Abstract
Increased nitric oxide (NO) production after burn injury is well established. However, there is little information relating to the reactions that occur as a consequence of NO generation under such circumstances. We have investigated the synthesis and function of NO in a rat model of local cutaneous thermal injury. We show that NO levels are elevated from 3 hours after injury with a concomitant increase in protein nitration. A selective inducible nitric oxide synthase (iNOS) inhibitor (1400W) significantly attenuated NO synthesis, protein nitration, and neutrophil accumulation in this model, but had no effect on edema formation. The results also indicate that NO synthesis and protein nitration occurred independently of neutrophil accumulation because these parameters were unaffected by depletion of circulating neutrophils. 3-Chlorotyrosine, a marker of neutrophil/myeloperoxidase-mediated protein damage was significantly increased from 1 hour after burn. Our observations provide evidence for the involvement of reactive species in the inflammatory response after burn. The use of selective iNOS inhibitors may represent a novel approach for the management of human burn injuries.
Cutaneous thermal injury initiates a pathophysiological response with a significant inflammatory component that involves several classes of chemical mediators. These mediators interact in a complex manner to cause the pain and secondary tissue damage associated with burn injury. The latter contributes to the development of systemic sequelae in human burn patients, eg, systemic inflammatory response syndrome and multiple organ dysfunction syndrome, a major cause of mortality in those with large injuries of this kind. 1 Pharmacological intervention has in general, been only marginally effective in controlling the progressive nature of thermal injury and a detailed knowledge of the contribution of individual mediator systems to the inflammatory response after burn is at present lacking.
The immediate cutaneous response to thermal injury comprises of pain and inflammatory swelling (edema formation). It is associated with progressive microvascular dysfunction and accompanied by neutrophil accumulation. 2 Our group, among others, has characterized the early phase of the inflammatory response after burn and provided evidence for the involvement of sensory neuropeptides and kinins, 3,4 but not neutrophils, 5,6 in mediating edema formation under such circumstances.
There is also some evidence to suggest that nitric oxide (NO) may contribute to the development of the inflammatory response after burn. Urinary excretion of NO metabolites is increased in rats 7 and humans 8 with severe burns and supraphysiological concentrations of NO metabolites have been detected in thermally injured cutaneous tissues. 9,10 Moreover, NO-mediated plasma extravasation within the burn wound has been demonstrated 9,10 and it is likely that inducible nitric oxide synthase (iNOS) contributes to this process because immunoreactive iNOS has been detected in thermally injured mouse 11 and human 12 skin. Indeed, iNOS inhibition has previously been reported to reduce microvascular leakage at 3, but not 1, hours after burn in the mouse. 9
The vasoactive properties of NO are mediated by direct interaction with guanylate cyclase, 13 however the importance of other NO pathways has become apparent. NO may react with superoxide (O2−) to form peroxynitrite (ONOO−). 14 ONOO− is a highly toxic oxidant that can interact with most classes of biomolecules to cause tissue damage. Nitration of protein tyrosine residues by ONOO− and/or other reactive nitrogen species (RNS) is particularly important because this results in the formation of 3-nitrotyrosine (3NT), a commonly measured marker of RNS generation in vivo and a possible route of RNS cytotoxicity. 15 ONOO− has been shown to cause edema formation in experimental animals 16,17 and is synthesized in vivo in response to a number of inflammatory stimuli. 18,19 Moreover, it has been reported that ONOO− is generated in human endothelial cells after ultraviolet B radiation 20 and that thermal injury induces the production of ONOO− in organs remote to the site of burn. 21,22 However, the generation of ONOO− in thermally injured skin has not been documented.
Polymorphonuclear neutrophils contain and release myeloperoxidase (MPO). This enzyme catalyzes the reaction of hydrogen peroxide (H2O2) and chloride ions (CI−) to form hypochlorous acid (HOCl). 23 HOCl, like ONOO−, can interact with a range of molecular targets and inactivate proteins. 24 HOCl can also chlorinate tyrosine residues to form 3-chlorotyrosine (3CT). Consequently, 3CT is regarded as a marker of neutrophil/MPO-mediated protein damage in vivo. 25 At present, there are no reports of protein chlorination within the burn wound or in the remote organs of experimental animals/human patients exposed to large burn injuries. Nonetheless, the high concentrations of MPO 26 and H2O2 27 released under these circumstances make protein chlorination a distinct possibility and may represent an important pathway of tissue injury after noxious heat exposure.
Thus, current understanding of NO function in acute burn injury is limited and to date, there have been no reports of protein nitration in thermally injured cutaneous tissues or on the consequences of enhanced NO synthesis on leukocyte function within the burn wound. The primary aim of this study was to examine the localized production and function of NO-derived reactive species and neutrophils in thermally injured rat skin. In addition, gas chromatography-mass spectrometry isotope dilution methods were developed to allow accurate quantification of 3CT.
Materials and Methods
Induction of Local Cutaneous Thermal Injury
All experiments were performed in accordance with the UK Animals (Scientific Procedures) Act 1986. Male Wistar rats (220 to 280 g) were prepared ∼24 hours before experimentation. For this, they were anesthetized with sodium pentobarbitone (50 mg kg−1, i.p.; May & Baker Ltd., Essex, UK) and the abdominal skin was shaven and depilated. On the day of experimentation, the animals were anesthetized in a nonrecovery procedure with thiopentone sodium (100 mg kg−1, i.p.; May & Baker Ltd.). Depth of anesthesia was assessed by the pedal reflex and maintenance doses were given as required. In prolonged experiments (≥3 hours), animals were intubated and body temperature was maintained at 37°C. Local cutaneous thermal injury was induced on the abdominal skin using a 1-cm-diameter temperature-controlled skin heater (Moor Instruments Ltd., Devon, UK) as previously described. 3 The probe temperature was maintained at 50°C for a 10-minute contact duration. Anesthesia was maintained throughout the experiment and the animals were killed by cervical dislocation 1 to 5 hours after induction of thermal injury. Control skin was taken from an adjacent, contralateral area of the abdomen in the same animal at the same time point under investigation. The location of skin used was alternated to remove any bias relating to regional skin heterogeneity. At the end of experiments, the abdominal skin was removed and thermally injured or control skin was excised with a 16-mm diameter punch. The skin biopsies were weighed, then either counted immediately for gamma emissions in the case of plasma extravasation experiments, fixed in formal saline for immunohistochemical examination, or frozen in liquid nitrogen and stored at −70°C before preparation for biochemical analyses.
