Abstract
Iron overload in the liver may occur in the clinical conditions hemochromatosis and transfusion-dependent thalassemia or by long-term consumption of large amounts of dietary iron. As iron concentrations increase in the liver, cirrhosis develops, and subsequently the normal architecture of the liver deteriorates. The underlying mechanisms whereby iron loading of hepatocytes leads to the pathology of the liver are not understood. Similarly, a direct relationship between the expression levels of paracellular junction genes and altered hepatocellular physiology has been reported; however, no relationship has been identified between iron loading and the expression of paracellular junction genes. Here, we report that the expression of numerous paracellular junction genes was decreased in iron-loaded hepatocytes, leading to increased cellular permeability, increased baculovirus-mediated gene transfer, and decreased gap junction communication. Iron loading of hepatocytes resulted in decreased E-cadherin promoter activity and subsequently decreased E-cadherin mRNA and protein expression. The data presented in this study describe a clear relationship between iron overload and decreased expression of paracellular junction genes in hepatic cells of rat and human origin.
Iron is essential for life and yet, inorganic unbound iron is toxic to all living cells. Iron transport and storage are regulated by well-defined mechanisms. 1 When the level of iron exceeds the capacity of these mechanisms, cells become iron-loaded. Apoferritin in cells enables them to survive the toxicity of free iron by binding iron. Ferritin regulates, by storage and release on demand, the amount of iron in the cell. However, most cells, other than erythroid precursors and macrophages, can only tolerate a few ferritin particles in the cytoplasm before they are considered to be iron-overloaded. 1 Iron overload is associated with liver pathogenicity in several human diseases, including hereditary hemochromatosis (HH) and transfusion-dependent thalassemia. HH is an autosomal recessive disorder that afflicts nearly one million Americans of European descent, making HH the most common genetic disorder among this population. 2-5 HH is a clinical condition in which excessive amounts of iron are sequestered in organs, primarily the liver. 6,7 HH is caused by a homozygous defect in the HFE gene; 8 however, the precise mechanism(s) that leads to iron deposition into hepatocytes has not been elucidated.
Iron deposition in hepatocytes of HH patients begins early in life and progresses at a constant rate, leading to liver fibrosis and cirrhosis as the normal architecture of the liver deteriorates. 7,9 Compared to normal cells, fibrotic and cirrhotic cells have an altered cellular morphology and histological differentiation, which is accompanied by changes in intercellular adhesion. Intercellular adhesion is mediated by paracellular junctions that include the calcium-dependent tight and adherens junctions, desmosomes, and calcium-independent gap junctions. 10 Tight junctions, partially composed of the intercellular occludin protein and the zona occludens (ZO)-1 plaque protein, are the apical-most paracellular junction complexes of an epithelial cell and, as such, they form continuous contacts that limit paracellular movement of molecules between adjacent cells. 11-13 The adherens junction is composed of the cadherin family of homophilic intercellular adhesion receptors. 10,14,15 E-cadherin, a 120-kd transmembrane glycoprotein that is highly expressed during development and thereafter in the epithelium, is the most predominant cadherin of the liver epithelium. The cytoplasmic domain of E-cadherin is directly associated with α- and β-catenin and through these proteins, E-cadherin is indirectly associated with several proteins including α-actinin and radixin that link the transmembrane E-cadherin to the actin cytoskeleton. 13 In the absence of E-cadherin, epithelial cells are not capable of stable intercellular adhesion. 10
Our laboratory previously reported that primary rat hepatocytes, plated on collagen-coated plastic dishes and fed chemically defined medium supplemented with dimethyl sulfoxide (DMSO), can be maintained in a differentiated state for longer than 1 year. 16,17 The morphological and biochemical characteristics of these cells are similar to those of hepatocytes in rat liver in vivo. Hepatocytes in culture secrete albumin and retain steady-state mRNA expression of liver-specific and common genes. As in the uninduced in vivo liver, the level of DNA synthesis in cells in these hepatocyte cultures is low and the hepatocytes do not undergo cell division, 16 but retain the capacity to synthesize DNA and divide. 18,19 During the first 3 days after plating, primary rat hepatocytes in this culture system form a single-cell monolayer. Shortly thereafter, the hepatocytes move on the collagen-coated dish into multicellular islands (HC Isom, unpublished data). 16,17 This dynamic motility is observed throughout the lifetime of the culture. As the cultures age, no space remains between hepatocytes within the islands and paracellular junctions between adjoining cells can be observed by electron microscopy. 16,20
Primary rat hepatocytes in long-term DMSO culture have been used to study the molecular mechanisms of albumin expression, 17,21 altered growth control including induction of DNA synthesis and proliferation, 22 immortalization and transformation, 23-25 as well as programmed cell death. 26 More recently, we have demonstrated that hepatocytes in long-term DMSO culture function as an ideal model system for studying chronic iron loading of hepatocytes. 19,20,27 The efficacy of various forms of iron to donate iron to long-term primary rat hepatocytes was examined and 3,5,5-trimethylhexanoyl ferrocene (TMH-ferrocene) was determined to be the most efficient iron donor of the compounds tested. 20 TMH-ferrocene is a chemically stable, nonionic form of iron that does not catalyze oxygen reactions under routine tissue culture conditions. TMH-ferrocene is lipophilic, and therefore readily diffuses into cultured hepatocytes, where a cytochrome p450-dependent reaction metabolizes the organic portion of the molecule and releases iron into the hepatocyte. 19 TMH-ferrocene was shown to load iron into hepatocytes in long-term DMSO culture in a concentration-dependent manner. 20 Iron from TMH-ferrocene was incorporated into ferritin cores within primary hepatocytes and ferritin protein levels were elevated in a physiological manner. 20 Changes in iron content, ferritin protein levels, and the expression of genes associated with iron regulation after chronic iron loading by TMH-ferrocene have also been studied using this system (E. E. Cable and H. C. Isom, unpublished results). Human hepatoma cells (Huh7s) are more difficult to iron-load than primary rat hepatocytes because of their rapid proliferation rates. However, acute responses to iron can be achieved by using the ferric nitrilotriacetate (FeNTA) iron donor. It has been previously demonstrated in human hepatic cell lines, that FeNTA induces the synthesis of ferritin by a transferrin-independent mechanism. 28-30 In the current study, the effect of iron loading on the expression of paracellular junction genes, specifically that of E-cadherin, was examined in iron-loaded hepatic cells of rat and human origin.
Materials and Methods
Cell Culture
Primary rat hepatocytes were maintained in Dulbecco’s modified Eagle’s medium/F12 (Life Technologies, Inc., Grand Island, NY) supplemented with insulin (0.06 μg/ml), glucagon (0.04 μg/ml), dexamethasone (0.4 μg/ml), transferrin (100 μg/ml), epidermal growth factor (25 ng/ml; Sigma Chemical Co., St. Louis, MO), 1 μmol/L [+]-α-tocopherol (Sigma) and 2% DMSO (Sigma) in a humidified incubator at 37°C with 5% CO2. 16,17 At 7 days after seeding and thereafter, primary rat hepatocyte cultures were maintained in Dulbecco’s modified Eagle’s medium/F12 supplemented as above (referred to as control medium) or control medium supplemented with 20 μmol/L of TMH-ferrocene. 31-33 For iron chelation experiments, primary rat hepatocytes that were maintained in either control medium or control medium supplemented with 20 μmol/L of TMH-ferrocene for 21 days were thereafter maintained in either control medium or control medium supplemented with 500 μmol/L of desferrioxamine (DFO). Huh7 human hepatoma cells were maintained in Dulbecco’s medium supplemented with 10% fetal calf serum. Huh7 cells were rinsed in phosphate-buffered saline (PBS) and fed serum-free medium before treatment with medium supplemented with 100 μmol/L of FeNTA.
Infection of Primary Rat Hepatocytes with Recombinant CMV-lacZ Baculovirus
The CMV-lacZ baculovirus (AcMNPV; Autographa californica multiple nucleopolyhedrovirus) was a kind gift of FM Boyce (Massachusetts General Hospital, Boston, MA) and baculovirus stocks and titers were prepared in Sf21 insect cells as described in Delaney and Isom. 34 Primary rat hepatocytes from male Fischer F344 rats (180 to 200 g; Charles River Breeding Laboratories, Wilmington, MA) were isolated and plated as described in Hu and colleagues. 21 At designated days of treatment with 20 μmol/L of TMH-ferrocene, duplicate plates of cells were trypsinized, and viable cell number was determined with a hemocytometer using Trypan blue exclusion. Average cell counts were calculated and used to determine the volume of high-titer CMV-lacZ baculovirus stock necessary to infect cells at the indicated multiplicity of infection. Baculovirus was diluted in Dulbecco’s modified Eagle’s medium/F12 (control medium) or control medium supplemented with 20 μmol/L of TMH-ferrocene to a final volume of 0.5 ml. Baculovirus was absorbed to primary rat hepatocytes for 1 hour at 37°C with gentle rocking every 15 minutes to ensure even distribution of the inoculum. After infection, cultures were washed twice in PBS, then refed with control medium or control medium supplemented with 20 μmol/L of TMH-ferrocene.
In Situ β-Galactosidase (β-gal) Staining with X-gal
At 24 hours after infection with CMV-lacZ baculovirus, primary rat hepatocyte cultures were washed once with PBS containing 2 mmol/L of MgCl2. Cultures were then fixed for 5 minutes in PBS containing 2% formaldehyde and 0.05% glutaraldehyde. Cultures were washed twice in PBS. Substrate/stain solution (PBS containing 5 mmol/L of potassium ferricyanide, 5 mmol/L of potassium ferrocyanide, 2 mmol/L of MgCl2, and 1 mg/ml of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal; Fisher Biotech, Fair Lawn, NJ) solubilized in dimethyl formamide was applied and cultures were incubated in a 37°C humidified incubator containing 5% CO2 for appropriate times to allow the appearance of β-gal activity. Cultures were then rinsed once in PBS and fixed at room temperature for 10 minutes in 10% phosphate-buffered formalin. After fixation, cultures were rinsed once in PBS and stored at 4°C in PBS containing 0.2% sodium azide. Cultures were observed for β-gal activity on an inverted microscope.
RNase Protection Assay
The RNase protection assay (RPA) was performed as described by Cable and colleagues. 27 RNA probes were synthesized from double-stranded polymerase chain reaction (PCR) products to ensure that each RNase probe was of a single and correct size before use in the RPA. The reverse PCR primer was designed to contain the bacterial T7 RNA polymerase promoter (5′-TAATACGACTCACTATAGGAGGP-3′). The underlined nucleotides correspond to the first five nucleotides of nascent RNA, whose presence has been shown to produce a highly efficient reaction. 35 The nonunderlined sequence is the T7 RNA polymerase promoter. The bold P represents the sequence of the reverse PCR primer. RNA was transcribed from the PCR template according to the manufacturer’s instructions (Life Technologies, Inc.). The primers used to produce the specific templates were: for rat E-cadherin, forward 5′-TTCAAAGTGTCTACAGATGGCGTC-3′ and reverse 5′-T7CCTCAAAGACCTCCTGGATAAACTC-3′; for rat protocadherin, forward 5′-AGTATCGCAAGAGTCAGTGCCAC-3′ and reverse 5′-T7CGGAGGAGTTCCTTTGTCTTTAGCC-3′; for rat junctional adhesion molecule (JAM), forward 5′-CCAGATCACAGTTCCCTATGCAGAC-3′ and reverse 5′-T7ATGCGTACAGCCTCTGACCTCATG-3′; for rat radixin, forward 5′-CACCTTGCCAAAGCACTCATACC-3′ and reverse 5′-T7TGATCCGTGTGACACCAGAATTAC-3′; for rat α-actinin, forward 5′-TTCATAGCCAGCAAAGGGGTC-3′ and reverse 5′-T7TCCGACGATGTCTTCTGCATCCAG-3′; for rat caveolin, forward 5′-CATGGCAGACGAGGTGAATGAG-3′ and reverse 5′-T7TGGACGTAGATGGAATAGACACGG-3′; for rat α-catenin, forward 5′-CCACGCAGGCAACATAAACTTC-3′ and reverse 5′-T7AGCATGGATCATCTGCAAACTCC-3′; for rat β-catenin, 5′-TGGACCACAAGCAGAGTGCTGAAG-3′; and reverse 5′-T7GGTTTCGGATCAATCCAACAGTTG-3′; for rat cyclophilin, forward 5′-ATGGTCAACCCCACCGTGTTCTTC-3′ and reverse 5′-T7ATGCCAGGACCTGTATGCTTCAGG-3′; and for rat glutaraldehyde phosphate dehydrogenase (GAPDH), forward 5′-TCTTCCACCTTTGATGCTGGG-3′ and reverse 5′-T7TTGTTATGGGGTCTGGGATGG-3′. The T7 sequence described above was added to each reverse primer to transcribe the anti-sense RNA. The probe templates were amplified from total rat liver RNA using the reverse transcriptase-PCR. The sizes of each of the PCR products were: rat E-cadherin, 583 nucleotides; rat protocadherin, 537 nucleotides; rat JAM, 459 nucleotides; rat radixin, 425 nucleotides; rat α-actinin, 393 nucleotides; rat caveolin, 366 nucleotides; rat α-catenin, 338 nucleotides; rat β-catenin, 538 nucleotides; rat cyclophilin, 290 nucleotides; and rat GAPDH, 269 nucleotides.
