Abstract
Cellular senescence has been suggested to play a role in the deterioration of renal graft function and has been linked to telomere shortening. We have investigated markers of cellular senescence in the F344 to LEW rat model of chronic renal transplant rejection. Syngeneic and LEW to F344 transplants were used as controls. Substantial telomere shortening was observed in all transplants, including allogeneic and syngeneic grafts from day 7 post-transplant onwards. Ischemia of native F344 kidneys was already sufficient to induce telomere shortening. It is known that shortened telomeres can activate cell cycle regulators, such as p21 and p16. Accordingly, all cases showed a transient p21 increase, with a maximum at day 7 and a sustained expression of p16. Importantly, senescence-associated β-galactosidase staining, a cytological marker for senescence, was only observed in tubular epithelial cells of chronically rejecting F344 allografts from day 30 post-transplantation onwards. Long-term surviving LEW allografts or syngeneic F344 grafts were negative for senescence-associated β-galactosidase. In conclusion, ischemia during transplantation results in telomere shortening and subsequent activation of p21 and p16, whereas senescence-associated β-galactosidase staining is only present in chronically rejecting kidney grafts.
Chronic allograft rejection (CR) is the most important cause of renal transplant loss. Various immunological and non-immunological factors have been implicated in its pathogenesis. 1 The pathological lesions observed in chronic rejection and allograft nephropathy overlap with the changes observed in aging kidneys 2 and it has been suggested that senescence of renal cells might contribute to the deterioration of graft function. 3
Somatic cells in vivo have a limit in their replicative capacity termed the Hayflick limit. 4 This limit has been ascribed to the loss of telomeric sequences at the end of chromosomes. Loss of telomeric repeats (TTAGGG) during sequential replications eventually compromises telomere function, leading to chromosomal instability and loss of genetic information. After cells have reached their maximal replicative potential they stop proliferating and may become senescent. Senescent cells are irreversibly arrested in the G1 phase of the cell cycle. Cells in senescence do not respond to various external stimuli, but remain metabolically active and contribute to an impaired tissue integrity and persistent inflammation. 5 In vivo cellular senescence has been proposed to act as a mechanism to prevent neoplastic transformation of cells. Furthermore, it is thought to act as a homeostatic mechanism to prolong the cellular lifespan. 6,7
Senescent cells display several characteristics, including shortened telomeres, increased expression of specific tumor suppressor genes and an increased activity of senescence-associated β-galactosidase (SA β-gal). 8,9 In addition, alterations in cell shape, altered matrix metalloproteinase and cytoskeletal collagen expression have been described. 10,11 Telomere erosion associated with senescence of somatic cells in culture has been extrapolated to the features of chronological aging, including a decrease in physiological capacity, loss of mass and decreased resistance to stress. Relatively short telomeres in old kidneys 2,12 may predispose to impaired graft outcome post-transplant. One important observation, in this respect, is that kidneys from older donors show worse graft survival. 13
The molecular mechanism by which telomere erosion limits proliferative potential has not been elucidated and a number of equivocal hypotheses have been proposed. One hypothesis is that critically shortened telomeres are unable to recruit sufficient telomeric proteins to form a functional nucleoprotein cap, which would expose a free broken DNA end as a consequence. Alternatively, a shortened telomere repeat stretch or an increase in the availability of free telomeric proteins arising through loss of substrate sites resulting in the necessary signals for senescence. 14
As cells become senescent there are accompanying changes in the expression of p21 and p16, which are involved in an arrest in the G1 phase of the cell cycle. These are induced in response to DNA damage (eg, via p53 activation), which subsequently may activate members of the cyclin dependent kinase (CDK) inhibitor family. 15 Activation of p53 results in activation of inhibitors of CDK4 (INK4 family, including p16ink4) and inhibitors of the cyclin E and A dependent kinases (Cip/Kip family, p21Cip1, Waf1, Sdi1). 16 Activation of INK4 and Kip family members results in inhibition of the cell cycle in the G1 phase via the retinoblastoma protein. An increased expression of p21 is involved in the induction of senescence whereas p16 accumulates in senescent cells and is involved in maintenance of senescence. 16-18 A marker suggested to be specific for senescent cells is accumulation of lysosomal senescence-associated β-galactosidase (SA β-gal) which is active at pH 6.0. 8,9 In vitro observations indicate that SA β-gal accumulates as cells senesce, though in vivo observations supporting this are limited. 19,20
In the present study, we used a rat model of human chronic transplant rejection to investigate to what extent the senescence markers are present in renal allografts. Transplantation of a F344 kidney into a LEW recipient results after a brief episode of acute rejection in chronic rejection. 21 Transplantation of a LEW kidney into a F344 recipient also results in acute rejection but chronic rejection does not occur. In this model we demonstrate telomere shortening, and subsequent expression of p21 and p16 proteins in both F344 to LEW and LEW to F344 allografts. However, SA β-gal staining was only found in tubular epithelial cells of F344 allografts with chronic rejection and not in syngeneic or LEW allografts. Thus, in this rat model of chronic rejection we found that telomere shortening is a first step for cellular senescence and occurs due to ischemia but additional processes are required for the induction of SA β-gal accumulation.
Materials and Methods
Animals
Nine-week-old male inbred Fisher (F344, RT1lv1) and Lewis (LEW, RT1l) rats were purchased from Harlan, Horst, The Netherlands. Animals had free access to water and standard rat chow. Animal care and experimentation were undertaken in accordance with the National Institutes of Health Guide for the care and use of laboratory animals.