Drug Treatment and Neutrophil Depletion Protocols
In some experiments, animals were treated with the highly selective iNOS inhibitor, 1400W 28 (Alexis Biochemicals Ltd., Nottingham, UK) or depleted of circulating neutrophils by pretreatment with a rabbit anti-rat neutrophil antiserum (Accurate Chemicals Corp., Westbury, NY). 29 1400W was dissolved in saline and administered to animals at a final dose of 40 μmol kg−1 (0.5 ml, s.c.), 5 minutes before the induction of experimental injury. The vehicle control group animals were pretreated with 0.5 ml of saline. The rabbit anti-rat neutrophil antiserum was administered at a dose of 2 ml kg−1 i.p., 17 hours before the induction of thermal injury. For these experiments, a control group of animals were pretreated in the same manner with an equivalent volume of rabbit irrelevant antiserum. To confirm that pretreatment of animals with the anti-neutrophil antiserum resulted in a selective depletion of circulating neutrophils, total and differential blood leukocyte counts were performed at the end of each experiment. For determination of total leukocyte counts, blood was collected via cardiac puncture with a heparinized (CP Pharmaceuticals, Wexham, UK) needle. The blood was diluted 1:10 with saline and then 1:20 with saline containing 0.1% crystal violet (Sigma Aldrich, Poole, UK) for blood counts. For differential leukocyte counts, blood smears were prepared on glass slides that were stained and fixed with Diff-Quik (Merck-BDH Ltd., Essex, UK), a commercial preparation.
Measurement of NO2−/NO3−
Frozen skin samples were homogenized in 1 ml of phosphate buffer (600 mmol/L NaCl, 600 mmol/L KH2PO4, 66 mmol/L Na2HPO4, pH 6.0). The homogenates were refrozen in liquid nitrogen, thawed to aid cell lysis, and centrifuged at 10,000 × g for 25 minutes at 4°C to remove tissue debris. The supernatant was then frozen in liquid nitrogen and stored at −70°C before assay. The NO2−/NO3− content of the homogenates was measured by the Greiss assay as an indicator of NO production in thermally injured and control rat skin. Homogenate NO2− levels were determined by mixing 100 μl of sample with 100 μl of Greiss reagent (1% w/v sulfanilic acid, 0.1% w/v with N-(1-naphthyl)-ethylenediamine in 5% phosphoric acid) and comparing absorbance (540 nm) readings to that of a NaNO2 standard (1 to 100 μmol/L). Combined NO2−/NO3− levels were determined by reducing NO3− to NO2− with nitrate reductase in the presence of NADPH (Sigma Chemical Company Ltd., Dorset, UK). For this 80 μl of sample were incubated with 10 μl of Aspergillus nitrate reductase (1 U ml−1; Sigma Chemical Company Ltd.) and 10 μl of NADPH 1 mmol L−1 for 1 hour at 37°C before the addition of Greiss reagent. All samples and standards were assayed in triplicate. Data are expressed as mean ± SEM of NO2−/NO3− (nmol g tissue−1).
Measurement of 3NT
Protein nitration in skin homogenates was measured by a commercial 3NT ELISA kit (TCS Biologicals Ltd., Claydon, UK) as previously described. 30 The assays were performed in 96-well microtiter plates precoated with nitrated bovine serum albumin (NBSA) (7.6 pmol/well−1). One hundred μl of each sample was incubated with 100 μl of rabbit anti-3NT primary antibody diluted 1:20,000 with 10 mmol/L of phosphate-buffered saline (PBS) for 2 hours at 37°C. The plate was then washed with 10 mmol/L of PBS containing 0.1% Tween to remove unbound primary antibody and 100 μl of donkey anti-rabbit secondary antibody (1:1000) conjugated to horseradish peroxidase was added to the appropriate wells. After a 1-hour incubation at 37°C, the plate was washed again and 100 μl of endogen, a peroxidase substrate, was added to each well. After a 30-minute incubation at room temperature, the reaction was stopped with 0.18 mol/L of sulfuric acid and absorbance was measured at 450 nm by a microplate reader (Anthos-Labtec Instruments GmbH, Salzberg, Austria). All samples and standards were assayed in triplicate. Data are expressed as mean ± SEM of NBSA equivalents (nmol g tissue−1).
Measurement of Neutrophil Accumulation
Tissue neutrophil levels were calculated by measurement of MPO activity. 6 Skin homogenates were prepared as above, except that 0.5% w/v hexadecyltrimethylammonium (Sigma Chemical Company Ltd.) was included in the homogenization buffer. The assay was performed in a 96-well microtiter plate in a total volume of 150 μl. Twenty-five μl of each sample was diluted 1:2 with phosphate buffer (60 mmol/L KH2PO4, 6.6 mmol/L Na2HPO4, 0.5% w/v hexadecyltrimethylammonium, pH 6.0) and incubated with 100 μl of K-Blue solution (a commercial preparation of stabilized H2O2 and tetramethylbenzadine; Neogen Corp., Lexington, KY) for 10 minutes at room temperature. After incubation, absorbance was measured at 620 nm by microplate reader. The absolute neutrophil content of each sample was determined by comparing absorbance readings with that of a rat neutrophil standard (0.08 to 2.50 × 106 cells/ml−1) as previously described. 6 All samples and standards were assayed in triplicate. Data are expressed as mean ± SEM of neutrophils/g tissue−1 (106).
Measurement of Plasma Extravasation
Plasma extravasation in thermally injured and control skin was measured as previously described. 3,6 Rats received 100 μl of saline containing 90 kBq of [125I]-albumin (ICN Pharmaceuticals Inc., Hampshire, UK) intravenously, 5 minutes before the induction of thermal injury. At 1 to 5 hours after burn, blood was obtained via cardiac puncture (≈2 ml) and centrifuged at 6000 × g for 4 minutes (MSE Ltd., West Sussex, UK) to obtain a plasma sample. The animals were then killed by cervical dislocation and skin samples excised. Gamma emissions from plasma, control and thermally injured skin were measured in a multichannel counter (EG&G Wallac Ltd., Milton Keynes, UK). Plasma extravasation was calculated by comparing the gamma emissions of individual skin sites with that of 100 μl of blood plasma. All data are expressed as mean ± SEM of plasma extravasated (μl g tissue−1).
