Abstract
The frequency of chronic renal failure increases with age, especially in women after menopause. Glomerulosclerosis is a common cause of chronic renal failure in aging. We reported that pre-menopausal female C57BL6 (B6) mice are resistant to glomerulosclerosis, irrespective of the type of injury. However, we now show that B6 mice develop progressive glomerulosclerosis after menopause. Glomerular lesions, first recognized in 18-month-old mice, consisted of hypertrophy, vascular pole sclerosis, and mesangial cell proliferation. Diffuse but moderate mesangial sclerosis and more marked hypertrophy were present at 22 months. At 28 to 30 months the glomerulosclerosis was diffuse and increased levels of type I and type IV collagen and transforming growth factor-β1 mRNA were present. Urine albumin excretion was significantly increased in 30-month-old mice. Mesangial cells isolated from 28-month-old mice retained their sclerotic phenotype in vitro. Comparison of the effects of uninephrectomy (Nx) in 20-month-old and 2.5-month-old mice revealed a 1.7-fold increase in urine albumin excretion, accelerated glomerulosclerosis, and renal function insufficiency in 20-month-old Nx mice, but not in 2.5-month-old Nx mice. Glycemic levels, glucose, insulin tolerance, and blood pressure were normal at all ages. Thus, B6 mice model the increased frequency of chronic renal failure in postmenopausal women and provide a model for studying the mechanism(s) of glomerulosclerosis in aging women.
Chronic renal failure is a major health problem world-wide and its prevalence has been rising. 1,2 According to the 1988–1994 Third National Health and Nutrition Examination Survey, 3% of noninstitutionalized adults have an increased serum creatinine level (corresponding to 5.6 million patients). Importantly, most of those affected are in the older age group, and women are over-represented. 2 These data are confirmed by the United States Renal Data System, and reveal that the incidence of chronic renal failure is low in young women compared to young men (aged 35 to 39 years, female/male = 0.6 in Caucasians), but that the incidence increases after menopause, where the female/male ratio reaches 1.08 in the 65 to 69 age group. 3
Glomerulosclerosis is the most common cause of chronic renal failure in aging. 3 Thus, the structural and functional abnormalities of glomeruli during biological aging may play a significant role. Although age-associated glomerular damage proceeds slowly in most individuals, intervening diseases such as diabetes mellitus, nephron number reduction, drug nephrotoxicity, or hypertension may accelerate this process. 2-7 Hemodynamic abnormalities, redox state imbalance, changes in sex hormone levels, as well as the accumulation of advanced glycation end products have all been implicated as important factors in the pathogenesis of aging-associated glomerulosclerosis. 3,8-13 However, the genetics and the cellular and molecular basis of the glomerulosclerosis in aging remains largely uncharacterized.
We found that young female C57BL6 (B6) mice are resistant to glomerulosclerosis in response to either reduction in nephron number or diabetes. 14-16 Herein, we examined the evolution of glomerular changes, including sclerosis, hypertrophy, cell turnover, and extracellular matrix composition in postmenopausal B6 mice. We found glomerular lesions at 18 to 20 months (early menopause) consisting of hypertrophy, mesangial cell proliferation, and vascular pole sclerosis. The mesangial lesions became more diffuse and prominent and the glomerular hypertrophy increased with age and were marked at 28 to 30 months (late menopause). The development of progressive glomerulosclerosis in old B6 mice was associated with increased cell turnover and extracellular matrix synthesis in intact glomeruli, and this phenotype was also present in mesangial cells isolated from 28-month-old mice. We found that nephrectomy induced renal changes in 20 months, but not 2.5 months. The abnormalities included albuminuria, severe glomerular lesions, and renal insufficiency. Thus, as in postmenopausal women with underlying glomerular diseases or hypertension, the introduction of renal stress in the postmenopausal period accelerated the development of chronic renal disease in B6 mice. Therefore, the B6 mouse strain provides a model for studying the pathogenetic mechanism(s) of glomerulosclerosis in aging women.
Materials and Methods
Mice
Female B6 mice were obtained from the National Institute of Aging, NIH. The life span of B6 mice is around 34 months under barrier conditions, ie, specific pathogen-free conditions in which they have free access to sterilized NIH 31 diet and water. Female B6 mice begin to have irregular, lengthened estrous cycles around 10 to 14 months of age and the cycles usually cease at 18 to 20 months of age (early menopausal period). 17 Since mice at 28 to 30 months may develop malignancies, we carefully examined all major organs grossly and took histological specimens from the spleen. Mice found to have tumors were excluded from the study. At least 6 mice from each age group (2.5 to 5 months, 8 months, 18 to 20 months, 22 months, and 28 to 30 months) were randomly chosen for the study.
Nephrectomy (Nx)
Nx was performed in 2.5- and 20-month-old female B6 mice (n = 4/group). Mice were anesthetized with ketamine and xylazine, provided by the Department of Veterinary Research, University of Miami School of Medicine. The left kidney was surgically exposed and the left adrenal gland was carefully freed from the upper pole of the renal capsule before ligating the renal artery and vein and removing the kidney. Nx mice were followed for one month, during which time they had free access to food and water.
Blood Pressure
Before the measurement of blood pressure, mice were acclimated to the restrainers for 1 hour/day for 5 days. On day 6, mice were placed in restrainers and rested for 10 to 15 minutes. An inflatable occlusion cuff was placed proximally on the tail and a sensor cuff was placed distally. Measurements were taken over a 1 hour period at 60-second intervals (Columbus Instruments, Columbus, OH). The systolic pressure was recorded as the mean of all stable measurements.
Glycemic Levels, Glucose, and Insulin Tolerance Test
Mice were fasted overnight (12 hours) with free access to water for the glucose tolerance test. After the measurement of baseline glycemic levels, mice were injected intraperitoneally with 2 g/kg of glucose, and blood glucose levels were monitored using a glucometer at 15 minutes, 30 minutes, 60 minutes, and 120 minutes. For the insulin tolerance test, mice were fasted for 3 hours, with free access to water, and then injected intraperitoneally with 0.5 unit/kg of regular insulin. Blood glucose levels were measured before, and 15 minutes, 30 minutes, and 60 minutes after insulin injection.
Serum Creatinine (Scr) and Blood Urea Nitrogen (BUN) Levels
Serum was prepared from blood collected at sacrifice. Scr and BUN levels were measured using kits obtained from Sigma (Saint Louis, MO), with adjustment of the sample volume (20 μl for Scr, 5 μl for BUN).
Urine Albumin
Albumin levels were measured in freshly voided morning urine samples by enzyme-linked immunosorbent assay (ELISA), using a kit from Bethyl Laboratory Inc. (Houston, TX). ELISA plates were first coated with goat anti-mouse albumin for 1 hour at room temperature. The plate was blocked with Postcoat solution for 30 minutes. After washing, 100 μl of urine samples (1:1000 diluted) or mice albumin standards (7.8–250 ng/ml) were added to the plate. The reaction was stopped 1 hour later and horseradish peroxidase antibody and the substrate for horseradish peroxidase were added. The amount of albumin in the urine was calculated from a standard curve based on purified mouse albumin as substrate. Urine creatinine levels were measured in the same samples and the urine albumin excretion rate was expressed as the ratio of albumin to creatinine.
Renal Histology
Kidneys were flushed first with saline and then perfused with 4% paraformaldehyde at mean arterial pressure levels to obtain in situ fixation. 18 Tissues were embedded in glycol methacrylate, and sections cut at a thickness of 4 μm were stained with periodic acid–Schiff (PAS).
