Abstract
We tested a hypothesis that interactions between fibronectin (FN), the major extracellular matrix component, and its integrin α4β1 receptor is important in the development of ischemia/reperfusion injury of steatotic liver transplants. We examined the effect of connecting segment-1 (CS1) peptide-facilitated blockade of FN-α4β1 interaction in a well-established steatotic rat liver model of ex vivo cold ischemia followed by iso-transplantation. In this model, CS1 peptides were administered through the portal vein of steatotic Zucker rat livers before and after cold ischemic storage. Lean Zucker recipients of fatty liver transplants received an additional 3-day course of CS1 peptides after transplant. CS1 peptide therapy significantly inhibited the recruitment of T lymphocytes, neutrophil activation/infiltration, and repressed the expression of proinflammatory tumor necrosis factor-α and interferon-γ. Moreover, it resulted in selective inhibition of inducible nitric oxide synthase expression, peroxynitrite formation, and hepatic necrosis. Importantly, CS1 peptide therapy improved function/histological preservation of steatotic liver grafts, and extended their 14-day survival in lean recipients from 40% in untreated to 100% in CS1-treated OLTs. Thus, CS1 peptide-mediated blockade of FN-α4β1 interaction protects against severe ischemia/reperfusion injury experienced otherwise by steatotic OLTs. These novel findings document the potential of targeting FN-α4β1 in vivo interaction to increase the transplant donor pool through modulation of marginal steatotic livers.
Orthotopic liver transplantation (OLT) is an effective therapeutic alternative for end-stage liver disease. The critical shortage of human donor livers has provided the rational to identify methods that would allow successful utilization of marginal steatotic grafts. It has been not fully elucidated as to why fatty livers exhibit a higher rate of dysfunction and primary nonfunction after transplantation, as compared with nonsteatotic OLTs. 1,2 Ischemia/reperfusion (I/R) insult suffered by OLTs is likely to influence a preferential damage of steatotic OLTs. 3,4 The migration of leukocytes through the vascular endothelium as well as through the extracellular matrix contributes to I/R injury. Fibronectin (FN), a large glycoprotein with a central role in cellular adhesion and migration, is likely a key extracellular matrix protein involved in these events. Indeed, a prominent feature of hepatic response to injury is the very early production of the so-called cellular FN by sinusoidal endothelial cells. 5 The importance of FN in cell migration and differentiation is underscored by the observation that mice made null for this extracellular matrix component die during embryonic development. 6,7 The role of FN in leukocyte adhesion, migration, and activation has been extensively reported. 8-10 FN has been implicated in many pathological states, including cutaneous wounds, 11 tumor metastasis, 12 rheumatoid arthritis, 13 cardiac allograft rejection, 14,15 and liver fibrosis. 5 Circulating leukocytes, such as T lymphocytes, use β1 integrins for adhesion and migration through endothelial cells and through distinct tissue microenvironments. 16,17 Of the β1 integrins, α4 has a central role, first, as a matrix receptor that binds to the alternatively spliced domain of FN, the connecting segment-1 (CS1), second, as a homing receptor that binds to the endothelial vascular cell adhesion molecule-1 (VCAM-1), recognizing a functionally different target sequence. 18,19 Interestingly, α4 integrin, initially thought to be restricted to mononuclear leukocytes and not to be expressed on neutrophils, also seems important in mediating neutrophil adhesion and migration. 20,21
The mechanisms of leukocyte recruitment to sites of inflammatory stimulation in liver, which is a venous-driven vascular bed with slow flow rates, may require a distinct cascade of adhesive events as compared with other organs with higher flow rates. The present study is the first to elucidate the significance of FN-α4β1-integrin interactions in steatotic liver I/R injury. We have developed a steatotic rat liver model of ex vivo cold ischemia followed by syngenic OLT in which steatotic liver grafts are characterized by severe I/R damage. 22 In this model, the blockade of FN-α4β1 integrin interactions with peptides that mimic the CS1-splicing domain of FN, significantly increased OLT survival rate from 40 to 100% (at 14 days). Moreover, our findings support the critical role for FN-α4β1-integrin interactions in the recruitment of T lymphocytes and neutrophils, important mediators of I/R injury, into steatotic OLTs.
Materials and Methods
Animals and Grafting Techniques
Genetically obese (fa−/fa−) male Zucker (230 to 275 g), lean (fa/−) Zucker (260 to 300 g), and male Sprague-Dawley (250 to 300 g) rats were obtained from Harlan Sprague Dawley, Inc. (Indianapolis, IN). Syngenic OLTs were performed using fatty and lean livers that were harvested, respectively, from obese Zucker and lean Sprague-Dawley rats. Steatotic and lean livers were then stored at 4°C in University of Wisconsin (UW) solution for 4 hours and 24 hours, respectively, before being iso-transplanted into lean Zucker or Sprague-Dawley recipients. The standard techniques of liver harvesting and orthotopic transplantation without hepatic artery reconstruction were performed according to the previously described Kamada’s and Caine’s 23,24 cuff technique, and an anhepatic phase of ≈16 to 20 minutes. Animals were fed a standard rodent diet and water ad libitum and cared for according to guidelines approved by the American Association of Laboratory Animal Care. Fatty Zucker rats of 230 g to 275 g body weight have >30% (≅40%) liver steatosis, which sets them as marginal donors. Oil Red-O staining confirmed the high content of fat in the steatotic donor livers, contrasting with livers harvested from lean rats in which no signs of steatosis were detected (Figure 1) ▶ .
Figure 1.
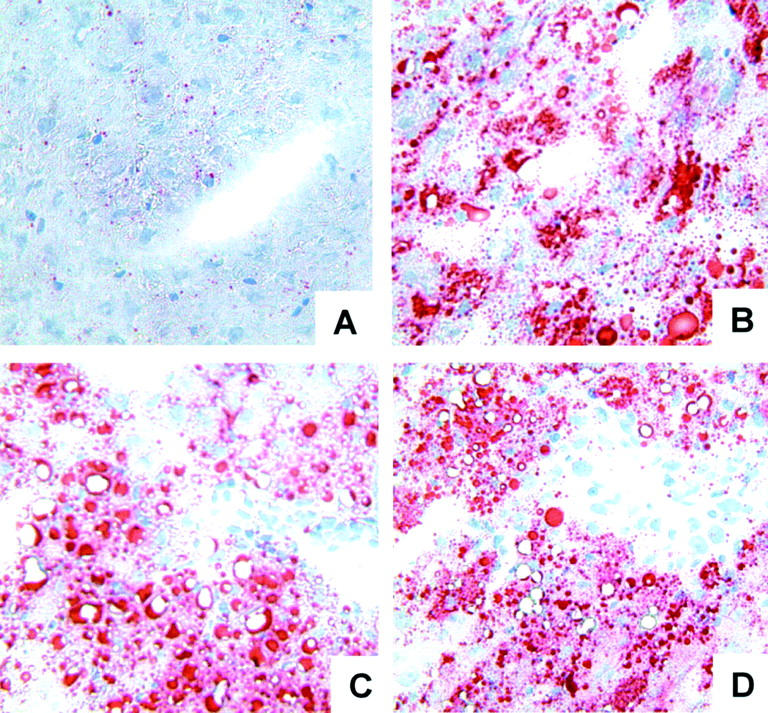
Representative Oil Red O staining in livers of lean and fatty Zucker rats. Steatotic naïve livers (B), controls (C), and CS1-treated (D) steatotic liver grafts at day 1 after OLT were characterized by >30% macrovesicular steatosis, whereas lean naïve livers (A) showed no signs of steatosis.