Immunohistochemistry
The streptavidin-biotin-immunoperoxidase technique was used in conjunction with a rabbit anti-iNOS polyclonal antibody (DAKO Ltd., Cambridgeshire, UK) for iNOS immunostaining. At the end of each in vivo experiment, control and thermally injured skin samples were fixed in formal saline. On the following day the samples were embedded in formalin-fixed paraffin wax and 5-μm sections were cut using a Reichert-Jung 2030 microtome (Mikrovid GmbH, Arnsberg, Germany). The sections were then deparaffinized with xylene and methylated spirit. To block endogenous peroxidase activity present within the tissues, sections were incubated with 1% H2O2 in methylated spirit for 15 minutes. The sections were then rehydrated with tap water and boiled for 2 minutes in 10 mmol/L of citric acid (pH 6.0) for the purposes of antigen retrieval. To prevent nonspecific antibody binding, 100 μl of 10% normal rabbit serum in 10 mmol/L of Tris-buffered saline (TBS) was applied directly to the sections for 10 minutes. The sections were then washed twice with 10 mmol/L of TBS, containing 0.05% Tween 20 and exposed to 100 μl of rabbit anti-iNOS polyclonal antibody diluted 1:100 with TBS. After 2 hours, the samples were washed again and exposed to 100 μl of porcine anti-rabbit biotinylated IgG (DAKO Ltd.) diluted 1:400 with TBS for 30 minutes. After washing, 100 μl of a commercial streptavidin-biotin-horseradish peroxidase complex (DAKO Ltd.) was applied to the sections for 30 minutes. After another TBS wash the sections were exposed to 100 μl of diaminobenzidine preparation and color development was allowed to take place for 5 minutes. The sections were washed once more, stained with hematoxylin, rinsed with tap water, and differentiated with acid alcohol. Finally the sections were dehydrated with methylated spirit and xylene and coverslips were fixed in place with dibutyl phlhalate xylene (DPX). Immunohistochemical detection of 3NT was performed in a similar manner except that a rabbit anti-3NT polyclonal antibody (DAKO Ltd.) was used as the primary antibody.
Measurement of 3CT
Supernatants from skin homogenates were prepared as described for measurement of NO2−/NO3− above. Fifty pmol of [13C9.15N1]-tyrosine (Cambridge Isotope Laboratories, Andover, MA) and -3CT internal standard (synthesized in house) was added to 1 ml of each sample and the resulting mixture was hydrolyzed by incubation with an equal volume of 6 mol/L of hydrobromic acid containing 1% phenol for 24 hours at 110°C. The hydrolyzed samples were then lyophilized by freeze-drying overnight. The following day samples were derivatized by adding 50 μl of acetonitrile and 50 μl of N-tert- butyl dimethylslyl-N-methyl trifluoraceramide (MTBSTFA) (Pierce Chemical Corp., Rockford, IL) for 30 minutes at 50°C in a nitrogen environment. The samples were then analyzed by gas chromatography-mass spectrometry (5890II gas chromatograph, 5971A mass spectrometer; Hewlett-Packard Corp; Lexington, MA). For this, 1 μl of derivatized sample was injected into the injection port and heated to 250°C with helium as a carrier gas (0.93 ml/min−1). Separations were performed on a fused silica capillary column (Agilent, Ultra 2: 12m × 0.2 mm I.D. × 0.33 μmol/L film thickness). Selective ion monitoring was performed using an electronionization mode of 70 eV, with the ion source maintained at 180°C. The m/z for tyrosine/3CT and their isotopically labeled standards was 302 and 305, respectively. The retention times for tyrosine/[13C9.15N1]-tyrosine and 3CT/[13C9.15N1]-3CT were 14.07 and 14.65 minutes, respectively. Spectra at m/z 302 were integrated to give levels of tyrosine or 3CT in skin samples. All data are expressed as mean ± SEM of the ratio of 3CT:tyrosine residues.
Statistical Analysis
All data were analyzed by analysis of variance and the Bonferroni’s modified t-test.
Results
Time Course of NO Synthesis, Protein Nitration, Neutrophil Accumulation, and Edema Formation after Thermal Injury
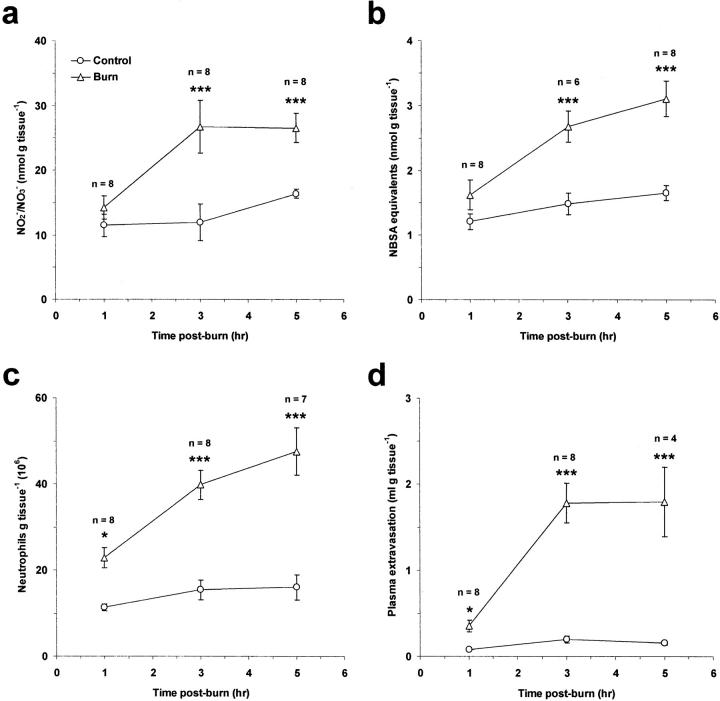
Tissue NO2−/NO3− and 3NT levels were measured in thermally injured and control skin as indicators of NO production and RNS-mediated protein nitration, respectively (Figure 1, a and b) ▶ . Thermal injury had no effect on these parameters when assessed at 1 hour after burn. However, at 3 and 5 hours after burn, a highly significant increase in both NO2−/NO3− and 3NT levels was observed compared to control tissue (P < 0.001) indicating a time dependency to this process. Similarly, thermal injury increased neutrophil accumulation and plasma extravasation in a manner that was progressive and dependent on time (Figure 1, c and d) ▶ . Thermal injury caused a significant increase in these parameters at 1 (P < 0.05), 3 (P < 0.001), and 5 hours (P < 0.001) after burn compared to control tissue, but was greater at 3 and 5 hours after injury than at 1 hour after burn.