A total of 100 μCi (33.3 pmol) of [32P]CTP (NEN Life Science Products, Inc., Boston, MA) was used to label the E-cadherin, protocadherin, JAM, radixin, α-actinin, caveolin, α-catenin, β-catenin, and cyclophilin RNA probes, whereas 50 μCi was used to label the GAPDH RNA probe. Cold nucleotides were added to each reaction to a final concentration of 500 μmol/L for each probe, except for CTP, which was added to a concentration of 175 μmol/L. After incubation at 37°C for 1 hour, the DNA template was removed from the reaction mixture by treatment with RNase-free DNase (Life Technologies, Inc.) and the nascent RNA was precipitated by the addition of 3 vol of ethanol/acetate and the addition of 20 μg of tRNA as a carrier. The precipitate was resuspended in formamide gel buffer and the probes purified using a 4% preparative urea-polyacrylamide gel electrophoresis gel. The portion of the gel containing the major product was excised from the gel and the RNA was extracted in 500 μl of dH2O by incubating at room temperature overnight. Contaminating urea and proteins were removed from the aqueous phase using standard phenol/chloroform extractions. The final product was again ethanol/acetate precipitated and redissolved in 100 μl of dH2O. A 1-μl aliquot was counted in a scintillation counter. Typically, a total of 1 × 107 to 5 × 107 cpm were obtained for each reaction product. The probes were stored at −80°C until used.
RNA was harvested from a single 60-mm dish of primary hepatocytes using the method of Chomczynski and Sacchi 36 Ten μg of total RNA was used for each reaction mixture. The RNA was hybridized to 16,000 cpm of each probe in a final volume of 12 μl of 5 mol/L of guanidine and 100 mmol/L of ethylenediaminetetraacetic acid (pH 7.4). The mixture was heated to 65°C for 5 minutes and incubated overnight (>16 hours) at room temperature. Unhybridized probe was removed by treating the hybridization mixture with RNase A (0.125 U) and RNase T1 (5 U; Ambion, Austin, TX) at 37°C for 1 hour in 350 μl of RNase buffer containing 10 mmol/L of HEPES (pH 7.3), 5 mmol/L of ethylenediaminetetraacetic acid, and 300 mmol/L of NaCl. The RNases were inactivated by treatment of the mixture with 10 μl of 20% sodium dodecyl sulfate (Sigma) and 0.06 U of proteinase K (Roche Molecular Biochemicals, Indianapolis, IN) at 37°C for 15 minutes. Protein was removed from the reaction mixture using phenol/chloroform and chloroform washes. The remaining protected RNA probes were precipitated using ethanol/acetate with Glycoblue (Ambion) as a co-precipitate. Standard positive and negative controls were included in each reaction set. The difference between the protected and unprotected probes was five nucleotides for all probes because of the inclusion of the GGAGG sequence. The protected probes were separated using a 4% urea-polyacrylamide gel electrophoresis sequencing gel and the fragments visualized and quantitated using a Phosphorimager and ImageQuant software (Molecular Dynamics, Sunnyvale, CA). The amount of protected probe in relation to the GAPDH internal control was calculated using the volume measurements from the Phosphorimager. Amounts of protected probe are expressed as fold difference compared to control medium.
Gene Array Analysis
Gene array analysis was performed by Incyte Genomics (Palo Alto, CA). The array used was the Rat Toxicology Chip (RatGem3). The array contains 8951 elements with 8456 unique genes.
Immunolocalization
After 21 days of treatment with TMH-ferrocene, cultures were fixed in 1% phosphate-buffered paraformaldehyde (Electron Microscopy Sciences, Fort Washington, PA) for 10 minutes at room temperature. Cultures were rinsed twice in PBS and permeabilized with either 0.2% (ZO-1, occludin, and connexin32) or 0.5% (E-cadherin) Triton X-100 (Sigma) for 10 minutes at room temperature. Cultures were then incubated for 1 hour in a block solution containing 10% bovine serum albumin and 0.1% Triton X-100 in PBS. Primary anti-ZO-1 antibody (a kind gift of Dr. T. W. Gardner, Penn State University College of Medicine, Hershey, PA) specific for rat, anti-E-cadherin antibody (Transduction Labs, Lexington, KY), anti-connexin32 (Chemicon International, Temecula, CA), or anti-occludin (Zymed Laboratories Inc., San Francisco, CA) was applied and incubated at room temperature for 2 hours. After five rinses in PBS and 0.1% Triton X-100, cultures were incubated for 1 hour in a Cy-2-conjugated secondary antibody specific for either rat (ZO-1) or mouse (E-cadherin, connexin32, and occludin) IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) diluted in PBS and 0.1% Triton X-100. Cultures were rinsed three times in PBS and 0.1% Triton X-100 and overlaid with coverslips. Immunolocalization for all proteins was observed by fluorescence microscopy.
Immunoblot Analysis
Protein was isolated from FeNTA-treated Huh7 cells or primary rat hepatocyte cultures maintained in control medium or medium supplemented with 20 μmol/L of TMH-ferrocene for 21 days (day 28 after seeding). Cultures were rinsed in triplicate with PBS and lysed in either membranous extraction buffer [150 mmol/L NaCl, 25 mmol/L Tris-Cl (pH 7.4), 2% Triton X-100 (Sigma), 2 μg/ml bovine serum albumin, and 50 μl/ml Protease Inhibitor Cocktail for Mammalian Cell Extracts (Sigma)] (E-cadherin, ferritin, and caveolin) or RIPA buffer [50 mmol/L Tris-Cl (pH 7.4), 150 mmol/L NaCl, 1% Nonidet P-40, 12 mmol/L deoxycholic acid, and 50 μl/ml Protease Inhibitor Cocktail for Mammalian Cell Extracts (Sigma)] (β-catenin, connexin32, and occludin). Lysates were centrifuged at 6400 × g for 10 minutes at 4°C. Protein concentrations were determined by the bicinchoninic acid assay 37 using bovine serum albumin protein standards (Sigma). Ten μg of protein were diluted in Laemmli buffer 38 and analyzed by sodium dodecyl sulfate-polyacrylamide (8 or 12%) gel electrophoresis under reducing conditions (3% β-mercaptoethanol). Proteins were transferred by electroblotting onto a nitrocellulose membrane (Osmonics, Minnetonka, MN) and stained with Ponceau S (Sigma) to verify equal loading of the gels. Blots were blocked in 5% milk in Tris-buffered saline-Tween for 1 hour at room temperature. Blots were incubated in primary anti-E-cadherin (Transduction Labs), anti-β-catenin (Cell Signaling Technology, Inc., Beverly, MA), anti-occludin (Zymed Laboratories, Inc.), anti-connexin32 (Chemicon International), anti-caveolin (Transduction Labs), or anti-ferritin (DAKO Corporation, Carpinteria, CA) antibody for 1 hour at room temperature. After three washings with Tris-buffered saline-Tween, secondary antibody conjugated to horseradish peroxidase was applied for 1 hour at room temperature, followed by an additional three washes in Tris-buffered saline-Tween. Bound antibodies were visualized by chemiluminescence.
Measurement of Gap Junction Intercellular Communication (GJIC)
GJIC was measured by the scrape-loading and dye-transfer technique as described previously. 39,40 Briefly, cultures maintained in control medium or medium supplemented with 20 μmol/L of TMH-ferrocene for 21 days (day 28 after seeding) were rinsed in triplicate. Two μl of 0.05% Lucifer yellow and 0.1% rhodamine dextran (Molecular Probes, Inc., Eugene, OR) dissolved in PBS were added to the cells and scrape-loaded using a rubber policeman. After a 2-minute incubation, the dye solution was removed and the cultures were rinsed four times with PBS to remove detached cells and excess dye. Subsequently, PBS was added to the cultures and to prevent drying. Because of its large size (molecular weight = 10,000), rhodamine dextran will only enter cells damaged by scrape-loading; however, the small size of Lucifer yellow (molecular weight = ∼450) allows it to freely diffuse through intact gap junctions of adjoining cells. The diffusion of the Lucifer yellow dye as compared to the nondiffusable rhodamine dextran was examined as a measure of GJIC by fluorescence microscopy.
Paracellular Junction Integrity/Membrane Permeability Assay
Loss of paracellular junction integrity and membrane permeability were measured by the inability of the primary rat hepatocytes in culture to retain the fluorescein metabolic product of fluorescein diacetate (FDA). Calcium-depleted or control cultures were washed four times in control medium. Cultures were then incubated for 45 minutes in Dulbecco’s modified Eagle’s medium/F12 containing 2 ng/ml of FDA (Molecular Probes) at 37°C. Cultures were then washed five times with PBS and overlayed with coverslips using Prolong Antifade mounting medium (Molecular Probes). Cultures were observed for fluorescein retention by fluorescence microscopy.
Reporter Assays
Primary rat hepatocytes were maintained for 7 days in control medium, after which, cultures were maintained in either control medium or medium supplemented with 20 μmol/L of TMH-ferrocene. Control and TMH-ferrocene-treated primary rat hepatocyte cultures or control and FeNTA-treated Huh7 cultures were transfected using the Effectene transfection reagent (Qiagen, Inc., Valencia, CA) with either: 1) CMV-renilla plasmid alone (a kind gift of Dr. B. Wigdahl, Penn State University College of Medicine, Hershey, PA); 2) CMV-renilla and pGL3 (Promega Corp., Madison, WI) plasmids; or 3) CMV-renilla and pGL3/PECAD (containing the firefly luciferase gene under control of the human E-cadherin promoter; a kind gift of Dr. Antonio Garcia de Herreros; Universitat Pompeu Fabra, Barcelona, Spain) plasmids. At 24 hours after transfection, cultures were harvested and analyzed for relative (firefly/renilla) luciferase activity using the Dual Luciferase Reporter Assay System (Promega Corp.).
Statistical Analyses
All RNase protection and luciferase reporter experiments used at least triplicate samples for each treatment condition studied and statistical analyses were performed with JMP 3.0.2 (SAS Institute, Cary, NC) using analysis of variance, then Dunnett’s test versus control, or Tukey-Kramer test for multiple comparisons as appropriate. The null hypothesis was rejected for P values less than or equal to 0.05.
