18 neurons) for patients with AD and 72.2 years (n = 18 neurons) for controls (Table 2) ▶ . Age adjustment of mean was made to the overall sample mean of 64.1 years (n = 36 neurons).
Figure 3.
There is a notable age-dependent decrease in microtubule density in pyramidal neurons among control individuals (P = 0.016; regression analysis, r2 = 0.9841). Error bars represent SEM.
Table 2.
Analysis of Covariance for Microtubule Length and Density with Age as a Covariate
| Group | n | Unadjusted mean | Student’s t test | Adjusted mean | Analysis of covariance with age — T value* |
|---|---|---|---|---|---|
| Length | |||||
| Nonpyramidal | |||||
| AD | 8 | 549 | P = 0.55 | 382 | −1.13 |
| Control | 5 | 424 | 705 | P = 0.2663 | |
| Pyramidal | |||||
| AD | 10 | 249 | P < 0.0001 | 105 | −4.37 |
| Control | 13 | 769 | 875 | P < 0.0001 | |
| Combined | |||||
| AD | 18 | 383 | P = 0.01 | 228 | −2.61 |
| Control | 18 | 673 | 828 | P = 0.0139 | |
| Density | |||||
| Nonpyramidal | |||||
| AD | 8 | 4.12 | P = 0.90 | 2.07 | −2.87 |
| Control | 5 | 3.94 | 7.37 | P = 0.0073 | |
| Pyramidal | |||||
| AD | 10 | 1.76 | P < 0.0001 | 0.01 | −7.32 |
| Control | 13 | 7.06 | 8.36 | P < 0.0001 | |
| Combined | |||||
| AD | 18 | 2.81 | P = 0.0002 | 0.92 | −5.03 |
| Control | 18 | 6.20 | 8.08 | P < 0.0001 |
*The T values and the corresponding P values are calculated on the age-adjusted differences in means ie, Alzheimer’s mean versus control mean, both adjusted to the overall mean age of 64.1 years.
The age-adjusted means for patients with AD for microtubule length per unit area and density were lower than the corresponding means for controls. However, difference in mean microtubule length was significant for pyramidal neurons (t = −4.37, P < 0.00001), but not for nonpyramidal neurons (t = −1.13, P = 0.3). The overall mean length of microtubules for neurons in AD was less than that for control neurons (t = −2.61, P = 0.01).
Difference in age-adjusted mean microtubule density was significant for both pyramidal neurons (t = −7.32, P < 0.0001) and for nonpyramidal neurons (t = −2.87, P = 0.01). Also, the overall mean microtubule density for neurons in AD was less than that for control neurons (t = −5.03, P < 0.0001). No gender differences in microtubule diminution were found.
Both pyramidal and nonpyramidal neurons in AD cases show a reduction in synaptic vesicle number in presynapses juxtaposed to them when compared to controls, although statistical significance was not met.
Discussion
In this study, we documented a clear microtubule deficit in pyramidal neurons in AD, one seen even in the absence of PHFs, and also with aging among normal individuals. The findings presented here are consistent with microtubule changes being concurrent with the mitochondrial abnormalities and increased oxidative damage and these changes occurring before any detectable changes in τ. 5,18-20 The findings are also consistent with previous work by one of the authors (MP-B) noting a microtubule decrease of near 50% in dendrites but that did not correlate the data with PHFs or age. 21 Our result showing a 55% reduction in microtubule density in normal patients from age 62 to age 80 years is consistent with a biochemical study reporting a 90% decrease in tubulin concentrations in normal human cerebral cortex from infants to age 90 years, 22 further buttressing the case that microtubule diminution is independent of τ abnormalities and closely linked to aging.
The finding of a small decrease in synaptic vesicle number in AD cases is consistent with past studies quantitating reduction of the vesicle-docking protein synaptophysin. 15,23 Based on these studies, Terry 7,24 proposed that reduced axoplasmic flow and resulting loss of synaptic connectivity underlie the cognitive deficits of AD. Further findings of Golgi disruption 25 and altered mitochondrial turnover 6 in vulnerable neurons in AD, organelles completely dependent on microtubules, are consistent with microtubule reduction. Our findings, in sum, suggest microtubule reduction in AD is marked, specifically limited to vulnerable pyramidal neurons, and not dependent on abnormalities in τ.
Footnotes
Address reprint requests to George Perry, Ph.D., Institute of Pathology, Case Western Reserve University, 2085 Adelbert Rd., Cleveland, OH 44106. E-mail: gxp7@po.cwru.edu.
Supported by the National Institutes of Health (grants NS38648 to M. A. S. and P50AG16570 to H. V. V.), the United Mitochondrial Disease Foundation (to G. P.), and the Alzheimer’s Association (grant IIRG-98-140 to G. P.).