Figure 1.
Time course of NO production (a), protein nitration (b), neutrophil accumulation (c), and edema formation (d) in rat skin after induction of local cutaneous thermal injury. Results are expressed as mean ± SEM. *, P < 0.05; ***, P < 0.001 compared to control skin at the corresponding time point.
Effect of 1400W on Burn Wound Pathophysiology
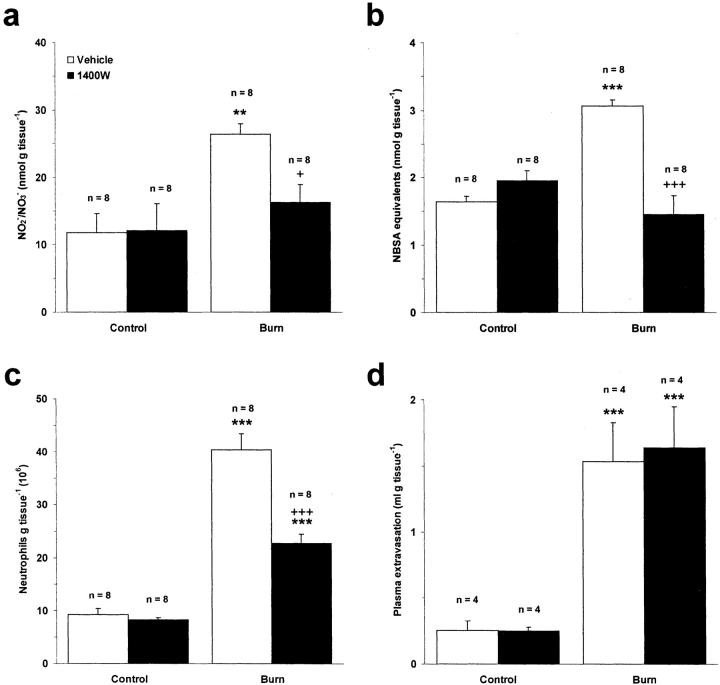
To elucidate the role of iNOS in this experimental system, rats were pretreated with the highly selective iNOS inhibitor, 1400W, or its vehicle control, saline. Heat injury caused a significant increase in NO production compared to control skin in saline-treated animals (P < 0.01) (Figure 2a) ▶ . Pretreatment of rats with 1400W (40 μmol kg−1, s.c.) abolished this increase when assessed at 3 hours after burn (P < 0.05). Similarly, protein nitration was attenuated in rats pretreated with 1400W indicating that iNOS is induced in this model and contributes to generation of RNS within the burn wound (P < 0.001) (Figure 2b) ▶ . 1400W had no effect on burn-induced plasma extravasation (Figure 2d) ▶ , but the neutrophil content of thermally injured skin was significantly reduced in rats pretreated with 1400W compared to those pretreated with saline (P < 0.001) (Figure 2c) ▶ .
Figure 2.
Effect of the iNOS inhibitor 1400W (40 μmol kg−1, s.c.) on NO production (a), protein nitration (b), neutrophil accumulation (c), and edema formation (d) in rat skin after induction of local cutaneous thermal injury at 3 hours after burn. Results are expressed as mean ± SEM. **, P < 0.01; ***, P < 0.001 compared to corresponding control skin. +, P < 0.05; +++, P < 0.001 compared to thermally injured skin in animals pretreated with the vehicle control, saline.
Effect of Neutrophil Depletion on Burn Wound Pathophysiology
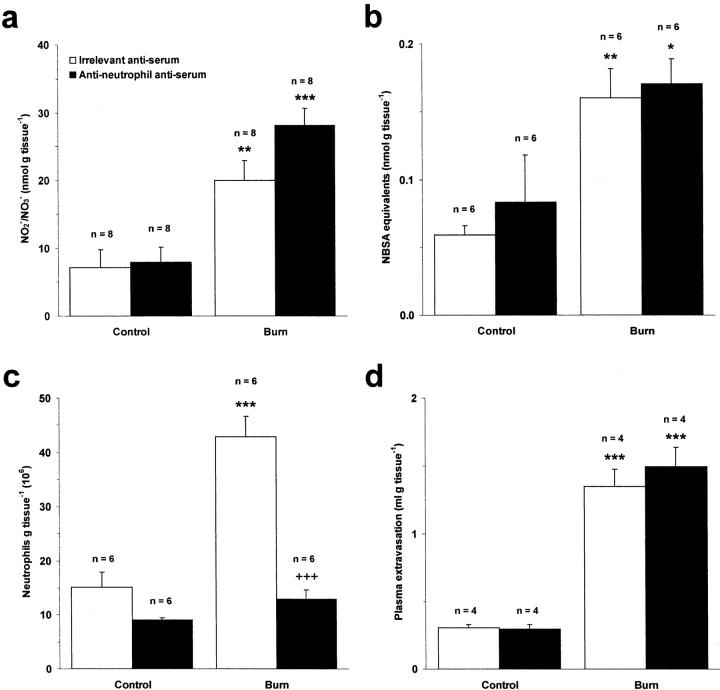
Pretreatment of rats with an anti-neutrophil antiserum (2 ml kg−1, i.p.) selectively depleted circulating neutrophils in these animals without affecting the viability of nonneutrophil leukocytes (data not shown). As expected, this treatment caused a profound reduction of neutrophil infiltration into thermally injured skin at 3 hours after burn when compared to animals treated with an irrelevant antiserum (P < 0.001) (Figure 3c) ▶ . In contrast, neutrophil depletion had no effect on plasma extravasation within the burn wound (Figure 3d) ▶ , indicating that neutrophils do not play an important role in mediating microvascular leakage after burn at this time point. The possibility that neutrophils constitute an important cellular source of NO within the burn wound was investigated by measuring NO2−/NO3− levels in neutrophil-depleted rats. NO production was similar to that observed in rats pretreated with the irrelevant antiserum (Figure 3a) ▶ . Furthermore, depletion of neutrophils had no inhibitory effect on protein nitration within the burn wound (Figure 3b) ▶ .
Figure 3.
Effect of neutrophil depletion with an anti-neutrophil antiserum (2 ml kg−1, i.p.) on NO production (a), protein nitration (b), neutrophil accumulation (c), and edema formation (d) in rat skin after induction of local cutaneous thermal injury at 3 hours after burn. Results are expressed as mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared to corresponding control skin. +++, P < 0.001 compared to thermally injured skin in animals pretreated with an irrelevant antiserum.
