Abstract
Glioblastoma multiforme comprises the majority of human brain tumors. Patients with glioblastoma multiforme have poor survival rates, with an average life expectancy of <1 year. To assess possible mechanisms and to potentially target invasive glioma cells, we previously measured the gene expression profiles of glioma cells under migration-activated or passive states. One of the genes identified was Fn14, which encodes a cell surface receptor for the tumor necrosis factor superfamily member named TWEAK. In this study, we show that Fn14 gene expression is induced in migration-activated glioma cells in vitro and significantly increases according to tumor grade in vivo (P < 0.01), with highest levels in glioblastoma tissue specimens. The in situ expression pattern of Fn14 mRNA and protein was confined to primary glioma cells and the vascular endothelium, with no detection in adjacent normal brain. Conversely, TWEAK mRNA levels are low in glioblastoma samples relative to normal brain tissue. In addition, activation of the Fn14 receptor by addition of recombinant TWEAK resulted in increased glioma cell migration in vitro. These results suggest a positive role for TWEAK and Fn14 in glioma progression and indicate that Fn14 gene expression may serve as a marker for invasive glioma cells.
Glioblastomas are the most common primary malignant brain tumors, biologically unique by virtue of their proclivity for local invasion into the adjacent normal brain tissue and the rarity of systemic metastasis. 1,2 Despite considerable efforts to improve neurosurgery and radiochemotherapy, only a subset of patients survive longer than 18 months. 3-5 Our focus has been on the invasive and migratory properties of malignant glioma cells, which are the main characteristics that distinguish benign from malignant disease; indeed, repopulation by these cells results in tumor regrowth and patient mortality. Even though the molecular mechanisms underlying the invasiveness of malignant glioma cells are still unclear, glioblastoma cells acquire increased invasive potential by a complex pathway that includes altered cell-substrate attachment, 6 cell-cell adhesion, 7 and an increase in both cell motility and survival. 8-10 Additionally, secreted proteases 11,12 and changes in extracellular matrix (ECM) composition 13 may accompany glioma invasion.
There is a direct association between glial tumor grades and migration and invasion, in which high-grade gliomas demonstrate more extensive migratory and invasive capacities. 9 During migration and invasion, tumor cells closely interact with the ECM. 14 The interaction of the cells and the ECM is a key factor that maintains the normal architecture of tissues. In the brain, a well-defined ECM only exists in the form of a true basement membrane around all cerebral blood vessels and at the glia limitans externa. 15 Most of the ECM proteins in the normal adult brain are localized to the perivascular space, in which collagen, fibronectin, and laminin constitute the three principal ECM proteins of the blood-brain barrier. 15 This perivascular region is one of the preferred invasion paths used by some glioma cells. 16
In vitro experiments suggest that several ECM components have the potential to act as permissive substrates for glioma cell migration. 10 Laminin stimulates migration of many human glioma cell lines; 8,10,17 in addition, the ECM produced by SF767 glioma cells strongly stimulates motility in most of the glioma cell lines tested. 10 Although the composition of this glioma matrix is not fully known, it contains tenascin C (unpublished data), and we speculate that glioma-derived ECM is a reasonable approximation of the in vivo invasion matrix. 13 Studies of both melanoma and glioma cells showed that there is a strong correlation between increased motility in vitro and increased invasiveness in vivo. 18,19 Because the in vivo invasive phenotype is difficult to study, glioma matrix produced by SF767 and other permissive ECM components such as laminin are used to induce cell migration in vitro as a model to study motility in relation to the invasiveness of glioblastoma. 8,10,19 Identifying and understanding the genes that mediate cellular migration and invasion could reveal potential therapeutical targets to reduce further spreading and specifically control the invading glioma cells.
Previously, we used cDNA microarray technology to investigate the spectrum of genes differentially expressed coincident to glioma migration. 20 Several anticipated and unanticipated gene candidates potentially involved in glioma migration emerged. In this study, we report the role of one gene candidate, the fibroblast growth factor-inducible 14 (Fn14) gene, in glioma migration. Fn14 is a member of the tumor necrosis factor (TNF) superfamily of receptors, and is characterized as a type Ia transmembrane receptor lacking a cytoplasmic death domain. 21-23 Fn14 is an immediate early response gene whose expression is directly activated after exposure to growth factors, fetal calf serum, and phorbol ester in fibroblasts. 21,22 Here, we demonstrate that Fn14 gene expression is up-regulated in migration-stimulated glioma cells in vitro. Additionally, Fn14 expression is minimal to absent in normal brain tissue but appears to increase with increasing tumor grade; in contrast, its ligand, TWEAK, is down-regulated in glioblastoma cells. In vitro activation of Fn14 by addition of recombinant TWEAK enhances cell motility. Therefore, Fn14 may serve as a therapeutic marker for glioma invasiveness.