Morphometry Analysis
Glomerular volume was assessed on sections of methacrylate-embedded kidney tissue using a digitizing tablet and video camera, as previously described. 19 50 glomeruli from each mouse were recorded. Glomerular surface area was obtained using the MetaMorph image analysis computer program (Universal Imaging Co., West Chester, PA). Glomerular volume was derived from the harmonic mean of the glomerular surface area. Glomerular allometry was calculated by the equation: y = bxα. Where y is glomerular volume, x is body weight, b is the value of y at x = 1, and α is the ratio of specific growth of y and x. α constitutes an index of differential growth. 20,21 The α value calculated from the glomerular volume and body weight of 5-month-old B6 female mice was given the value of 1. Values of α greater than 1 indicate a positive differential increase of y relative to x. The mesangial area was measured by capturing the nucleus-free, PAS-positive, area within each glomerulus. The nuclei of 50 glomeruli were counted to determine the relative glomerular cell number. 15
Glomerular Labeling Index
One hour before sacrifice, mice were injected intraperitoneally with 0.25 mg/g bromodeoxyuridine (BrdU). Samples of kidney and small intestine (as a control tissue) were fixed with 4% paraformaldehyde. Paraffin sections were processed and used for BrdU staining. Antigen retrieval was performed by placing the slides in 0.01 mol/L citrate buffer (pH 6) at 100°C for 10 minutes. Endogenous peroxidase was blocked by incubating the sections with 1% H2O2 for 20 minutes. Slides were further incubated with DakoCytomation (Fort Collins, CO) free protein blocking solution for 10 minutes before incubating overnight with sheep anti-BrdU antibody (1:100, Biodesign International, Saco, ME) at 4°C. The slides were rinsed with PBS and incubated with a biotinylated rabbit anti-sheep IgG second antibody for 1.5 hours. ABC Vector Elite and DAB were used to reveal BrdU nuclear staining. The number of positively stained nuclei was counted in 40 to 50 successive glomeruli in each section and the labeling index is expressed as the ratio of positive cells/total glomerular cell number × 100.
Glomerular Type I and Type IV Collagen, TGF-β1 mRNA Levels
Glomeruli were microdissected from B6 mice and glomerular RNA was extracted and reverse-transcripted as previously described. 22 Type I collagen mRNA levels were determined by competitive PCR using the primers 5′-GTGAACCTGGCAAACAAGGT (sense) and 5′-CTGGAGACCAGAAGCCAC (antisense). The α1 type I collagen mutant was created by deletion of a 140-bp fragment from the original PCR product using the primers 5′-GAATCTGGACGTGAAAGAATGGCGATCG (sense) and 5′-CGATCGCCATTCTTTCACGTCCAGATTC (antisense). Competitive PCR for α1 type I collagen and GAPDH was performed by addition of decreasing amounts of mutant to sample cDNA. Data are expressed as the ratio of type I collagen to GAPDH mRNA. Glomerular type IV collagen and TGF-β1 mRNA levels were measured by real-time PCR. 23 Briefly, after glomerular RNA isolation, equal amounts of RNA from each sample were reverse-transcripted with α1 type IV collagen and TGF-β1 specific primers and amplified using the TaqMan one step RT-PCR master mix reagents kit and ABI Prism 7700 sequence detection system (Perkin Elmer Applied Biosystems, Foster City, CA). The primers were: forward, 5′-CACCATAGAGAGAAGCGAGATGTTC; reverse, 5′-GGCTGACGTGTGTTCGC, for α1 type IV collagen; and forward, 5′-ACTGGAGTTGTACGGCAGTGG; reverse, 5′-GCAGTGAGCGCTGAATCGA, for TGF-β1. VIC labeled 5′-AAGCCCACGCCATCCACCTTGA was used as the probe for α1 type IV collagen amplification. 6-carboxyfluorescein (FAM) labeled 5′-TGAACCAAGGAGACGGAATACAGGGCT was used as the probe for TGF-β1 amplification. The TaqMan ribosomal RNA control reagents kit was used to measure the expression of 18S ribosomal RNA genes. The levels of α1 type IV collagen and TGF-β1 mRNA expression were normalized to 18S mRNA levels in samples from uninephrectomized mice. However, since we found that 18S mRNA levels were increased 2.2-fold in glomeruli from 30-month-old mice compared to that from 5-month-old mice (data not shown), glomerular α1 type IV collagen mRNA levels in these mice were normalized to GAPDH mRNA levels, which were measured by competitive PCR.
Immunofluorescence Microscopy
Frozen kidney sections were cut at a thickness of 5 μm and processed as described. 15 Briefly, sections were incubated with cold acetone for 10 minutes and coated with rabbit anti-type IV collagen (Biodesign International) followed by biotin-conjugated goat anti-rabbit IgG (Sigma) and streptavidin-conjugated fluorescein isothiocyanate (Zymed Laboratories, Inc., San Francisco, CA). For the identification of macrophages, sections were stained with a rat anti-CD68 antibody (Serotec Inc., Raleigh, NC). Spleen sections were used as a positive control.
Mesangial Cell Culture
Mesangial cells were isolated from 5- and 28-month-old female B6 mice and characterized as previously described. 24 Cells were grown in DMEM-Ham’s F-12 mixture supplemented with 20% fetal bovine serum (FBS), 100 U/ml streptomycin, and 100 U/ml penicillin. Cells at passage 5–8 were used for all experiments. Total RNA was collected from cells at 80% confluence in 75 mm2 flask. The levels of TGF-β1, α1 type I collagen and α1 type IV collagen mRNAs in mesangial cells were measured by quantitative RT-PCR as described above. To compare the levels of type I collagen production between mesangial cells isolated from 5-month-old and 28-month-old mice, cells in 24-well plates were incubated with medium containing 0.1% FCS and 80 μg/ml β-aminopropionitrile. Supernatants and cell layers were collected and the amount of type I collagen was determined by ELISA as previously described. 25
Transfection and Type I Collagen and TGF-β Reporter Genes Assays
Transient transfection was performed in cultured mesangial cells using Transfast (Promega, Madison, WI). A type I collagen reporter construct (kindly provided by Drs. A.C. Poncelet and H.W. Schnaper 26 ) or a TGF-β reporter construct p3TP-Lux (a gift from Dr. J. Massague) was introduced, together with a β-galactosidase vector, in the presence or absence of 2 ng/ml TGF-β1 (R&D Systems, Minneapolis, MN). Luciferase and β-galactosidase activity were measured 24 hours after transfection. 25
Statistical Analyses
All values are expressed as mean ± SE. One-way analysis of variance was used to evaluate the differences among 2.5-month, 5-month, 18- to 20-month, 22-month, and 28- to 30-month age groups. The two-tailed unpaired t-test was used to analyze differences in glomerular and mesangial cell mRNA levels between 5-month-old and 28- to 30-month-old mice.
Results
Body Weight, Heart Weight, Kidney Weight, and Blood Pressure
Body weight slowly increased to 8 months and remained stable thereafter (table 1) ▶ . The ratio heart and kidney weight/body weight was similar at 4, 18, and 20–22 months. However, at 30 months kidney weight/body weight ratio was decreased.
Table 1.
Body Weight, Heart Weight, Kidney Weight, and Blood Pressure in Female B6 Mice
| 5 months (n = 6) | 22 months (n = 6) | 28 months (n = 4) | 30 months (n = 4) | |
|---|---|---|---|---|
| Body weight (g) | 22.4 ± 1.3** | 25.7 ± 1.4 | 24.9 ± 2.1 | 26 ± 2.1 |
| Heart weight (g) | 0.11 ± 0.01 | 0.17 ± 0.02 | 0.18 ± 0.01 | 0.12 ± 0.04 |
| HW/BW* | 0.005 ± 0.003 | 0.006 ± 0.0007 | 0.007 ± 0.004 | 0.004 ± 0.001 |
| Kidney weight (g) | 0.2 ± 0.02 | 0.22 ± 0.01 | 0.25 ± 0.02 | 0.18 ± 0.02 |
| KW/BW* | 0.009 ± 0.0001 | 0.009 ± 0.001 | 0.01 ± 0.001 | 0.007 ± 0.001## |
| SBP (mmHg)* | 113.6 ± 3.6 | 114.3 ± 6.4 | 104.8 ± 3.9 | 103.7 ± 2.4 |
*HW/BW, heart weight/body weight; KW/BW, kidney weight/body weight; SBP, systolic blood pressure.
**P <0.01, vs. other age group of mice;
##P <0.01, vs. 5-month-old mice.