Kidney Transplantation
Kidney transplantations were performed as previously described. 22 Rats were 9 to 11 weeks of age at the time of transplantation. The left kidney of the recipient was removed and a donor kidney was transplanted in the orthotopic position. The remaining native right kidney was removed 7 days after transplantation. Postoperatively, animals received 1 mg/kg body weight of Temgesic subcutanously (buprenorphine-hydrochlorid, Schering-Plough B.V., Amstelveen, The Netherlands) for pain relief. Cold ischemia times varied between 30 and 45 minutes.
LEW rats that received a F344 kidney graft were sacrificed on days 7, 14, 30, 60, and 100 after transplantation and kidneys were perfused with PBS and snap-frozen in liquid nitrogen. Similarly, F344 rats received a LEW kidney and were sacrificed on days 7, 14, 30, 60, and 100. In addition syngeneic transplants, both F344 to F344 and LEW to LEW, were performed and sacrificed on day 14 and day 60 post-transplantation (post-Tx). All experiments were performed in the absence of immunosuppression.
Ischemia-Reperfusion Experiments
Male F344 rats (9 weeks of age) were operated under halothane anesthesia. The renal artery was clamped using small artery clamps for 45 minutes (warm ischemia). From each rat both kidneys were clamped for 45 minutes, one kidney was removed after 45 minutes of ischemia, the second kidney was reperfused for 2 hours. Kidneys were harvested and snap-frozen in liquid nitrogen.
Histology
Tissue samples were fixed in methyl Carnoy’s solution (50% methanol, 30% chloroform, 20% acetic acid), paraffin-embedded, and stained with periodic acid-Schiff (Merck, Darmstadt, Germany) or hematoxylin and eosin (Klinipath, Duiven, The Netherlands). Sections were scored blindly by a pathologist according to the Banff working classification. 23
Telomere Length Assay
To determine total telomeric DNA content the TeloREAD assay was used (Promega Corporation, WI, USA) as described. 24 Briefly, total genomic DNA was isolated according to standard phenol/chloroform extraction methods. Ten nanograms of DNA were denatured and incubated with TeloDetection enzymes and a telomere- specific probe; pyrophosphorolysis was performed at 55°C for 60 minutes. Finally, ENLITEN reagent was added and light output was measured in a luminometer. Telomere lengths calculated by the TeloREAD assay were verified by comparison with independent control samples of known telomere lengths determined by standard Southern blot analyses of telomere restriction fragment lengths (TRF).
Protein Isolation and Western Blot Analysis
To detect protein levels of the cell cycle regulators p21 and p16 in renal allografts, Western blot analysis was performed. Pieces of kidney cortex were homogenized using an ultra turrax (IKA Labortechnik, Staufen, Germany) and lysed in lysis buffer, containing 20 mmol/L Tris-HCl (pH 7.4), 137 mmol/L NaCl, 10% glycerol, 1% Triton X-100, 2 mmol/L ethylene diamine tetra acetic acid (EDTA), 1 mmol/L phenylmethylsulfanylfluoride (PMSF), 2 μg/ml leupeptin, 2 μg/ml antipain, 2 μg/ml chymostatin and 5 units/ml trasylol. The amount of protein was determined using the bicinchoninic acid (BCA) protein assay (Pierce Chemical Co., Rockford, IL) and 70 μg of protein was applied under reducing conditions to 12% (p21) or 15% (p16) SDS-PAGE gels. After electrophoresis, proteins were blotted semi-dry to polyvinyldene fluoride (PVDF) membranes (Immobilon-P, Millipore, Bedford, USA). Membranes were blocked with PBS/0.05% Tween 20/2% casein before incubation with the primary antibody. For staining of p21 protein we used goat antibodies directed against p21 (sc-397G, Santa Cruz Biotechnology, CA, USA) that were subsequently incubated with a HRP-conjugated rabbit anti-goat antiserum (Dako, Glostrup, Denmark). To detect p16 protein we used a mouse monoclonal antibody (sc-1661, Santa Cruz) followed by a HRP conjugated goat-anti-mouse antiserum (Dako). Finally, development of the blots was performed with Supersignal (Pierce) and exposure to Hyperfilm films (Amersham Pharmacia Biotech, UK).
Blots were stripped using Restore Western Blot Stripping buffer (Pierce) for 20 minutes at 37°C. Subsequently blots were incubated with a mouse monoclonal antibody reactive with actin (sc-8432, Santa Cruz) followed by a HRP conjugated goat-anti-mouse antiserum (Dako) to quantify the amount of proteins in all samples. Expression was quantified using Stratagene-EagleSight software (Stratagene, Amsterdam, The Netherlands).
Immunohistochemistry
Detection of p16 protein expression was performed with immunohistochemistry using acetone fixed, 3-μm thin cryostat sections. First the endogenous peroxidase activity and non-specific protein binding were blocked using 0.6% H2O2/0.01% NaN3 in PBS and PBS/1% BSA/1% normal goat serum respectively. The primary antibody, a mouse monoclonal antibody against p16 (Santa Cruz) diluted in PBS/1% BSA was applied overnight. After washing, the sections were incubated with the secondary antibody, HRP-conjugated goat-anti-mouse immunoglobulins (Dako). Finally, the sections were stained with diaminobenzidine hydrochloride (DAB, Sigma) and embedded in entellan (Merck, Darmstadt, Germany).
Senescence Staining
Staining for senescence-associated β-galactosidase (SA β-gal) activity was performed as previously described 8 with some modifications. Briefly, cryostat sections (3 μm) of snap-frozen post-Tx and normal kidneys were fixed with 0.2% glutaraldehyde and 2% formaldehyde. After washing with PBS, sections were incubated for 18 hours with freshly prepared stain solution at 37°C (no CO2). The stain solution contained 2 mg/ml 5-bromo-4-chloro-3-indolyl β-D-galactopyranoside (X-gal, Sigma Chemical Company, St. Louis, MI, USA) in 40 mmol/L citric acid/sodium phosphate (pH 6.0), 5 mmol/L potassium ferrocyanide, 5 mmol/L potassium ferricyanide, 150 mmol/L NaCl, 30 mmol/L MgCl2. Subsequently, sections were washed and counterstained with eosin.