Materials and Methods
Cell Culture Conditions and ECM Preparation
Human astrocytoma cell lines T98G (American Type Culture Collection, Manassas, VA), G112, 24 U118MG (American Type Culture Collection), and SF767 25 were maintained in minimum essential medium (Invitrogen Corp., Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (Hyclone Laboratories, Inc., Logan, UT) in a 37°C, 5% CO2 atmosphere at constant humidity. Laminin from human placenta was obtained from Invitrogen Corp. Astrocytoma-derived ECM was prepared as described previously. 10 Briefly, a confluent monolayer of SF767 cells was maintained in culture for 3 to 5 days for ECM deposit. The cells were lysed from the ECM with 0.5% Triton X-100 for 30 minutes at 25°C followed by incubation with 0.1 mol/L of NH4OH for 5 minutes at 25°C and the residual ECM was thoroughly rinsed with phosphate-buffered saline (PBS). 26
RNA Isolation and Northern Blot Analysis
Total RNA was extracted using the RNeasy kit (Qiagen Inc., Valencia, CA) according to the manufacturer’s protocols. Ten μg of each RNA sample were separated by electrophoresis in 1.2% agarose gels containing 2.2 mol/L of formaldehyde, transferred onto Zetabind nylon membranes (Cuno Inc., Meriden, CT) by electroblotting, and then crosslinked to the membrane by UV light irradiation using a Stratalinker (Stratagene, La Jolla, CA). Membrane prehybridization, cDNA probe radiolabeling, hybridization, and washes were performed as previously described. 21 The cDNA hybridization probes were 1) human Fn14, ∼1.0-kb EcoRI/XhoI fragment of pBluescript/hFn14; 22 2) human TWEAK, ∼1.0-kb BglII/XbaI fragment of pBluescript/hTWEAK (gift from T. Zheng, Biogen Inc., Cambridge, MA); and 3) human GAPDH, ∼0.8-kb PstI/XbaI fragment of pHcGAP (American Type Culture Collection).
Quantitative Reverse Transcription-Polymerase Chain Reaction (QRT-PCR)
Real-time quantitative PCR was performed using a LightCycler (Roche Diagnostics, Indianapolis, IN) with SYBR green fluorescence signal detection after each cycle of amplification as described previously. 27 Briefly, total RNA was isolated from human brain or brain tumor tissues or cultured glioma cells using the RNeasy kit. cDNA was synthesized from 1 μg of DNase I-treated total RNA in a 20-μl reaction volume using the Retroscript kit (Ambion Inc., Austin, TX) for 60 minutes at 42°C. PCR was performed on 2 μl of the cDNA in a final volume of 20 μl using the following primers: Fn14, sense 5′-CCA AGC TCC TCC AAC CAC AA-3′; anti-sense 5′-TGG GGC CTA GTG TCA AGT CT-3′ (amplicon size, 242 bp); TWEAK, sense 5′-CCC TGC GCT GCC TGG AGG AA-3′; anti-sense 5′-AGA CCA GGG CCC CTC AGT GA-3′ (amplicon size, 200 bp); histone H3.3, sense 5′-CCA CTG AAC TTC TGA TTC GC-3′; anti-sense 5′-GCG TGC TAG CTG GAT GTC TT-3′ (amplicon size, 215 bp). The PCR data were analyzed with the LightCycler analysis software as previously described. 27-30 Quantification was based on the number of cycles necessary to produce a detectable amount of product above background. The difference in the cycle number (d) was normalized to the housekeeping gene histone H3.3 and then used to calculate the fold difference in copy number according to the formula f = 2(d), where f = fold difference in specific gene expression and d = cycle number difference between compared sources of mRNA (corrected for differences in histone). 27 To ensure specificity of the PCR product amplification, the melting curves for standard and sample products were analyzed, 30 along with electrophoresis of the PCR products in a 2% agarose gel, followed by ethidium bromide staining.
Preparation of Tissues and Nonradioactive in Situ Hybridization
Under an institutional review board-approved protocol, human frozen glioma biopsy tissue specimens were obtained at the time of surgery or autopsy, frozen in liquid nitrogen, and stored at −80°C. Frozen sections, 6-μm thick, were fixed with 4% paraformaldehyde, and permeabilized with pepsin. Sections were then incubated with digoxigenin-labeled anti-sense RNA probe (200 ng/ml) overnight at 55°C according to procedures by St. Croix and colleagues. 31 Digoxigenin-labeled anti-sense or sense Fn14 RNA probes were generated by PCR amplification of 500-bp product and incorporation of a T7 promoter into the following primers: anti-sense 5′-AGA CGA TGC CGC AGG AGA GA-3′; sense 5′-CCT AGT GTC AAG TCT GCC CC-3′. In vitro transcription was performed with digoxigenin RNA-labeling reagents and T7 RNA polymerase (Ambion). A horseradish peroxidase-conjugated rabbit anti-digoxigenin antibody (DAKO, Carpinteria, CA) was used for signal amplification after treatment with biotin-tyramide (GenPoint kit, DAKO). Enhanced signal amplification was done by addition of horseradish peroxidase-conjugated rabbit anti-biotin (DAKO), biotin-tyramide, and then alkaline phosphatase-conjugated rabbit anti-biotin (DAKO). The alkaline phosphatase substrate Fast Red TR/napthol AS-MS (Sigma, St. Louis, MO) was then used to detect the hybridization signals. The sections were counterstained with Mayer’s hematoxylin.