Glycemic Levels, Glucose, and Insulin Tolerance
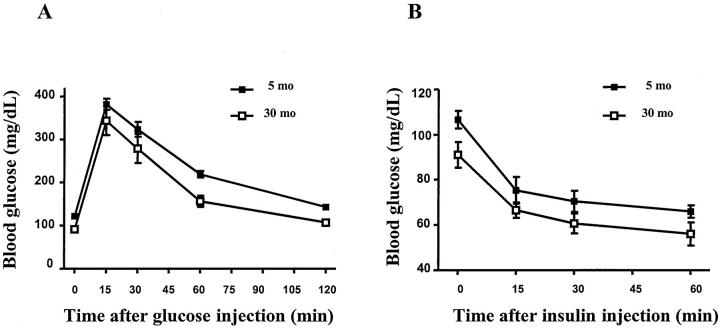
Glycemic levels were normal in all mice (Figure 1) ▶ . Glucose tolerance (GTT) was normal at both 5-month-old and 30-month-old mice. Glycemic levels returned to normal in both groups of mice 2 hours after a glucose challenge (2 g/kg). The response to insulin (ITT) in both 5- and 30-month-old mice was also normal (Figure 1) ▶ .
Figure 1.
Glucose and insulin tolerance tests. Tests were performed in mice as described in materials and methods. The responses to glucose (A) and insulin (B) did not differ from 5 months to 30 months in B6 mice.
Albuminuria
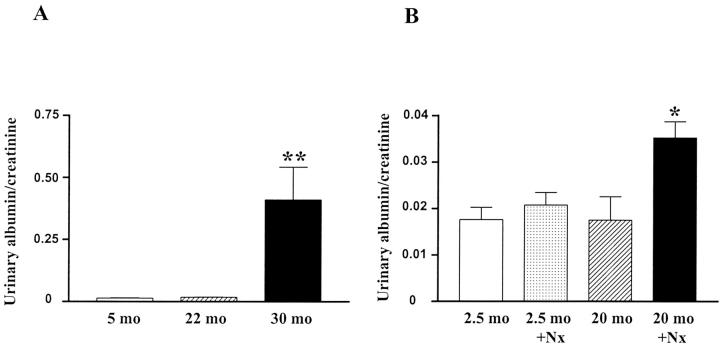
The urinary albumin/creatinine ratio remained normal in mice from 4 to 22 months but was increased nearly 19-fold in 30-month-old mice (Figure 2A) ▶ . A 50% reduction in nephron number by Nx in 20-month-old mice induced a 1.7-fold increase in the urine albumin excretion rate, but albumin excretion did not change in 2.5-month-old Nx mice (Figure 2B) ▶ .
Figure 2.
Urine albumin excretion. A: Mice at different ages. (**, P < 0.01 vs. mice at 5 months or 22 months). B: Uninephrectomized mice compared to age-matched controls. (*, P < 0.05 vs. uninephrectomized mice at 2.5 months).
Serum Creatinine and Blood Urea Nitrogen
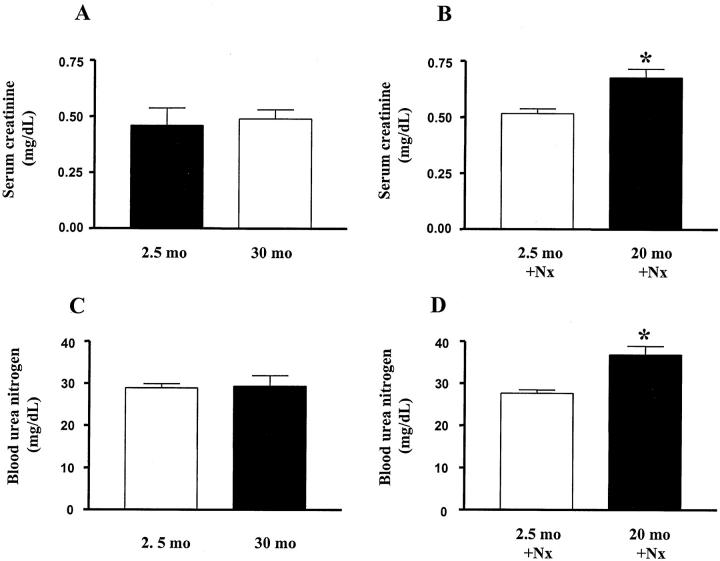
Serum creatinine and urea nitrogen levels remained normal in 22- and 30-month-old B6 mice. Nx induced a significant increase in serum creatinine and urea nitrogen levels one month after Nx in 20-month-old mice, but these values remained normal after Nx in 2.5-month-old mice (Figure 3) ▶ .
Figure 3.
Serum creatinine and blood urea nitrogen levels. A: Scr levels in 5-month-old and 30-month-old mice; B: Increased Scr levels in 20-month-old uninephrectomized mice. (P < 0.05 vs. 2.5-month-old uninephrectomized mice). C: BUN levels in 5-month-old and 30-month-old mice. D: Increased BUN levels in 20-month-old uninephrectomized mice. (P < 0.05 vs. 2.5-month-old uninephrectomized mice).
Histology
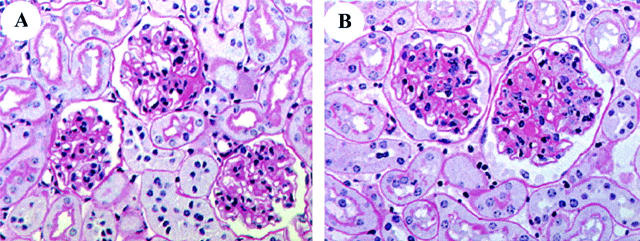
5 months: There were no glomerular lesions by light microscopy (Figure 4A) ▶ .
Figure 4.
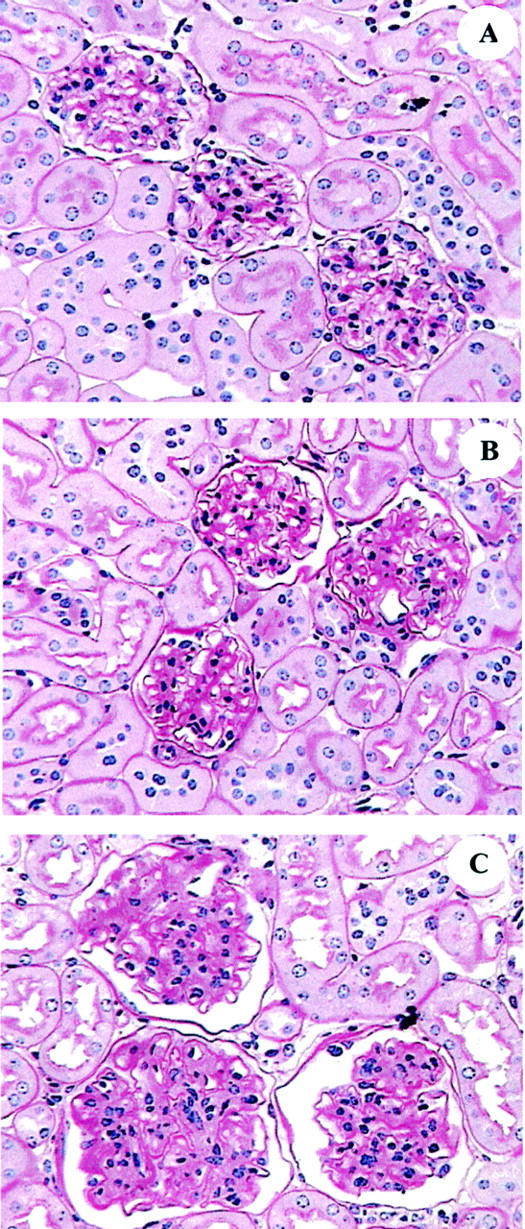
Glomerular histology (PAS × 200). A: 5 months. Glomeruli are normal. B: 22 months. The mesangial area is markedly expanded. C: 30 months. Glomeruli are large. Cell number is increased. The mesangial area is increased, compared to 22 months.
18 and 20 months: Glomerular hypertrophy was present, and this was associated with an increase in PAS-positive material at the vascular pole. However, the peripheral mesangial areas remained relatively normal. There were no tubulointerstitial lesions.