Treatment with CS1 Peptides
CS1 peptides were synthesized on a Beckman System 990 peptide synthesizer (Beckman Instruments, Inc., Fullerton, CA) and purified by high-performance liquid chromatography, as described. 15 The sequence of the peptides used in the study corresponded to the alternatively spliced CS1 variant of FN (25-mer: DELPQLVTLPHPNLHGPEILDVPST) and have been shown in vitro and in vivo to block the interaction between α4β1 integrin and its FN ligand. 25-30 Most of the adhesion functions mediated by α4β1 integrin are attributed to interactions with its two ligands, FN and VCAM-1. Although it is possible that CS1 peptide may interfere with α4β1-VCAM-1 interactions, α4β1 integrin has been shown to have distinct ligand-binding sites for VCAM-1 (QIDSPL) and FN (LDV), 25 which suggest that CS1 peptide that binds to α4β1 does not likely inhibit the binding by VCAM-1. Moreover, although VCAM-1 and CS1-FN may share spatial overlapping binding sites on α4β1, the concentration of FN peptides that interfere with the α4β1-VCAM-1 binding is several-fold higher than that required for the α4β1-FN blockade. 28 Cellular FN is almost absent in naïve steatotic livers, and it is up-regulated very early in the liver vasculature after cold storage and OLT (Figure 2) ▶ . Based on these observations and in our previous studies of CS1-facilitated blockade of α4β1-FN interactions in cardiac transplants, 31 we designed a therapeutic regimen in which the treatment group received two doses of CS1 peptides (500 μg/rat) intraportally during procurement and before OLT. In addition, OLT recipients received a 3-day course of CS1 peptides (1 mg/rat/day, i.v.) and were followed for survival and sGOT levels. Control recipients received scrambled peptides or remained untreated. Separate groups of rats were sacrificed at 6 hours, and 1, 3, 7, and 100 days after OLT, and liver samples were collected for further analyses.
Figure 2.
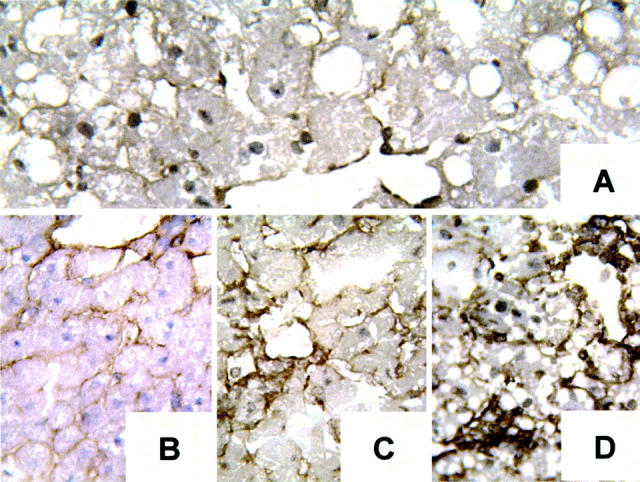
Cellular FN expression in steatotic livers. Cellular FN is almost absent in naïve steatotic livers (A) and it is up-regulated in the liver vasculature at 4 hours of cold storage in UW solution (before OLT) (B), and at 6 (C) and 24 (D) hours after OLT (n = 4 to 5/group). Original magnifications, ×200.
Myeloperoxidase (MPO) Assay
MPO is a naturally occurring constituent of neutrophils and is used as a marker for neutrophil infiltration in the liver. 32 Frozen tissue is thawed and suspended in an iced solution of 0.5% hexadecyltrimethyl-ammonium (Sigma, St. Louis, MO) and 50 mmol/L of potassium phosphate buffer solution (Sigma) with the pH adjusted to 5. Samples were homogenized for 30 seconds and centrifuged at 15,000 rpm for 15 minutes at 4°C. Supernatant (0.1 ml) is mixed in a solution of hydrogen peroxide-sodium acetate and tetramethyl benzidine (Sigma). The change in absorbance at 460 nm was measured with a Beckman DU spectrophotometer (Beckman Institute, Fullerton, CA). The quantity of enzyme degrading 1 μmol/L of peroxide per minute at 25° C per g of tissue was defined as 1 U of MPO activity.
Histology and Immunohistochemistry
Liver specimens were fixed in a 10% buffered formalin solution and embedded in paraffin. Sections were made at 4 μm and stained with hematoxylin and eosin (H&E). The previously published criteria of Suzuki and colleagues 33 was modified to reflect the histological severity of I/R injury in our OLT model. In this classification, sinusoidal congestion, hepatocyte necrosis, and ballooning degeneration are graded from 0 to 4. 22 No necrosis, congestion, or centrilobular ballooning is given a score of 0, whereas severe congestion and ballooning degeneration as well as >60% lobular necrosis is given a value of 4. Two pathologists blindly evaluated the histological samples.
OLTs were also examined serially by naphthol AS-D chloroacetate esterase staining for neutrophil infiltration and by immunohistochemistry for mononuclear cell (MNC) infiltrate, activation, FN deposition, nitric oxide synthase (NOS) expression, and nitrotyrosine detection. 34 Briefly, liver tissue was embedded in Tissue Tec OCT compound (Miles, Elkhart, IN), snap-frozen in liquid nitrogen, and stored at −70°C. Cryostat sections (5 μm) were fixed in acetone and then endogenous peroxidase activity was inhibited with 0.3% H2O2 in phosphate-buffered saline (PBS). Appropriate primary mouse antibody against rat T cells (R73), monocytes/macrophages (ED1), interleukin (IL)-2R+ cells (CD25) (Harlan Bioproducts, Indianapolis, IN), inducible NOS (iNOS), endothelial NOS (eNOS) (Santa Cruz Biotechnology, Santa Cruz, CA), anti-nitrotyrosine (Upstate Biotechnology, Lake Placid, NY), and anti-cellular FN (IST-9; Accurate Chemical, Westbury, NY) were added at optimal dilutions. Bound primary antibody was detected using biotinylated anti-mouse IgG and streptavidin peroxidase-conjugated complexes (DAKO, Carpinteria, CA). Negative controls included sections in which the primary antibody was replaced with either dilution buffer or normal mouse serum. Control sections from inflammatory tissues known to be positive for each stain were included as positive controls. The peroxidase reaction was developed with 3,3-diaminobenzidine tetrahydrochloride (Sigma). The sections were evaluated blindly by counting the labeled cells in triplicates by within 10 high-power fields per section. In the case of continuous labeling, some antigens were analyzed in a semiquantitative manner in which the relative abundance of each one was judged as −, negative; +, little; ++, moderately abundant; and +++, >200 cells/10 high-power fields or very abundant.
RNA Extraction and Reverse Transcriptase-Polymerase Chain Reaction
For evaluation of NOS and cytokine gene expression OLTs were harvested serially and RNA was extracted with TRIzol (Life Technologies, Inc., Grand Island, NY) using a Polytron RT-3000 (Kinematica AG, Littau-Luzem, Switzerland), as previously described. 35 Reverse transcription was performed using 4 μg of total RNA in a first-strand cDNA synthesis reaction with SuperScript II RNaseH Reverse Transcriptase (Life Technologies, Inc.) as recommended by the manufacturer. One μl of the resulting reverse transcriptase product was used for polymerase chain reaction amplification.
Western Blots
Protein was extracted from liver tissue samples withPBSTDS buffer (50 mmol/L Tris, 150 mmol/L NaCl, 0.1% sodium dodecyl sulfate, 1% sodium deoxycholate, and 1% Triton X-100, pH 7.2). Proteins (30 μg/sample) in sodium dodecyl sulfate-loading buffer (50 mmol/L Tris, pH 7.6, 10% glycerol, 1% sodium dodecyl sulfate) were subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA). The gel was then stained with Coomassie blue to document equal protein loading. The membrane was blocked with 3% dry milk and 0.1% Tween 20 (USB, Cleveland, OH) in PBS and incubated with specific primary antibodies against iNOS, eNOS, BclII, Bag-1, caspase-3, and actin (Santa Cruz Biotechnology, Santa Cruz, CA). The filters were washed and then incubated with horseradish peroxidase donkey anti-rabbit Ab (Amersham, Arlington Heights, IL). After development membranes were striped and re-blotted with an antibody against actin (Santa Cruz Biotechnology). Relative quantities of protein were determined using a densitometer (Kodak Digital Science 1D Analysis Software, Rochester, NY).