Immunohistochemical Detection of iNOS and 3NT
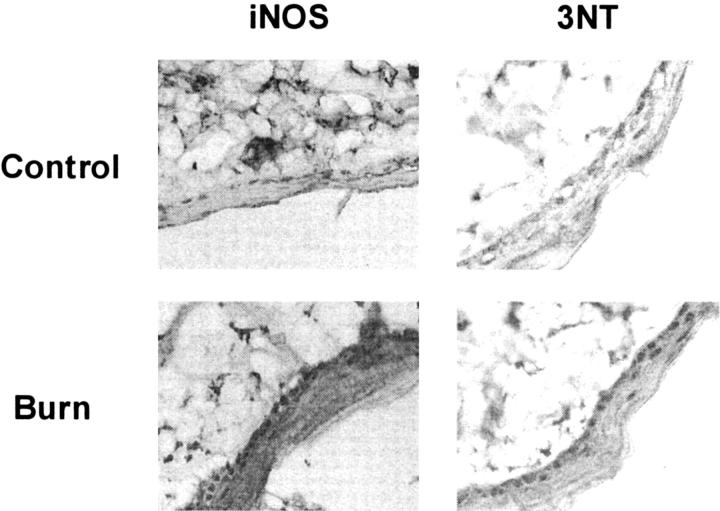
A greater distribution and intensity of iNOS and 3NT staining was detected throughout the epidermis and in some dermal structures of thermally injured rat skin compared to control, untreated skin when examined at 3 hours after burn (Figure 4) ▶ .
Figure 4.
Immunohistochemical localization of iNOS and 3NT in control and thermally injured rat skin at 3 hours after burn. Original magnification, ×40.
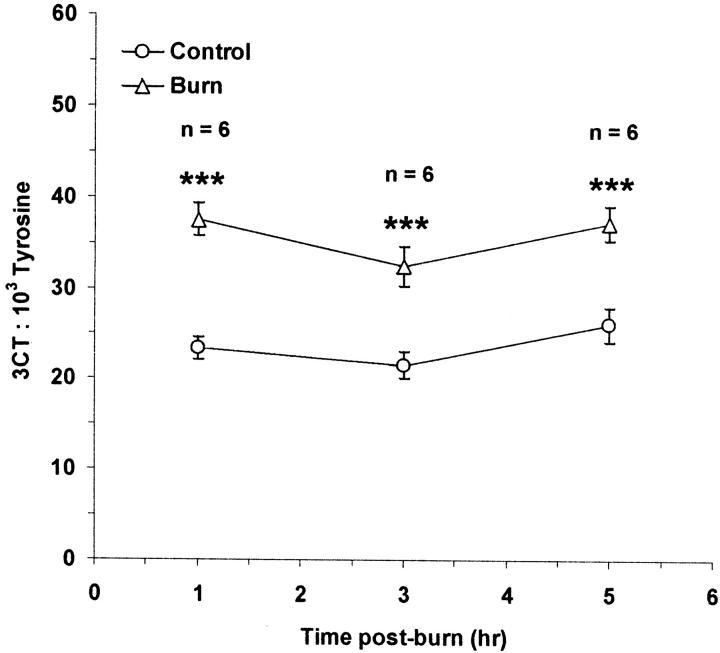
Time Course of Protein Chlorination after Thermal Injury
3CT was measured in thermally injured and control skin as an indicator of neutrophil/MPO-mediated protein chlorination in vivo (Figure 5) ▶ . An approximate 1.5-fold increase in the 3CT content of thermally injured skin was detected at 1, 3, and 5 hours after burn compared to untreated, control tissue (P < 0.001).
Figure 5.
Time course of protein chlorination of rat skin after induction of local cutaneous thermal injury. Results are expressed as mean ± SEM. ***, P < 0.001 compared to control skin at the corresponding time point.
Discussion
It has been suggested that pharmacological therapies are unlikely to be of benefit in preventing the development of the inflammatory response after burn because of the complex nature of the chemical mediators involved in this process. 31 However, we have demonstrated that a selective iNOS inhibitor, 1400W, attenuated NO production, protein nitration, and neutrophil accumulation in response to burn injury. Evidence of neutrophil-mediated protein chlorination was also obtained, but interestingly, neutrophil depletion did not affect either NO production or protein nitration. In addition, we did not obtain any evidence that NO contributes to the development of burn-associated edema formation in our model.
1400W abolished elevated protein nitration in thermally injured tissues, indicating that in this instance, the generation of RNS was entirely dependent on iNOS activity. There is much circumstantial evidence to associate iNOS induction with protein nitration, 15 but few studies demonstrate a functional relationship between iNOS activity and protein nitration in vivo. Interestingly, iNOS inhibition has previously been reported to prevent protein nitration in the gastrointestinal tract of rats subjected to a large total body surface area burn injury. 21 Thus, iNOS may contribute to remote organ dysfunction as well as burn wound pathophysiology under such circumstances. The neutrophil could well have a central role in this context, as it has been proposed that these cells are activated at the site of burn, then circulate to initiate and amplify responses in remote organs. 32 The inhibitory effect of 1400W on neutrophil accumulation could therefore be beneficial in treating both local and systemic events. In addition, there is growing evidence to suggest that iNOS-derived NO and/or associated species promote leukocyte accumulation. Previous reports demonstrate that 1400W attenuates eosinophil accumulation in the inflamed murine lung 33 and other iNOS inhibitors have been shown to exhibit similar activity in vivo. 34 Moreover, neutrophil infiltration is suppressed in iNOS knockout mice. 35 The mechanism by which iNOS-derived NO promotes neutrophil accumulation is unclear. It has been suggested that NO increases the delivery of leukocytes to inflamed tissues through its effect on microvascular blood flow. 29 Alternatively, it has been proposed that iNOS-derived NO promotes neutrophil accumulation by enhancing the synthesis of MIP-2 and KC and it is of interest to note that the levels of both chemokines are elevated after thermal injury. 36 Furthermore, one cannot discount the possibility that ONOO− is involved in this process because iNOS inhibition attenuated protein nitration, as well as neutrophil accumulation in this model. Indeed, noncytotoxic concentrations of ONOO− have been shown to enhance the surface expression of l-selectin, Mac-1, and the fMLF receptor in human neutrophils. 37 The lack of effect of 1400W on plasma extravasation conflicts with earlier studies that show iNOS inhibitors to attenuate this parameter in rodent models of local cutaneous thermal injury. 9 However, it is well established that multiple factors mediate burn-induced plasma extravasation and as such, NO-associated pathways may have been redundant at the time of sampling in our model.