22 months: There was both increased glomerular size and diffused but moderate mesangial sclerosis, and some vascular poles contained PAS-positive deposits (Figure 4B) ▶ . Glomerular cell number was normal. There were a small number of glomeruli with wrinkled basement membranes, mesangial atrophy and thickening of Bowman’s capsule. The interstitium was normal.
28 to 30 months: There were severe glomerular lesions (Figure 4C) ▶ . The glomeruli were large, the vascular spaces were reduced in size and obsolescent glomeruli were present. There was a prominent increase in PAS-positive material in the mesangial areas and the basement membranes were thickened and had irregular subepithelial spikes. Occasional glomeruli contained capillary loops with areas of aneurysmal dilatation associated with mesangiolysis. There were PAS-positive amorphous deposits, inflammatory cell infiltration, and nuclear debris in many capillary loops. There were also prominent tubulointerstitial lesions, especially in the medullary areas, consisting of thickened tubular basement membranes, tubular atrophy, and casts. Uninephrectomy in 20-month-old mice: There were glomerular lesions that were similar to those seen in 28-month-old mice (Figure 5A) ▶ .
Figure 5.
Glomerular histology of uninephrectomized mice. (PAS × 200). A: 2.5 months. There is increase in glomerular volume but not mesangial area. B: 20 months. There is significant increase in glomerular mesangial area.
Uninephrectomy in 2.5-month-old mice: The glomeruli appeared normal except for the increase in size (Figure 5B) ▶ .
Glomerular Volume
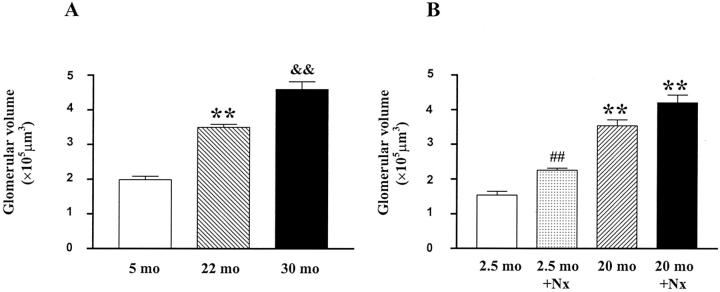
Glomerular volume progressively increased with age (5 months, 1.9 ± 0.15 × 105 μm3; 22 months, 3.5 ± 0.08 × 105 μm3; 30 months, 4.6 ± 0.35 × 105 μm3. Figure 6A ▶ ). Thus, glomerular allometric growth was abnormal. Glomerular allometric growth was calculated based on the equation y = bxα. The α exponent in 5-month-old mice, arbitrarily given a value of 1, was 1.14 in 22-month-old mice and 1.23 in 30-month-old mice. While Nx was associated with increased glomerular volume in 2.5-month-old mice, the value was lower than that in 20-month-old (2.5-month Nx, 2.26 ± 0.06 × 105 μm3 vs. 20-month, 3.55 ± 0.06 × 105 μm3, P < 0.01). There was a trend of further increase in glomerular volume in 20-month-old mice one month after Nx (P = 0.06, Figure 6B ▶ ).
Figure 6.
Glomerular volume. A: Progressive increase in glomerular volume as mice age. (**, P < 0.01 vs. 5 months; &&, P < 0.01 vs. 22 months). B: There is a significant increase in glomerular volume in 2.5-month-old uninephrectomized mice, but the values are still lower than at 20 months. (##, P < 0.01 vs. age-matched controls; **, P < 0.01 vs. 2.5-month-old uninephrectomized mice).
Glomerular Cell Number and Turnover
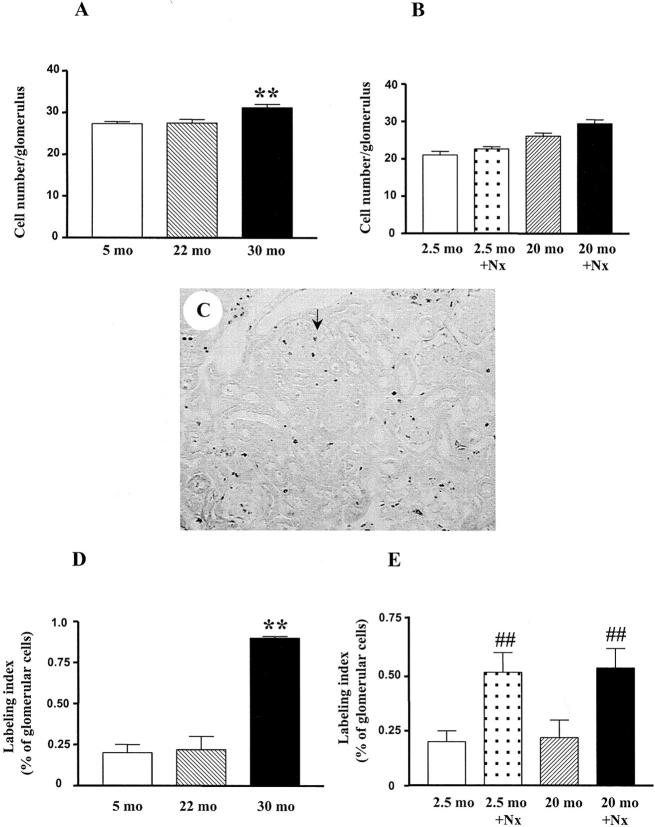
There was no difference in glomerular cell number between 5-month-old and 22-month-old mice. Likewise, glomerular cell turnover did not differ between 5-month-old (0.2 ± 0.05%) and 22-month-old (0.22 ± 0.08%) mice. However, glomerular cell number was significantly increased in 30-month-old mice (31.2 ± 0.8/glomerulus), as was glomerular cell turnover (0.9 ± 0.01% at 30 months, P < 0.01, Figure 7,A and B ▶ ). Immunofluorescence staining revealed that the number of macrophages was increased in 30-month-old mice glomeruli (3.5/glomerulus vs. 0.3/glomerulus in 5-month-old mice, P < 0.01).
Figure 7.
Glomerular cell number and labeling index. A: Increased glomerular cell number in 30-month-old mice. (**, P < 0.01 vs. 5 months or 22 months). B: No significant change of glomerular cell number in 2.5-month-old and 20-month-old uninephrectomized mice. C: Representative area of renal cortex showing BrdU staining (from 30-month-old mouse, ×250). Positive nuclear staining is seen within glomeruli (shown with arrow), tubules, and interstitium. D: Increased BrdU labeling of glomerular cells in 30-month-old mice. (**, P < 0.01 vs. 5 months or 22 months). E: Increased BrdU labeling of glomerular cells in uninephrectomized mice. (##, P < 0.01 vs. respective age-matched controls).
Nx resulted in increased glomerular cell turnover in both 2.5-month-old (0.52 ± 0.09%) and 20-month-old Nx mice (0.54 ± 0.09%). Total glomerular cell number remained unchanged in both groups compared to non-Nx controls (data not shown).
Mesangial Area
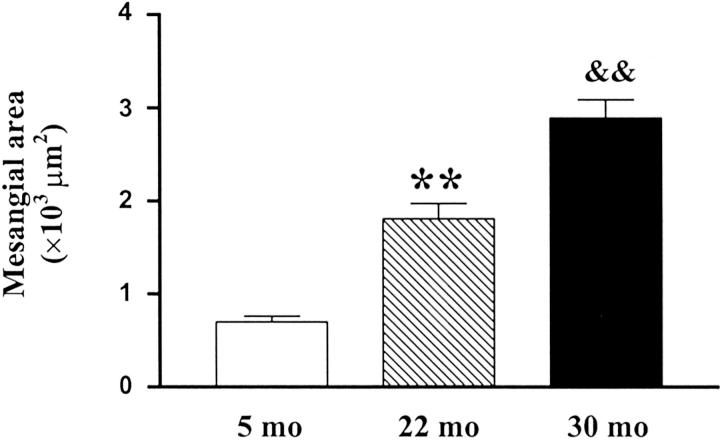
Glomerular mesangial area progressively increased with age (5 months, 701.9 ± 59.3 μm2; 22 months, 1808 ± 165 μm2; 30 months, 2888 ± 200.2 μm2; P < 0.01, Figure 8 ▶ ).