Results
Baculovirus-Mediated Gene Transfer Efficiency to Iron-Loaded Hepatocytes
We recently reported that baculovirus-mediated gene delivery to long-term (>20 days) primary rat hepatocytes was markedly increased when cultures were treated with calcium-free medium supplemented with 100 mmol/L of EGTA before and during infection with baculovirus. 41 Under control medium conditions, baculovirus-mediated transfer of the lacZ reporter gene was limited to peripheral cells of multicellular hepatocyte islands and hence, baculovirus-mediated gene transfer efficiency was extremely low. However, examination of β-gal staining in cultures after calcium-depletion by treatment in calcium-free medium or calcium-free medium supplemented with EGTA revealed that gene transfer was no longer limited to peripheral cells and was homogeneous throughout the hepatocyte islands. 41 For example, at a multiplicity of 1600 pfu/cell, the percent β-gal-positive internal cells increased from 1.2% in medium containing calcium to 75.3% in calcium-free medium supplemented with 100 μmol/L of EGTA. 41 Detailed analyses demonstrated that depletion of extracellular calcium enhanced baculovirus entry into internal hepatocytes and subsequent gene transfer by disrupting paracellular junction integrity. Paracellular junction integrity was restored at 12 hours after the readdition of calcium to the cultures, therefore conferring no permanent effects on the cultures. 42
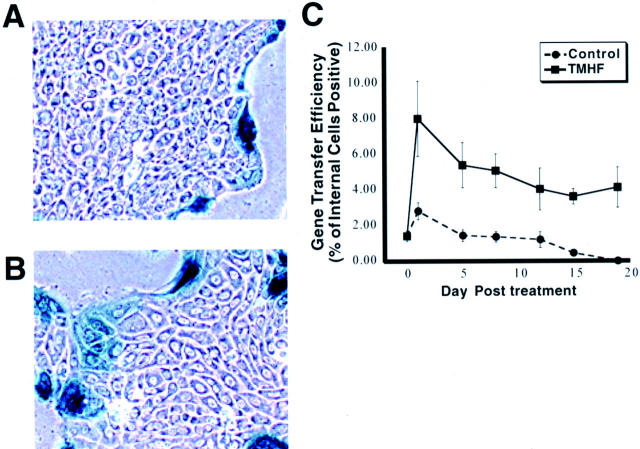
As discussed above in the introduction, hepatocytes in long-term DMSO culture can be chronically overloaded with iron. Because it is important to be able to introduce exogenous genetic information into a high percentage of iron-loaded hepatocytes, we designed experiments to determine whether transient depletion of calcium had the same effects on baculovirus-mediated gene delivery to iron-loaded primary rat hepatocytes as we had observed for control hepatocyte cultures. The concentration of TMH-ferrocene and time point selected were based on data obtained from previous studies in our laboratory. 19,20,27 Specifically, when hepatocytes were treated with 20 μmol/L of TMH-ferrocene, a linear increase in total iron content was observed for 21 days (E. E. Cable and H. C. Isom, unpublished data). Compared to untreated control cultures, total iron content in cultures treated with 20 μmol/L of TMH-ferrocene for 21 days was increased by >20-fold. Cultures treated for 21 days with 20 μmol/L of TMH-ferrocene were morphologically indistinguishable from control cultures (Figure 1 ▶ , compare A and B). No rounding of cells or cytopathology was observed as a result of treatment with 20 μmol/L of TMH-ferrocene. In addition, total intracellular protein levels were not significantly different in iron-loaded compared to control cultures (data not shown).
Figure 1.
Internal hepatocytes stained positive for baculovirus-mediated gene transfer in iron-loaded cultures. Primary rat hepatocytes were maintained for 7 days in control medium, after which, cultures were maintained in either control medium or medium supplemented with 20 μmol/L of TMH-ferrocene. Primary rat hepatocytes maintained in control medium or control medium supplemented with 20 μmol/L of TMH-ferrocene were infected for 1 hour with 400 pfu of CMV-lacZ baculovirus/cells at the indicated day after treatment. At 24 hours after infection, cultures were fixed and stained for β-gal activity. Control cultures show no β-gal activity in internal hepatocytes (A), whereas cultures treated with 20 μmol/L of TMH-ferrocene for 21 days display an increased percentage of both internal and peripheral cells positive for β-gal activity (B). C: Time-course analysis indicating the percentage of internal cells positive for β-gal activity at 24 hours after infection with 400 pfu of CMV-lacZ baculovirus/cells plotted against time of treatment with 20 μmol/L of TMH-ferrocene. Each marker represents the mean percentage with SD of β-gal-positive internal primary rat hepatocytes at the indicated time after treatment. Original magnifications, ×100.
Control cultures and cultures treated with 20 μmol/L of TMH-ferrocene for 20 days were infected with 400 pfu CMV-lacZ baculovirus/cell. At 24 hours after infection, cultures were fixed and stained for β-gal activity (Figure 1, A and B) ▶ , demonstrating that baculovirus-mediated gene delivery could be achieved in iron-loaded primary rat hepatocytes. However, we made the unexpected observation that although the majority of gene transfer was restricted to the peripheral cells, β-gal activity was also observed in internal iron-loaded hepatocytes. Indeed, the pattern of gene transfer in iron-loaded cultures was reminiscent of what was observed for control cultures pretreated and infected in calcium-free medium. 41 A detailed time-course analysis showed that by day 2 after treatment with TMH-ferrocene, the percentage of internal hepatocytes expressing the transgene was higher in iron-loaded cultures compared to control cultures (Figure 1C) ▶ . At day 20 after treatment with TMH-ferrocene, the percentage of β-gal-positive internal hepatocytes was ∼5%, whereas in control cultures, no internal hepatocytes expressed the β-gal transgene. Indeed, the pattern of increased baculovirus-mediated gene transfer after iron treatment mimicked the effects of calcium depletion. Having recently demonstrated that calcium depletion appears to enhance baculovirus entry into internal hepatocytes by disrupting paracellular junction complexes, 41,42 we wanted to determine whether iron loading of primary rat hepatocytes alters paracellular junction integrity.
Decreased Expression of Paracellular Junction Complex Gene mRNA Levels in Iron-Loaded Hepatocytes
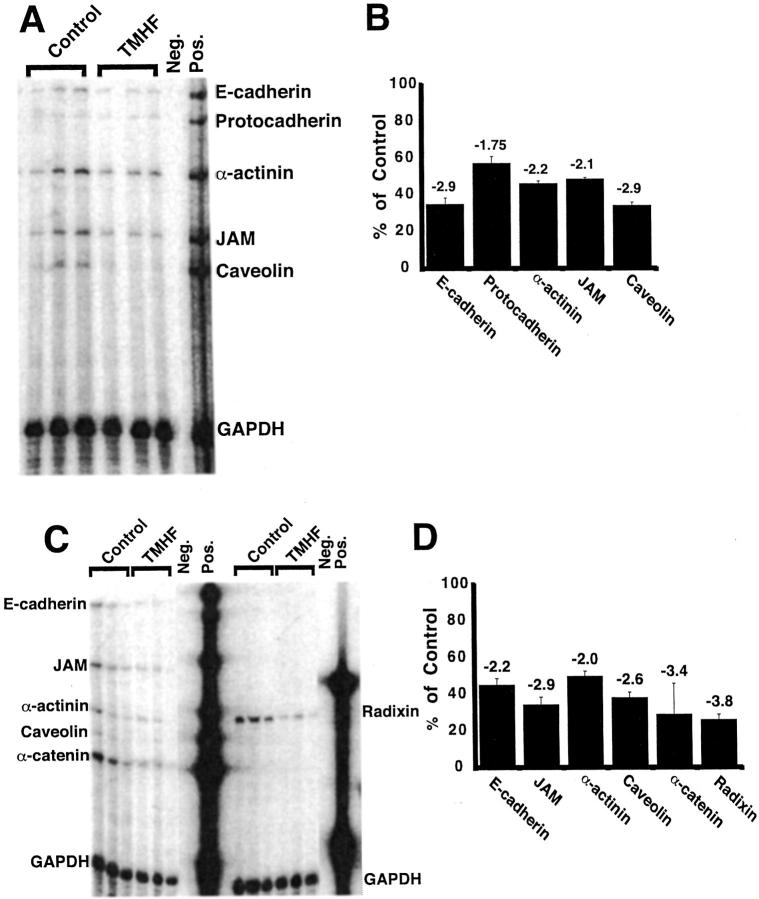
Gene array analysis was performed on RNA isolated from control cultures and cultures treated with 20 μmol/L of TMH-ferrocene for 21 days. The expression of numerous genes, including several paracellular junction genes, was altered in iron-loaded primary rat hepatocyte cultures compared to control cultures. The data revealed that the number of transcripts whose levels were increased was 258, whereas the number of transcripts whose levels were decreased was 627. A change of 1.7 or greater was considered significantly different in this analysis. The levels of E-cadherin, caveolin, JAM, protocadherin, and α-catenin mRNA expression were reduced by 2.1- to 3.6-fold in iron-loaded compared to control cultures of primary rat hepatocytes (Table 1) ▶ . RPAs were also performed on RNA from control and TMH-ferrocene-treated hepatocyte cultures (Figure 2) ▶ . In these studies, the expression of E-cadherin, protocadherin, α-actinin, JAM, and caveolin mRNAs was reduced by 2.9-, 1.75-, 2.2-, 2.1-, and 2.9-fold in iron-loaded compared to control cultures of primary rat hepatocytes, respectively (Figure 2, A and B) ▶ . The results closely matched the gene array analysis. We next extended the RPA analysis to two additional paracellular junction genes, α-catenin and radixin. Decreased expression of α-catenin was detected in the gene array analysis and radixin gene expression was not measured in the gene array analysis (Table 1) ▶ . The same concentration of TMH-ferrocene (20 μmol/L) and time point of treatment (21 days) were selected for these analyses. In addition, the effects on expression of E-cadherin, JAM, caveolin, and α-actinin were re-evaluated. We conclude from these studies, that α-catenin and radixin mRNA levels were decreased by 3.4- and 3.8-fold, respectively, in iron-loaded compared to control hepatocyte cultures (Figure 2, C and D) ▶ . In addition, the previously observed decreases in E-cadherin, JAM, caveolin, and α-actinin mRNA expression were reproduced.
Table 1.
Gene Array Analysis of Iron-Related Gene Expression in Long-Term Iron-Loaded Primary Rat Hepatocytes
| Gene | Fold expression |
|---|---|
| E-cadherin | −3.6 |
| Caveolin | −3.2 |
| JAM | −2.9 |
| Protocadherin | −2.1 |
| α-catenin | −2.1 |
Primary rat hepatocytes were either untreated or treated with 20 μmol/L TMHF for 21 days. Total RNA was harvested and gene expression was analyzed using gene array analysis. Decreases in transcript levels are expressed as the negative inverse of the ratio of treated normalized to control.
Figure 2.
RPA analysis demonstrated decreased expression of paracellular junction genes in primary rat hepatocytes after treatment with TMH-ferrocene. Primary rat hepatocytes were maintained for 7 days in control medium, after which, cultures were maintained in either control medium or medium supplemented with 20 μmol/L of TMH-ferrocene. After 21 days of treatment, control and iron-loaded cultures were harvested for total RNA. RPA analyses for the selected paracellular junction genes indicated in each figure (A and C) were performed on RNA from control (control) and iron-loaded (TMHF) cultures. Quantitative analyses of expression of selected paracellular junction genes indicated in each figure (B and D) were performed for RPA analyses for RNA from control (control) and iron-loaded (TMHF) cultures. As a control for sample processing and gel loading, the data were normalized to GAPDH expression. Each bar represents the mean percentage with SD of mRNA expression in iron-loaded cultures compared to control cultures. The fold-decrease in mRNA expression in iron-loaded cultures relative to control cultures is displayed above each bar.
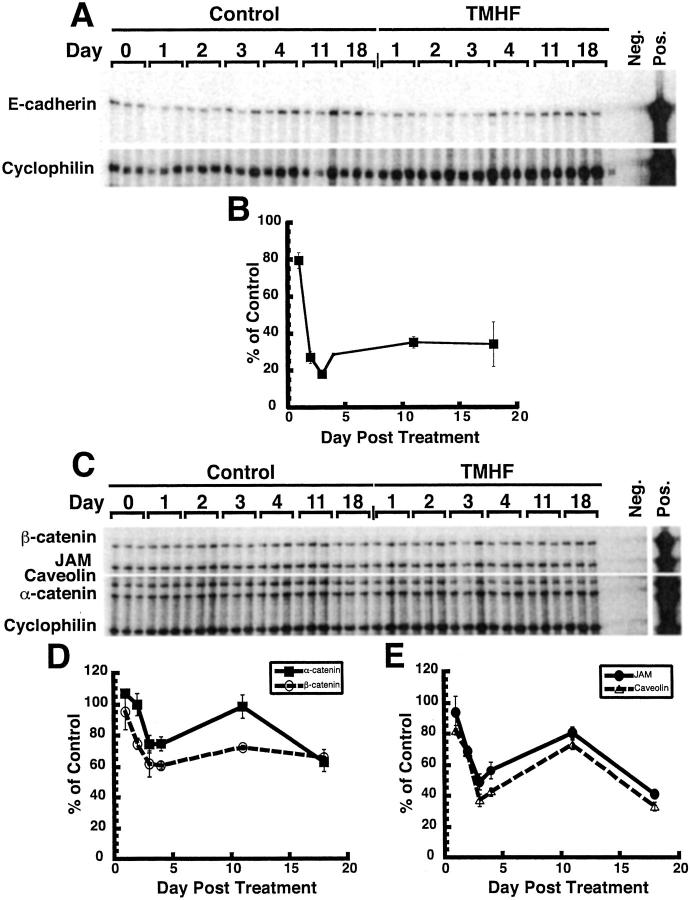
The effect of iron loading throughout time on the expression of numerous paracellular junction genes was also analyzed. At 1, 2, 3, 4, 12, and 18 days of treatment with TMH-ferrocene, cultures were harvested for total RNA. Time-course analyses demonstrated that by day 3 after treatment with TMH-ferrocene, the expression of E-cadherin mRNA levels decreased to ∼20% of the control level and remained between 20% and 40% of the control level through 18 days of treatment (Figure 3, A and B) ▶ . Additionally, time-course analysis demonstrated that by day 3 after treatment with TMH-ferrocene, the mRNA expression of α-catenin, β-catenin, JAM, and caveolin was ∼75%, 60%, 50%, and 40% of control, respectively (Figure 3 ▶ ; C, D, and E). At day 12 after treatment with 20 μmol/L of TMH-ferrocene, mRNA expression increased slightly over day 3 levels; however, by day 18 after treatment, the expression of α-catenin, β-catenin, JAM, and caveolin was ∼60%, 60%, 35%, and 40% of control cultures, respectively (Figure 3 ▶ ; C, D, and E).