Migration and Viability Assays
Quantitation of cellular migration was performed using a microliter scale migration assay as described in detail previously. 8 Briefly, 10-well slides were coated with 0.1 μg/ml of human laminin at 37°C for 1 hour and washed with PBS to enhance cell attachment, without promoting migration. Approximately 2000 serum-starved glioma cells were plated onto each well using a cell sedimentation manifold (CSM Inc., Phoenix, AZ) to establish a confluent monolayer of 1 mm in diameter. Cells were then allowed to migrate for 24 to 48 hours in serum-free medium in the absence or presence of 50 ng/ml of recombinant human TWEAK (PeproTech Inc., Rocky Hill, NJ) or 100 ng/ml of recombinant human TRAIL (Alexis Corp., San Diego, CA). In certain experiments, 2.5 μg/ml of soluble Fn14-Fc receptor 32 or control mouse IgG (Sigma) was preincubated with 50 ng/ml of TWEAK at 37°C for 15 minutes before starting the migration assay. The average migration rate of five replicates was calculated as the increasing radius of the entire cell population throughout time. At the end of the migration interval, the slides were washed three times with PBS and subjected to the Live/Dead Viability/Cytotoxicity assay (Molecular Probes, Eugene, OR), followed by incubation in Live/Dead solution for 40 minutes. This assay provides a two-color fluorescence: calcein AM (green fluorescence for living cells) and ethidium homodimer-1 (red fluorescence for dead cells), and percentage of live/dead cells were enumerated under epifluorescent microscopy.
Western Blot Analysis
Monolayers of cells were washed in PBS containing 1 mmol/L of phenylmethyl sulfonyl fluoride and then lysed in 2× sodium dodecyl sulfate-sample buffer (0.25 mol/L Tris-HCl, pH 6.8, 2% sodium dodecyl sulfate, 25% glycerol) containing 10 μg/ml aprotinin, 10 μg/ml leupeptin, 0.7 μg/ml pepstatin, 20 mmol/L NaF, and 1 mmol/L phenylmethyl sulfonyl fluoride. Protein concentrations were determined using the BCA assay procedure (Pierce Chemical Co., Rockford, IL), with bovine serum albumin as a standard. Thirty μg of total cellular protein were loaded per lane, separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and then transferred to nitrocellulose (Schleicher & Schuell, Keene NH) by electroblotting. The nitrocellulose membrane was blocked with 5% nonfat dry milk in Tris-buffered saline, pH 8.0, with 0.1% Tween-20 before addition of anti-Fn14 polyclonal serum 21 and then horseradish peroxidase-conjugated anti-rabbit IgG (Promega, Madison, WI). Bound secondary antibodies were detected using a chemiluminescence system (NEN, Boston, MA).
Immunohistochemistry
Paraffin-embedded tissue blocks were sectioned (6-μm thick) onto slides and then deparaffinized. Sections were quenched with 3% hydrogen peroxide in methanol for 15 minutes, microwaved 5 minutes in H2O, and blocked for 1 hour with Tris-buffered saline (0.05 mol/L Tris-HCl, pH 7.6, 0.25 mol/L NaCl) containing 3% goat serum and 0.1% Triton X-100. Slides were incubated in rabbit anti-Fn14 antisera 21 or rabbit preimmune sera (1:10 dilution) overnight at 4°C. The secondary antibody (biotin-conjugated goat anti-rabbit IgG; Jackson Laboratories, Bar Harbor, ME) was applied at a 1:200 dilution in Tris-buffered saline containing 1% goat serum, followed by streptavidin-conjugated horseradish peroxidase (1:500 dilution; Amersham Biosciences, Piscataway, NJ). Sections were exposed to diaminobenzidine peroxidase substrate (Sigma) for 5 minutes and counterstained with Mayer’s hematoxylin.
Statistical Analyses
The Student’s t-test and the Kruskal-Wallis correlation test were used to compare the ratios of gene expression. Differences among the unpaired brain tumor groups were analyzed relative to the null hypothesis with 95% confidentiality.