Figure 8.
Progressive increase in glomerular mesangial area with age. (**, P < 0.01 vs. 5 months; &&, P < 0.01 vs. 22 months).
Mesangial area in Nx 20-month-old mice was comparable to that found in 28- to 30-month-old mice (data not shown). However, the mesangial area in 2.5-month-old Nx mice remained comparable to normal controls.
Type I and Type IV Collagen and TGF-β1 mRNA Levels in Glomeruli
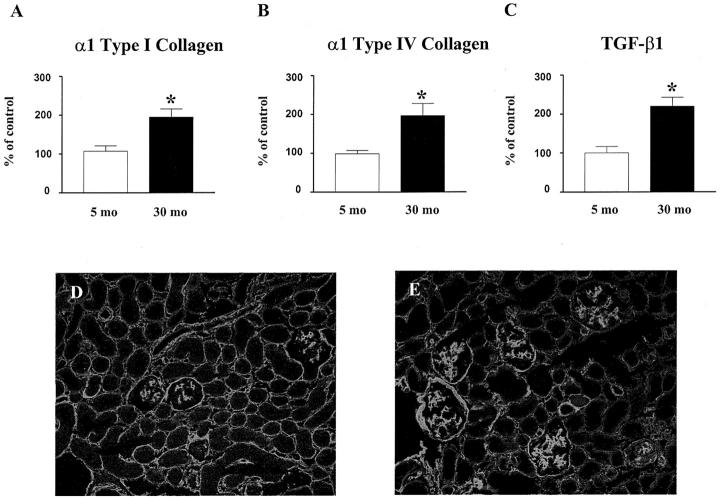
The values of mRNA at 22 and 30 months were expressed as the percentage of the values of 5-month-old mice (considered to be premenopausal controls). α1 type I collagen mRNA levels were significantly increased in 30-month-old mice, as determined by competitive PCR (Figure 9A) ▶ . Glomerular α1 type IV collagen mRNA levels, measured by real-time PCR, were similar in female B6 mice at 2.5, 5 and 8 months of age (data not shown). There were no differences in the levels of glomerular α1 type IV collagen mRNA between 5-month-old and 22-month-old mice (118 ± 12% vs. 5-month, 100 ± 9%, P = ns). Since we found that 18S mRNA levels were increased, while GAPDH mRNA levels remained stable in glomeruli of 30-month-old mice, GAPDH was selected as a housekeeping gene control. There was a significant increase in glomerular α1 type IV collagen mRNA levels in 30-month-old, compared to both 5-month-old and 22-month-old mice (Figure 9B) ▶ . There was also a significant increase in glomerular TGF-β1 mRNA levels in 30-month-old compared to 5-month-old mice (Figure 9C) ▶ . There were no differences in the levels of glomerular TGF-β1 mRNA among 5-month, 18-month and 22-month-old mice (5-month, 100 ± 19%; 18-month, 117 ± 34%; 22-month 109 ± 21%. P = ns).
Figure 9.
Type I and type IV collagen and TGF-β1 expression. The levels of type I and type IV collagen and TGF-β1 mRNA were corrected by the levels of GAPDH mRNA. Data obtained from 5-month-old mice glomeruli were defined as 100% (Control). A: Increased α1 (I) collagen mRNA expression in glomeruli of 30-month-old mice. (*, P < 0.05 vs. 5 months). B: Increased α1 (IV) collagen mRNA expression in glomeruli of 30-month-old mice. (*, P < 0.05 vs. 5 months). C: Increased TGF-β1 mRNA expression in glomeruli of 30-month-old mice. (*, P < 0.05 vs. 5 months). D and E: Immunofluorescence microscopy of type IV collagen. Compared to 5-month-old mice (D), there is prominent accumulation of type IV collagen in glomeruli of 30-month-old mice glomeruli (E). Magnification, ×250.
Uninephrectomized 20-month-old mice had significantly increased levels of type IV collagen and TGF-β1 mRNA (Figure 10, A and B) ▶ . There were no differences in α1 type IV collagen and TGF-β1 mRNA levels between 2.5-month-old Nx and age-matched controls.
Figure 10.
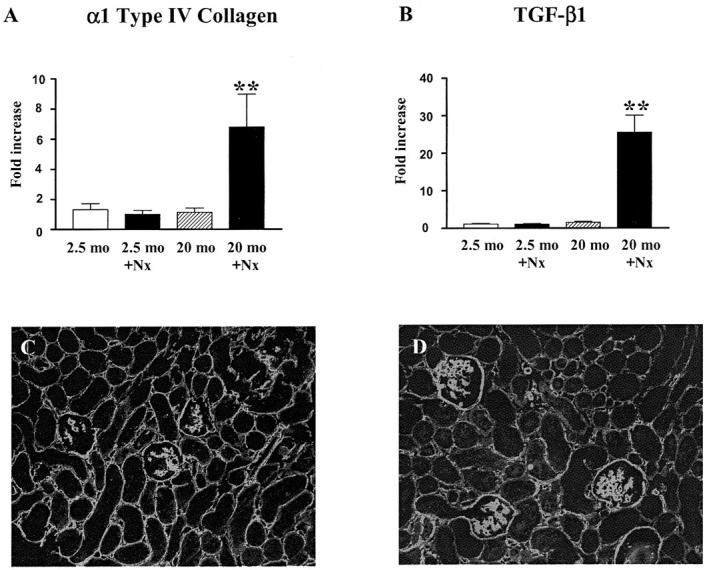
Type IV collagen and TGF-β1 expression. A: Increased α1 (IV) collagen mRNA expression in glomeruli of 20-month-old uninephrectomized mice. Note that there were no differences in glomerular α1 (IV) collagen mRNA expression in female B6 mice at 2.5 months and 20 months. α1 (IV) collagen mRNA levels were normalized to 18S mRNA and expressed as fold increase compared to 2.5-month-old uninephrectomized mice. (**, P < 0.01 vs. 2.5-month-old uninephrectomized mice). B: Increased TGF-β1 mRNA expression in glomeruli of 20-month-old uninephrectomized mice. Note that there were no differences in glomerular TGF-β1 mRNA levels in B6 mice at 2.5 months and 20 months. TGF-β1 mRNA levels were normalized to 18S mRNA and expressed as fold of increase compared to 2.5-month-old uninephrectomized mice. **, P < 0.01 vs. 2.5-month-old uninephrectomized mice. C and D: Immunofluorescence microscopy of type IV collagen. While the pattern of type IV collagen distribution in glomeruli of 2.5-month-old uninephrectomized mice was similar to 5-month-old mice (C), the amount and distribution of type IV collagen were compatible in 20-month-old uninephrectomized and 30-month-old mice (D). Magnification, × 250.
Immunofluorescence Microscopy
Although α1 type IV collagen mRNA levels were not increased at 22 months, immunofluorescence microscopy revealed a moderate increase in type IV collagen in the mesangial areas and more marked accumulation of type IV collagen in the glomeruli of 30-month-old mice (Figure 9, D and E) ▶ . The intensity and distribution of type IV collagen staining were comparable in 20-month-old Nx and 30-month-old intact mice (Figure 10, C and D) ▶ .