Figure 3.
Time-course analysis of paracellular junction gene mRNA expression in primary rat hepatocyte cultures during iron loading by TMH-ferrocene. Primary rat hepatocytes were maintained for 7 days in control medium, after which, cultures were maintained in either control medium or medium supplemented with 20 μmol/L of TMH-ferrocene. At 1, 2, 3, 4, 12, and 18 days of treatment with TMH-ferrocene, cultures were harvested for total RNA. RPA analysis probing for E-cadherin (A) and α-catenin, β-catenin, JAM, and caveolin (C) mRNA expression throughout time after TMH-ferrocene treatment was performed on RNA from control (control) and iron-loaded (TMHF) cultures. Quantitative analyses of the expression of E-cadherin (B), α- and β-catenin (D), and JAM and caveolin (E) mRNA in iron-loaded primary rat hepatocyte cultures compared to control cultures. As a control for sample processing and gel loading, the data were normalized to cyclophilin expression. Each marker represents the mean percentage with SD of mRNA expression in iron-loaded cultures compared to the control cultures. Where no error bar is observed, the error falls within the size of the marker.
Decreased Expression of Paracellular Junction Complex Proteins in Iron-Loaded Hepatocytes
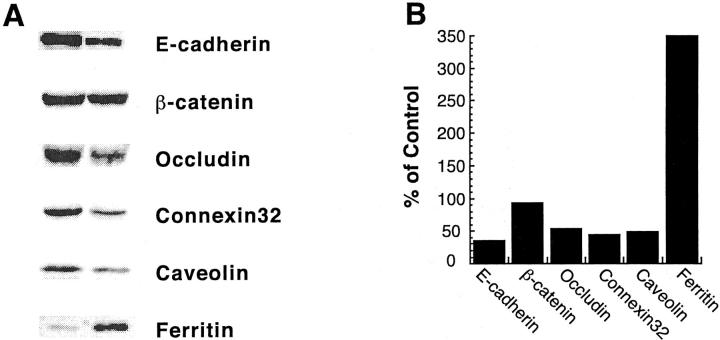
To determine whether decreased mRNA expression of paracellular junction genes translated into a decrease in expression of paracellular junction proteins, immunoblot analyses were performed on cellular lysate from primary rat hepatocyte cultures treated with 20 μmol/L of TMH-ferrocene for 21 days. E-cadherin protein levels in primary rat hepatocyte cultures treated with 20 μmol/L of TMH-ferrocene for 20 days were ∼35% of control cultures (Figure 4, A and B) ▶ , which corresponded to the decrease observed in the mRNA levels. Decreases in protein expression were also observed for the tight junction protein, occludin (54%); the gap junction protein, connexin32 (45%); and caveolin (50%), which was just recently demonstrated to associate at paracellular junctions (Figure 4, A and B) ▶ . 43 Conversely, no significant change in β-catenin protein expression was observed in iron-loaded cultures compared to control cultures. The protein expression of ferritin increased to ∼350% of the level in control cultures, indicating that iron-loading by TMH-ferrocene induced a known iron-responsive gene (Figure 4, A and B) ▶ .
Figure 4.
Protein expression of numerous paracellular junction genes was decreased in iron-loaded primary rat hepatocytes. Primary rat hepatocytes were maintained for 7 days in control medium, after which, cultures were maintained in either control medium or medium supplemented with 20 μmol/L of TMH-ferrocene. After 21 days of treatment, control and TMH-ferrocene-treated cultures were harvested for protein and subjected to immunoblot analysis. A: Blots were analyzed for E-cadherin, β-catenin, occludin, connexin32, caveolin, and ferritin expression. B: The percent expression of each paracellular junction protein analyzed in TMH-ferrocene-treated (TMHF) cultures compared to control cultures.
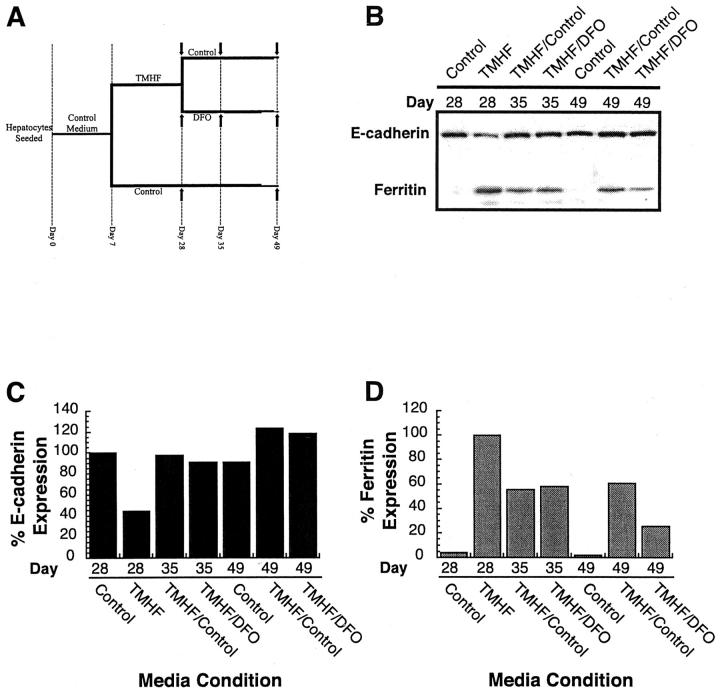
To determine whether removal of iron from cultures maintained in medium supplemented with 20 μmol/L of TMH-ferrocene restored E-cadherin protein levels to that of control cultures, intracellular iron was chelated by treatment with 500 μmol/L of DFO. As displayed in Figure 5A ▶ , primary rat hepatocytes were maintained in control medium for 7 days after seeding (day 7), after which, cultures were either maintained in control medium or in control medium supplemented with 20 μmol/L of TMH-ferrocene for 21 days (day 28). At day 28, cultures on control medium were maintained in control medium; however, iron-loaded cultures were now either further maintained in control medium or control medium supplemented with 500 μmol/L of DFO for an additional 21 days (day 49). At days 28, 35, and 49 of the experiment, protein was isolated and immunoblot analyses were performed for E-cadherin and ferritin. At day 28 (corresponding to 21 days of treatment with 20 μmol/L of TMH-ferrocene), E-cadherin protein levels were ∼40% of control cultures (Figure 5, B and C) ▶ and ferritin protein expression was ∼20-fold higher than in control cultures (Figure 5, B and D) ▶ . The effects of placing cells in medium free of TMH-ferrocene or supplemented with the chelator DFO were as expected. Specifically, placing cells in control media reduced the level of ferritin expression. When cells were treated with DFO, ferritin levels were reduced and with time (day 49) to lower levels than when cells were simply cultured in iron-free medium. After removal of iron from the medium for 7 days (day 35) by either feeding with control or DFO-supplemented medium, E-cadherin protein levels returned to that of control cultures consistently maintained in control medium throughout the experiment. Twenty-one days after the removal of iron from the cultures (day 49), E-cadherin levels exceeded that of the control cultures. The results of this experiment demonstrate that the effects of iron on E-cadherin are reversible, further indicating a direct regulatory role of iron on E-cadherin expression.
Figure 5.
E-cadherin protein expression after release from iron-loading and addition the iron chelator DFO. A: Experimental schematic for iron loading and removal of iron from primary rat hepatocyte cultures. Isolated primary rat hepatocytes were plated onto collagen-coated tissue culture dishes and fed control medium for a period of 7 days (days 0 through 7). Seven days after plating, cultures were either refed in control medium (control) or medium supplemented with 20 μmol/L of TMH-ferrocene (TMHF). Cultures were refed three times per week for 21 days (days 7 through 28), after which, cultures were either refed three times per week with either control medium (control or TMHF/control) or medium supplemented with 500 μmol/L of DFO (TMHF/DFO). Protein samples were harvested at days 28, 35, and 49 after removal of TMH-ferrocene from the media or chelation using medium supplemented with DFO (arrows). B: Immunoblot analysis for E-cadherin and ferritin protein levels in primary rat hepatocyte cultures after removal of iron and addition of DFO. Quantitative analyses of immunoblots for E-cadherin (C) and ferritin (D) were performed by densitometry.
E-Cadherin Promoter Activity Was Decreased in Iron-Loaded Primary Rat Hepatocytes
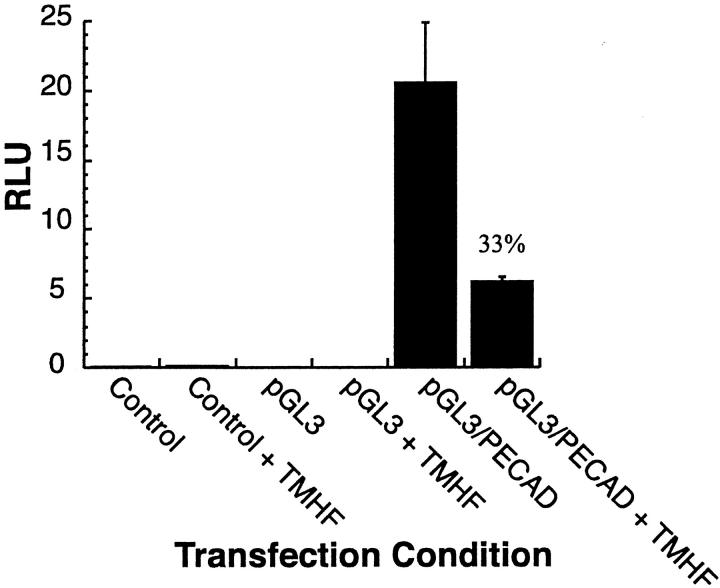
Primary rat hepatocytes were maintained for 7 days in control medium, after which, cultures were maintained in either control medium or medium supplemented with 20 μmol/L of TMH-ferrocene. After 12 days of treatment, control and TMH-ferrocene-treated cultures were transfected with either: 1) CMV-renilla plasmid alone (control); 2) CMV-renilla and pGL3 plasmids (pGL3); or 3) CMV-renilla and pGL3/PECAD (containing the firefly luciferase gene under control of the human E-cadherin promoter) plasmids. At 24 hours after transfection, cultures were harvested and analyzed for relative (firefly/renilla) luciferase activity (Figure 6) ▶ . E-cadherin promoter activity in iron-loaded primary rat hepatocyte cultures (+TMHF) was ∼33% of that observed in control cultures. No luciferase activity was observed in control or pGL3 transfections. Therefore, iron regulation of the E-cadherin promoter may be wholly or partially responsible for the decreased E-cadherin mRNA and protein levels observed in iron-loaded primary rat hepatocytes.
Figure 6.
E-cadherin promoter activity was decreased in iron-loaded primary rat hepatocyte cultures. Primary rat hepatocytes were maintained for 7 days in control medium, after which, cultures were maintained in either control medium or medium supplemented with 20 μmol/L of TMH-ferrocene. After 12 days of treatment, control and TMH-ferrocene-treated (+TMHF) cultures were transfected with either: CMV-renilla plasmid alone (control); CMV-renilla and pGL3 plasmids (pGL3); or CMV-renilla and pGL3/PECAD plasmids. At 24 hours after transfection, cultures were harvested and analyzed for relative (firefly/renilla) luciferase activity (RLU).
Immunolocalization of Paracellular Junction Proteins Was Altered in Iron-Loaded Cultures of Primary Rat Hepatocytes
As previously described, E-cadherin mRNA and protein expression were reduced after iron loading of primary rat hepatocytes. To determine whether the localization of E-cadherin was affected by iron loading of primary rat hepatocytes, cultures treated with 20 μmol/L of TMH-ferrocene for 21 days were examined for E-cadherin immunolocalization. In control cultures, E-cadherin localized to the membranes of adjoining cells (Figure 7A) ▶ , whereas, in agreement with the immunoblot analysis, immunolocalization of E-cadherin to the membranes of adjoining cells was reduced in iron-loaded cultures (Figure 7B) ▶ . Interestingly, iron-loaded cultures displayed a localization of E-cadherin into distinct intracellular punctate regions.
Figure 7.