Results
Induction of Fn14 mRNA and Protein Expression in Glioma Cells on Migration-Promoting Substrate
Previous data from cDNA microarray analysis suggested that Fn14 gene expression is induced in migrating glioma cells. 20 To confirm the microarray data, Fn14 mRNA levels were examined in glioma cell lines that were plated either in standard culture flasks or in glioma-derived ECM-precoated flasks. Glioma-derived ECM has been previously shown to enhance the motility behavior of these cells. 10,20,33 To compare the Fn14 gene expression level among different cell lines, Fn14 mRNA levels were normalized to the level found in G112 cells, where Fn14 gene expression was initially characterized. 20 Northern blot data indicated that the ∼1.2-kb human Fn14 transcript was detected in four glioma cell lines under standard culture conditions (Figure 1A) ▶ . However, culture of these glioma cells on motility-promoting substrate resulted in a twofold to threefold induction of Fn14 mRNA expression. Thus, this data confirms the microarray analysis verifying the correlation between the induction of Fn14 mRNA and migration-activation of glioma cells.
Figure 1.
Fn14 mRNA and protein expression in glioma cell lines cultured on tissue culture plastic or glioma-derived substrate. A: Northern blot analysis using RNA from each of the indicated cell lines cultured on tissue culture plastic (P) or glioma-derived ECM (M) was performed using the indicated cDNA probes. GAPDH was used as loading control and relative Fn14 mRNA expression was normalized to GAPDH and presented as fold induction over G112 cells cultured on tissue culture plastic. B: Immunoblot analysis of Fn14 expression in glioma cell lines. Total cell lysates were prepared from the indicated cell lines and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblot analysis using Fn14 polyclonal antiserum.
To determine whether Fn14 protein expression also increases in migration-activated glioma cells, we measured Fn14 protein levels in the four glioma cell lines under passive or migration-activated states. Human glioma cell lines were grown either in standard culture flasks or in flasks precoated with glioma-derived ECM. Total cellular lysates were prepared and Fn14 was detected by Western blot analysis. Densitometric analysis of the Fn14 signal intensities revealed approximately twofold induction of Fn14 protein expression in all four glioma cells cultured on migration promoting ECM (Figure 1B) ▶ , consistent with the Northern blot findings.
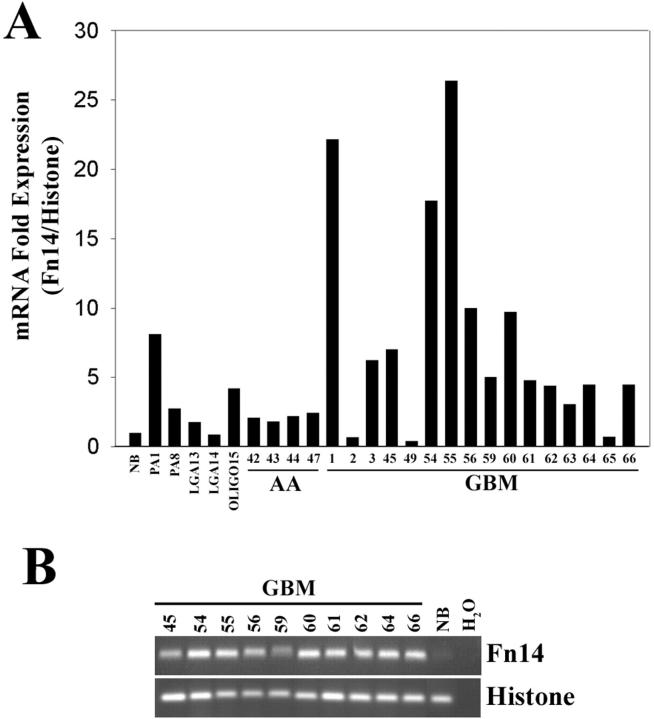
Fn14 mRNA Overexpression in GBM Tissue Samples
Motility may increase with grade of astrocytoma. 19 Because expression of Fn14 mRNA is higher in migration activated glioma cells, we assessed whether Fn14 expression varied with histopathological grade of glial neoplasms. Total RNA was extracted from 29 snap-frozen human brain biopsy specimens: 2 pilocytic astrocytomas, 2 low-grade astrocytomas, 1 oligodendroglioma, 4 anaplastic astrocytomas, 16 glioblastomas, and 3 normal adult brain tissues. Fn14 mRNA levels in these tissues were measured by real-time quantitative RT-PCR and SYBR green fluorogenic detection. Glial neoplasms demonstrated increased expression levels of Fn14 mRNA relative to the average level of Fn14 found in pooled mRNA from three normal brain tissue samples (Figure 2A) ▶ . Separate QRT-PCR for Fn14 in these three separate specimens concurred within 5% of each other. In the pilocytic astrocytoma tissues, Fn14 mRNA was eightfold overexpressed in one sample and twofold overexpressed in the second sample. Fn14 mRNA was fourfold overexpressed in the oligodendroglioma specimen, whereas the two low-grade astrocytomas displayed only slight Fn14 mRNA induction compared to normal brain. Analysis of four anaplastic astrocytomas showed a twofold overexpression of Fn14 mRNA compared to normal brain. The highest overexpression of Fn14 mRNA was observed in GBM tissues, with five(+)-fold induction of Fn14 mRNA in ∼68% of the GBM specimens. Amplicon identity was confirmed by analysis of the melting curve of the DNA using the LightCycler software program (data not shown) and DNA fragment size was verified by agarose gel electrophoresis. Briefly, the PCR assays for both Fn14 and histone mRNA were terminated during the exponential phase, and the amplification products were separated on a 2% agarose gel and then stained with ethidium bromide. An Fn14 PCR product of the appropriate size was observed in the GBM tissue samples concordant with the SYBR green fluorescent quantitation, whereas minimal expression of Fn14 transcript was detected in normal brain tissue (Figure 2B) ▶ . Thus, it appears that Fn14 mRNA is overexpressed in many advanced glial neoplasms, and most dramatically in GBM. A statistical analysis of Fn14 gene expression among normal brain, low-grade astrocytoma, anaplastic astrocytoma, and GBM was performed using the Kruskal-Wallis analysis of variance on ranks. Expression of Fn14 mRNA significantly increased according to tumor grade (P < 0.01). No statistical difference between Fn14 mRNA expression in normal brain and low-grade astrocytoma was observed; in contrast, Fn14 expression in anaplastic astrocytoma differs from that in glioblastoma multiforme (P < 0.05).