It was hypothesized that the neutrophil leukocyte plays an important role in mediating RNS synthesis and protein nitration within the burn wound. MPO catalyzes tyrosine nitration by ONOO− 38 in addition to generating HOCl. 39 However, depletion of circulating neutrophils with an anti-neutrophil antiserum had no effect on burn-induced protein nitration in this model. It would therefore appear that the neutrophil is not required for protein nitration within the burn wound. Furthermore, neutrophil-derived HOCl has the potential to react with nitrite (NO2−) to form nitryl chloride (NO2Cl), another endogenous nitrating agent. 39 Because NO2Cl is the only nitrating agent other than ONOO− or ONOO−-derived species likely to be formed outside of highly acidic physiological environments (eg, the stomach), then one could conclude that ONOO− is the most probable initiator of protein nitration in the studies presented here and appears to be generated from NO and O2− of nonneutrophil origin in an MPO-independent manner. We have recently shown that the neutrophil plays an essential role in mediating protein nitration in a rat model of nonallergic, nonseptic zymosan-induced inflammation. 40 The results presented here would appear to be at variance with this finding. A recent study of acute inflammatory responses in eosinophil peroxidase and MPO-knockout mice shows similar contrasting results. 41 In this study, leukocyte peroxidases were shown to participate in protein nitration in mice treated with Candida albicans, but not those treated with Mesocestoides corti/zymosan or thioglycolate/zymosan and it was suggested that these findings could be explained by thus far unidentified differences in the chemical milieu surrounding inflamed tissue. Regardless, we emphasize the importance of iNOS-derived NO in the generation of RNS during inflammation, as in both the present thermal injury study and our previous investigation, 40 protein nitration was abolished by an iNOS inhibitor.
If as suggested, ONOO− is generated within the burn wound then this may have important implications for its pathophysiology. For example, ONOO− has been shown to inactivate superoxide dismutase 42 and α1-anti-proteinase 43 via tyrosine nitration. If this process occurs within the burn wound, it may partially explain why superoxide dismutase activity is markedly diminished 44 and elastase activity is enhanced in this microenvironment. 45 Moreover, ONOO− can cause DNA strand breakage 46 and directly induce lipid peroxidation. 47 The relevance of DNA damage to acute burn injury is not immediately apparent, however, this process can lead to poly-ADP ribose synthetase-related energy depletion in inflamed tissue 48 and may therefore contribute to the ATP deficiencies commonly observed in severely burned patients. 49 Indeed, poly-ADP ribose synthetase inhibition has been shown to be of benefit in preventing cellular NAD+ losses in animal models of shock. 50 With respect to lipid peroxidation, ONOO− may be an important initiator of this process within the burn wound, because O2− has poor reactivity with lipids, 51 yet exogenous superoxide dismutase mimetics attenuate this parameter in thermally injured humans. 52 Furthermore, this is the first demonstration of protein chlorination within the burn wound, as measured by a quantitative gas chromatography-mass spectrometry assay. The findings are interesting because 3CT levels were highest at 1 hour after burn, whereas neutrophil accumulation continued to rise from 1 to 5 hours after injury. As such, one could conclude that a relatively small threshold of neutrophil activity is required to cause maximal protein chlorination within the burn wound. The significance of HOCl generation in vivo is poorly understood, however, like ONOO−, this molecule can inactivate proteins 24 and cause endothelial dysfunction in vitro. 53 Further studies are therefore required to identify the functional importance of protein chlorination within the burn wound.
In conclusion, we have used a rat model of deep partial thickness burn injury to demonstrate the involvement of iNOS-derived NO in promoting RNS synthesis and enhancing neutrophil accumulation within thermally injured cutaneous tissues. We also provide evidence to show that neutrophil/MPO-mediated protein modifications occur within the burn wound, although depletion of these cells did not alter NO synthesis or subsequent RNS generation. Interestingly, neither iNOS-derived NO nor neutrophils appear to have a critical role in mediating plasma extravasation in this model. Although these studies have provided insight into the synthesis and function of NO and RNS in thermal injury, further investigations are required to determine precise molecular pathologies associated with iNOS induction under such circumstances. Nonetheless, therapeutic iNOS inhibition may prove to be of benefit in limiting the secondary tissue damage caused by neutrophil- and nonneutrophil-derived reactive species in humans with thermal skin trauma and therefore limit the requirement for wound excision and subsequent skin grafting under such circumstances. In addition, we consider the relationship between iNOS induction in thermally injured skin and the development of potentially fatal systemic sequelae (eg, systemic inflammatory response syndrome and multiple organ dysfunction syndrome) worthy of closer examination.
Footnotes
Address reprint requests to Professor S. D. Brain, Centre for Cardiovascular Biology and Medicine, King’s College London, New Hunt’s House, Guy’s Campus, London SE1 1UL, UK. E-mail: sue.brain@kcl.ac.uk.
Supported by grants from the Medical Research Council (United Kingdom).