Quantification of SA β-gal staining was performed using a Zeiss microscope equipped with a full-color 3 CCD camera. Images were analyzed with KS-400 image analysis software from Zeiss Kontron to quantify the amount of blue staining within the cells. Damaged tissue, vessels, and glomeruli were excluded from the analysis manually. All sections analyzed were stained at the same time and all analysis parameters were kept identical.
Results
Telomere Shortening Is Found in Both F344 and LEW Allografts from Day 7 Post-Transplant
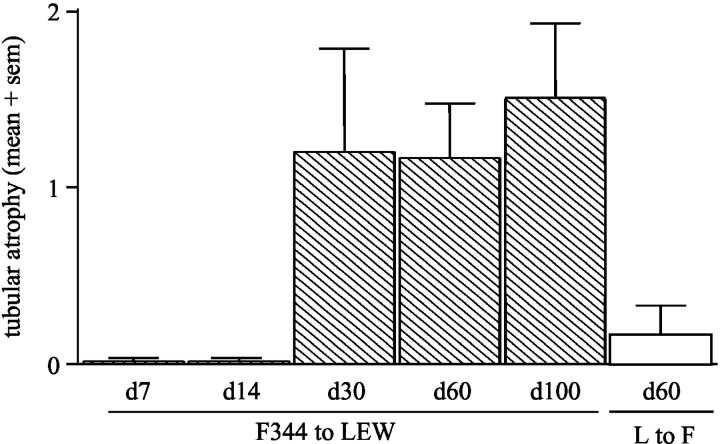
F344 renal allografts removed from LEW recipients at various time points post-Tx showed interstitial infiltrate and various glomerular lesions. Tubular atrophy and vascular lesions were present in kidneys removed at day 30 post-Tx and at later time points (Figure 1) ▶ . In contrast, LEW allografts removed from F344 recipients did not show tubular atrophy or vascular abnormalities at any time point.
Figure 1.
Tubular atrophy in F344 to LEW renal allografts at day 7, 14, 30, 60, and 100 post-Tx according to Banff criteria (0, no tubular atrophy; 1, tubular atrophy in <25% of cortical tubules; 2, tubular atrophy in 26 to 50% of cortical tubules; 3, tubular atrophy in >51% of cortical tubules). LEW to F344 allografts are shown in day 60 post-Tx.
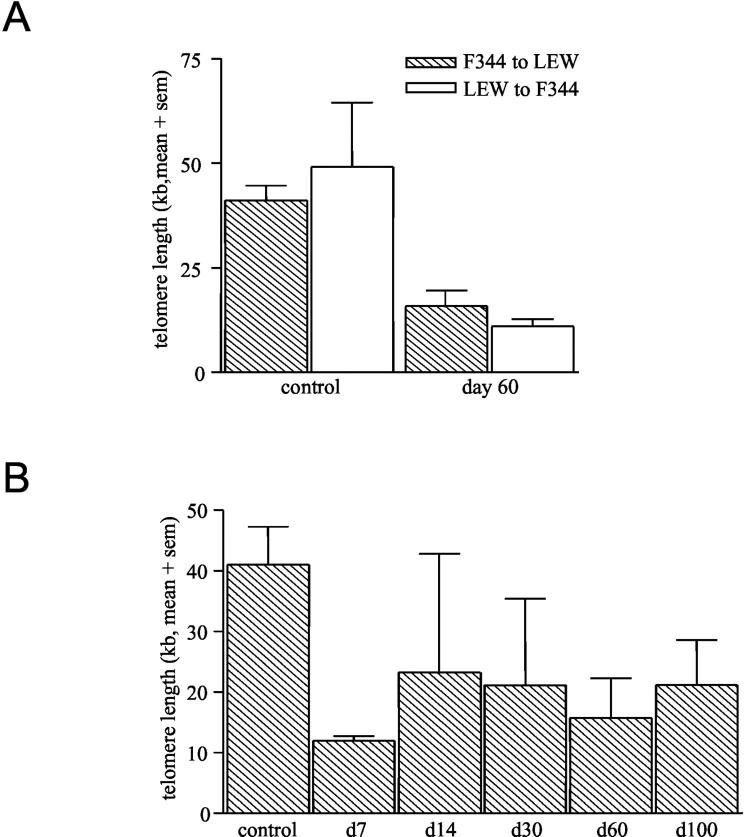
Telomere lengths were measured using the TeloREAD assay 24 in F344 to LEW and LEW to F344 renal allografts and compared these to control, non-transplanted LEW, and F344 kidneys. Normal rat kidneys have median telomere lengths of about 45 kb (Figure 2A) ▶ . F344 to LEW and LEW to F344 allografts removed at day 60 post-Tx both have shortened telomeres of about 10 to 20 kb (Figure 2A) ▶ . Furthermore, F344 to LEW renal allografts removed at day 7 post-Tx already have shortened telomeres (Figure 2B) ▶ . The shortening of telomeres found at all later time points (including day 100) was comparable to telomere lengths found in day 7 kidneys (Figure 2B) ▶ . LEW allografts removed from F344 rats at comparable time points showed similar telomere shortening (data not shown).
Figure 2.
Telomere restriction fragment (TRF) length as measured with the TeloREAD assay in normal F344 and LEW kidneys or in F344 and LEW allografts on day 60 (A). Telomere length in F344 to LEW renal allografts in time after transplantation (B). Values are expressed as mean of 3 samples + SEM (sem).