Figure 2.
Fn14 mRNA expression in various glial tissues. A: Quantitation of Fn14 mRNA expression using real-time RT-PCR. Total RNA was extracted from various glial tissues and analyzed by quantitative PCR for Fn14 mRNA and normalized to histone H3.3 expression. Fn14 expression for various glial tissues was then normalized to expression in normal brain. (NB, average Fn14 expression in three normal brain samples; PA, pilocytic astrocytoma; LGA, low-grade astrocytoma; OLIGO, oligodendroglioma; AA, anaplastic astrocytoma; GBM, glioblastoma multiforme). B: Assessment of Fn14 and histone H3.3 amplicon specificities after QRT-PCR. The PCR reaction was stopped at the exponential log phase and reaction products were separated on an agarose gel and detected by ethidium bromide staining.
Fn14 mRNA and Protein Are Expressed in Tumor Cells and Endothelial Cells of GBM Tissues
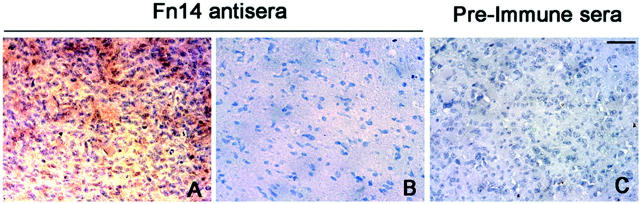
To assess the expression of Fn14 in tumor tissue, a highly sensitive nonradioactive in situ mRNA hybridization method that detects transcripts in frozen sections of human tissues was performed. 31 Positive localization of Fn14 mRNA in glioblastoma tissue was detected in six of six different glioma biopsies. Fn14 mRNA expression was observed in the cells residing in the tumor core and at the invading edge; however, adjacent normal brain within the same section failed to stain for Fn14 mRNA expression (data not shown). In addition, peroxidase-based immunohistochemistry studies on paraffin-embedded GBM tissues showed Fn14 staining confined to GBM tumor cells (Figure 3A) ▶ as compared to controlled rabbit preimmune sera (Figure 3C) ▶ . No Fn14 immunoreactivity was detected in the adjacent normal brain region (Figure 3B) ▶ . Fn14 mRNA and protein expression was also detected in vascular endothelial cells throughout the tissue sections irrespective of proximity to tumor (data not shown), arguing for a dissociation of Fn14 as a marker of angiogenesis. These data corroborate the Fn14 PCR findings in the glioma tissues and indicate that the predominant cellular source of the Fn14 mRNA was tumor cells; however, some Fn14 mRNA is also likely to be derived from the vascular endothelium.
Figure 3.
Immunohistochemical analysis of Fn14 expression in human glioblastoma tissue biopsies. Serial sections of paraffin-embedded glioma biopsies were used for peroxidase-based immunostaining for Fn14. Localization of Fn14 protein was detected in glioma cells (A) but not in normal adjacent brain (B). No immunoreactivity was detected when glioma biopsy tissue was stained with rabbit preimmune serum (C). Scale bar, 100 μm.