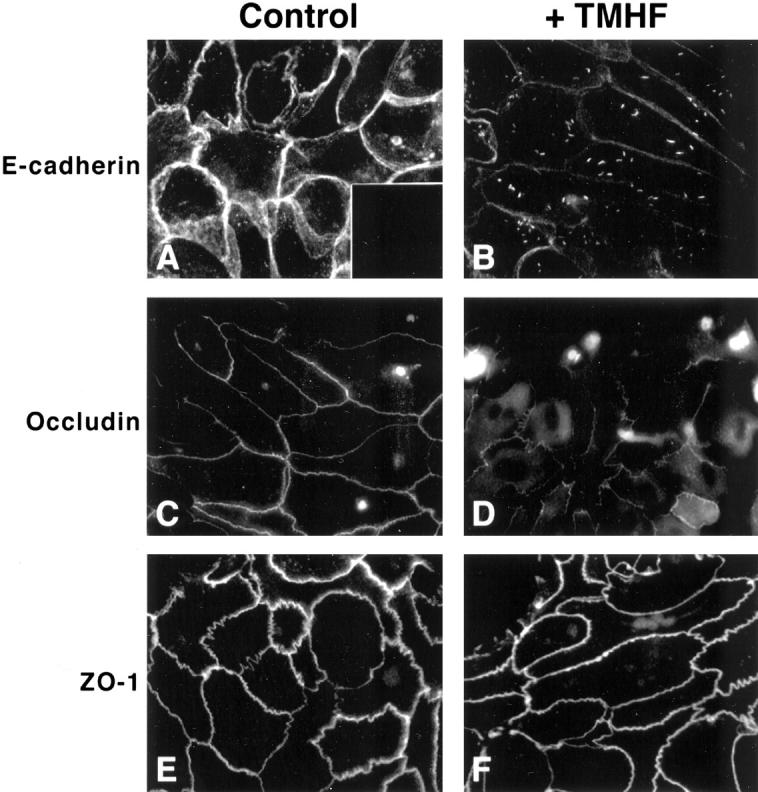
Immunolocalization of paracellular junction proteins after iron loading of primary rat hepatocytes. Primary rat hepatocytes were maintained for 7 days in control medium, after which, cultures were maintained in either control medium or medium supplemented with 20 μmol/L of TMH-ferrocene for 21 days. After 21 days of iron loading, cultures were fixed and immunofluorescence was performed for E-cadherin (A and B), occludin (C and D), and ZO-1 (E and F) on control (control) and iron-loaded (+TMHF) cultures. (A, inset) No immunofluorescence was observed when cultures were incubated in control mouse IgG antibody rather than primary antibody. Original magnifications, ×600.
To determine whether treatment of primary rat hepatocyte cultures with 20 μmol/L of TMH-ferrocene for 21 days affected paracellular tight junctions, control and iron-loaded primary rat hepatocyte cultures were examined for immunolocalization of occludin and the tight junction protein, ZO-1. In control cultures, occludin localized to the membranes of adjoining cells (Figure 7C) ▶ , whereas, in agreement with the immunoblot analysis, immunolocalization of occludin to the membranes of adjoining cells was reduced in iron-loaded cultures (Figure 7D) ▶ . Occludin also localized intracellularly after iron loading, which was not observed in control cultures. In contrast, ZO-1 immunolocalization was not altered by iron loading (Figure 7, E and F) ▶ , indicating that the effects of TMH-ferrocene treatment on paracellular protein localization were limited to certain paracellular junction proteins. It should be noted that this finding was in contrast to what we observed for transient calcium depletion, in which EGTA-treatment caused ZO-1 internalization. 42
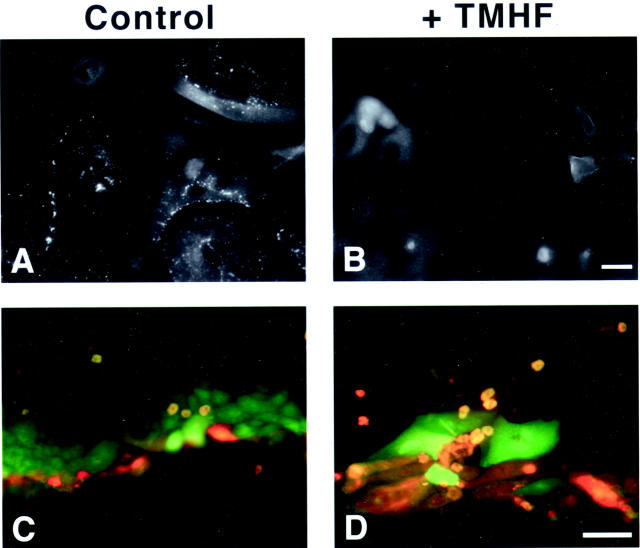
Iron loading of primary rat hepatocytes with 20 μmol/L of TMH-ferrocene for 21 days also altered the localization of the gap junction protein, connexin32 (Figure 8, A and B) ▶ . Whereas in both control and iron-loaded cultures, connexin32 was observed diffusely throughout the cytoplasm, punctate staining was observed in control cultures, indicative of connexin32 oligomerization into hemichannels, and subsequently gap junctions, at the membranes of adjoining cells. This decrease in gap junction formation translated into a decrease in GJIC as measured by the scrape-loading and dye-transfer technique. Diffusion of Lucifer yellow (green) dye was decreased in iron-loaded cultures, diffusing into only one cell layer (Figure 8D) ▶ beyond the scratch line indicated by rhodamine dextran (red). Conversely, Lucifer yellow diffused through several cell layers beyond the rhodamine dextran scratch line in control cultures (Figure 8C) ▶ .
Figure 8.
Immunolocalization of gap junction proteins and decreased GJIC in iron-loaded primary rat hepatocytes. Primary rat hepatocytes were maintained for 7 days in control medium, after which, cultures were maintained in either control medium or medium supplemented with 20 μmol/L of TMH-ferrocene for 21 days. After 21 days of iron loading, cultures were fixed and immunofluorescence was performed for connexin32 (A and B) on control (control) and iron-loaded (+TMHF) cultures. GJIC was analyzed by the scrape-loading and dye-transfer technique on control (control) and iron-loaded (+TMHF) cultures after 21 days of iron loading (C and D). Note that in control cultures (C), Lucifer yellow (green) diffused into multiple layers of cells, demonstrating functional GJIC, whereas in iron-loaded cultures (D), Lucifer yellow only diffused into a single layer of cells, demonstrating inhibition of GJIC. Original magnifications: ×400 (A, B); ×200 (C, D). Scale bars: 20 μm (A, B); 100 μm (C, D).
Paracellular Junction Integrity Was Compromised and Membrane Permeability Was Increased in Iron-Loaded Primary Rat Hepatocytes
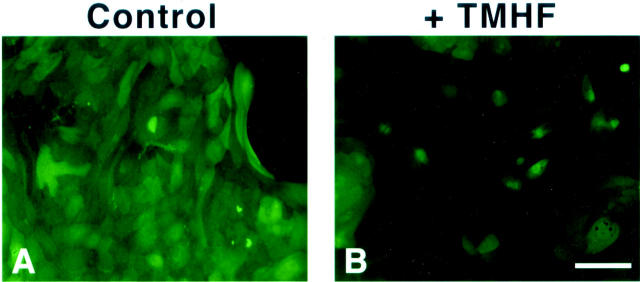
To examine if decreases in paracellular junction gene expression after iron loading of primary rat hepatocytes altered paracellular junction integrity and membrane permeability, FDA staining was performed on control cultures and cultures treated with 20 μmol/L of TMH-ferrocene for 21 days. FDA, a nonpolar nonfluorescent fatty acid ester, freely enters living cells and is hydrolyzed by cellular esterases to produce fluorescein. In cells with intact cell membranes, the polar fluorescein accumulates intracellularly, a phenomenon known as fluorochromasia. 44,45 The accumulation or release of fluorescein after hydrolysis of FDA is dependent on the cell membrane permeability and integrity. 45-48 When membrane permeability is increased, fluorescein is able to passively diffuse out of the cells into intercellular clefts. The fluorescein is not retained intercellularly when paracellular junctions are compromised and consequently, the fluorescein diffuses into the medium. The level of fluorescein retention within the hepatocytes decreased after iron loading (Figure 9, A and B) ▶ , suggesting that iron loading increases membrane permeability and intercellular leakage of fluorescein resulting from loss of paracellular junction integrity.
Figure 9.
Paracellular junction integrity and membrane permeability in iron-loaded primary rat hepatocyte cultures. Primary rat hepatocytes were maintained for 7 days in control medium, after which, cultures were maintained in either control medium (control, A) or medium supplemented with 20 μmol/L of TMH-ferrocene (+TMHF, B). After 21 days of treatment, control and TMH-ferrocene-treated cultures were stained with 2 ng/ml of FDA for 45 minutes at 37°C. Cultures were then rinsed five times with PBS and observed by fluorescence microscopy. Original magnifications, ×200. Scale bar, 100 μm.
Iron Treatment of Human Hepatic Cells with the FeNTA Iron Donor Decreased E-Cadherin Protein Expression
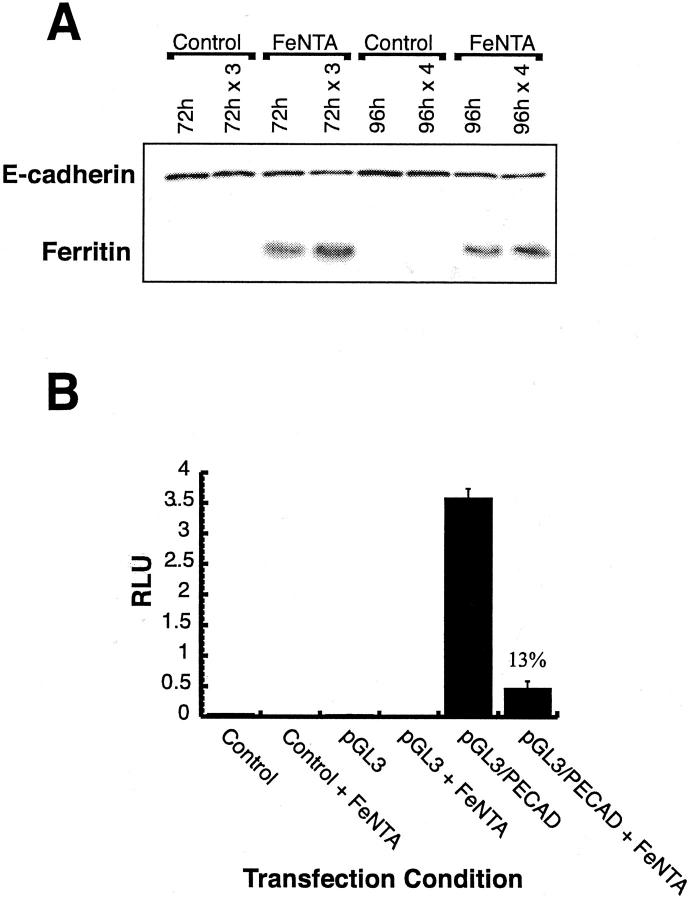
To determine whether the decreased E-cadherin expression observed in iron-loaded primary rat hepatocytes could be duplicated in a human hepatic culture system, the Huh7 human hepatoma cell line was treated with 100 μmol/L of FeNTA for 72 or 96 hours. Twenty-four hours after plating, Huh7 cells were treated, in the absence of fetal bovine serum, with control medium or control medium supplemented with 100 μmol/L of FeNTA (Figure 10A) ▶ . Cultures were either not refed (72 hours or 96 hours) or refed every 24 hours (72 hours × 3 or 96 hours × 4) with the appropriate medium. After 72 or 96 hours, cultures were harvested for protein and analyzed by immunoblotting for E-cadherin or ferritin protein expression. All treatments demonstrated a significant decrease in E-cadherin protein expression over control cultures, with the greatest decrease occurring in the 96 hours × 4 treatment. Ferritin expression was significantly increased over controls for each FeNTA treatment condition. This study demonstrates that iron regulation of E-cadherin and ferritin expression occurs in human as well as rat cells of hepatic origin.
Figure 10.