In Situ Cell Death
Apoptosis was detected by the terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) method. This tailing reaction is especially sensitive to the type of DNA fragmentation occurring in apoptotic rather than necrotic cell death. 36 The TUNEL assay was performed on 8-μm cryostat sections using the In Situ Cell Death Detection kit (Roche Diagnostics, Indianapolis, IN) according to the manufacturer’s protocol. TUNEL+ cells were detected under light microscopy. Terminal transferase was omitted as a negative control. Positive controls were generated by treatment with DNase 1 (30 U/ml in 40 mmol/L of Tris-·Cl, pH 7.6, 6 mmol/L MgCl2, and 2 mmol/L CaCl2 for 30 minutes).
Statistical Analysis
Statistical comparisons between groups were performed by Prism 3.0 (Graph Pad Software Inc., San Diego, CA) and Student’s t-test. P values of less than 0.05 were considered statistically significant.
Results
CS1 Peptides Prolong OLT Survival and Improve Fatty Zucker Hepatic Function
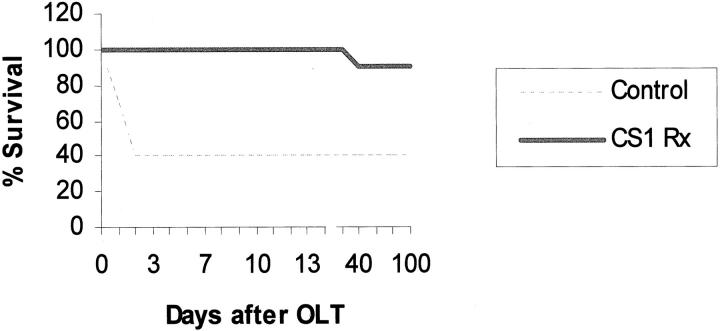
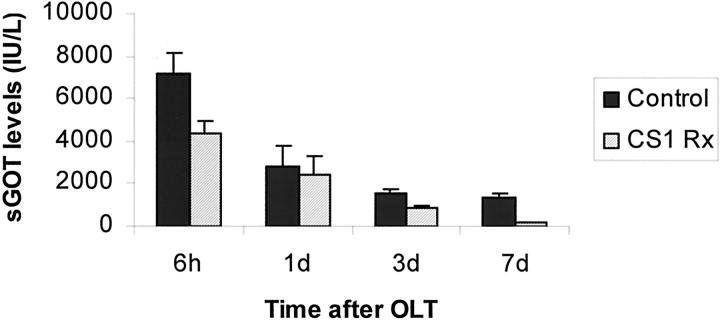
To determine the effects of CS1 facilitated blockade of α4β1- FN interactions in an in vivo transplant setting, OLTs were performed using steatotic livers that were harvested and cold-stored for 4 hours before being transplanted into syngenic lean Zucker rat recipients. CS1 peptides were administered to donor steatotic livers during procurement and before OLT, and an additional 3-day course of peptides was given to OLT recipients. The in vivo blockade of α4β1-FN interactions with CS1 significantly increased the 14-day OLT survival rate. Indeed, CS1-treated recipients had a 14-day survival rate of 100% (10 of 10), contrasting with the respective controls in which 40% (4 of 10) (P < 0.004) survived >14 days (Figure 3) ▶ . With the exception of one CS1-treated recipient that died at 33 days, apparently unrelated to OLT, all of the remaining survived >100 days. Such a striking improvement in the survival rate observed in the CS1-treated OLTs correlated with improved liver function, as measured serially by sGOT levels (Figure 4) ▶ . Indeed, sGOT levels at 6 hours (4374 ± 581 versus 7179 ± 1021, P < 0.04), day 3 (830 ± 130 versus 1600 ± 190, P < 0.02), and day 7 (148 ± 78 versus 1325 ± 247, P < 0.01), were significantly decreased after CS1 therapy, as compared with respective controls. Moreover, control livers were characterized by central vein congestion, and several centrilobular ischemic areas with neutrophil infiltration adjacent to necrotic tissue, contrasting with CS1-treated livers that showed only mild periportal ballooning without significant vascular congestion or necrosis (score: 2.7 ± 0.5 versus 0.6 ± 0.5, P < 0.02; day 1 after OLT) (Figure 5) ▶ . In addition, MPO activity in CS1-treated livers at 6 hours (7.2 ± 0.4 versus 9.8 ± 0.9, P < 0.04) and day 1 (5.3 ± 0.5 versus 8.9 ± 0.3, P < 0.008) after transplant was also significantly decreased as compared with respective controls (Figure 6) ▶ . A few numbers of neutrophils were detected by naphthol AS-D chloroacetate esterase staining in hepatic triads of all studied livers, including naïve steatotic livers. However, neutrophils were rarely found in the hepatic parenchyma of the CS1-treated livers whereas in the respective controls they were noticed in moderated numbers adjacent to necrotic areas (Figure 6) ▶ .
Figure 3.
Survival of steatotic OLT recipients. Lean Zucker rats were transplanted with livers harvested from obese Zucker donors. Steatotic livers received a dose of CS1 peptides at the time of liver procurement and at the time of transplantation. OLT recipients received an additional 3-day course of CS1 peptides. Control animal survival at 14 days was 40% versus 100% in the CS1 peptide-treated group, respectively (P < 0.004; n = 10 rats/group).
Figure 4.
sGOT levels of steatotic OLT recipients. CS1-mediated blockade of α4β1-FN interactions improved liver function as evidenced by sGOT levels. Levels of sGOT were significantly lower at 6 hours (P < 0.04), day 3 (P < 0.02), and day 7 (P < 0.01) in the CS1 peptide-treated recipients as compared with respective controls (n = 4 to 5 rats/group).
Figure 5.
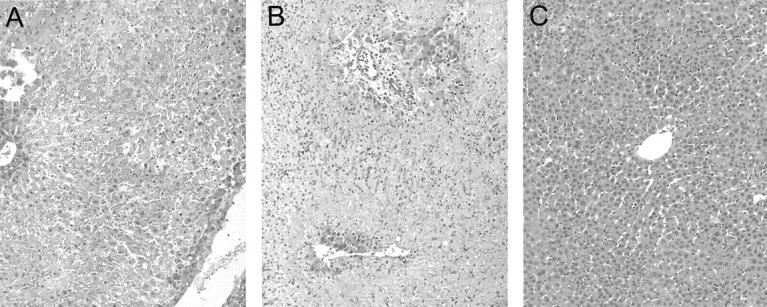
Representative histology of steatotic liver grafts harvested at day 1 after OLT. Untreated (A) and scrambled peptide-treated control (B) OLTs were characterized by extensive centrilobular pallor and necrosis with central vein vascular congestion throughout the entire biopsy. Neutrophilic infiltration was noted adjacent to the necrotic tissue. In contrast, CS1-treated OLTs (C) showed mild periportal and central ballooning changes with virtually absence of vascular congestion or necrosis (n = 3 to 4/group). H&E stain; original magnifications, ×100.
Figure 6.
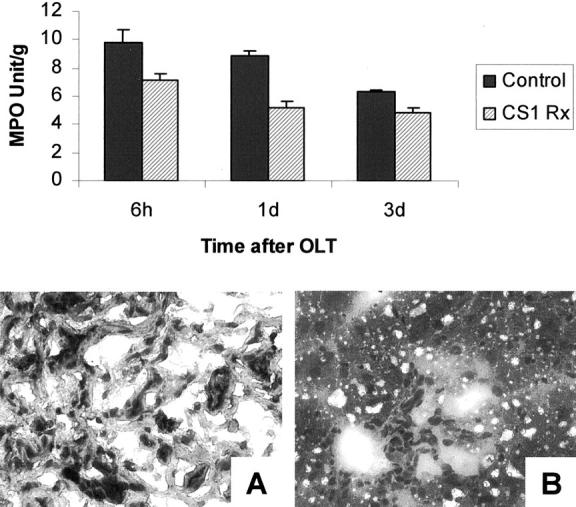
MPO activity and naphthol AS-D chloroacetate esterase staining in steatotic OLTs. MPO activity (top) in CS1-treated livers at 6 hours (P < 0.04) and day 1 (P < 0.008) after transplant was significantly decreased as compared with respective controls. Representative naphthol AS-D chloroacetate esterase staining at day 1 after OLT (bottom) illustrates a moderated presence of neutrophils in necrotic areas of control OLTs (A) contrasting with virtual absence of those cells in the parenchyma of CS1-treated liver grafts (n = 4 to 5/group). Original magnifications, ×200.