Transient Expression of p21 and Subsequent Expression of p16 in Both F344 and LEW Allografts
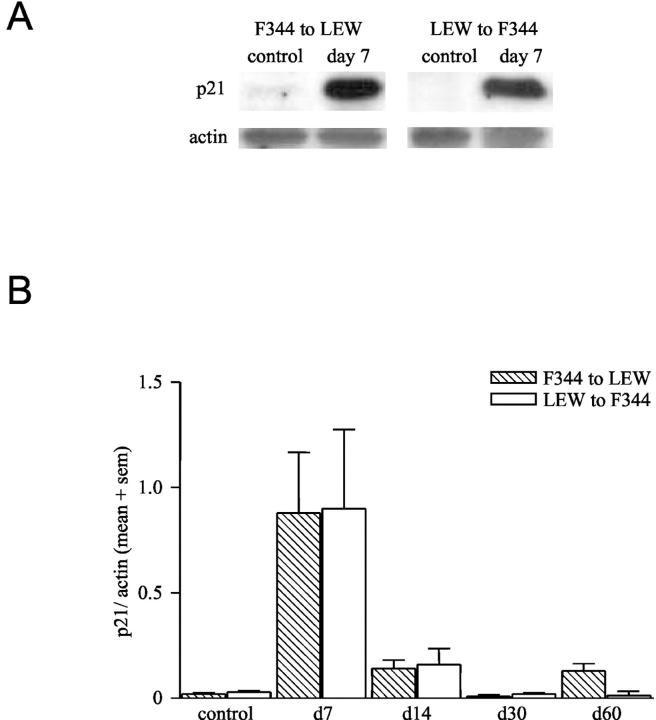
Expression of the cell cycle regulatory proteins, like p21, has been described to be the consequence of telomere shortening. Therefore we performed Western blot analysis on lysates of F344 and LEW renal allografts and determined the p21 protein levels. In normal F344 or LEW kidneys no p21 protein was detected by Western blot analysis (Figure 3A) ▶ , whereas in both cases p21 protein level was increased by day 7 after Tx and decreased thereafter (Figure 3B) ▶ .
Figure 3.
A: Western blot analysis of p21 and actin expression in lysates of normal and F344 to LEW renal allografts (day 7). Samples are representative for 3 samples in each group. B: Densitometry of p21 Western blots of F344 and LEW renal allografts at various time points after transplantation, corrected for actin content of the samples. Data are expressed as mean + SEM (sem). (n = 3 samples for all groups).
Expression of p16 has been suggested to increase after p21 protein levels decreased and to remain elevated during senescence. 17,18 Normal F344 or LEW kidneys do not show p16 expression. In F344 to LEW renal allografts nuclear expression of p16 was found in tubular epithelial cells (Figure 4A) ▶ . In addition, tubular basement membrane staining is found, but this is also observed in a control without the primary antibody and therefore considered non-specific (Figure 4A) ▶ . Expression of p16 in the F344 to LEW renal allografts was observed from day 7 and increased with time up to day 60 (Figure 4B) ▶ . In LEW to F344 allografts p16 was detectable from day 14 and increased with time, remaining at an elevated level up to day 60 (Figure 4B) ▶ .
Figure 4.

A: Immunohistochemical staining of nuclear p16 protein in normal F344 (left) and F344 to LEW allograft at day 60 (middle). No nuclear staining is observed in the absence of the primary antibody (right). (×400) B: Densitometry of total p16 staining on Western blot of F344 and LEW renal allografts on various time points after transplantation, corrected for actin content of the samples. Data are expressed as mean + SEM (sem). (n = 3 samples for all groups).
SA β-Gal Staining Parallels with Chronic Rejection
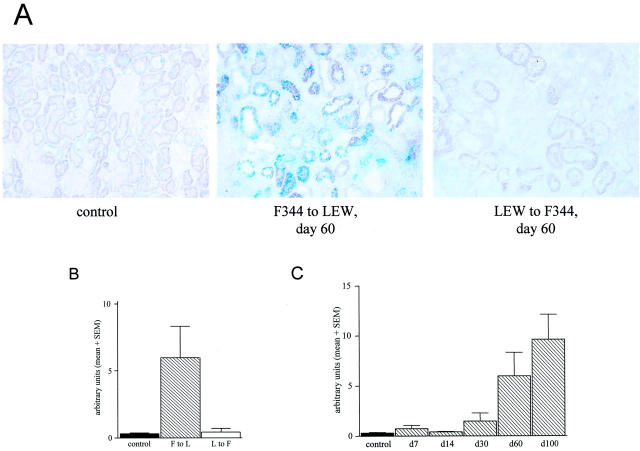
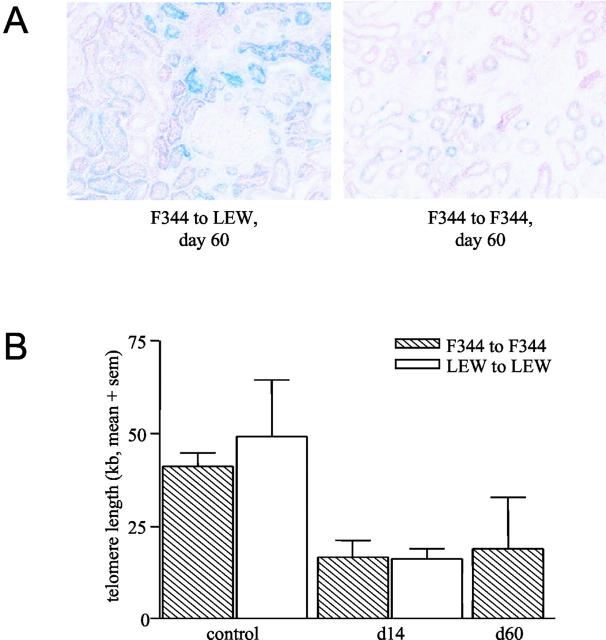
F344 to LEW renal allografts and LEW to F344 allografts were stained with SA β-gal at pH 6.0. In normal F344 kidneys SA β-gal-positive cells were found sporadically whereas in F344 to LEW renal allografts by day 60 after Tx increased numbers of SA β-gal-positive cells were present (Figure 5A) ▶ . In F344 allografts SA β-gal positivity was mainly observed in tubular epithelial cells and never in glomerular cells. In contrast, LEW to F344 renal allografts by day 60 after Tx showed hardly any SA β-gal-positive cells. Quantification of the amount of SA β-gal staining showed that LEW to F344 and normal kidneys had comparable numbers of SA β-gal-positive cells. The F344 to LEW renal allografts had an increased number of SA β-gal-positive cells at day 60 (Figure 5B) ▶ .