E-cadherin expression and promoter activity in iron-loaded human hepatic cell cultures. A: E-cadherin protein expression was decreased in Huh7 cells treated with FeNTA. The Huh7 human hepatoma cell line was treated with 100 μmol/L of FeNTA for 72 or 96 hours. Twenty-four hours after plating, Huh7 cells were treated, in the absence of fetal bovine serum, with control medium or medium supplemented with 100 μmol/L of FeNTA. Cultures were either not refed (72 hours or 96 hours) or refed every 24 hours (72 hours × 3 or 96 hours × 4) with the appropriate medium. After 72 hours or 96 hours, cultures were harvested for protein and analyzed for E-cadherin or ferritin protein expression. B: E-cadherin promoter activity was decreased in FeNTA-treated human hepatic cells. Huh7 cells were transfected with either CMV-renilla plasmid alone (control), CMV-renilla and pGL3 plasmids (pGL3), or CMV-renilla and pGL3/PECAD (pGL3/PECAD) plasmids in serum-free medium in the absence or presence of 100 μmol/L of FeNTA (+FeNTA). At 24 hours after transfection, cultures were harvested and analyzed for relative (firefly/renilla) luciferase activity (RLU).
E-Cadherin Promoter Activity Was Decreased in FeNTA-Treated Human Hepatic Cells
To determine whether the E-cadherin promoter activity was altered by iron treatment in a human cell culture system, Huh7 cells were transfected in serum-free medium in the absence or presence of 100 μmol/L of FeNTA with either: 1) CMV-renilla plasmid alone (control); 2) CMV-renilla and pGL3 plasmids (pGL3); or 3) CMV-renilla and pGL3/PECAD plasmids. At 24 hours after transfection, cultures were harvested and analyzed for relative (firefly/renilla) luciferase activity (Figure 10B) ▶ . At 24 hours after transfection, cultures were harvested and analyzed for relative (firefly/renilla) luciferase activity. E-cadherin promoter activity was ∼13% in FeNTA-treated (+FeNTA) compared to untreated cultures. No luciferase activity was observed in control or pGL3 transfections. This study further demonstrates iron-dependent regulation of the E-cadherin promoter and suggests the existence of a novel transcriptional regulatory mechanism present in both human and rat hepatic cell culture systems.
Discussion
Based on the results presented in this article, we conclude the following: 1) paracellular junction complex junction integrity was compromised and membrane permeability was increased in iron-loaded primary rat hepatocytes. 2) Expression of numerous paracellular junction genes was decreased in a time-dependent manner in primary rat hepatocytes treated with 20 μmol/L of TMH-ferrocene throughout 21 days. 3) The expression of numerous paracellular junction proteins was also decreased in iron-loaded hepatocytes. 4) The effects of iron on E-cadherin were reversible as indicated by the findings that protein expression of E-cadherin returned to levels comparable to control cultures after TMH-ferrocene was removed from the media and/or iron was chelated from the cells by exposure to DFO. 5) Localization of the adherens junction protein, E-cadherin, the tight junction protein, occludin, and the gap junction protein, connexin32, was altered in iron-loaded primary rat hepatocytes. 6) Gap junction communication was decreased in primary rat hepatocytes after iron loading. 7) E-cadherin protein expression was decreased in FeNTA-treated human hepatic cells. 8) E-cadherin promoter activity was decreased in iron-loaded primary rat hepatocytes and FeNTA-treated human hepatic cells. 9) Baculoviruses infected a greater proportion of internal hepatocytes in iron-loaded compared to control long-term nondividing hepatocyte cultures most likely because iron loading reduces expression of paracellular junction complex genes and hence compromises paracellular junction complex integrity.
Hepatocytes in long-term DMSO culture are highly differentiated and an excellent in vitro model for studying growth, gene expression, and pathological changes in adult hepatocytes. The long-term DMSO hepatocyte culture system has several notable advantages that have made it possible for our laboratory and other laboratories to use the system to study differentiated hepatocyte function. 49-54 Some of these characteristics are of particular importance to the experiments reported in this study. It was possible to examine the effect of iron loading on paracellular junction complexes because hepatocytes in long-term DMSO culture have intercellular junctions and express paracellular junction complex genes. In addition, the way in which the hepatocytes are maintained in this system made it possible to use techniques such as FDA staining to measure paracellular junction integrity and membrane permeability, gap junction communication, and immunofluorescence staining for paracellular junction proteins. Specifically, the cells are homogeneous because DMSO essentially eliminates contaminating nonparenchymal liver cells normally present in primary rat hepatocyte cultures and there is no need to add an exogenous source of contaminating cells for co-culture. The hepatocytes are plated on rat-tail collagen-surfaced plates and therefore, the morphology of the cells is easily visualized because the cells are not in a matrix or co-cultured. Another advantage of the long-term DMSO culture system that made these studies possible is that enough viable cells can be obtained (5 × 105 to 1 × 106 cells per 60-mm dish) to perform most molecular studies including RPA and gene array analyses.
It is important to note that the studies in this article have also added to our knowledge regarding the properties of primary rat hepatocytes in long-term DMSO culture. Specifically, we report here for the first time that these cells retain the ability to express paracellular junction genes including: E-cadherin, protocadherin, α-actinin, JAM, caveolin, α-catenin, β-catenin, radixin, occludin, connexin32, and ZO-1. In addition, we have demonstrated that this culture system can be used to study gap junction intercellular communication.
In this study, the effects of iron loading on different genes that play a role in paracellular junction integrity were measured. Paracellular junction complexes of hepatocytes include the tight and adherens junctions, desmosomes, and gap junctions. 55-60 Tight junctions, partially composed of occludin and ZO-1 and ZO-2, form continuous contacts and limit paracellular movement of molecules. 11-13 The tight junction complex is stabilized by the binding of JAM to ZO-1. 61 We showed in this study that iron-loading did not alter ZO-1 immunolocalization, which may be because of the interaction of ZO-1 and cytoskeletal elements. However, we know that alteration of ZO-1 immunolocalization can be achieved in hepatocytes in long-term DMSO culture; we recently demonstrated that calcium depletion induces a rapid alteration in the cellular localization of ZO-1 and that this effect can be readily reversed when calcium is returned to the hepatocyte cultures. 42 Although iron loading did not alter ZO-1 immunolocalization, iron loading did lead to reduced expression of JAM mRNA and this reduction was detected in both the gene array and RPA analyses.
Adherens junctions, composed of cadherins or nonclassical cadherins known as protocadherins, are adhesive contacts located below the tight junctions. Epithelial intercellular contact at the adherens junction is mediated by calcium-dependent intercellular binding of interdigitated E-cadherin dimers that form a continuous zipper-like structure. 14,62-64 The binding of cadherins to cytoplasmic α- and β-catenins, in association with the actin cytoskeleton through proteins such as α-actinin and radixin, couples cell-cell adhesion to tissue morphology and signal transduction. 14,65-70 The results of the gene array analysis comparing RNA from iron-loaded and control cultures of hepatocytes indicated that the expression of E-cadherin, protocadherin, and α-catenin mRNAs were all significantly reduced in iron-loaded hepatocytes. These three genes were also found to be significantly reduced when RNA from iron-loaded compared to control hepatocytes was subjected to RPA analysis. One strength of this study is that the data obtained initially by gene array analysis was verified by a second technique, RPA. The complementarity of the data are of interest because not all changes detected by gene array analysis can be validated by a second procedure. It is also noteworthy that the data obtained by RPA was highly reproducible using hepatocytes isolated from different animals and cultured independently. Other laboratories have reported in various different experimental conditions that decreased E-cadherin expression can be caused by transcriptional down-regulation or mutations in the E-cadherin gene. 71-77 In this study, gene array, RPA analyses, and E-cadherin promoter reporter assays indicate that transcriptional down-regulation of the E-cadherin gene may be occurring in both iron-loaded primary rat hepatocytes and FeNTA-treated human hepatic cells in culture.
Additional RPA analysis demonstrated that β-catenin, α-actinin, and radixin mRNA levels were also decreased in iron-loaded cultures; however, β-catenin protein levels were not altered in iron-loaded primary rat hepatocytes. One possible explanation for this may be found in the characteristics of β-catenin degradation. Degradation of β-catenin is regulated by a multisubunit complex composed of constitutively active GSK3β, axin, and adenomatous polyposis coli. 78-80 This complex regulates the level of β-catenin protein accumulation in a cell to maintain homeostasis of β-catenin protein levels and prevent subsequent tumorigenesis that can result from overaccumulation of β-catenin. 67,81-83 β-Catenin protein expression in iron-loaded primary rat hepatocytes may be similar to control cultures in that the degradation of β-catenin may be inhibited to maintain the appropriate levels of β-catenin in the hepatocytes.
Recently, the roles of E-cadherin and α- and β-catenin in signal transduction were demonstrated to be dependent on the lipid-rich scaffolding caveolae membranes. 84 Caveolae are composed of caveolin family members (caveolin-1, -2, and -3), cholesterol, sphingolipids, and signaling molecules, which enable them to function in signal transduction. 84 We conclude from both the gene array and RPA analyses that iron loading reduced expression of caveolin mRNA levels. Direct cell-cell communication is mediated through gap junctions, which are channels composed of oligomerized connexins that connect neighboring cells. Molecular movement through these channels is relatively nonspecific and occurs by passive diffusion. 85 Gap junction communication was also decreased, consistent with decreases observed in connexin32 protein levels and gap junction formation, in iron-loaded primary rat hepatocyte cultures. The disruption of paracellular junctions after iron loading also resulted in the increased intercellular leakage and membrane permeability of fluorescein and subsequent loss of fluorescein accumulation within the hepatocytes.
Iron overload is associated with liver pathogenicity in several human diseases including genetic HH and thalassemia. 86-94 Iron deposition in hepatocytes of those afflicted with genetic HH begins early in life and progresses at a constant rate. 7,9 As iron concentrations increase in the liver, fibrosis begins to expand the portal tracts. At later stages, iron loading is found throughout the liver acinus. Cirrhosis begins to develop as the normal architecture of the liver is destroyed, marking a decrease in life expectancy and an increased risk of development of hepatocellular carcinoma. 95-98 Increased development and severity of hepatocellular carcinoma has also been associated with decreased expression of the invasion-suppressor gene, E-cadherin. 76,99 The relationship between membranous E-cadherin levels and the tumor grade and patient prognosis is well established for carcinomas of the liver, as well as the esophagus and colon. 100-108 Although much evidence is available that relates iron loading of hepatocytes or decreased E-cadherin expression with the incidence and severity of hepatocellular carcinoma, the relationship between iron loading and decreased paracellular junction gene expression has not been previously investigated. The results presented in this report describe the down-regulation of paracellular junction mRNA expression and specifically, decreased E-cadherin expression at the mRNA and protein levels, in iron-loaded rat and human hepatic cells in vitro. These studies suggest that in HH and other iron-loaded patients, iron loading of hepatocytes may decrease paracellular junction gene expression, leading to increased risk for developing hepatocellular carcinoma. Our experiments in rat and human hepatic cells demonstrate that iron loading decreased the E-cadherin promoter activity and expression of numerous paracellular junction genes and altered cellular localization of several paracellular junction proteins, increasing membrane permeability and inhibiting intercellular communication, potentially predisposing hepatocytes to carcinogenesis by additional yet undefined mechanisms.
Acknowledgments
We thank Colleen Kelley, Nidhi Mahendru, and Tom Miller for their excellent technical assistance; Dr. T. W. Gardner, Penn State University College of Medicine, Hershey, PA, for providing the anti-ZO-1 antibody; Dr. F. R. Boyce, Department of Neurology, Massachusetts General Hospital, Boston, MA, for providing the CMV-lacZ baculovirus; Dr. B. Wigdahl, Penn State University College of Medicine, Hershey, PA, for providing the pRL-Tk renilla plasmid; Dr. Antonio Garcia de Herreros, Universitat Pompeu Fabra, Barcelona, Spain, for providing the pGL3/PECAD plasmid; and Nicole Harhaj for helpful discussions.
Footnotes
Address reprint requests to Harriet C. Isom, Ph.D., Department of Microbiology and Immunology, H107, Penn State College of Medicine, Penn State Milton S. Hershey Medical Center, P.O. Box 850, 500 University Dr., Hershey, PA 17033-0850. E-mail: hisom@psu.edu.
Supported by the National Institutes of Health (research grants CA23931, DK53430, and DK54482 to H. C. I.).