References
- 1.Alzheimer A: Über eine eigneartige Ehrankung der Himrinde. Allg Z Psychiatr Psychish-Gerichtliche Med 1907, 64: 146–148. Translated in Neurological Classics in Modern Translation. Edited by DA Rottenberg, FH Hochberg. New York, Hafner Press, 1977 pp 41–43
- 2.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI: Abnormal phosphorylation of the microtubule-associated protein τ (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA 1986, 83:4913-4917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyasaka T, Morishima-Kawashima M, Ravid R, Heutink P, van Swieten JC, Nagashima K, Ihara Y: Molecular analysis of mutant and wild-type-tau deposited in the brain affected by the FTDP-17 R406W mutation. Am J Pathol 2001, 158:373-379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki K, Terry RD: Fine structural localization of acid phosphatase in senile plaques in Alzheimer’s presenile dementia. Acta Neuropathologica 1967, 8:276-284 [DOI] [PubMed] [Google Scholar]
- 5.Praprotnik D, Smith MA, Richey PL, Vinters HV, Perry G: Filament heterogeneity within the dystrophic neurites of senile plaques suggests blockage of fast axonal transport in Alzheimer’s disease. Acta Neuropathol 1996, 91:226-235 [DOI] [PubMed] [Google Scholar]
- 6.Hirai K, Aliev G, Nunomura A, Figioka H, Russdl RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PLR, Jones PK, Peterson RB, Perry G, Smith MA: Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci 2001, 21:3017-3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terry RD: The pathogenesis of Alzheimer disease: an alternative to the amyloid hypothesis. J Neuropathol Exp Neurol 1996, 55:1023-1025 [PubMed] [Google Scholar]
- 8.Scheff SW, DeKosky ST, Price DA: Quantitative assessment of cortical synaptic density in Alzheimer’s disease. Neurobiol Aging 1990, 11:29-37 [DOI] [PubMed] [Google Scholar]
- 9.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R: Physical basis of cognitive alterations in Alzheimer disease: synapse loss is a major correlate of cognitive impairment. Ann Neurol 1991, 30:572-580 [DOI] [PubMed] [Google Scholar]
- 10.Praprotnik D, Smith MA, Richey PL, Vinters HV, Perry G: Plasma membrane fragility in dystrophic neurites in senile plaques of Alzheimer’s disease: an index of oxidative stress. Acta Neuropathol 1996, 91:1-5 [DOI] [PubMed] [Google Scholar]
- 11.McKhann GD, Drachman DA, Folstein MF, Katzman R, Price D, Stadlan EM: Clinical diagnosis of Alzheimer disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services task force on Alzheimer’s disease. Neurology 1984, 34:939-944 [DOI] [PubMed] [Google Scholar]
- 12.Porter KR: Cytoplasmic microtubules and their functions. Wolstenholme GEW O’Connor M eds. Principles of Biomolecular Organization. 1966:pp 308-345 CIBA Foundation Symposium, J and A Churchill London
- 13.Lawrence ME, Possingham JV: Observations of microtubule-like structures within spinach plastids. Biol Cell 1984, 52:77-82 [Google Scholar]
- 14.Carpenter MB, Sutin J: Human Neuroanatomy, ed 8 1983. Williams and Wilkins Baltimore
- 15.DeKosky ST, Scheff SW: Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol 1990, 27:457-464 [DOI] [PubMed] [Google Scholar]
- 16.Perry G, Lipphardt S, Mulvihill P, Kancherla M, Mijares M, Gambetti P, Sharma S, Maggiora L, Cornette J, Lobl T, Greenberg B: Amyloid precursor protein in senile plaques of Alzheimer disease. Lancet 1998, ii:746. [DOI] [PubMed] [Google Scholar]
- 17.Sternberger LA, Sternberger NH: Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci USA 1983, 80:6126-6130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA: Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol 2001, 60:759-767 [DOI] [PubMed] [Google Scholar]
- 19.Nunomura A, Perry G, Pappolla MA, Wade R, Hirai K, Chiba S, Smith MA: RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer’s disease. J Neurosci 1999, 19:1959-1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nunomura A, Perry G, Papolla MA, Friedland RP, Hirai K, Chiba S, Smith MA: Neuronal oxidative stress precedes amyloid-β deposition in Down Syndrome. J Neuropathol Exp Neurol 2000, 59:1011-1017 [DOI] [PubMed] [Google Scholar]
- 21.Paula-Barbosa M, Tavares MA, Cadete-Leite A: A quantitative study of frontal cortex dendritic microtubules in patients with Alzheimer disease. Brain Res 1987, 417:139-142 [DOI] [PubMed] [Google Scholar]
- 22.Yan SB, Hwang S, Rustan TD, Frey WH: Human brain tubulin purification: decrease in soluble tubulin with age. Neurochem Res 1985, 10:1-18 [DOI] [PubMed] [Google Scholar]
- 23.DeKosky ST, Scheff SW, Styren SD: Structural correlates of cognition in dementia: quantification and assessment of synapse change. Neurodegeneration 1996, 5:417-421 [DOI] [PubMed] [Google Scholar]
- 24.Terry RD, Katzman R: Life span and synapses: will there be a primary senile dementia? Neurobiol Aging 2001, 22:347-348 [DOI] [PubMed] [Google Scholar]
- 25.Stieber A, Mourelatos Z, Gonatas NK: In Alzheimer’s disease the Golgi apparatus of a population of neurons without neurofibrillary tangles is fragmented and atrophic. Am J Pathol 1996, 148:415-426 [PMC free article] [PubMed] [Google Scholar]