CS1 Peptides Protect against I/R Injury in a Lean Syngenic OLT Model
To determine the efficacy of CS1 peptide-facilitated blockade of α4β1-FN interactions in an alternated model of lean I/R injury, we performed OLTs using livers that were harvested from nonsteatotic Sprague-Dawley donors, stored at 4°C for 24 hours, and then transplanted into syngenic recipients. In this well-established model of lean liver I/R injury, 37.5% of the untreated recipients die within the first 48 hours after OLT. 37 The in vivo CS1 peptide-facilitated therapy significantly increased the 14-day lean OLT survival rate. Indeed, CS1 peptide-treated Sprague-Dawley recipients had a 14-day survival rate of 100% (six of six), contrasting with the respective controls in which only 50% (three of six) (P < 0.007) survived >14 days. The effectiveness of CS1 peptide therapy in improving OLT survival was also correlated with prevention of hepatocellular damage and significant improvement in liver function as assessed by the significant decrease in sGOT levels (1251 ± 195 versus 3733 ± 455, P < 0.01) as compared with the respective lean control OLTs.
CS1 Peptide Therapy Decreases Positioning of MNCs in Portal Areas of Steatotic OLTs
Our earlier studies have shown that infiltrating MNCs localize selectively in FN-rich areas in rejecting cardiac allografts. 14,34 Further, we have shown that the blockade of α4β1-FN interactions abrogates acute rejection and decreases MNC infiltration in cardiac allografts. 31 In the present study, we evaluated the role of CS1-mediated therapy on leukocyte infiltration in steatotic OLTs. As shown in Figure 7 ▶ and Table 1 ▶ , T-cell infiltration was minimal in CS1-treated livers and significantly increased in the respective controls both at day 1 (5.0 ± 1 versus 12.7 ± 2, P < 0.02) and day 3 (5.0 ± 1.4 versus 13.7 ± 3.2, P < 0.02) after OLT. Indeed, the role of CS1 peptide therapy in depressing T-lymphocyte infiltration was observed as early as 6 hours after OLT. Focal T-cell infiltration was detected near the liver microvasculature of 6-hour controls and absent in CS1-treated livers (not shown). Macrophage infiltration was moderate to high in both CS1-treated and control livers at day 1 (++/+++ versus +++). In contrast, by day 3 after OLT, macrophages resumed to almost normal levels in CS1-treated grafts whereas respective controls were characterized by a persistent ongoing intragraft infiltration of these cells (94.2 ± 16 versus 169.0 ± 20, P < 0.003). Indeed, longer term OLT controls, such as day 7, were characterized by massive MNC infiltrates (not shown). IL2-R, a marker of leukocyte activation, was also found to be markedly decreased after CS1 peptide-targeted therapy (Figure 7 ▶ , Table 1 ▶ ).
Figure 7.
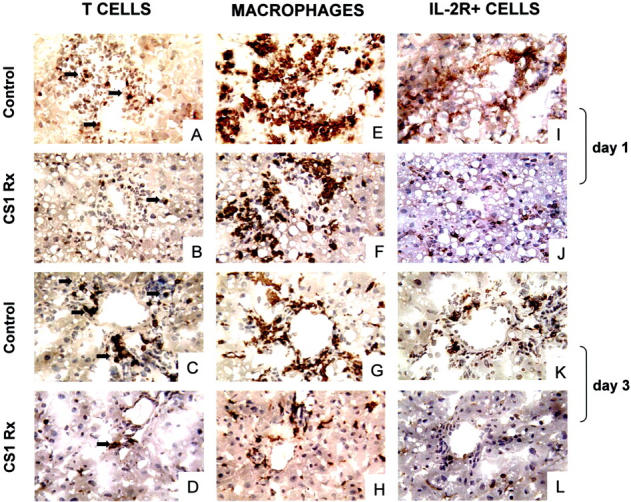
MNC infiltration and activation in steatotic OLTs. Immunoperoxidase staining of T lymphocytes (A–D), monocytes/macrophages (E–H), and IL-2R+ cells (I–L) in fatty liver grafts at day 1 (A, B, E, F, I, and J) and day 3 (C, D, G, H, K, and L) after OLT. CS1-treated livers were characterized by a significant decrease in T infiltration as compared with respective controls both at day 1 (B and A, respectively) and day 3 (D and C, respectively). Monocyte/macrophage infiltration was moderate to high in both CS1 and control liver grafts (F and E, respectively). In contrast, at day 3, macrophages resumed to almost normal levels in CS1-treated OLTs while remaining high in the respective controls (H and G, respectively). In addition, blockade of the α4β1-FN interactions was associated with a decrease in intragraft IL-2R+ cells (J and L versus I and K, respectively) (n = 3 to 5/group). Original magnifications, ×200.
Table 1.
Sequential Immunohistochemical Analysis of Infiltrating Cells in Steatotic OLTs
| Days after OLT | Immunostaining (mean ± SD) | |||||
|---|---|---|---|---|---|---|
| TCR+ lymphocytes | ED1+ macrophages | IL-2R+ cells | ||||
| Control | CS1 Rx | Control | CS1 Rx | Control | CS1 Rx | |
| 1 | 12.7 ± 2.0 | 5.0 ± 1.0 | +++ | ++/+++ | 60.0 ± 2.1 | 13.3 ± 6.6 |
| 3 | 13.7 ± 3.2 | 5.0 ± 1.4 | 169.0 ± 20.5 | 94.2 ± 16.0 | 21.3 ± 4.5 | 10.2 ± 1.5 |
The results of immunostaining are expressed as mean ± SD of data from 10 high-power fields per section per rat and three to five rats per group. Staining was evaluated in a semi-quantitative fashion on a scale from + (weak) to +++ (strong) when continuous labeling of the antigen was observed.
CS1 Peptide Therapy Decreases iNOS Expression by Macrophages in Steatotic OLTs
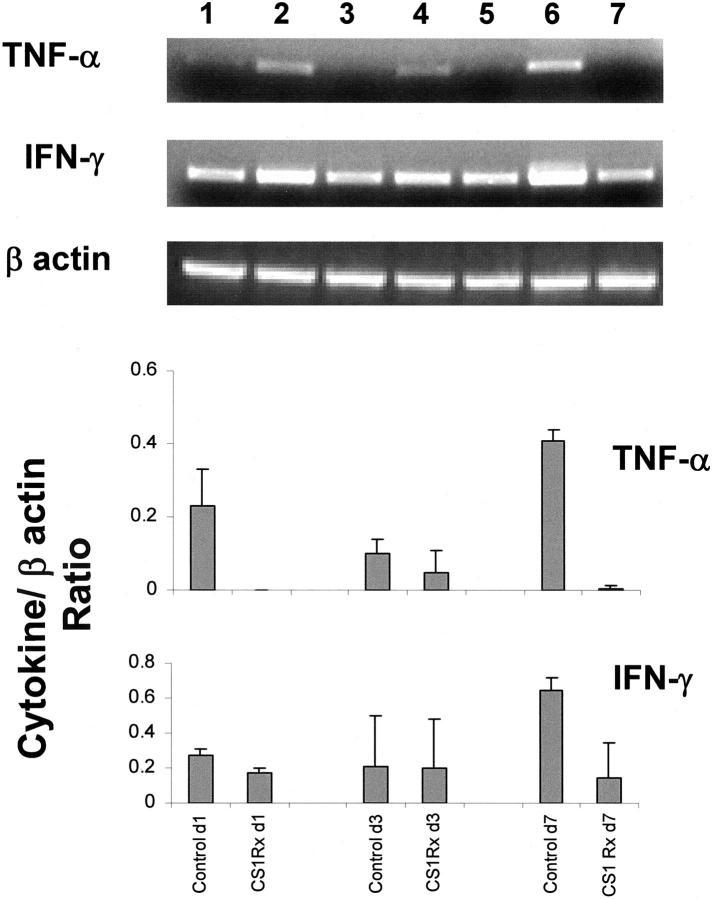
There is a growing body of evidence indicating that nitric oxide (NO) has an important role in ischemia injury. To determine whether CS1 peptide therapy affected endothelial nitric oxide synthase (eNOS) and inducible nitric oxide synthase (iNOS) expression in steatotic OLTs, we performed reverse transcriptase-polymerase chain reaction, Western blot, and immunohistological analyses.Hepatic sinusoidal lining cells were equally positive for e-NOS in control and CS1-treated groups. As shown in Figure 8 ▶ , all groups expressed similar levels of e-NOS at day 1 after OLT by Western blots. In contrast, untreated and control-scrambled peptide-treated control OLTs were characterized by elevated levels of iNOS expression, predominantly at day 1 after OLT, whereas CS1-treated OLTs were associated with almost absence of iNOS induction, both at mRNA and protein levels (Figure 8) ▶ . Moreover, infiltrating MNCs were the predominant source of iNOS expression in injured control OLTs. Indeed, infiltrating MNCs in control livers were moderately to highly positive for iNOS whereas in well-preserved CS1 livers those cells were nearly iNOS-negative (++/+++ versus −/+) (Figure 8) ▶ . Tumor necrosis factor (TNF)-α and interferon (IFN)-γ, two proinflammatory cytokines known to up-regulate iNOS expression by macrophages, were significantly depressed by CS1-mediated blockade of FN-α4β1 integrin interactions in steatotic OLTs (Figure 9) ▶ .