Figure 5.
A: Senescence-associated β-galactosidase staining (SA β-gal) (pH 6.0) in frozen sections of normal F344 kidney, F344 to LEW renal allograft at day 60, and a LEW to F344 renal allograft at day 60. Biopsies are representative for 6 biopsies in each group. (×250) B: Quantification of SA β-gal staining using Zeiss ks400 analysis for normal F344 kidneys, F344 and LEW renal allografts on day 60 post-Tx. Data are expressed as mean + SEM (sem) (n = 6 for all groups). C: Quantification of SA β-gal staining using Zeiss ks400 analysis. F344 to LEW day 7 (n = 3), day 14 (n = 3), day 30 (n = 3), day 60 (n = 6) and day 100 (n = 3), data expressed as mean + SEM (sem).
Since the F344 to LEW renal allografts showed an increased SA β-gal staining at day 60 post-Tx, we investigated several other time points. F344 allografts have an increased SA β-gal staining from day 30 post-Tx, which further increased until day 100. Quantification of this staining clearly shows that there is an increase in SA β-gal-positive cells in time after Tx in rejecting F344 allografts (Figure 5C) ▶ .
Syngeneic F344 and LEW Grafts Do Not Show SA β-Gal Staining, Ischemia Is Sufficient to Induce Telomere Shortening
In addition to allogeneic transplantations we performed syngeneic F344 to F344 and LEW to LEW renal transplantations and determined TRF lengths and SA β-gal staining. Syngeneic F344 to F344 transplants showed hardly any SA β-gal-positive cells at day 60 after Tx (Figure 6A) ▶ . However, the TRF length of syngeneic renal grafts was as short as the TRF length of allogeneic F344 or LEW grafts at day 60 (Figure 6B) ▶ .
Figure 6.
A: SA β-gal staining of F344 to LEW allograft (left) and F344 to F344 syngraft (right) on day 60 post-Tx. (×250). B: Telomere restriction fragment (TRF) length of normal F344 and LEW kidneys (n = 3) compared with F344 to F344 (day 14 and 60, n = 3) and LEW to LEW syngrafts (day 14, n = 3), expressed as mean + SEM (sem).
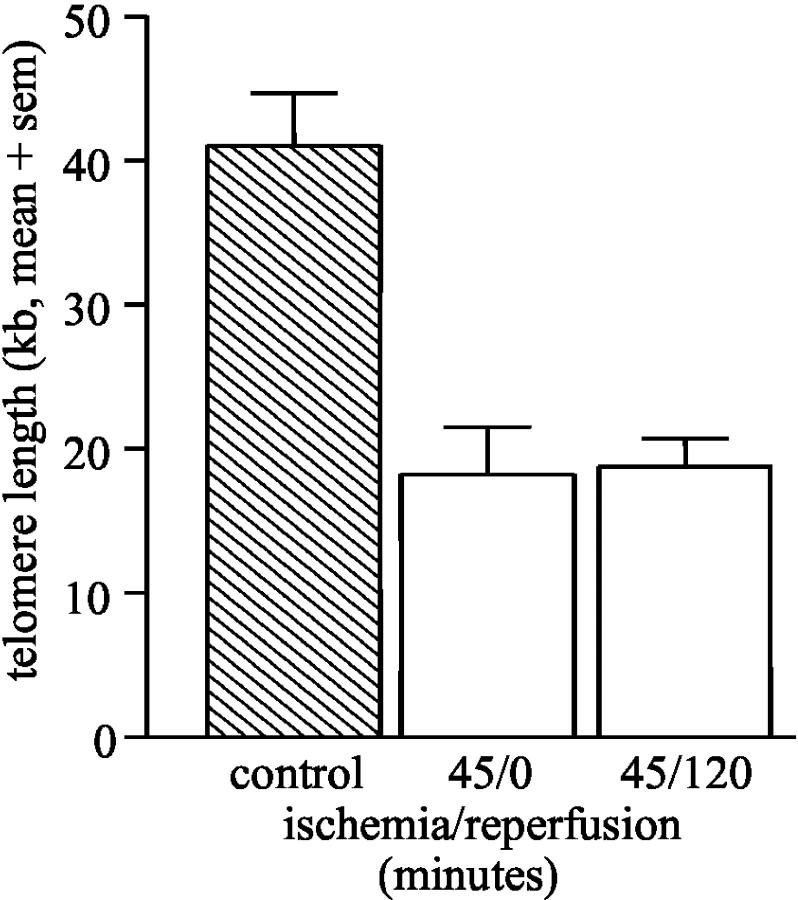
Since both allogeneic and syngeneic transplants already at day 7 post-transplant were characterized by shortened telomeres, we investigated whether ischemia or reperfusion injury was sufficient to induce telomere shortening. Clamping of the renal artery for 45 minutes was enough to induce substantial telomere shortening which was not further increased by subsequent reperfusion (Figure 7) ▶ .