The Fn14 Ligand, TWEAK, Is Not Overexpressed in Migrating Glioma Cells and Is Down-Regulated in GBM Tissues
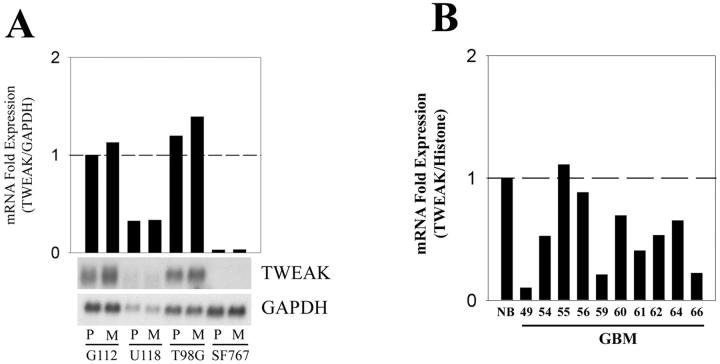
The TNF-related cytokine TWEAK was recently identified as a ligand for Fn14. 23 To determine whether the levels of TWEAK mRNA change as a consequence of cell migration, we assayed TWEAK mRNA levels in four glioma cell lines plated on a nonmotile substrate or a motility-promoting glioma-derived ECM substrate. Total RNA was isolated and subjected to Northern blot analysis for TWEAK expression. Expression of TWEAK mRNA was detected in two of the four glioma cell lines (G112 and T98G), and the level of expression did not change on motility-promoting substrate (Figure 4A) ▶ . Thus, migration-activation of glioma cells does not lead to up-regulation of TWEAK mRNA levels.
Figure 4.
TWEAK mRNA expression in glioma cell lines and glioma tissues. A: Northern blot analysis using RNA from each of the indicated cell lines cultured on tissue culture plastic (P) or glioma-derived ECM (M) was performed using the indicated cDNA probes. GAPDH was used as loading control and relative TWEAK mRNA expression was normalized to GAPDH and presented as fold induction over G112 cells cultured on tissue culture plastic. B: Total RNA was extracted from various glioma biopsy tissues and real-time quantitative RT-PCR was performed to analyze TWEAK mRNA expression. TWEAK mRNA expression was normalized to histone H3.3 mRNA expression, and the TWEAK expression in glioma tissues was further normalized to the average of TWEAK mRNA expression levels in three normal brain samples.
To initiate exploration of a possible autocrine activation pathway of Fn14, TWEAK mRNA expression in normal brain and GBM tissue samples was measured using real-time RT-PCR. Relative to the levels of TWEAK mRNA expression detected in the pooled mRNA of three normal brain tissue samples, 9 of 10 GBM samples had a lower level of TWEAK mRNA expression (Figure 4B) ▶ . The average expression in GBM specimens was ∼50% that in normal brain, with a range of 5 to 105% (Student’s t-test, P < 0.01). These results indicate that TWEAK mRNA is down-regulated in GBM tissues.
TWEAK Stimulates Glioma Cell Migration
Because Fn14 gene expression is up-regulated in migration-activated glioma cells, we investigated whether activation of this receptor by addition of human recombinant TWEAK affected cell motility. Human glioma cells were deposited using a cell sedimentation manifold onto 10-well glass slides previously coated with 0.1 μg/ml of laminin. This low concentration of laminin was used to promote cell attachment to glass slides but did not stimulate cell migration (data not shown). Cells were serum-deprived for 8 hours before addition of TWEAK (50 ng/ml). Migration throughout 48 hours was quantified by measuring the incremental increase in the circular area occupied by the cell population, using the size on day 0 as reference. In G112 and T98G, two cell lines that constitutively express TWEAK mRNA, addition of exogenous TWEAK did not significantly change the cellular migration rate (Figure 5A ▶ , compare lanes a and b and lanes e and f). However, in the two cell lines that express very low or undetectable levels of TWEAK mRNA, SF767 and U118, TWEAK addition enhanced the rate of cell migration approximately twofold (Figure 5A ▶ , compare lanes i and j and m and n). To confirm whether TWEAK-induced migration of glioma cells was a result of receptor-ligand interactions on the cell surface, we used a recombinant soluble murine Fn14-Fc decoy receptor. 32 Preincubation of TWEAK with the Fn14-Fc decoy receptor before application to the cells led to reduced cell migration in TWEAK-nonexpressing cells SF767 and U118 (Figure 5A ▶ , lanes k and o), as well as reduced migration rates of TWEAK-expressing cells G112 and T98G (Figure 5A ▶ , lanes c and g). Preincubation of TWEAK with control mouse IgG did not inhibit TWEAK-stimulated glioma migration (Figure 5A ▶ , lanes d, h, l, p). In addition, cell migration studies after Fn14-Fc decoy washout showed recovery of cell migration in the presence of TWEAK in all four cell lines (Figure 5B ▶ , lanes d, h, l, p) similar to the migration rates observed in TWEAK treatment alone (Figure 5B ▶ , lanes b, f, j, n). These results suggest that TWEAK stimulates glioma cell motility via activation of the Fn14 receptor.
Figure 5.