Figure 8.
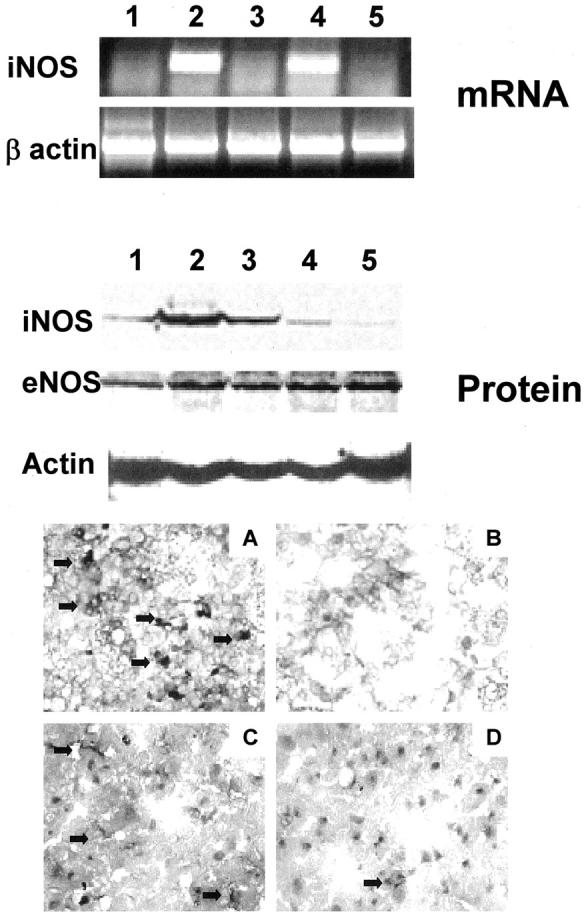
iNOS expression and peroxynitrite formation in steatotic OLTs. Top: iNOS expression at mRNA level in steatotic naïve livers (lane 1) and OLTs at day 1 (lanes 2 and 3) and day 3 (lanes 4 and 5) after transplantation. Control livers (lanes 2 and 4) showed high levels of iNOS mRNA expression in particular at day 1 after OLT (lane 2). In contrast, iNOS mRNA expression was virtually absent in naïve (lane 1) and CS1-treated OLTs at day 1 (lane 3) and day 3 (lane 5). Middle: A similar result at the protein level. Control day 1 OLTs expressed high levels of iNOS (lanes 2 and 3) whereas iNOS was depressed in naïve fatty livers (lane 1) and day 1 CS1-treated OLTs (lanes 4 and 5). In contrast to iNOS, eNOS was expressed in comparable amounts between control (lanes 2 and 3) and CS1 (lanes 4 and 5) OLTs at day 1. Bottom: iNOS (A and B) and nitrotyrosine (C and D) staining in day 1 control (A and C) and CS1-treated (B and D) OLTs. iNOS staining was highly positive in infiltrating MNCs of control OLTs and almost absent in CS1-treated liver grafts. Nitrotyrosine staining was moderated and mild in control and CS1-treated OLTs, respectively (n = 3/group). Original magnifications, ×200 (A and B).
Figure 9.
Proinflammatory cytokine gene expression in steatotic OLTs. TNF-α and IFN-γ were highly expressed in control OLTs and markedly depressed in CS1-treated liver grafts at day 1 (lanes 2 and 3, respectively), day 3 (lanes 4 and 5, respectively), and day 7 (lanes 6 and 7, respectively). Indeed the levels of these cytokines in the CS1-treated livers were comparable to those from nave livers (lane 1). Bottom: The ratios of TNF-α/β-actin (day 1, 0.23 ± 0.1 versus 0, P < 0.04; day 7, 0.41 ± 0.03 versus 0.01 ± 0.01, P < 0.001) and IFN-γ/β-actin (day 1, 1.4 ± 0.1 versus 0.8 ± 0.2, P < 0.04; day 7, 3.3 ± 0.4 versus 0.8 ± 1, P < 0.04) in control and CS1-treated OLTs, respectively (n = 3/group).
High levels of iNOS expression have been associated with production of peroxynitrite, a potent oxidant associated with the NO-mediated tissue damage. 38 Nitric oxide interacts in vivo with superoxide (O2−) to produce peroxynitrite (ONOO−), and peroxynitrite formation can be detected by nitrotyrosine staining. In our experimental settings, nitrotyrosine deposition was predominantly present in moderated amounts in vascular lining cells of control OLTs (++) contrasting with minimal deposition in CS1-treated livers (+) (Figure 8) ▶ .
CS1 Peptide Therapy Prevents Hepatic Cell Death in Steatotic OLTs
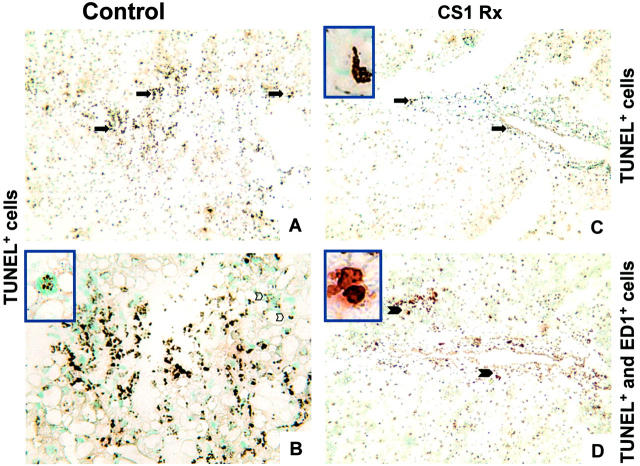
To address a possible link between iNOS expression and cell death, we assessed the expression of anti-apoptotic (Bcl-2, Bag-1) and proapoptotic (caspase-3) gene products in OLTs by Western blots. In addition, we have also used the TUNEL cell-staining assay to identify apoptotic cells and H&E to detect liver necrosis. Interestingly, we didn’t find significant differences between Bcl-2 (Bcl-2/actin ratio, 1.1 versus 0.9), Bag-1 (Bag-1/actin ratio, 0.7 versus 0.8), or caspase-3 (p20/actin ratio, 0.5 versus 0.6; p11/actin ratio, 0.7 versus 1.0) expression between CS1 peptide-treated and control livers at day 1 after OLT. Both CS1-treated and control OLTs were characterized by the presence of apoptotic cells at day 1 (Figure 10) ▶ . Although day 1 controls showed extensive labeling of TUNEL+ cells (+++) (Figure 10, A and B) ▶ , CS1-treated livers presented a moderated number of cells undergoing apoptosis (++) (Figure 10C) ▶ . At later time points after liver transplantation (days 3 and 7), TUNEL+ labeling was considerably reduced in CS1-treated OLTs (+) contrasting with a constant moderated to elevated presence of TUNEL + cells in the respective control OLTs (++/+++). In an attempt to identify cell types undergoing apoptosis in steatotic liver grafts, TUNEL-labeled tissue sections were double-labeled with antibodies against MNCs. As shown in Figure 10D ▶ , cells undergoing apoptosis in CS1-treated livers were in majority ED1-positive, indicating that apoptosis occurred preferentially in infiltrating monocytes/macrophages. We did not detect any hepatocyte apoptosis in CS1-treated OLTs. In damaged control livers, most of the TUNEL+ cells were also infiltrating macrophages, whereas only a very small number of hepatocytes were TUNEL+. However, control OLTs were characterized by extensive hepatic necrosis contrasting with CS1-treated livers, which had virtually no signs of necrosis (Figure 5) ▶ . Taken together those results suggest that possible negative effects of iNOS/NO in our steatotic liver model of I/R injury were preferentially associated to necrosis rather than apoptosis.