Figure 7.
Telomere restriction fragment length of F344 kidneys after 45 minutes of ischemia (n = 3) and 45 minutes of ischemia followed by 2 hours of reperfusion (n = 3), expressed as mean + SEM (sem).
Discussion
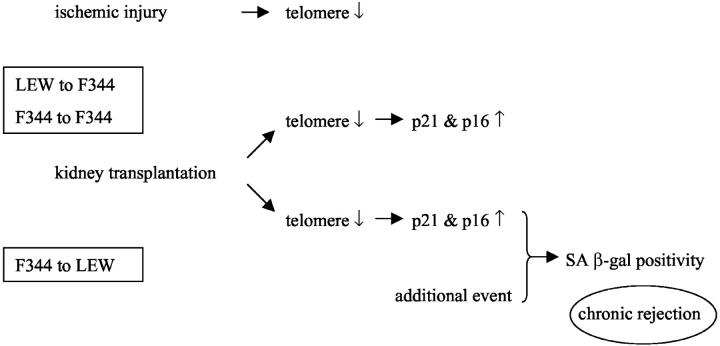
We investigated characteristics of cellular senescence in the F344 to LEW model of chronic renal graft rejection. In both F344 and LEW allografts, as well as syngeneic grafts, telomere shortening occurred. This resulted in activation of the cell cycle regulators p21 and p16 in F344 and LEW allografts. Interestingly, only F344 allografts show SA β-gal staining, the cytological marker for cellular senescence. LEW allografts or syngeneic transplants do not show SA β-gal staining despite comparable telomere shortening. Therefore we postulate that an additional (perhaps immunological/physiological) event contributes to the development of cellular senescence. Although telomere shortening can be a trigger for senescence, it is not sufficient for the induction of SA β-gal staining in a rat kidney transplantation model (Figure 8) ▶ .
Figure 8.
Schematic representation of events as observed in the F344 to LEW and LEW to F344 renal allografts. Transplantation results in shortening of telomeres in all combinations investigated, this results in activation of p21 and p16. However, only in F344 to LEW renal allografts SA β-gal staining is observed, this suggests that an additional event is required to induce cellular senescence.
These transplantation data are remarkable, both for the degree of observed telomere loss and the rapidity of its onset. The degree of loss is too great to be accounted for by proliferation. It most likely reflects DNA fragmentation due to reactive oxygen species, as it is observed after only 45 minutes of ischemia.
Ischemia and reperfusion during transplantation result in a transient increase of reactive oxygen species in the organ, which are potent inducers of DNA breaks. The ability of reactive oxygen species to damage DNA may be related to site-specific Fenton reactions. Telomeres are G-rich and therefore particularly sensitive to DNA oxidation and thus single-strand breaks. 25,26 In the F344 to LEW rat model we observed a decrease in TRF length, from approximately 45 kb to 20 kb after ischemia. This loss of approximately 25 kb is consistent with rapid telomere shortening as a consequence of oxidative damage and disruption of nucleoprotein complexes. 27,28 This situation is exacerbated by the fact that telomere binding proteins are also involved in DNA repair. General chromosomal breaks induced by oxidative stress might attract these proteins from the telomeres, resulting in stabilization of telomeres.
Such rapid and specific telomere loss is not without precedent. Similar massive telomere loss has also been reported as an early event in DNA damage-induced apoptosis in lymphocytes. 29 Although we cannot compare lymphocytes and tubular epithelial cells directly, similar mechanisms might occur also in tubular epithelial cells. Apoptosis of tubular epithelial cells has been found after ischemia and reperfusion of the kidney. 30
The SA β-gal staining seems to parallel with the histological and functional presence of chronic rejection in the F344 to LEW model. SA β-gal staining was observed only in F344 renal allografts transplanted in LEW recipients and not in LEW allografts transplanted in F344 rats. In addition, F344 syngeneic grafts did not show SA β-gal staining, although both LEW to F344 and syngeneic grafts have shortened telomeres. Apparently acute rejection episodes that occur in both F344 to LEW and LEW to F344 transplants are not enough to induce cells to go into senescence (defined as SA β-gal positivity), even when they already have decreased TRF lengths. F344 to LEW renal transplants develop signs of CR after day 30, the time frame within which the first SA β-gal-positive cells were detected. Since LEW to F344 allografts do not show increased SA β-gal positivity we believe that additional (immunological/physiological) events are required. Previously, we have demonstrated an humoral response against kidney proteins in LEW recipients of F344 grafts, that is not present in F344 recipients of LEW allografts or in recipients of syngeneic grafts. 22 We cannot exclude that factors involved in the induction of these immunological responses contribute to the accumulation of SA β-gal, but the presence of telomere erosion, p16, and p21 expression are consistent with its induction as a function of cellular senescence.
In the F344 to LEW renal allografts, tubular atrophy, a histopathological feature of CR, was present from day 30 post-Tx. Furthermore, SA β-gal accumulation is mainly found in tubular epithelial cells and not in glomeruli. In patients, ischemia/reperfusion injury can result in delayed graft function. Recently it has been shown that a defect in tubular function is responsible for delayed graft function. 31 This supports the hypothesis that tubular epithelial cells are extremely sensitive to oxidative damage. In addition, in aging human kidneys the decrease in telomere length is faster in cortex compared to medulla. 2 In the TRF length assay cortical DNA was used, which consists predominantly of tubular cell DNA. Therefore the decreased TRF length is most likely a result of loss of tubular telomeric DNA. This implies that predominantly tubular epithelial cells are damaged by oxidative damage and that this contributes to the deterioration of graft function.