References
- 1.Kripner J, Broz L, Konigova R, Bouska I: Mortality in pediatric burns in the Prague Burns Centre (1994–1997). Acta Chir Plast 1998, 40:79-82 [PubMed] [Google Scholar]
- 2.Jakobsson OP, Benediktsson G, Arturson G: Early post-burn oedema in leucocyte-free rats. Burns Incl Therm Inj 1985, 12:16-21 [DOI] [PubMed] [Google Scholar]
- 3.Siney L, Brain SD: Involvement of sensory neuropeptides in the development of plasma extravasation in rat dorsal skin following thermal injury. Br J Pharmacol 1996, 117:1065-1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rawlingson A, Gerard NP, Brain SD: Interactive contribution of NK1 and kinin receptors to the acute inflammatory oedema observed in response to noxious heat stimulation: studies in NK1 receptor knockout mice. Br J Pharmacol 2001, 134:1805-1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waller J, Siney L, Hoult JR, Brain SD: A study of neurokinins and other oedema-inducing mediators and mechanisms in thermal injury. Clin Exp Pharmacol Physiol 1997, 24:861-863 [DOI] [PubMed] [Google Scholar]
- 6.Pinter E, Brown B, Hoult JR, Brain SD: Lack of evidence for tachykinin NK1 receptor-mediated neutrophil accumulation in the rat cutaneous microvasculature by thermal injury. Eur J Pharmacol 1999, 369:91-98 [DOI] [PubMed] [Google Scholar]
- 7.Becker WK, Shippee RL, McManus AT, Mason AD, Jr, Pruitt BA, Jr: Kinetics of nitrogen oxide production following experimental thermal injury in rats. J Trauma 1993, 34:855-862 [DOI] [PubMed] [Google Scholar]
- 8.Gamelli RL, George M, Sharp-Pucci M, Dries DJ, Radisavljevic Z: Burn-induced nitric oxide release in humans. J Trauma 1995, 39:869-877 [DOI] [PubMed] [Google Scholar]
- 9.Sozumi T: The role of nitric oxide in vascular permeability after a thermal injury. Ann Plast Surg 1997, 39:272-277 [DOI] [PubMed] [Google Scholar]
- 10.Yonehara N, Yoshimura M: Interaction between nitric oxide and substance P on heat-induced inflammation in rat paw. Neurosci Res 2000, 36:35-43 [DOI] [PubMed] [Google Scholar]
- 11.Inoue H, Ando K, Wakisaka N, Matsuzaki K, Aihara M, Kumagai N: Effects of nitric oxide synthase inhibitors on vascular hyperpermeability with thermal injury in mice. Nitric Oxide 2001, 5:334-342 [DOI] [PubMed] [Google Scholar]
- 12.Paulsen SM, Wurster SH, Nanney LB: Expression of inducible nitric oxide synthase in human burn wounds. Wound Repair Regen 1998, 6:142-148 [DOI] [PubMed] [Google Scholar]
- 13.Davis KL, Martin E, Turko IV, Murad F: Novel effects of nitric oxide. Annu Rev Pharmacol Toxicol 2001, 41:203-236 [DOI] [PubMed] [Google Scholar]
- 14.Carreras MC, Pargament GA, Catz SD, Poderoso JJ, Boveris A: Kinetics of nitric oxide and hydrogen peroxide production and formation of peroxynitrite during the respiratory burst of human neutrophils. FEBS Lett 1994, 341:65-68 [DOI] [PubMed] [Google Scholar]
- 15.Greenacre SA, Ischiropoulos H: Tyrosine nitration: localisation, quantification, consequences for protein function and signal transduction. Free Radic Res 2001, 34:541-581 [DOI] [PubMed] [Google Scholar]
- 16.Rachmilewitz D, Stamler JS, Karmeli F, Mullins ME, Singel DJ, Loscalzo J, Xavier RJ, Podolsky DK: Peroxynitrite-induced rat colitis—a new model of colonic inflammation. Gastroenterology 1993, 105:1681-1688 [DOI] [PubMed] [Google Scholar]
- 17.Greenacre S, Ridger V, Wilsoncroft P, Brain SD: Peroxynitrite: a mediator of increased microvascular permeability? Clin Exp Pharmacol Physiol 1997, 24:880-882 [DOI] [PubMed] [Google Scholar]
- 18.Salvemini D, Wang ZQ, Wyatt PS, Bourdon DM, Marino MH, Manning PT, Currie MG: Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br J Pharmacol 1996, 118:829-838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costantino G, Cuzzocrea S, Mazzon E, Caputi AP: Protective effects of melatonin in zymosan-activated plasma-induced paw inflammation. Eur J Pharmacol 1998, 363:57-63 [DOI] [PubMed] [Google Scholar]
- 20.Hattori Y, Nishigori C, Tanaka T, Uchida K, Nikaido O, Osawa T, Hiai H, Imamura S, Toyokuni S: 8-hydroxy-2[prime]-deoxyguanosine is increased in epidermal cells of hairless mice after chronic ultraviolet B exposure. J Invest Dermatol 1996, 107:733-737 [DOI] [PubMed] [Google Scholar]
- 21.Chen LW, Hsu CM, Wang JS, Chen JS, Chen SC: Specific inhibition of iNOS decreases the intestinal mucosal peroxynitrite level and improves the barrier function after thermal injury. Burns 1998, 24:699-705 [DOI] [PubMed] [Google Scholar]
- 22.Soejima K, Traber LD, Schmalstieg FC, Hawkins H, Jodoin JM, Szabo C, Szabo E, Varig L, Salzman A, Traber DL: Role of nitric oxide in vascular permeability after combined burns and smoke inhalation injury. Am J Respir Crit Care Med 2001, 163:745-752 [DOI] [PubMed] [Google Scholar]
- 23.Weiss SJ: Tissue destruction by neutrophils. N Engl J Med 1989, 320:365-376 [DOI] [PubMed] [Google Scholar]
- 24.Favero TG, Colter D, Hooper PF, Abramson JJ: Hypochlorous acid inhibits Ca2+-ATPase from skeletal muscle sarcoplasmic reticulum. J Appl Physiol 1998, 84:425-430 [DOI] [PubMed] [Google Scholar]
- 25.Hazen SL, Heinecke JW: 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest 1997, 99:2075-2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baskaran H, Yarmush ML, Berthiaume F: Dynamics of tissue neutrophil sequestration after cutaneous burns in rats. J Surg Res 2000, 93:88-96 [DOI] [PubMed] [Google Scholar]
- 27.Hansbrough JF, Wikstrom T, Braide M, Tenenhaus M, Rennekampff OH, Kiessig V, Bjursten LM: Neutrophil activation and tissue neutrophil sequestration in a rat model of thermal injury. J Surg Res 1996, 61:17-22 [DOI] [PubMed] [Google Scholar]
- 28.Garvey EP, Oplinger JA, Furfine ES, Kiff RJ, Laszlo F, Whittle BJ, Knowles RG: 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J Biol Chem 1997, 272:4959-4963 [DOI] [PubMed] [Google Scholar]