References
- 1.Harford JB, Rouault TA, Huebers HA, Klausner RD: Molecular mechanisms of iron metabolism. Stamatoyannopoulos G Nienhuis AW Majerus PW Varmus H eds. The Molecular Basis of Blood Diseases. 1994:pp 351-397 W. B. Saunders, Philadelphia
- 2.Philpott CC: Molecular aspects of iron absorption: insights into the role of HFE in hemochromatosis. Hepatology 2002, 35:993-1001 [DOI] [PubMed] [Google Scholar]
- 3.Levy JE, Montross LK, Andrews NC: Genes that modify the hemochromatosis phenotype in mice. J Clin Invest 2000, 105:1209-1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards CQ, Griffen LM, Goldgar D, Drummond C, Skolnick MH, Kushner JP: Prevalence of hemochromatosis among 11,065 presumably healthy blood donors. N Engl J Med 1988, 318:1355-1362 [DOI] [PubMed] [Google Scholar]
- 5.Prows CA: Hereditary hemochromatosis. Nur Clin N Am 2000, 35:707-717 [PubMed] [Google Scholar]
- 6.Bonkovsky HL: Iron and the liver. Am J Med Sci 1991, 301:32-43 [DOI] [PubMed] [Google Scholar]
- 7.Bothwell TH, Charlton RW, Motulsky AG: Hemochromatosis. Scriver CR Beaudet AL Sly WS Valle D eds. The Metabolic and Molecular Basis of Inherited Disease. 1995:pp 2237-2269 McGraw-Hill, New York
- 8.Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, Dormishian F, Domingo R, Jr, Ellis MC, Fullan A, Hinton LM, Jones NL, Kimmel BEKGS, Lauer P, Lee VK, Loeb DB, Mapa FA, McClelland E, Meyer NC, Mintier GA, Moeller N, Moore T, Morikang E, Prass CE, Quintana L, Starnes SM, Schatzman RC, Brunke KJ, Drayna DT, Risch NJ, Bacon BR, Wolff RK: A novel MHC class I-like gene is mutated in patients with hereditary hemochromatosis. Nat Genet 1996, 13:399-408 [DOI] [PubMed] [Google Scholar]
- 9.Deugnier YM, Loreal O, Turlin B, Guyader D, Jouanolle H, Moirand R, Jacquelinet C, Brissot P: Liver pathology in genetic hemochromatosis: a review of 135 homozygous cases and their bioclinical correlations. Gastroenterology 1992, 102:2050-2059 [DOI] [PubMed] [Google Scholar]
- 10.Gumbiner BM: Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 1996, 84:345-357 [DOI] [PubMed] [Google Scholar]
- 11.Wang G, Zabner J, Deering C, Launspach J, Shao J, Bodner M, Jolly DJ, Davidson BL, McCray PB, Jr: Increasing epithelial junction permeability enhances gene transfer to airway epithelia in vivo. Am J Resp Cell Mol 2000, 22:129-138 [DOI] [PubMed] [Google Scholar]
- 12.Denker BM, Nigam SK: Molecular structure and assembly of the tight junction. Am J Physiol 1998, 274:F1-F9 [DOI] [PubMed] [Google Scholar]
- 13.Mitic LL, Anderson JM: Molecular architecture of tight junctions. Annu Rev Physiol 1998, 60:121-142 [DOI] [PubMed] [Google Scholar]
- 14.Yap AS, Brieher WM, Gumbiner BM: Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol 1997, 13:119-146 [DOI] [PubMed] [Google Scholar]
- 15.Birchmeier W, Behrens J: Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta 1994, 1198:11-26 [DOI] [PubMed] [Google Scholar]
- 16.Isom HC, Secott T, Georgoff I, Woodworth C, Mummaw J: Maintenance of differentiated rat hepatocytes in primary culture. Proc Natl Acad Sci USA 1985, 82:3252-3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isom H, Georgoff I, Salditt-Georgieff M, Darnell JE, Jr: Persistence of liver-specific messenger RNA in cultured hepatocytes: different regulatory events for different genes. J Cell Biol 1987, 105:2877-2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serra R, Isom HC: Stimulation of DNA synthesis and protooncogene expression in primary rat hepatocytes in long-term DMSO culture. J Cell Physiol 1993, 154:543-553 [DOI] [PubMed] [Google Scholar]
- 19.Cable EE, Isom HC: Metabolism of 3,5,5-trimethylhexanoyl-ferrocene by rat liver: release of iron from 3,5,5-trimethylhexanoyl-ferrocene by a microsomal, phenobarbital-inducible cytochrome P-450. Drug Metab Dispos 1999, 27:255-260 [PubMed] [Google Scholar]
- 20.Cable EE, Connor JR, Isom HC: Accumulation of iron by primary rat hepatocytes in long-term culture: changes in nuclear shape mediated by non-transferrin-bound forms of iron. Am J Pathol 1998, 152:781-792 [PMC free article] [PubMed] [Google Scholar]
- 21.Hu JM, Camper SA, Tilghman SM, Miller T, Georgoff I, Serra R, Isom HC: Functional analyses of albumin expression in a series of hepatocyte cell lines and in primary hepatocytes. Cell Growth Differ 1992, 3:577-588 [PubMed] [Google Scholar]
- 22.Cable EE, Isom HC: Exposure of primary rat hepatocytes in long-term DMSO culture to selected transition metals induces hepatocyte proliferation and formation of duct-like structures. Hepatology 1997, 26:1444-1457 [DOI] [PubMed] [Google Scholar]
- 23.Woodworth C, Secott T, Isom HC: Transformation of rat hepatocytes by transfection with simian virus 40 DNA to yield proliferating differentiated cells. Cancer Res 1986, 46:4018-4026 [PubMed] [Google Scholar]
- 24.Woodworth CD, Isom HC: Transformation of differentiated rat hepatocytes with adenovirus and adenovirus DNA. J Virol 1987, 61:3570-3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woodworth CD, Isom HC: Regulation of albumin gene expression in a series of rat hepatocyte cell lines immortalized by simian virus 40 and maintained in chemically defined medium. Mol Cell Biol 1987, 7:3740-3748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bour ES, Ward LK, Cornman GA, Isom HC: Tumor necrosis factor-alpha-induced apoptosis in hepatocytes in long-term culture. Am J Pathol 1996, 148:485-495 [PMC free article] [PubMed] [Google Scholar]
- 27.Cable EE, Miller TG, Isom HC: Regulation of heme metabolism in rat hepatocytes and hepatocyte cell lines: δ-aminolevulinic acid synthase and heme oxygenase are regulated by different heme-dependent mechanisms. Arch Biochem Biophys 2000, 384:280-295 [DOI] [PubMed] [Google Scholar]
- 28.Parkes JG, Randell EW, Olivieri NF, Templeton DM: Modulation by iron loading and chelation of the uptake of non-transferrin-bound iron by human liver cells. Biochim Biophys Acta 1995, 1243:373-380 [DOI] [PubMed] [Google Scholar]
- 29.Lescoat G, Loreal O, Moirand R, Dezier JF, Pasdeloup N, Deugnier Y, Brissot P: Iron induction of ferritin synthesis and secretion in human hepatoma cell (Hep G2) cultures. Liver 1989, 9:179-185 [DOI] [PubMed] [Google Scholar]
- 30.Goto Y, Paterson M, Listowsky I: Iron uptake and regulation of ferritin synthesis by hepatoma cells in hormone-supplemented serum-free media. J Biol Chem 1983, 258:5248-5255 [PubMed] [Google Scholar]
- 31.Dullmann J, Wulfhekel U, Nielsen P, Heinrich HC: Iron overload of the liver by trimethylhexanoylferrocene in rats. Acta Anat 1992, 143:96-108 [DOI] [PubMed] [Google Scholar]
- 32.Nielsen P, Heinelt S, Dullmann J: Chronic feeding of carbonyl-iron and TMH-ferrocene in rats. Comparison of two iron-overload models with different iron absorption. Comp Pharmacol Toxicol 1993, 106:429-436 [DOI] [PubMed] [Google Scholar]
- 33.Nielsen P, Heinrich HC: Metabolism of iron from (3,5,5-trimethylhexanoyl)ferrocene in rats. A dietary model for severe iron overload. Biochem Pharmacol 1993, 45:385-391 [DOI] [PubMed] [Google Scholar]
- 34.Delaney WE, IV, Isom HC: Hepatitis B virus replication in human HepG2 cells mediated by hepatitis B virus recombinant baculovirus. Hepatology 1998, 28:1134-1146 [DOI] [PubMed] [Google Scholar]
- 35.Maslak M, Martin CT: Kinetic analysis of T7 RNA polymerase transcription initiation from promoters containing single-stranded regions. Biochemistry 1993, 32:4281-4285 [DOI] [PubMed] [Google Scholar]
- 36.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 37.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC: Measurement of protein using bicinchoninic acid. Anal Biochem 1985, 150:76-85 [DOI] [PubMed] [Google Scholar]
- 38.Laemmli UK: Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 1970, 227:680-685 [DOI] [PubMed] [Google Scholar]
- 39.El-Fouly MH, Trosko JE, Chang CC: Scrape-loading and dye transfer. A rapid and simple technique to study gap junctional intercellular communication. Exp Cell Res 1987, 168:422-430 [DOI] [PubMed] [Google Scholar]
- 40.Kojima T, Mitaka T, Mizuguchi T, Mochizuki Y: Effects of oxygen radical scavengers on connexins 32 and 26 expression in primary cultures of adult rat hepatocytes. Carcinogenesis 1996, 17:537-544 [DOI] [PubMed] [Google Scholar]
- 41.Bilello JP, Delaney WE, IV, Boyce FM, Isom HC: Transient disruption of intercellular junctions enables baculovirus entry into nondividing hepatocytes. J Virol 2001, 75:9857-9871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bilello JP, Cable EE, Myers RL, Isom HC: Role of paracellular junction complexes in baculovirus-mediated gene transfer to non-dividing rat hepatocytes. Gene Ther (in press) [DOI] [PubMed]
- 43.Nusrat A, Parkos CA, Verkade P, Foley CS, Liang TW, Innis-Whitehouse W, Eastburn KK, Madara JL: Tight junctions are membrane microdomains. J Cell Sci 2000, 113:1771-1781 [DOI] [PubMed] [Google Scholar]
- 44.Persidsky MD, Baillie GS: Fluorometric test of cell membrane integrity. Cryobiology 1977, 14:322-331 [DOI] [PubMed] [Google Scholar]
- 45.Rotman B, Papermaster BW: Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proc Natl Acad Sci USA 1966, 55:134-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barth CA, Schwarz LR: Transcellular transport of fluorescein in hepatocyte monolayers: evidence for functional polarity of cells in culture. Proc Natl Acad Sci USA 1982, 79:4985-4987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aeschbacher M, Reinhardt CA, Zbinden G: A rapid cell membrane permeability test using fluorescent dyes and flow cytometry. Cell Biol Toxicol 1986, 2:247-255 [DOI] [PubMed] [Google Scholar]
- 48.Prosperi E: Intracellular turnover of fluorescein diacetate. Influence on membrane ionic gradients on fluorescein efflux. Histochem J 1990, 22:227-233 [DOI] [PubMed] [Google Scholar]
- 49.De La Vega FM, Mendoza-Figueroa T: Dimethyl sulfoxide enhances lipid synthesis and secretion by long-term cultures of adult rat hepatocytes. Biochimie 1991, 73:621-624 [DOI] [PubMed] [Google Scholar]
- 50.Jeong TC, Jeong HG, Yang KH: Induction of cytochrome P-450 by dimethyl sulfoxide in primary cultures of adult rat hepatocytes. Toxicol Lett 1992, 61:275-281 [DOI] [PubMed] [Google Scholar]
- 51.Lindsay CK, Chenery RJ, Hawksworth GM: Primary culture of rat hepatocytes in the presence of dimethyl sulfoxide. A system to investigate the regulation of cytochrome P450 IA. Biochem Pharmacol 1991, 42:S17-S25 [DOI] [PubMed] [Google Scholar]
- 52.Muakkassah-Kelly SF, Bieri F, Waechter F, Bentley P, Staubli W: Long-term maintenance of hepatocytes in primary culture in the presence of DMSO: further characterization and effect of nafenopin, a peroxisome proliferator. Exp Cell Res 1987, 171:37-51 [DOI] [PubMed] [Google Scholar]
- 53.Muakkassah-Kelly SF, Bieri F, Waechter F, Bentley P, Staubli W: The use of primary cultures of adult rat hepatocytes to study induction of enzymes and DNA synthesis: effect of nafenopin and electroporation. Experientia 1988, 44:823-827 [DOI] [PubMed] [Google Scholar]
- 54.Villa P, Arioli P, Guaitani A: Mechanism of maintenance of liver-specific functions by DMSO in cultures rat hepatocytes. Exp Cell Res 1991, 194:157-160 [DOI] [PubMed] [Google Scholar]