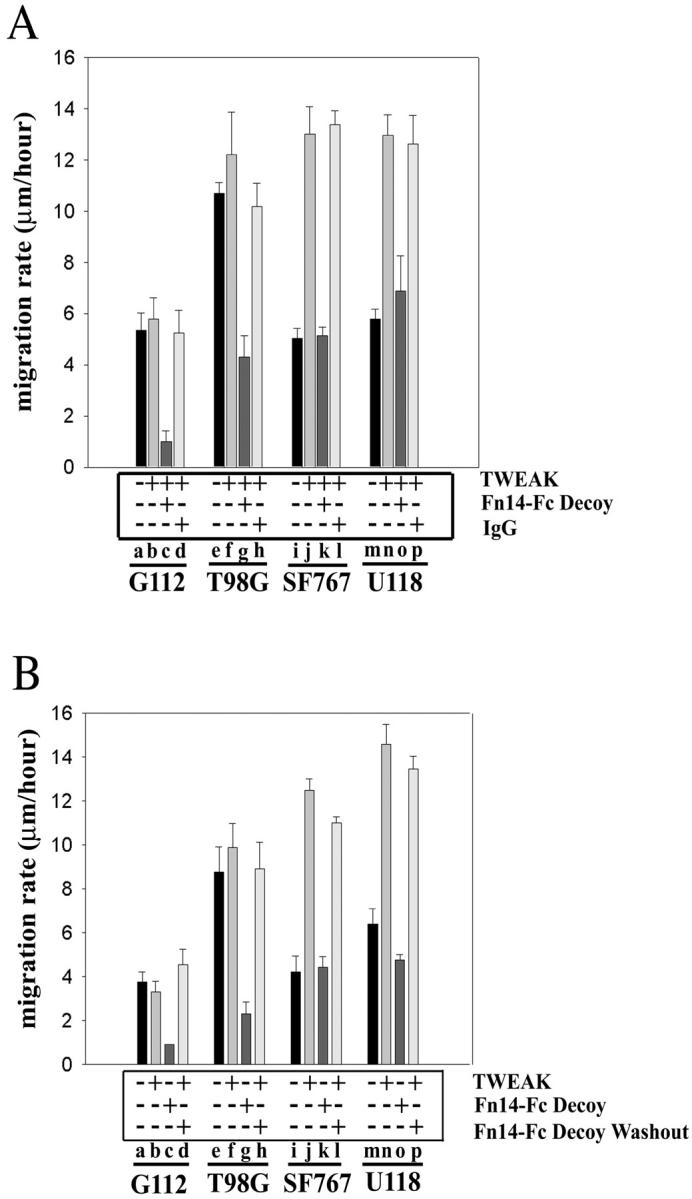
TWEAK enhances glioma cell migration via Fn14 receptor activation. A: Glioma cells were plated onto 10-well glass slides precoated with laminin and then serum-deprived for 8 hours before addition of TWEAK. In some experiments, TWEAK was preincubated with Fn14-Fc decoy receptor or control mouse IgG before addition to cell monolayer. Cell migration was assessed throughout 48 hours. B: Glioma cells were treated with TWEAK and/or Fn14-Fc protein as described above and then the cells were washed with PBS and reincubated with TWEAK. Cell migration was assessed 24 hours later.
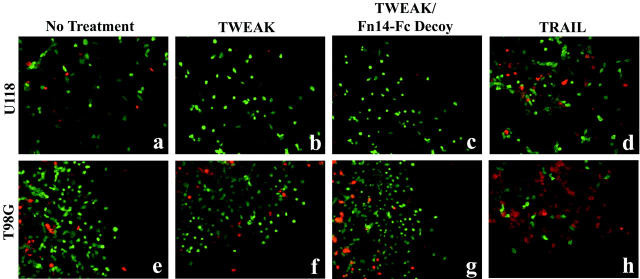
To ensure that the migration arrest effect of the Fn14-Fc decoy receptor was not because of cell toxicity, studies were performed to determine cell viability and death after TWEAK and/or Fn14-Fc treatment. At the end of the migration time intervals, cells were fixed and cell viability was determined using the Live/Dead assay. Treatment of cells with TWEAK alone (Figure 6, b and f) ▶ or TWEAK and Fn14-Fc decoy receptor (Figure 6, c and g) ▶ showed no cytotoxic effect as indicated by green fluorescent cells as compared to untreated cells (Figure 6, a and e) ▶ . In contrast, cells treated with TRAIL, a proapoptotic TNF family member, 34 showed cytotoxicity, as expected, as indicated by the presence of red fluorescent cells (Figure 6, d and h) ▶ .
Figure 6.
Effect of TWEAK and Fn14-Fc on glioma cell viability. U118 and T98G cells were plated onto 10-well glass slides precoated with laminin and then serum-starved for 8 hours before addition of TWEAK and/or Fn14-Fc decoy receptor. In some experiments, cells were treated with TRAIL as a control for the cell viability assay. At the end of the migration intervals, the cells were washed three times with PBS and incubated with the Live/Dead solution. Live cells fluoresce green and dead cells fluoresce red. a and e, No treatment; b and f, TWEAK; c and g, TWEAK plus Fn14-Fc decoy; d and h, TRAIL.