Figure 10.
Representative apoptosis in steatotic liver grafts at day 1 after OLT. Control (A and B) and CS1-treated (C and D) OLTs were characterized by an elevated number of TUNEL+ cells (brown, arrows indicate positive labeling). Most of the cells undergoing apoptosis stained also for the monocyte/macrophage marker ED1 (D, arrows indicate double-positive brown and red labeling, and enlargements of those areas are provided in the top left inset). Only a few TUNEL+-stained hepatocytes were detected in control OLTs (B, top left inset illustrates an enlarged TUNEL+ hepatocyte). Original magnifications, ×100 (A, C, and D); ×200 (B).
Discussion
We report here the results of our studies on the effects of the blockade of α4β1-FN interactions on the development of I/R injury in a well-established model of steatotic OLTs. CS1 peptide-facilitated blockade of α4β1-FN interactions in steatotic OLTs: 1) significantly increased the 14-day OLT survival rate from 40 to 100%; 2) ameliorated cardinal histological features of hepatocyte injury and improved liver function; 3) diminished intragraft MNC infiltration/activation and decreased MPO activity; 4) inhibited TNF-α and IFN-γ expression; 5) down-regulated iNOS expression as well as peroxynitrite formation; and 6) prevented hepatic necrosis. This is the first demonstration that blockade of α4β1-FN interactions prevents severe I/R insult in steatotic livers.
The deleterious role of cytokine and reactive oxygen species released by leukocytes at the graft site has been documented. However the mechanisms involved in the leukocyte recruitment to sites of inflammatory stimulation in liver are far from being entirely understood. Leukocyte recruitment to organ transplants requires a sequential cascade of adhesive events that may differ with the type of organ transplanted. The observation that all of the CS1 peptide-treated liver recipients stayed alive during the 14-day survival period, with very good histological liver preservation, strongly supports our hypothesis of a critical role for FN-α4β1 integrin interactions in steatotic liver I/R injury. T lymphocytes have been shown to play a key role in liver I/R injury. 39,40 In our model of steatotic OLTs, FN-α4β1 integrin blockade was associated with almost abolition of intragraft T-cell infiltration. A role for FN-mediated T-lymphocyte migration has been previously shown by others in skin inflammation 41 and rheumatoid arthritis, 42 and by us in cardiac transplants. 31,43 However, the role of the interactions between FN and its α4β1 integrin receptor on T-cell recruitment may be of particular relevance in steatotic OLTs. Indeed, infusion of a soluble form of P selectin glycoprotein ligand-1 (sPSGL-1), a ligand for the CD62 selectin family, failed to affect the initial recruitment of T into steatotic OLTs (A. J. Coito, unpublished data). Interestingly, selectins are not an essential step for leukocyte recruitment into inflamed liver microvasculature, as suggested by studies in animals lacking both endothelial CD62E and CD62P. 44 Perhaps, lower shear rates lead to selectin-independent leukocyte rolling and adhesion. 45 Moreover, it has been shown that steatosis decreases sinusoidal blood flow by ∼50% in humans and rats. 46,47 All together, these findings highlight the role of alternative pathways of leukocyte recruitment in injured fatty livers and emphasize the role that FN-α4β1 integrin interactions may have in steatotic OLTs. Neutrophils, other important mediators of I/R injury, 48 were also depressed by CS1 therapy. Our results support the novel concept that α4β1 integrin is an important mediator in neutrophil adhesion and migration. 20,21,49 Surprisingly, CS1 peptide therapy was not very effective in decreasing the initial numbers of macrophages infiltrating steatotic liver grafts. Indeed, despite a partial decrease in macrophage infiltration in the CS1 peptide-treated livers, both control and CS1-treated OLTs were characterized by elevated numbers of those mononuclear leukocytes at day 1 after OLT. The recruitment of monocytes/macrophages at the graft site may in part be mediated by FN-α4β1-independent interactions. However, the inhibition of T-cell infiltration by CS1 therapy may have impaired the required signals leading to macrophage activation in steatotic OLTs. Indeed, TNF-α expression, a marker of macrophage activation was almost abolished in CS1-treated livers and readily expressed in the respective controls.
The emerging evidence that nitric oxide (NO) has an important role in ischemia injury has attracted the attention to nitric oxide synthases (NOS). There are at least three different isoforms of NOS able to generate NO from l-arginine. Neuronal NOS (nNOS) and endothelial NOS (eNOS) are constitutively expressed and generate only small amounts of NO that most of the cases are sufficient for cell signaling. 50-52 Inducible NOS (iNOS) is being triggered in many cell types by cytokines such as TNF-α or IFN-γ, and in contrast to eNOS, generates NO in a sustained manner for prolonged periods of time, leading to large amounts of NO. 53 Hence, we evaluated whether or not the blockade of FN-α4β1 interactions affects the expression of eNOS and iNOS in steatotic OLTs. Our results indicate that blockade of FN-α4β1 interactions selectively inhibited iNOS expression, most likely by down-regulating TNF-α and IFN-γ expression, without affecting eNOS deposition. Moreover, the presence of nitrotyrosine, a marker of peroxynitrite formation, in injured control livers suggests that overexpression of iNOS in this model was associated with NO release. These observations support the concept that moderated levels of NO might be beneficial, whereas high NO levels associate with liver pathological conditions. Indeed, it has been shown that iNOS expression is critical in immune-mediated liver injury, 54 and iNOS inhibition with selective inhibitors ameliorates liver I/R injury. 55 There is a growing number of reports illustrating that iNOS and NO promote 56,57 and protect against apoptosis. 58,59 Interestingly, we were not able to find a correlation between apoptosis and iNOS overexpression in our experimental model. Indeed, in both well-preserved CS1-treated livers and damaged control livers the majority of cells undergoing apoptosis were infiltrating MNCs. Only a very few number of hepatocytes were undergoing apoptosis in the severely injured control livers. Instead, necrosis was the preferential form of hepatocyte death in those livers. Control steatotic OLTs were characterized by massive necrosis whereas CS1-treated steatotic grafts showed nearly no signs of necrosis. Our findings in cold ischemia and reperfusion support and extend others in models of warm I/R indicating that hepatocellular necrosis is the dominant mechanism of cell death in fatty livers, 60 as well as in nonsteatotic lean livers. 61 Moreover, it provides an indication of a possible iNOS/NO-mediated role on hepatic necrosis in steatotic liver grafts.
In conclusion, this work provides evidence of a critical role mediated by FN-α4β1 integrin interactions in the development of I/R injury of steatotic liver grafts. CS1 peptide-facilitated blockade of FN-α4β1 integrin interactions significantly inhibited T-lymphocyte and neutrophil infiltration in steatotic OLTs, repressed the expression of proinflammatory cytokines, and clearly improved liver histological preservation and steatotic OLT recipient survival. Moreover, our results indicated a possible FN-α4β1 integrin-mediated up-regulation of iNOS expression and necrosis in steatotic liver grafts. Further investigations using this model should clarify whether necrosis in steatotic OLTs is indeed iNOS/NO-dependent.