Mesangial Cell Type I and Type IV Collagen Transcription and mRNA and Protein Levels
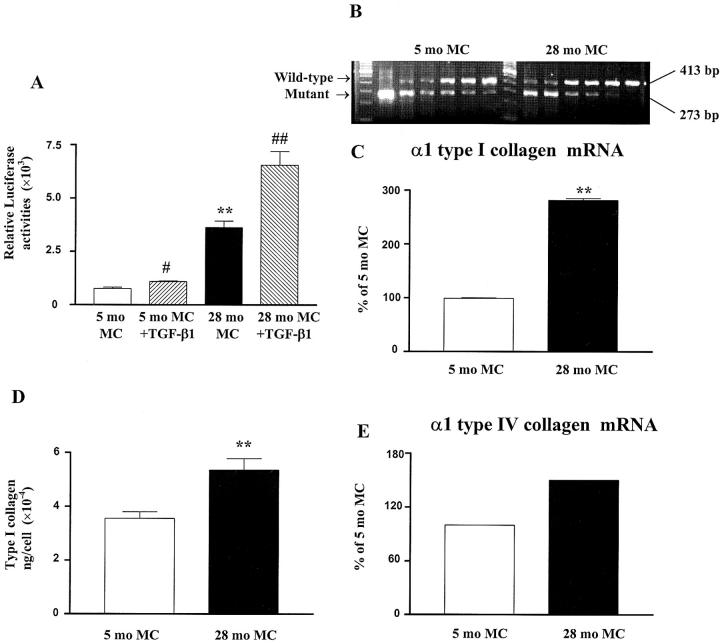
At baseline, type I collagen transcription was 4.7-fold higher in mesangial cells isolated from 28-month-old mouse than those in cells isolated from 5-month-old mouse (Figure 11A) ▶ . Type I collagen transcription was increased by TGF-β1 in cells from both ages (Figure 11A) ▶ . Similarly, α1 type I collagen mRNA and protein levels were higher in cells isolated from 28-month-old mouse (Figure 11, B–D) ▶ . α1 type IV collagen mRNA levels were also increased 1.5-fold in 28-month-old compared to 5-month-old mesangial cells (Figure 11E) ▶ .
Figure 11.
Type I collagen transcriptional activities and type I and type IV collagen expression in mesangial cells. A: Increased type I collagen transcriptional activity in mesangial cells isolated from 28-month-old mouse (28-month MC). Basal and TGF-β1-mediated activation of type I collagen transcription was assessed in mesangial cells transfected with 250 ng of a type I collagen promoter reporter construct and 250 ng of a Rous sarcoma virus β-galactosidase plasmid in the presence or absence of 2 ng/ml TGF-β1. (**, P < 0.01 vs. cells isolated from a 5-month-old mouse (5-month MC); #, P < 0.05, ##, P < 0.01 vs. basal activities). B: Increased α1 (I) collagen mRNA expression in mesangial cells isolated from 28-month-old mouse. α1 (I) collagen and GAPDH mRNA levels were measured by competitive PCR using total RNA isolated from mesangial cells. Results are expressed as the ratio of α1 (I) collagen to GAPDH mRNA. Mesangial cells from 28-month-old mouse had higher α1 (I) collagen mRNA levels as shown by competitive PCR. cDNAs from mesangial cells cultured from 5-month and 28-month-old mice were amplified with decreasing amounts of competing mutant (from left to right: 0.1−0.00125 attomol). C: α1 (I) collagen mRNA levels were increased in mesangial cells isolated from 28-month-old mouse. (**, P < 0.01 vs. cells isolated from a 5-month-old mouse). D: Increased type I collagen in mesangial cells isolated from 28-month-old mouse. The amount of type I collagen produced by mesangial cells was determined by ELISA and corrected by the cell number. (**, P < 0.01 vs. cells isolated from 5-month-old mouse). E: Increased α1 (IV) collagen mRNA expression in mesangial cells isolated from 28-month-old mouse. α1 (IV) collagen mRNA levels were measured by real-time PCR and corrected by the GAPDH mRNA levels. The value obtained from mesangial cells isolated from 5-month-old mouse was arbitrarily defined as 100%.
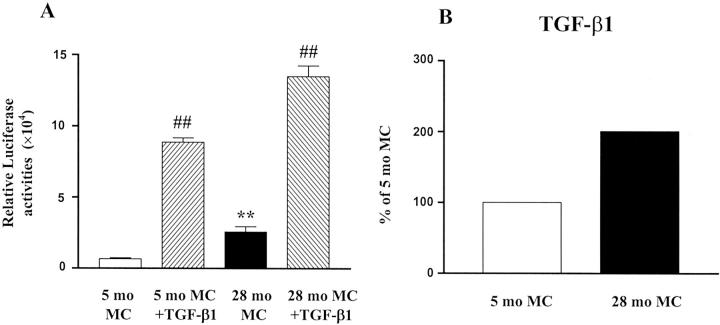
Mesangial Cell TGF-β1
TGF-β transcriptional activities, which were induced by TGF-β1 in cells of both ages, were 3.9-fold higher in 28-month-old mesangial cells (Figure 12A) ▶ . TGF-β1 mRNA levels were also significantly higher in 28-month-old mesangial cells compared to 5-month-old cells (Figure 12B) ▶ .
Figure 12.
TGF-β transcriptional activities and mRNA expression in mesangial cells. A: Increased TGF-β transcriptional activities in mesangial cells isolated from 28-month-old mouse (28-month MC). Basal and TGF-β1-mediated activation of TGF-β responses were assessed in mesangial cells transfected with 250 ng of a TGF-β reporter construct and 250 ng of a Rous sarcoma virus β-galactosidase plasmid in the presence or absence of 2 ng/ml TGF-β1. (**, P < 0.01 vs. cells isolated from 5-month-old mouse (5-month MC); ##, P < 0.01 vs. basal activities). B: Increased TGF-β1 mRNA expression in mesangial cells isolated from 28-month-old mouse. TGF-β1 mRNA levels were measured by real-time PCR and corrected by the GAPDH mRNA levels. The value obtained from mesangial cells isolated from 5-month-old mouse was arbitrarily defined as 100%.
Discussion
Aging is associated with decreased function and structural damage of various organs, including the kidney, where gross anatomical changes in the kidney include decreased overall weight and cortical thinning. 27 There are also microscopic lesions in the glomeruli consisting of hypertrophy, mesangial expansion, and thickening of the peripheral basement membranes. We previously found that premenopausal female B6 mice were resistant to glomerulosclerosis. 14-16 However, the current data show that these B6 mice develop progressive glomerulosclerosis after menopause. These data agree with previous observations. 28
The life span of B6 mice is approximately 34 months under barrier and specific pathogen-free conditions. B6 mice develop irregular, lengthened estrous cycles at 10 to 14 months and the cycles most often cease at the age of 18 to 20 months. 17 We found that glomerular lesions in early menopausal mice were restricted to the vascular pole and consisted of hypertrophy without an increase in mesangial matrix and cell number. Diffuse, moderate glomerulosclerosis was first seen at 22 months.
Normal control of glomerular allometric growth was lost after menopause namely glomerular size was disproportionately increased in proportion to body weight. The increase in glomerular size was first present shortly after menopause and continued to progress through 30 months, the end of the study. Since kidney weight remained stable from 5 to 22 months and was decreased in 30-month-old mice, the control of glomerular size, but not total kidney weight, appeared to become dysregulated with age. Despite increased glomerular size and extracellular matrix, there was no increase in urine albumin excretion in 22-month-old female B6 mice. The increase in glomerular size was associated with structural and functional changes, namely, B6 mice in the late menopausal period spontaneously developed severe, diffused glomerulosclerosis. There was also a notable thickening of the glomerular basement membranes and an increase in urine albumin excretion (19-fold). Thus, our data in postmenopausal B6 mice demonstrate the development of both histological and functional changes characteristic of progressive glomerulosclerosis.
Glomerular hypertrophy has been found in various forms of progressive glomerular diseases suggesting that glomerular hypertrophy may be a marker of the propensity to develop glomerulosclerosis. 29,30 However, we previously showed that the association of hypertrophy and the development of glomerulosclerosis in the premenopausal period depended on the genetic background. 14-16 For instance, we found that glomerular size was enlarged in both young ROP and young B6 mice that carried the Os mutation (which induces a 50% reduction in nephron number). However, glomerulosclerosis occurred only in ROP Os/+ mice. Furthermore, glomerular size reached a plateau in B6 Os/+ mice and was not further increased by uninephrectomy or by diabetes induction. On the other hand, glomerular size continued to increase with age in ROP Os/+ mice, and this was accentuated by both uninephrectomy and diabetes induction. 14-16 Thus, dysregulation in the control of glomerular size may be associated with glomerulosclerosis. This hypothesis is supported by the current study, where a continuing increase in glomerular size after menopause was associated with the development of progressively evolving glomerulosclerosis.