Senescence of tubular epithelial cells implies an inappropriate response to damage signals, resulting in persistent inflammation and thereby scarring. In case of senescence, injured tissue cannot be replaced by healthy epithelium and fibrosis may be a consequence. 32 Recently, expression of p21 has been associated with early chronic liver allograft rejection. P21 expression was predominantly found in biliary epithelial cells and was increased in patients with early CR. 33 Replicative senescence of the epithelial cells was responsible for the characteristic phenotypic changes observed. In our model, p21 expression is transiently found in both F344 to LEW and LEW to F344 allografts, however this increase was not sufficient to induce SA β-gal accumulation. Tubular epithelial cells in F344 grafts with CR become SA β-gal-positive, thus impaired function of tubular epithelial cells might contribute to decreased renal function.
In humans, in vivo telomere shortening with age has been demonstrated and in human renal transplantation older donor kidneys have an inferior prognosis. 12,13 Since the transplantation procedure might induce telomere erosion, it is conceivable that if telomeres are already shortened in aging kidneys and the kidneys receive an additional stress during ischemia, the outcome will be worse. However data obtained in rodents cannot be extrapolated to humans without any precaution. Although rodents are frequently used as experimental models to study human diseases it is known that they are different in some ways. In senescence research mice and man have been shown to have a different pathogenesis, 34 whereas in rats it is not yet clear.
Patients with CR have increased oxidative stress markers and decreased anti-oxidants suggesting that oxidative stress plays a role in the development or progression of CR. 35,36 Ischemia/reperfusion of organ transplants results in generation of free radicals, inducing DNA breaks and telomere erosion. To prevent telomere erosion and thereby the first prerequisite for the senescent phenotype, generation of free radicals should be prevented. The usage of antioxidants in kidney preservation solutions might be helpful in preventing oxidative damage in transplant organs and influence long-term function. 37,38 Expression of anti-oxidants can prevent lesions of CR after antibody transfer. However, the timing of anti-oxidant expression is important. 39
In conclusion, oxidative damage during transplantation results in damage to genomic DNA, leading to a decreased telomere length and thereby activation of the cell cycle regulators. Shortening of telomeres will not be solely sufficient for cells to become SA β-gal-positive, though this will be affected by yet unknown factors.
Acknowledgments
We thank Dr. H. Benediktsson for scoring of the kidney histology, Dr. M.G.A. van Dixhoorn for helpful discussions, and Prof. Dr. M.R. Daha for critical reading of the manuscript.
Footnotes
Address reprint requests to Simone A. Joosten, M.Sc., Department of Nephrology, C3P Leiden University Medical Center Albinusdreef 2 2333 ZA Leiden, The Netherlands. E-mail: S.A.Joosten@LUMC.nl
Supported by a grant from the Dutch Kidney Foundation (grant C98.1783), C.N. is in receipt of a British Transplantation training fellowship, and P.S. and A.J. are supported by Darlinda’s Charitable trust.
References
- 1.Paul LC: Chronic renal transplant loss. Kidney Int 1995, 47:1491-1499 [DOI] [PubMed] [Google Scholar]
- 2.Melk A, Ramassar V, Helms LM, Moore R, Rayner D, Solez K, Halloran PF: Telomere shortening in kidneys with age. J Am Soc Nephrol 2000, 11:444-453 [DOI] [PubMed] [Google Scholar]
- 3.Melk A, Halloran PF: Cell senescence and its implications for nephrology. J Am Soc Nephrol 2001, 12:385-393 [DOI] [PubMed] [Google Scholar]
- 4.Hayflick L, Moorhead PS: The serial cultivation of human diploid cell strains. Exp Cell Res 1961, 25:585-621 [DOI] [PubMed] [Google Scholar]
- 5.Serrano M, Blasco MA: Putting the stress on senescence. Curr Opin Cell Biol 2001, 13:748-753 [DOI] [PubMed] [Google Scholar]
- 6.Shiels PG, Nolan CE, Sklavounou E: Cellular senescence, organ aging, and renal pathogenesis. Am J Transplant 2003, in press
- 7.Shiels PG: Somatic cell nuclear transfer as a tool for investigating aging processes in mammals. Gene Ther Mol Biol 1999, 4:11-22 [Google Scholar]
- 8.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O: A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 1995, 92:9363-9367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurz DJ, Decary S, Hong Y, Erusalimsky JD: Senescence-associated (β)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J Cell Sci 2000, 113:3613-3622 [DOI] [PubMed] [Google Scholar]
- 10.Dai CY, Enders GH: p16 INK4a can initiate an autonomous senescence program. Oncogene 2000, 19:1613-1622 [DOI] [PubMed] [Google Scholar]
- 11.Linskens MH, Feng J, Andrews WH, Enlow BE, Saati SM, Tonkin LA, Funk WD, Villeponteau B: Cataloging altered gene expression in young and senescent cells using enhanced differential display. Nucleic Acids Res 1995, 23:3244-3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L, Suwa T, Wright WE, Shay JW, Hornsby PJ: Telomere shortening and decline in replicative potential as a function of donor age in human adrenocortical cells. Mech Ageing Dev 2001, 122:1685-1694 [DOI] [PubMed] [Google Scholar]