Acknowledgments
We thank Prof. Maria de Sousa for invaluable discussions on the role of extracellular matrix proteins and Dr. Liz Messersmith for providing the CS1 peptides.
Footnotes
Address reprint requests to Dr. Ana J. Coito, M.D., Dumont-UCLA Transplant Center, Division of Liver and Pancreas Transplant, 10833 Leconte Ave., Rm. 77-120 CHS, Box 957054, Los Angeles, CA 90095-7054. E-mail: acoito@mednet.ucla.edu.
Supported by grants from the American Association for the Study of Liver Diseases/American Liver Foundation (AASLD/ALF) (grant 01072059 to A. J. C.), American Liver Transplantation (AST) (grant 01114026 to A. J. C.), the American Heart Association (grant 0030006N to A. J. C.), the National Institutes of Health (grants RO1 A142223 and RO1 AI23847 to J. W. K. W.), and by the Dumont Research Foundation.
F. A. and X.-D. S. contributed equally to this work.
References
- 1.Alexander JW, Vaughn WK: The use of “marginal” donors for organ transplantation. The influence of donor age on outcome. Transplantation 1991, 51:135-141 [DOI] [PubMed] [Google Scholar]
- 2.Lehmann TG, Wheeler MD, Schwabe RF, Connor HD, Schoonhoven R, Bunzendahl H, Brenner DA, Jude SR, Zhong Z, Thurman RG: Gene delivery of Cu/Zn-superoxide dismutase improves graft function after transplantation of fatty livers in the rat. Hepatology 2000, 32:1255-1264 [DOI] [PubMed] [Google Scholar]
- 3.Strasberg SM, Howard TK, Molmenti EP, Hertl M: Selecting the donor liver: risk factors for poor function after orthotopic liver transplantation. Hepatology 1994, 20:829-838 [DOI] [PubMed] [Google Scholar]
- 4.Koneru B, Dikdan G: Hepatic steatosis and liver transplantation current clinical and experimental perspectives. Transplantation 2002, 73:325-330 [DOI] [PubMed] [Google Scholar]
- 5.Jarnagin WR, Rockey DC, Koteliansky VE, Wang SS, Bissell DM: Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J Cell Biol 1994, 127:2037-2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO: Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development 1993, 119:1079-1091 [DOI] [PubMed] [Google Scholar]
- 7.Georges-Labouesse EN, George EL, Rayburn H, Hynes RO: Mesodermal development in mouse embryos mutant for fibronectin. Dev Dyn 1996, 207:145-156 [DOI] [PubMed] [Google Scholar]
- 8.Shimizu Y, van Seventer GA, Horgan KJ, Shaw S: Regulated expression and binding of three VLA (beta 1) integrin receptors on T cells. Nature 1990, 345:250-253 [DOI] [PubMed] [Google Scholar]
- 9.Hauzenberger D, Klominek J, Sundqvist KG: Functional specialization of fibronectin-binding beta 1-integrins in T lymphocyte migration. J Immunol 1994, 153:960-971 [PubMed] [Google Scholar]
- 10.Coito AJ, de Sousa M, Kupiec-Weglinski JW: Fibronectin in immune responses in organ transplant recipients. Dev Immunol 2000, 7:239-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown LF, Dubin D, Lavigne L, Logan B, Dvorak HF, Van De WL: Macrophages and fibroblasts express embryonic fibronectins during cutaneous wound healing. Am J Pathol 1993, 142:793-801 [PMC free article] [PubMed] [Google Scholar]
- 12.Humphries MJ: Fibronectin and cancer: rationales for the use of antiadhesives in cancer treatment. Semin Cancer Biol 1993, 4:293-299 [PubMed] [Google Scholar]
- 13.Elices MJ, Tsai V, Strahl D, Goel AS, Tollefson V, Arrhenius T, Wayner EA, Gaeta FC, Fikes JD, Firestein GS: Expression and functional significance of alternatively spliced CS1 fibronectin in rheumatoid arthritis microvasculature. J Clin Invest 1994, 93:405-416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coito AJ, Binder J, de Sousa M, Kupiec-Weglinski JW: The expression of extracellular matrix proteins during accelerated rejection of cardiac allografts in sensitized rats. Transplantation 1994, 57:599-605 [PubMed] [Google Scholar]
- 15.Coito AJ, Onodera K, Kato H, Busuttil RW, Kupiec-Weglinski JW: Fibronectin-mononuclear cell interactions regulate type 1 helper T cell cytokine network in tolerant transplant recipients. Am J Pathol 2000, 157:1207-1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wayner EA, Garcia-Pardo A, Humphries MJ, McDonald JA, Carter WG: Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol 1989, 109:1321-1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan JL, Hynes RO: Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor alpha 4 beta 1. Cell 1990, 60:53-61 [DOI] [PubMed] [Google Scholar]
- 18.Komoriya A, Green LJ, Mervic M, Yamada SS, Yamada KM, Humphries MJ: The minimal essential sequence for a major cell type-specific adhesion site (CS1) within the alternatively spliced type III connecting segment domain of fibronectin is leucine-aspartic acid-valine. J Biol Chem 1991, 266:15075-15079 [PubMed] [Google Scholar]
- 19.Osborn L, Vassallo C, Browning BG, Tizard R, Haskard DO, Benjamin CD, Dougas I, Kirchhausen T: Arrangement of domains, and amino acid residues required for binding of vascular cell adhesion molecule-1 to its counter-receptor VLA-4 (alpha 4 beta 1). J Cell Biol 1994, 124:601-608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinhardt PH, Elliott JF, Kubes P: Neutrophils can adhere via alpha4beta1-integrin under flow conditions. Blood 1997, 89:3837-3846 [PubMed] [Google Scholar]
- 21.Johnston B, Kubes P: The alpha4-integrin: an alternative pathway for neutrophil recruitment? Immunol Today 1999, 20:545-550 [DOI] [PubMed] [Google Scholar]
- 22.Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen XD, Zhao D, Zaky J, Melinek J, Lassman CR, Kolls JK, Alam J, Ritter T, Volk HD, Farmer DG, Ghobrial RM, Busuttil RW, Kupiec-Weglinski JW: Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest 1999, 104:1631-1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamada N, Calne RY: Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation 1979, 28:47-50 [PubMed] [Google Scholar]
- 24.Kamada N, Calne RY: A surgical experience with five hundred thirty liver transplants in the rat. Surgery 1983, 93:64-69 [PubMed] [Google Scholar]
- 25.Elices MJ, Osborn L, Takada Y, Crouse C, Luhowskyj S, Hemler ME, Lobb RR: VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell 1990, 60:577-584 [DOI] [PubMed] [Google Scholar]
- 26.Elices MJ, Tsai V, Strahl D, Goel AS, Tollefson V, Arrhenius T, Wayner EA, Gaeta FC, Fikes JD, Firestein GS: Expression and functional significance of alternatively spliced CS1 fibronectin in rheumatoid arthritis microvasculature. J Clin Invest 1994, 93:405-416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wahl SM, Allen JB, Hines KL, Imamichi T, Wahl AM, Furcht LT, McCarthy JB: Synthetic fibronectin peptides suppress arthritis in rats by interrupting leukocyte adhesion and recruitment. J Clin Invest 1994, 94:655-662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makarem R, Newham P, Askari JA, Green LJ, Clements J, Edwards M, Humphries MJ, Mould AP: Competitive binding of vascular cell adhesion molecule-1 and the HepII/IIICS domain of fibronectin to the integrin alpha 4 beta 1. J Biol Chem 1994, 269:4005-4011 [PubMed] [Google Scholar]