- 29.Ridger VC, Pettipher ER, Bryant CE, Brain SD: Effect of the inducible nitric oxide synthase inhibitors aminoguanidine and L-N6-(1-iminoethyl)lysine on zymosan-induced plasma extravasation in rat skin. J Immunol 1997, 159:383-390 [PubMed] [Google Scholar]
- 30.Greenacre SA, Evans P, Halliwell B, Brain SD: Formation and loss of nitrated proteins in peroxynitrite-treated rat skin in vivo. Biochem Biophys Res Commun 1999, 262:781-786 [DOI] [PubMed] [Google Scholar]
- 31.Arturson G: Pathophysiology of the burn wound and pharmacological treatment. The Rudi Hermans Lecture, 1995. Burns 1996, 22:255-274 [DOI] [PubMed] [Google Scholar]
- 32.Piccolo MT, Wang Y, Verbrugge S, Warner RL, Sannomiya P, Piccolo NS, Piccolo MS, Hugli TE, Ward PA, Till GO: Role of chemotactic factors in neutrophil activation after thermal injury in rats. Inflammation 1999, 23:371-385 [DOI] [PubMed] [Google Scholar]
- 33.Koarai A, Ichinose M, Sugiura H, Yamagata S, Hattori T, Shirato K: Allergic airway hyperresponsiveness and eosinophil infiltration is reduced by a selective iNOS inhibitor, 1400W, in mice. Pulm Pharmacol Ther 2000, 13:267-275 [DOI] [PubMed] [Google Scholar]
- 34.Nishida K, Ohta Y, Ishiguro I: Contribution of NO synthases to neutrophil infiltration in the gastric mucosal lesions in rats with water immersion restraint stress. FEBS Lett 1998, 425:243-248 [DOI] [PubMed] [Google Scholar]
- 35.Ajuebor MN, Virag L, Flower RJ, Perretti M, Szabo C: Role of inducible nitric oxide synthase in the regulation of neutrophil migration in zymosan-induced inflammation. Immunology 1998, 95:625-630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faunce DE, Llanas JN, Patel PJ, Gregory MS, Duffner LA, Kovacs EJ: Neutrophil chemokine production in the skin following scald injury. Burns 1999, 25:403-410 [DOI] [PubMed] [Google Scholar]
- 37.Rohn TT, Nelson LK, Sipes KM, Swain SD, Jutila KL, Quinn MT: Priming of human neutrophils by peroxynitrite: potential role in enhancement of the local inflammatory response. J Leukoc Biol 1999, 65:59-70 [DOI] [PubMed] [Google Scholar]
- 38.Sampson JB, Rosen H, Beckman JS: Peroxynitrite-dependent tyrosine nitration catalyzed by superoxide dismutase, myeloperoxidase, and horseradish peroxidase. Methods Enzymol 1996, 269:210-218 [DOI] [PubMed] [Google Scholar]
- 39.Eiserich JP, Cross CE, Jones AD, Halliwell B, van der Vliet A: Formation of nitrating and chlorinating species by reaction of nitrite with hypochlorous acid. A novel mechanism for nitric oxide-mediated protein modification. J Biol Chem 1996, 271:19199-19208 [DOI] [PubMed] [Google Scholar]
- 40.Greenacre SAB, Rocha FAC, Rawlingson A, Meinerikandathevan S, Poston RN, Ruiz E, Halliwell B, Brain SD: Protein nitration in cutaneous inflammation in the rat: essential role of inducible nitric oxide synthase and polymorphonuclear leukocytes. Br J Pharmacol 2002, 136:985-994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brennan ML, Wu W, Fu X, Shen Z, Song W, Frost H, Vadseth C, Narine L, Lenkiewicz E, Borchers MT, Lusis AJ, Lee JJ, Lee NA, Abu-Soud HM, Ischiropoulos H, Hazen SL: A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J Biol Chem 2002, 277:17415-17427 [DOI] [PubMed] [Google Scholar]
- 42.Grzelak A, Soszynski M, Bartosz G: Inactivation of antioxidant enzymes by peroxynitrite. Scand J Clin Lab Invest 2000, 60:253-258 [DOI] [PubMed] [Google Scholar]
- 43.Gole MD, Souza JM, Choi I, Hertkorn C, Malcolm S, Foust RF, III, Finkel B, Lanken PN, Ischiropoulos H: Plasma proteins modified by tyrosine nitration in acute respiratory distress syndrome. Am J Physiol 2000, 278:L961-L967 [DOI] [PubMed] [Google Scholar]
- 44.Kawai S, Komura J, Asada Y, Niwa Y: Experimental burn-induced changes in lipid peroxide levels, and activity of superoxide dismutase and glutathione peroxidase in skin lesions, serum, and liver of mice. Arch Dermatol Res 1988, 280:171-175 [DOI] [PubMed] [Google Scholar]
- 45.Barisoni D, Bellavite P, Sorio A, Bonazzi ML, Zermani R, Bortolani A: Monitoring of elastase in plasma of burned patients in relation to other inflammation parameters. Burns 1991, 17:141-146 [DOI] [PubMed] [Google Scholar]
- 46.Epe B, Ballmaier D, Roussyn I, Briviba K, Sies H: DNA damage by peroxynitrite characterized with DNA repair enzymes. Nucleic Acids Res 1996, 24:4105-4110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radi R, Beckman JS, Bush KM, Freeman BA: Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys 1991, 288:481-487 [DOI] [PubMed] [Google Scholar]
- 48.Szabo C, Ohshima H: DNA damage induced by peroxynitrite: subsequent biological effects. Nitric Oxide 1997, 1:373-385 [DOI] [PubMed] [Google Scholar]
- 49.Yu YM, Tompkins RG, Ryan CM, Young VR: The metabolic basis of the increase in energy expenditure in severely burned patients. J Parenter Enteral Nutr 1999, 23:160-168 [DOI] [PubMed] [Google Scholar]
- 50.Cuzzocrea S, Zingarelli B, Caputi AP: Peroxynitrate-mediated DNA strand breakage activates poly(ADP-ribose) synthetase and causes cellular energy depletion in a nonseptic shock model induced by zymosan in the rat. Shock 1998, 9:336-340 [DOI] [PubMed] [Google Scholar]
- 51.Bielski BH, Arudi RL, Sutherland MW: A study of the reactivity of HO2/O2− with unsaturated fatty acids. J Biol Chem 1983, 258:4759-4761 [PubMed] [Google Scholar]
- 52.Thomson PD, Till GO, Woolliscroft JO, Smith DJ, Prasad JK: Superoxide dismutase prevents lipid peroxidation in burned patients. Burns 1990, 16:406-408 [DOI] [PubMed] [Google Scholar]
- 53.Tatsumi T, Fliss H: Hypochlorous acid and chloramines increase endothelial permeability: possible involvement of cellular zinc. Am J Physiol 1994, 267:H1597-H1607 [DOI] [PubMed] [Google Scholar]