- 13.De Fijter JW, Mallat MJ, Doxiadis II, Ringers J, Rosendaal FR, Claas FH, Paul LC: Increased immunogenicity and cause of graft loss of old donor kidneys. J Am Soc Nephrol 2001, 12:1538-1546 [DOI] [PubMed] [Google Scholar]
- 14.Karlseder J, Smogorzewska A, De Lange T: Senescence induced by altered telomere state, not telomere loss. Science 2002, 295:2446-2449 [DOI] [PubMed] [Google Scholar]
- 15.Itahana K, Dimri G, Campisi J: Regulation of cellular senescence by p53. Eur J Biochem 2001, 268:2784-2791 [DOI] [PubMed] [Google Scholar]
- 16.Sherr CJ, Roberts JM: CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 1999, 13:1501-1512 [DOI] [PubMed] [Google Scholar]
- 17.Robles SJ, Adami GR: Agents that cause DNA double-strand breaks lead to p16INK4a enrichment and the premature senescence of normal fibroblasts. Oncogene 1998, 16:1113-1123 [DOI] [PubMed] [Google Scholar]
- 18.Stein GH, Drullinger LF, Soulard A, Dulic V: Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol Cell Biol 1999, 19:2109-2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I: Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 2002, 105:1541-1544 [DOI] [PubMed] [Google Scholar]
- 20.Chkhotua A, Shohat M, Tobar A, Magal N, Kaganovski E, Shapira Z, Yussim A: Replicative senescence in organ transplantation: mechanisms and significance. Transplant Immunol 2002, 9:165-171 [DOI] [PubMed] [Google Scholar]
- 21.Paul LC: Experimental models of chronic renal allograft rejection. Transplant Proc 1995, 27:2126-2128 [PubMed] [Google Scholar]
- 22.Joosten SA, Van Dixhoorn MGA, Borrias MC, Benediktsson H, Van Veelen PA, Van Kooten C, Paul LC: Antibody response against perlecan and collagen types IV and VI in chronic renal allograft rejection in the rat. Am J Pathol 2002, 160:1301-1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y: The Banff 97 working classification of renal allograft pathology. Kidney Int 1999, 55:713-723 [DOI] [PubMed] [Google Scholar]
- 24.Learish RD, Shultz J, Ho S, Bulleit RF: Small-scale telomere repeat sequence content assay using pyrophosphorolysis coupled with ATP detection. Biotechniques 2002, 33:1349-1353 [DOI] [PubMed] [Google Scholar]
- 25.Von Zglinicki T, Saretzki G, Docke W, Lotze C: Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp Cell Res 1995, 220:186-193 [DOI] [PubMed] [Google Scholar]
- 26.Von Zglinicki T: Oxidative stress shortens telomeres. Trends Biochem Sci 2002, 27:339-344 [DOI] [PubMed] [Google Scholar]
- 27.Honda S, Hjelmeland LM, Handa JT: Oxidative stress–induced single-strand breaks in chromosomal telomeres of human retinal pigment epithelial cells in vitro. Invest Ophthalmol Vis Sci 2001, 42:2139-2144 [PubMed] [Google Scholar]
- 28.Proctor CJ, Kirkwood TB: Modeling telomere shortening and the role of oxidative stress. Mech Ageing Dev 2002, 123:351-363 [DOI] [PubMed] [Google Scholar]
- 29.Ramirez R, Carracedo J, Jimenez R, Canela A, Herrera E, Aljama P, Blasco MA: Massive telomere loss is an early event of DNA damage-induced apoptosis. J Biol Chem 2003, 278:836-842 [DOI] [PubMed] [Google Scholar]
- 30.Daemen MA, De Vries B, Buurman WA: Apoptosis and inflammation in renal reperfusion injury. Transplantation 2002, 73:1693-1700 [DOI] [PubMed] [Google Scholar]
- 31.El Maghraby TA, Boom H, Camps JA, Blokland KA, Zwinderman AH, Paul LC, Pauwels EK, De Fijter JW: Delayed graft function is characterized by reduced functional mass measured by (99m)technetium-mercaptoacetyltriglycine renography. Transplantation 2002, 74:203-208 [DOI] [PubMed] [Google Scholar]
- 32.Halloran PF, Melk A, Barth C: Rethinking chronic allograft nephropathy: the concept of accelerated senescence. J Am Soc Nephrol 1999, 10:167-181 [DOI] [PubMed] [Google Scholar]
- 33.Lunz JG, III, Contrucci S, Ruppert K, Murase N, Fung JJ, Starzl TE, Demetris AJ: Replicative senescence of biliary epithelial cells precedes bile duct loss in chronic liver allograft rejection: increased expression of p21(WAF1/Cip1) as a disease marker and the influence of immunosuppressive drugs. Am J Pathol 2001, 158:1379-1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sherr CJ, DePinho RA: Cellular senescence: mitotic clock or culture shock? Cell 2000, 102:407-410 [DOI] [PubMed] [Google Scholar]
- 35.Simic-Ogrizovic S, Simic T, Reljic Z, Markovic S, Blagojevic R, Radivojevic D, Lezaic V, Djukanovic L, Mimic-Oka J: Markers of oxidative stress after renal transplantation. Transplant Int 1998, 11(Suppl 1):S125-S129 [DOI] [PubMed] [Google Scholar]
- 36.Cristol JP, Vela C, Maggi MF, Descomps B, Mourad G: Oxidative stress and lipid abnormalities in renal transplant recipients with or without chronic rejection. Transplantation 1998, 65:1322-1328 [DOI] [PubMed] [Google Scholar]
- 37.Land W, Schneeberger H, Schleibner S, Illner WD, Abendroth D, Rutili G, Arfors KE, Messmer K: The beneficial effect of human recombinant superoxide dismutase on acute and chronic rejection events in recipients of cadaveric renal transplants. Transplantation 1994, 57:211-217 [DOI] [PubMed] [Google Scholar]
- 38.Land W: Postischemic reperfusion injury and allograft dysfunction: is allograft rejection the result of a fateful confusion by the immune system of danger and benefit? Transplant Proc 1999, 31:332-336 [DOI] [PubMed] [Google Scholar]
- 39.Hancock WW, Buelow R, Sayegh MH, Turka LA: Antibody-induced transplant arteriosclerosis is prevented by graft expression of anti-oxidant and anti-apoptotic genes. Nat Med 1998, 4:1392-1396 [DOI] [PubMed] [Google Scholar]