The etiology of progressive glomerulosclerosis and glomerular hypertrophy in postmenopausal B6 mice is not clear. We found that glomerular number remained stable, and did not differ between premenopausal and late-menopausal mice, in agreement with Davies et al who reported that glomerular number remained stable in B6 mice from 6 to 34 months. 31 This suggests that decreased nephron number did not play a role in the induction of glomerulosclerosis. Since we found that aging B6 mice had normal glycemic levels and normal responses to a glucose challenge and insulin infusion and remained normotensive, the glomerular changes were not due to causal factors such as diabetics, insulin resistance, or hypertension. While premenopausal B6 mice were resistant to sclerosis, we recently found that B6 mice ovariectomized at 9 months develop glomerulosclerosis within 3 months thereafter (data not shown). Together with the presence of progressive glomerulosclerosis in postmenopausal B6 mice, these data suggest that estrogen may play a key role in conferring the resistance to glomerulosclerosis in premenopausal B6 mice. 23,32 This may also contribute to the relative resistance of young women to the development of glomerulosclerosis, and the loss of this protection after menopause.
Mesangial cells play a central role in the pathogenesis of glomerulosclerosis. 33-35 Although all three types of glomerular cells: endothelial, epithelial, and mesangial cells are involved in glomerular extracellular matrix (ECM) turnover, mesangial cells appear to be a major source of pathological glomerular ECM. An imbalance between ECM synthesis and degradation by mesangial cells contributes directly to the development and progression of glomerulosclerosis. 36,37 Interestingly, we found that mesangial cells isolated from three different strains of mice with glomerulosclerosis (ROP, diabetic NOD, and B6 mice transgenic for bovine growth hormone) all exhibited a stable sclerotic phenotype characterized by increased type I and type IV collagen, and/or decreased metalloproteinase-9 expression. 23,24,37,38 Herein, we demonstrate that similar phenotypic changes, including increased type I and type IV collagen expression, were present in mesangial cells isolated from sclerotic 28-month-old B6 mice. Therefore, mesangial cell phenotypic changes may be common to the process of, and/or may lead to, glomerulosclerosis. Aging-associated glomerular lesions appear to proceed very slowly, so that renal function remains adequate even at an advanced age. 27 Our data suggest that in the presence of intervening disease or injury stimuli, the aged kidney may have an increased susceptibility to the development of severe renal dysfunction and glomerulosclerosis. This conclusion is based on a comparison between the consequences of uninephrectomy in 2.5-month-old and 20-month-old female B6 mice. 20-month-old Nx mice developed severe glomerular lesions and progressive glomerular hypertrophy, comparable to those seen in intact 28-month-old mice. Note that the glomeruli in intact 20-month-old B6 mice showed only glomerular hypertrophy and modest glomerulosclerosis. In addition, 20-month-old Nx mice had increased urine albumin excretion and mild renal insufficiency. Since the levels of glomerular type IV collagen mRNA were higher in 20-month-old Nx mice but the levels of protein expression were comparable to those in 30-month-old mice, the increase in sclerosis at 30 months likely includes changes in extracellular matrix degradation. The only change found in 2.5-month-old Nx B6 mice was an increase in glomerular size.
TGF-β is an important regulator of extracellular matrix genes. Elevated glomerular TGF-β1 levels have been found to contribute significantly to the accumulation of type IV and type I collagen in glomerular lesions of various forms of progressive glomerular diseases. 39-41 An increase in TGF-β levels has also been implicated in the pathogenesis of aging-related glomerulosclerosis in rats. 42 We found increased TGF-β1 mRNA levels in the glomeruli of 30-month-old mice and in 20-month-old old Nx mice. The concomitant increase in TGF-β1 and type I and type IV collagen mRNA levels suggests that TGF-β1 may contribute to the up-regulation of type I and type IV collagen. The findings that TGF-β1 increased type I collagen transcriptional responses in mesangial cells, and that the increases in type I and type IV collagen expression in mesangial cells isolated from 28-month-old sclerotic mice was associated with increased TGF-β1 mRNA levels and transcriptional responses, further supports the postulate that TGF-β1 may play a role in the pathogenesis of progressive glomerulosclerosis in aging. However, since there were no differences in the glomerular levels of TGF-β1 mRNA among 5-month, 18-month and 22-month-old mice with two kidneys, TGF-β1 may not be the key player in the early glomerular stages of hypertrophy and glomerulosclerosis in this model.
In conclusion, progressive glomerulosclerosis and glomerular hypertrophy begin in the early menopausal period in B6 mice, and uninephrectomy markedly accelerated these processes. These mice provide a new model to investigate progressive renal disease in the postmenopausal period.
Acknowledgments
We thank Drs. A.C. Poncelet and W.H. Schnaper for the α2 type I collagen promoter reporter construct and Drs. B. Beaton and Y.J. Zeng for excellent technical assistance with kidney tissue preparation and BrdU staining.
Footnotes
Address reprint requests to: Feng Zheng, M.D., Department of Medicine, Vascular Biology Institute, University of Miami School of Medicine, 1600 N.W. 10th Avenue, RMSB, Rm 1043-R104, Miami, FL 33136. E-mail: fzheng@med.miami.edu.
Supported by the National Institutes of Health (grant NIA R01, AG19366–01 to G.E.S. and NIA R01, AG17170–01 to L.J. S.).
F.Z. and A.R.P. contributed equally to this work.
References
- 1.Berthoux F, Jones E, Gellert R, Mendel S, Saker L, Briggs D: Epidemiological data of treated end-stage renal failure in the European Union (EU) during the year 1995: report of the European Renal Association Registry and the National Registries. Nephrol Dial Transplant 1999, 14:2332-2342 [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Wei GL, McQuillan G, Brancati FL, Levey AS, Jones C, Klag MJ: Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med 2001, 161:1207-1216 [DOI] [PubMed] [Google Scholar]
- 3.: U. S. Renal Data System: USRDS 1999 annual data report. 1999. NIDDK The National Institutes of Health
- 4.Brancati FL, Whelton PK, Randall BL, Neaton JD, Stamler J, Klag MJ: Risk of end-stage renal disease in diabetes mellitus: a prospective cohort study of men screened for MRFIT. Multiple Risk Factor Intervention Trial. JAMA 1997, 278:2069-2074 [PubMed] [Google Scholar]
- 5.Striker GE: Kidney disease and hypertension in blacks. Am J Kidney Dis 1992, 20:673. [PubMed] [Google Scholar]
- 6.Muhlberg W, Platt D: Age-dependent changes of the kidneys: pharmacological implications. Gerontology 1999, 45:243-253 [DOI] [PubMed] [Google Scholar]
- 7.Striker GE, Nagle RB, Kohnen PW, Smuckler EA: Response to unilateral nephrectomy in old rats. Arch Pathol 1969, 87:439-442 [PubMed] [Google Scholar]
- 8.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J: End-stage renal disease in African-American and white men. 16-year MRFIT findings. JAMA 1997, 277:1293-1298 [PubMed] [Google Scholar]