- 29.Molossi S, Elices M, Arrhenius T, Diaz R, Coulber C, Rabinovitch M: Blockade of very late antigen-4 integrin binding to fibronectin with connecting segment-1 peptide reduces accelerated coronary arteriopathy in rabbit cardiac allografts. J Clin Invest 1995, 95:2601-2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molossi S, Elices M, Arrhenius T, Diaz R, Coulber C, Rabinovitch M: Blockade of very late antigen-4 integrin binding to fibronectin with connecting segment-1 peptide reduces accelerated coronary arteriopathy in rabbit cardiac allografts. J Clin Invest 1995, 95:2601-2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coito AJ, Korom S, Graser E, Volk HD, Van De WL, Kupiec-Weglinski JW: Blockade of very late antigen-4 integrin binding to fibronectin in allograft recipients: I. Treatment with connecting segment-1 peptides prevents acute rejection by suppressing intragraft mononuclear cell accumulation, endothelial activation, and cytokine expression. Transplantation 1998, 65:699-706 [DOI] [PubMed] [Google Scholar]
- 32.Kato A, Yoshidome H, Edwards MJ, Lentsch AB: Regulation of liver inflammatory injury by signal transducer and activator of transcription-6. Am J Pathol 2000, 157:297-302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D: Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation 1993, 55:1265-1272 [DOI] [PubMed] [Google Scholar]
- 34.Coito AJ, Brown LF, Peters JH, Kupiec-Weglinski JW, Van De WL: Expression of fibronectin splicing variants in organ transplantation: a differential pattern between rat cardiac allografts and isografts. Am J Pathol 1997, 150:1757-1772 [PMC free article] [PubMed] [Google Scholar]
- 35.Coito AJ, Shaw GD, Li J, Ke B, Ma J, Busuttil RW, Kupiec-Weglinski JW: Selectin-mediated interactions regulate cytokine networks and macrophage heme oxygenase-1 induction in cardiac allograft recipients. Lab Invest 2002, 82:61-70 [DOI] [PubMed] [Google Scholar]
- 36.Gold R, Schmied M, Giegerich G, Breitschopf H, Hartung HP, Toyka KV, Lassmann H: Differentiation between cellular apoptosis and necrosis by the combined use of in situ tailing and nick translation techniques. Lab Invest 1994, 71:219-225 [PubMed] [Google Scholar]
- 37.Amersi F, Nelson SK, Shen XD, Kato H, Melinek J, Kupiec-Weglinski JW, Horwitz LD, Busuttil RW, Horwitz MA: Bucillamine, a thiol antioxidant, prevents transplantation-associated reperfusion injury. Proc Natl Acad Sci USA 2002, 99:8915-8920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rees DD, Cellek S, Palmer RM, Moncada S: Dexamethasone prevents the induction by endotoxin of a nitric oxide synthase and the associated effects on vascular tone: an insight into endotoxin shock. Biochem Biophys Res Commun 1990, 173:541-547 [DOI] [PubMed] [Google Scholar]
- 39.Zwacka RM, Zhang Y, Halldorson J, Schlossberg H, Dudus L, Engelhardt JF: CD4(+) T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J Clin Invest 1997, 100:279-289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Moine O, Louis H, Demols A, Desalle F, Demoor F, Quertinmont E, Goldman M, Deviere J: Cold liver ischemia-reperfusion injury critically depends on liver T cells and is improved by donor pretreatment with interleukin 10 in mice. Hepatology 2000, 31:1266-1274 [DOI] [PubMed] [Google Scholar]
- 41.Ferguson TA, Mizutani H, Kupper TS: Two integrin-binding peptides abrogate T cell-mediated immune responses in vivo. Proc Natl Acad Sci USA 1991, 88:8072-8076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laffon A, Garcia-Vicuna R, Humbria A, Postigo AA, Corbi AL, de Landazuri MO, Sanchez-Madrid F: Upregulated expression and function of VLA-4 fibronectin receptors on human activated T cells in rheumatoid arthritis. J Clin Invest 1991, 88:546-552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coito AJ, Binder J, Brown LF, de Sousa M, Van De WL, Kupiec-Weglinski JW: Anti-TNF-alpha treatment down-regulates the expression of fibronectin and decreases cellular infiltration of cardiac allografts in rats. J Immunol 1995, 154:2949-2958 [PubMed] [Google Scholar]
- 44.Wong J, Johnston B, Lee SS, Bullard DC, Smith CW, Beaudet AL, Kubes P: A minimal role for selectins in the recruitment of leukocytes into the inflamed liver microvasculature. J Clin Invest 1997, 99:2782-2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaboury JP, Kubes P: Reductions in physiologic shear rates lead to CD11/CD18-dependent, selectin-independent leukocyte rolling in vivo. Blood 1994, 83:345-350 [PubMed] [Google Scholar]
- 46.Hayashi M, Tokunaga Y, Fujita T, Tanaka K, Yamaoka Y, Ozawa K: The effects of cold preservation on steatotic graft viability in rat liver transplantation. Transplantation 1993, 56:282-287 [DOI] [PubMed] [Google Scholar]
- 47.Gao W, Connor HD, Lemasters JJ, Mason RP, Thurman RG: Primary nonfunction of fatty livers produced by alcohol is associated with a new, antioxidant-insensitive free radical species. Transplantation 1995, 59:674-679 [DOI] [PubMed] [Google Scholar]
- 48.Lemasters JJ, Thurman RG: Reperfusion injury: a role for neutrophils. Hepatology 1991, 14:954-955 [DOI] [PubMed] [Google Scholar]
- 49.Werr J, Xie X, Hedqvist P, Ruoslahti E, Lindbom L: Beta1 integrins are critically involved in neutrophil locomotion in extravascular tissue in vivo. J Exp Med 1998, 187:2091-2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harrison DG: Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest 1997, 100:2153-2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Christopherson KS, Bredt DS: Nitric oxide in excitable tissues: physiological roles and disease. J Clin Invest 1997, 100:2424-2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Christopherson KS, Bredt DS: Nitric oxide in excitable tissues: physiological roles and disease. J Clin Invest 1997, 100:2424-2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nathan C: Inducible nitric oxide synthase: what difference does it make? J Clin Invest 1997, 100:2417-2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sass G, Koerber K, Bang R, Guehring H, Tiegs G: Inducible nitric oxide synthase is critical for immune-mediated liver injury in mice. J Clin Invest 2001, 107:439-447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Isobe M, Katsuramaki T, Hirata K, Kimura H, Nagayama M, Matsuno T: Beneficial effects of inducible nitric oxide synthase inhibitor on reperfusion injury in the pig liver. Transplantation 1999, 68:803-813 [DOI] [PubMed] [Google Scholar]
- 56.Forrester K, Ambs S, Lupold SE, Kapust RB, Spillare EA, Weinberg WC, Felley-Bosco E, Wang XW, Geller DA, Tzeng E, Billiar TR, Harris CC: Nitric oxide-induced p53 accumulation and regulation of inducible nitric oxide synthase expression by wild-type p53. Proc Natl Acad Sci USA 1996, 93:2442-2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Messmer UK, Reed UK, Brune B: Bcl-2 protects macrophages from nitric oxide-induced apoptosis. J Biol Chem 1996, 271:20192-20197 [DOI] [PubMed] [Google Scholar]
- 58.Genaro AM, Hortelano S, Alvarez A, Martinez C, Bosca L: Splenic B lymphocyte programmed cell death is prevented by nitric oxide release through mechanisms involving sustained Bcl-2 levels. J Clin Invest 1995, 95:1884-1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dimmeler S, Haendeler J, Nehls M, Zeiher AM: Suppression of apoptosis by nitric oxide via inhibition of interleukin-1beta-converting enzyme (ICE)-like and cysteine protease protein (CPP)-32-like proteases. J Exp Med 1997, 185:601-607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Selzner M, Rudiger HA, Sindram D, Madden J, Clavien PA: Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology 2000, 32:1280-1288 [DOI] [PubMed] [Google Scholar]
- 61.Gujral JS, Bucci TJ, Farhood A, Jaeschke H: Mechanism of cell death during warm hepatic ischemia-reperfusion in rats: apoptosis or necrosis? Hepatology 2001, 33:397-405 [DOI] [PubMed] [Google Scholar]