- 55.Borrmann CM, Mertens C, Schmidt A, Langbein L, Kuhn C, Franke WW: Molecular diversity of plaques of epithelial-adhering junctions. Ann NY Acad Sci 2000, 915:144-150 [DOI] [PubMed] [Google Scholar]
- 56.Fujikura Y, Ohta H, Hirai T, Fukumoto T: Immunohistochemical analysis of rat liver using a monoclonal antibody (HAM8) against gap junction. Anat Rec 1993, 235:335-341 [DOI] [PubMed] [Google Scholar]
- 57.Fujimoto K: Freeze-fracture replica electron microscopy combined with SDS digestion for cytochemical labeling of integral membrane proteins. Application to the immunogold labeling of intercellular junctional complexes. J Cell Sci 1995, 108:3443-3449 [DOI] [PubMed] [Google Scholar]
- 58.Novikoff PM, Ikeda T, Hixson DC, Yam A: Characterizations of and interactions between bile ductule cells and hepatocytes in early stages of rat hepatocarcinogenesis induced by ethionine. Am J Pathol 1991, 139:1351-1368 [PMC free article] [PubMed] [Google Scholar]
- 59.Tateno C, Yoshizato K: Long-term cultivation of adult rat hepatocytes that undergo multiple cell divisions and express normal parenchymal phenotypes. Am J Pathol 1996, 148:383-392 [PMC free article] [PubMed] [Google Scholar]
- 60.Tsukita S, Tsukita S: Isolation of cell-to-cell adherens junctions from rat liver. J Cell Biol 1989, 108:31-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bazzoni G, Martinez-Estrada OM, Orsenigo F, Cordenonsi M, Citi S, Dejana E: Interaction of junctional adhesion molecule with the tight junction components Zo-1, cingulin, and occludin. J Biol Chem 2000, 275:20520-20526 [DOI] [PubMed] [Google Scholar]
- 62.Heymann R, Kallenbach S, Alonso S, Carroll P, Mitsiadis TA: Dynamic expression patterns of the new protocadherin families CNRs and Pcdh-gamma during mouse odontogenesis: comparison with reelin expression. Mech Dev 2001, 106:181-184 [DOI] [PubMed] [Google Scholar]
- 63.Suzuki ST: Recent progress in protocadherin research. Exp Cell Res 2000, 261:13-18 [DOI] [PubMed] [Google Scholar]
- 64.Wijnhoven BP, Dinjens WN, Pignatelli M: E-cadherin-catenin cell-cell adhesion complex and human cancer. Br J Surg 2000, 87:992-1005 [DOI] [PubMed] [Google Scholar]
- 65.Nishiyama M, Ozturk M, Frohlich M, Mafune K, Steele G, Jr, Wands JR: Expression of human alpha-actinin in human hepatocellular carcinoma. Cancer Res 1990, 50:6291-6294 [PubMed] [Google Scholar]
- 66.Nollet F, Berx G, van Roy F: The role of the E-cadherin/catenin adhesion complex in the development and progression of cancer. Mol Cell Biol Res Com 1999, 2:77-85 [DOI] [PubMed] [Google Scholar]
- 67.Park WS, Oh RR, Park JY, Kim PJ, Shin MS, Lee JH, Kim HS, Lee SH, Kim SY, Park YG, An WG, Kim HS, Jang JJ, Yoo NJ, Lee JY: Nuclear localization of beta-catenin is an important prognostic factor in hepatoblastoma. J Pathol 2001, 193:483-490 [DOI] [PubMed] [Google Scholar]
- 68.Williams BO, Barish GD, Klymkowsky MW, Varmus HE: A comparative evaluation of beta-catenin and plakoglobin signaling activity. Oncogene 2000, 19:5720-5728 [DOI] [PubMed] [Google Scholar]
- 69.Tsukada N, Ackerley CA, Phillips MJ: The structure and organization of the bile canalicular cytoskeleton with special reference to actin and actin-binding proteins. Hepatology 1995, 21:1106-1113 [PubMed] [Google Scholar]
- 70.Hiscox S, Jiang WG: Ezrin regulates cell-cell and cell-matrix adhesion, a possible role with E-cadherin/beta-catenin. J Cell Sci 1999, 112:3081-3090 [DOI] [PubMed] [Google Scholar]
- 71.Birchmeier W, Weidner KM, Behrens J: Molecular mechanisms leading to loss of differentiation and gain of invasiveness in epithelial cells. J Cell Sci 1993, 17:159-164 [DOI] [PubMed] [Google Scholar]
- 72.Birchmeier W, Weidner KM, Hulsken J, Behrens J: Molecular mechanisms leading to cell junction (cadherin) deficiency in invasive carcinomas. Semin Cancer Biol 1993, 4:231-239 [PubMed] [Google Scholar]
- 73.Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A: The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2000, 2:84-89 [DOI] [PubMed] [Google Scholar]
- 74.Hajra KM, Ji X, Fearon ER: Extinction of E-cadherin expression in breast cancer via a dominant repression pathway acting on proximal promoter elements. Oncogene 1999, 18:7274-7279 [DOI] [PubMed] [Google Scholar]
- 75.Hennig G, Lowrick O, Birchmeier W, Behrens J: Mechanisms identified in the transcriptional control of epithelial gene expression. J Biol Chem 1996, 271:595-602 [DOI] [PubMed] [Google Scholar]
- 76.Hennig G, Behrens J, Truss M, Frisch S, Reichmann E, Birchmeier W: Progression of carcinoma cells is associated with alterations in chromatin structure and factor binding at the E-cadherin promoter in vivo. Oncogene 1995, 11:475-484 [PubMed] [Google Scholar]
- 77.Behrens J: Cadherins and catenins: role in signal transduction and tumor progression. Cancer Metastasis Rev 1999, 18:15-30 [DOI] [PubMed] [Google Scholar]
- 78.Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A: Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J 1998, 17:1371-1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fagotto F, Jho Eh, Zeng L, Kurth T, Joos T, Kaufmann C, Costantini F: Domains of axin involved in protein-protein interactions, Wnt pathway inhibition, and intracellular localization. J Cell Biol 1999, 145:741-756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sakanaka C, Weiss JB, Williams LT: Bridging of beta-catenin and glycogen synthase kinase-3beta by axin and inhibition of beta-catenin-mediated transcription. Proc Natl Acad Sci USA 1998, 95:3020-3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Behrens J: Control of beta-catenin signaling in tumor development. Ann NY Acad Sci 2000, 910:21-33 [DOI] [PubMed] [Google Scholar]
- 82.Jeng YM, Wu MZ, Mao TL, Chang MH, Hsu HC: Somatic mutations of beta-catenin play a crucial role in the tumorigenesis of sporadic hepatoblastoma. Cancer Lett 2000, 152:45-51 [DOI] [PubMed] [Google Scholar]
- 83.Wagenaar RA, Crawford HC, Matrisian LM: Stabilized β-catenin immortalizes colonic epithelial cells. Cancer Res 2001, 61:2097-2104 [PubMed] [Google Scholar]
- 84.Galbiati F, Volonte D, Brown AM, Weinstein DE, Ben-Ze’ev A, Pestell RG, Lisanti MP: Caveolin-1 expression inhibits Wnt/beta-catenin/Lef-1 signaling by recruiting beta-catenin to caveolae membrane domains. J Biol Chem 2000, 275:23368-23377 [DOI] [PubMed] [Google Scholar]
- 85.Kumar NM, Gilula NB: The gap junction communication channel. Cell 1996, 84:381-388 [DOI] [PubMed] [Google Scholar]
- 86.Kontoghiorghes GJ, Weinberg ED: Iron: mammalian defense systems, mechanisms of disease, and chelation therapy approaches. Blood Rev 1995, 9:33-45 [DOI] [PubMed] [Google Scholar]
- 87.Weinberg ED: Iron depletion: a defense against intracellular infection and neoplasia. Life Sci 1992, 50:1289-1297 [DOI] [PubMed] [Google Scholar]
- 88.Weinberg ED: Iron in neoplastic disease. Nutr Cancer 1983, 4:223-233 [DOI] [PubMed] [Google Scholar]
- 89.Weinberg ED: Iron loading and disease surveillance. Emerg Infect Dis 1999, 5:346-352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weinberg ED: Iron withholding: a defense against infection and neoplasia. Physiol Rev 1984, 64:65-102 [DOI] [PubMed] [Google Scholar]
- 91.Weinberg ED: Roles of iron in infection and neoplasia. J Pharmacol Paris 1985, 16:358-364 [PubMed] [Google Scholar]
- 92.Weinberg ED: Roles of iron in neoplasia. Promotion, prevention, and therapy. Biol Trace Elem Res 1992, 34:123-140 [DOI] [PubMed] [Google Scholar]
- 93.Reizenstein P: Iron, free radicals and cancer. Med Oncol Tumor Pharmacol 1991, 8:229-233 [DOI] [PubMed] [Google Scholar]
- 94.Engelhardt R, Langkowski JH, Fischer R, Nielsen P, Kooijman H, Keinrich HC, Bucheler E: Liver iron quantification: studies in aqueous iron solutions, iron overloaded rats, and patients with hereditary hemochromatosis. Magn Reson Imaging 1994, 12:999-1007 [DOI] [PubMed] [Google Scholar]
- 95.Niederau C, Fischer R, Sonnenberg A, Stremmel W, Trampisch HJ, Strohmeyer G: Survival and causes of death in cirrhotic and noncirrhotic patients with primary hemochromatosis. N Engl J Med 1985, 313:1256-1262 [DOI] [PubMed] [Google Scholar]
- 96.Bomford A, Williams R: Long term results of venesection therapy in idiopathic haemochromatosis. Q J Med 1976, 45:611-623 [PubMed] [Google Scholar]
- 97.Fargion S, Mandelli C, Piperno A, Cesana B, Fracanzani AL, Fraquelli M, Bianchi PA, Fiorelli G, Conte D: Survival and prognostic factors in 212 Italian patients with genetic hemochromatosis. Hepatology 1992, 15:655-659 [DOI] [PubMed] [Google Scholar]
- 98.Tiniakos G, Williams R: Cirrhotic process, liver cell carcinoma and extrahepatic malignant tumors in idiopathic haemochromatosis. Study of 71 patients treated with venesection therapy. Appl Pathol 1988, 6:128-138 [PubMed] [Google Scholar]
- 99.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA: The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2000, 2:76-83 [DOI] [PubMed] [Google Scholar]
- 100.Huang GT, Lee HS, Chen CH, Sheu JC, Chiou LL, Chen DS: Correlation of E-cadherin expression and recurrence of hepatocellular carcinoma. Hepatogastroenterology 1999, 46:1923-1927 [PubMed] [Google Scholar]
- 101.Nuruki K, Toyoyama H, Ueno S, Hamanoue M, Tanabe G, Aikou T, Ozawa M: E-cadherin but not N-cadherin expression is correlated with the intracellular distribution of catenins in human hepatocellular carcinomas. Oncol Rep 1998, 5:1109-1114 [DOI] [PubMed] [Google Scholar]
- 102.Endo K, Ueda T, Ueyama J, Ohta T, Terada T: Immunoreactive E-cadherin, alpha-catenin, beta-catenin, and gamma-catenin proteins in hepatocellular carcinoma: relationships with tumor grade, clinicopathologic parameters, and patients’ survival. Hum Pathol 2000, 31:558-565 [DOI] [PubMed] [Google Scholar]
- 103.Monga SP, Pediaditakis P, Mule K, Stolz DB, Michalopoulos GK: Changes in WNT/beta-catenin pathway during regulated growth in rat liver regeneration. Hepatology 2001, 33:1098-1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ashida K, Terada T, Kitamura Y, Kaibara N: Expression of E-cadherin, alpha-catenin, beta-catenin, and CD44 (standard and variant isoforms) in human cholangiocarcinoma: an immunohistochemical study. Hepatology 1998, 27:974-982 [DOI] [PubMed] [Google Scholar]
- 105.Ihara A, Koizumi H, Hashizume R, Uchikoshi T: Expression of epithelial cadherin and alpha- and beta-catenins in nontumoral livers and hepatocellular carcinomas. Hepatology 1996, 23:1441-1447 [DOI] [PubMed] [Google Scholar]
- 106.Ikeguchi M, Makino M, Kaibara N: Clinical significance of E-cadherin-catenin complex expression in metastatic foci of colorectal carcinoma. J Surg Oncol 2001, 77:201-207 [DOI] [PubMed] [Google Scholar]
- 107.Krishnadath KK, Tilanus HW, van Blankenstein M, Hop WC, Kremers ED, Dinjens WN, Bosman FT: Reduced expression of the cadherin-catenin complex in oesophageal adenocarcinoma correlates with poor prognosis. J Pathol 1997, 182:331-338 [DOI] [PubMed] [Google Scholar]
- 108.Hao X, Palazzo JP, Ilyas M, Tomlinson I, Talbot IC: Reduced expression of molecules of the cadherin/catenin complex in the transition from colorectal adenoma to carcinoma. Anticancer Res 1997, 17:2241-2247 [PubMed] [Google Scholar]