- 9.Hackbarth H, Harrison DE: Changes with age in renal function and morphology in C57BL/6, CBA/HT6, and B6CBAF1 mice J Gerontol 1982, 37:540-547 [DOI] [PubMed] [Google Scholar]
- 10.Anderson S, Brenner BM: Effects of aging on the renal glomerulus. Am J Med 1986, 80:435-442 [DOI] [PubMed] [Google Scholar]
- 11.Ruiz-Torres P, Lucio J, Gonzalez-Rubio M, Rodriguez-Puyol M, Rodriguez-Puyol D: Oxidant/antioxidant balance in isolated glomeruli and cultured mesangial cells. Free Radic Biol Med 1997, 22:49-56 [DOI] [PubMed] [Google Scholar]
- 12.Baylis C: Age-dependent glomerular damage in the rat. Dissociation between glomerular injury and both glomerular hypertension and hypertrophy male gender as a primary risk factor. J Clin Invest 1994, 94:1823-1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vlassara H, Bucala R, Striker L: Pathogenic effects of advanced glycosylation: biochemical, biologic, and clinical implications for diabetes and aging. Lab Invest 1994, 70:138-151 [PubMed] [Google Scholar]
- 14.Esposito C, He CJ, Striker GE, Zalups RK, Striker LJ: Nature and severity of the glomerular response to nephron reduction is strain-dependent in mice. Am J Pathol 1999, 154:891-897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He C, Esposito C, Phillips C, Zalups RK, Henderson DA, Striker GE, Striker LJ: Dissociation of glomerular hypertrophy, cell proliferation, and glomerulosclerosis in mouse strains heterozygous for a mutation (Os) which induces a 50% reduction in nephron number. J Clin Invest 1996, 97:1242-1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng F, Striker GE, Esposito C, Lupia E, Striker LJ: Strain differences rather than hyperglycemia determine the severity of glomerulosclerosis in mice. Kidney Int 1998, 54:1999-2007 [DOI] [PubMed] [Google Scholar]
- 17.Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE: A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol Reprod 1982, 27:327-339 [DOI] [PubMed] [Google Scholar]
- 18.Yang CW, Vlassara H, Peten EP, He CJ, Striker GE, Striker LJ: Advanced glycation end products up-regulate gene expression found in diabetic glomerular disease. Proc Natl Acad Sci USA 1994, 91:9436-9440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doi T, Striker LJ, Gibson CC, Agodoa LY, Brinster RL, Striker GE: Glomerular lesions in mice transgenic for growth hormone and insulinlike growth factor-I. I. Relationship between increased glomerular size and mesangial sclerosis. Am J Pathol 1990, 137:541-552 [PMC free article] [PubMed] [Google Scholar]
- 20.Yang CW, Striker LJ, Pesce C, Chen WY, Peten EP, Elliot S, Doi T, Kopchick JJ, Striker GE: Glomerulosclerosis and body growth are mediated by different portions of bovine growth hormone. Studies in transgenic mice. Lab Invest 1993, 68:62-70 [PubMed] [Google Scholar]
- 21.DeHoff RT, Rhines FN: Method of estimating size of discrete objects. DeHoff RT Rhines FN eds. Quantitative Microscopy. 1968:pp 75 McGraw-Hill New York
- 22.Peten EP, Garcia-Perez A, Terada Y, Woodrow D, Martin BM, Striker GE, Striker LJ: Age-related changes in α1- and α2-chain type IV collagen mRNAs in adult mouse glomeruli: competitive PCR. Am J Physiol 1992, 263:F951-F957 [DOI] [PubMed] [Google Scholar]
- 23.Potier M, Karl M, Zheng F, Elliot SJ, Striker GE, Striker LJ: Estrogen-related abnormalities in glomerulosclerosis-prone mice: reduced mesangial cell estrogen receptor expression and prosclerotic response to estrogens. Am J Pathol 2002, 160:1877-1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacot TA, Striker GE, Stetler-Stevenson M, Striker LJ: Mesangial cells from transgenic mice with progressive glomerulosclerosis exhibit stable, phenotypic changes including undetectable MMP-9 and increased type IV collagen. Lab Invest 1996, 75:791-799 [PubMed] [Google Scholar]
- 25.Zheng F, Fornoni A, Elliot SJ, Guan Y, Breyer MD, Striker LJ, Striker GE: Upregulation of type I collagen by TGF-β in mesangial cells is blocked by PPARγ activation. Am J Physiol Renal Physiol 2002, 282:F639-F648 [DOI] [PubMed] [Google Scholar]
- 26.Poncelet AC, Schnaper HW: Sp1 and Smad proteins cooperate to mediate transforming growth factor-β 1-induced α2 (I) collagen expression in human glomerular mesangial cells. J Biol Chem 2001, 276:6983-6992 [DOI] [PubMed] [Google Scholar]
- 27.Baylis C, Schmidt R: The aging glomerulus. Semin Nephrol 1996, 16:265-276 [PubMed] [Google Scholar]
- 28.Yumura W, Sugino N, Nagasawa R, Kubo S, Hirokawa K, Maruyama N: Age-associated changes in renal glomeruli of mice. Exp Gerontol 1989, 24:237-249 [DOI] [PubMed] [Google Scholar]
- 29.Fogo A, Ichikawa I: Evidence for a pathogenic linkage between glomerular hypertrophy and sclerosis. Am J Kidney Dis 1991, 17:666-669 [DOI] [PubMed] [Google Scholar]
- 30.MacKay K, Striker LJ, Stauffer JW, Agodoa LY, Striker GE: Relationship of glomerular hypertrophy and sclerosis: studies in SV40 transgenic mice. Kidney Int 1990, 37:741-748 [DOI] [PubMed] [Google Scholar]
- 31.Davies I, Fotheringham AP, Faragher BE: Age-associated changes in the kidney of the laboratory mouse. Age Ageing 1989, 18:127-133 [DOI] [PubMed] [Google Scholar]
- 32.Potier M, Elliot SJ, Tack I, Lenz O, Striker GE, Striker LJ, Karl M: Expression and regulation of estrogen receptors in mesangial cells: influence on matrix metalloproteinase-9. J Am Soc Nephrol 2001, 12:241-251 [DOI] [PubMed] [Google Scholar]
- 33.Johnson RJ, Floege J, Yoshimura A, Iida H, Couser WG, Alpers CE: The activated mesangial cell: a glomerular “myofibroblast”? J Am Soc Nephrol 1992, 2:S190-S197 [DOI] [PubMed] [Google Scholar]
- 34.Schocklmann HO, Lang S, Sterzel RB: Regulation of mesangial cell proliferation. Kidney Int 1999, 56:1199-1207 [DOI] [PubMed] [Google Scholar]
- 35.Cornacchia F, Fornoni A, Plati AR, Thomas A, Wang Y, Inverardi L, Striker LJ, Striker GE: Glomerulosclerosis is transmitted by bone marrow-derived mesangial cell progenitors. J Clin Invest 2001, 108:1649-1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lupia E, Elliot SJ, Lenz O, Zheng F, Hattori M, Striker GE, Striker LJ: IGF-1 decreases collagen degradation in diabetic NOD mesangial cells: implications for diabetic nephropathy. Diabetes 1999, 48:1638-1644 [DOI] [PubMed] [Google Scholar]
- 37.Fornoni A, Striker LJ, Zheng F, Striker GE: Reversibility of glucose-induced changes in mesangial cell extracellular matrix depends on the genetic background. Diabetes 2002, 51:499-505 [DOI] [PubMed] [Google Scholar]
- 38.Elliot SJ, Striker LJ, Hattori M, Yang CW, He CJ, Peten EP, Striker GE: Mesangial cells from diabetic NOD mice constitutively secrete increased amounts of insulin-like growth factor-I. Endocrinology 1993, 133:1783-1788 [DOI] [PubMed] [Google Scholar]
- 39.Poncelet AC, Schnaper HW: Regulation of human mesangial cell collagen expression by transforming growth factor-β1. Am J Physiol 1998, 275:F458-F466 [DOI] [PubMed] [Google Scholar]
- 40.Ketteler M, Noble NA, Border WA: Increased expression of transforming growth factor-β in renal disease. Curr Opin Nephrol Hypertens 1994, 3:446-452 [DOI] [PubMed] [Google Scholar]
- 41.Isono M, Cruz MC, Chen S, Hong SW, Ziyadeh FN: Extracellular signal-regulated kinase mediates stimulation of TGF-β1 and matrix by high glucose in mesangial cells. J Am Soc Nephrol 2000, 11:2222-2230 [DOI] [PubMed] [Google Scholar]
- 42.Ma LJ, Nakamura S, Whitsitt JS, Marcantoni C, Davidson JM, Fogo AB: Regression of sclerosis in aging by an angiotensin inhibition-induced decrease in PAI-1. Kidney Int 2000, 58:2425-2436 [DOI] [PubMed] [Google Scholar]