Discussion
We described in an earlier report a genetic profiling analysis of passive versus motility-stimulated glioma cells in vitro. 20 These experiments revealed induction of the Fn14 gene, which encodes a member of the TNF receptor superfamily. 23 In this report, we confirmed that Fn14 mRNA and protein levels increase in migration-activated glioma cells. In addition, quantitation of Fn14 mRNA levels by real-time RT-PCR showed abundant expression of Fn14 transcripts in primary glioblastomas, whereas minimal detection of Fn14 mRNA was observed in normal brain. Fn14 mRNA and protein in GBM samples were expressed by both glioblastoma cells and the vascular endothelium. These results suggest that Fn14 may be a unique marker for invasive glioblastoma.
Human Fn14 is a type-I transmembrane protein of 129 amino acids in length, making it the smallest member of the TNF receptor superfamily identified to date. 21-23 It has ∼82% amino acid sequence identity to murine Fn14 and has been mapped to human chromosome 16p13.3. 22 The human Fn14 gene encodes an ∼1.2-kb mRNA that is up-regulated in fibroblasts treated with polypeptide mitogens, fetal bovine serum, or phorbol 12-myristate 13-acetate (PMA). 22 Furthermore, Fn14 mRNA expression has been detected in a variety of tissues, with highest expression in heart, placenta, kidney, lung, and pancreas, and relatively low expression in brain and liver. 22 Fn14 gene expression is activated during murine liver regeneration and is uniquely elevated in both human and murine hepatocellular carcinomas relative to the low expression in normal liver. 22 Fn14 gene induction is not associated with all cancerous tissues; up-regulation of Fn14 gene expression was not observed in a small sample of human breast, ovary, or kidney tumor specimens. 22 Our results demonstrate that a relatively high level of Fn14 gene expression is present in glial tumors, specifically, GBM. Detection of Fn14 mRNA and protein were limited to the glioblastoma cells and the vascular endothelium in vivo and was absent from adjacent normal brain regions. Endothelial cell expression of the Fn14 receptor had been reported earlier by Wiley and colleagues. 23 These results demonstrate that Fn14 expression and activation may have important and specific roles in the progression of brain cancer.
Studies on glioma cells have shown that there is a strong correlation between migration rate in vitro and invasive behavior in vivo. 18,19 We previously reported that exposure of glioma cells to glioma-derived ECM or laminin enhances the motility of these cells. 8,20 In this study, we found overexpression of Fn14 mRNA and protein in the glioma cell lines activated to migrate by exposure to glioma-derived ECM. However, it is possible that proteins in the ECM itself could affect Fn14 expression. To ensure that up-regulated Fn14 expression is a migration-associated phenomenon, we determined the gene expression profiles of cells migrating at the outer edge (rim) in comparison to nonmotile cells in the core of the radial motility assay. Interesting, Fn14 is one of the candidates that is highly expressed in cells migrating at the rim after 48 hours of migration-time interval (unpublished data). This finding corroborates our notion that up-regulation of Fn14 is, in part, a migration-associated effect.
Recently, TWEAK was identified to be a ligand for Fn14, and TWEAK mRNA has been detected in a number of different tissues including the brain. 35,36 TWEAK is a type II membrane protein and a member of the TNF ligand superfamily. 36 Analysis of an embryonic kidney cell line transfected with a human TWEAK expression construct revealed that TWEAK is cleaved early during its production and secreted as an 18-kd protein, 36 suggesting a prominent role for the soluble form. Soluble TWEAK was reported to weakly induce cell death in a restricted number of cell lines such as breast carcinoma MCF-7, cervical carcinoma HeLa S3, and colon carcinoma HT-29. 36,37 The biological function of TWEAK in mediating apoptosis in these cells was dependent on the presence of interferon-γ or inhibition of protein synthesis. 37 Alternatively, TWEAK mediates chemokine induction and induces proliferation in endothelial cells. 38 Our observation that Fn14 gene expression was up-regulated in human glioblastoma tumors led us to investigate the expression pattern of TWEAK in the glioma tissue samples. First, we investigated TWEAK mRNA levels in glioma cell lines and its relative expression in relation to migratory phenotype. The results indicated that although two of four glioma cell lines expressed TWEAK, the level of TWEAK mRNA did not change when cells were cultured on a migration-promoting substrate. Second, we examined TWEAK mRNA levels in GBM tissue samples or regions from normal brain. Quantitation of TWEAK gene expression using real-time RT-PCR indicated that, unlike Fn14 expression, TWEAK expression is readily detected in normal brain, and is consistently low in GBM samples.
We have shown that TWEAK enhances cell migration in the two glioma cell lines that express low levels of TWEAK mRNA. The migratory phenotype induced by TWEAK appears to be the result of Fn14 activity, consistent with the observation that preincubation of an Fn14-Fc decoy receptor with recombinant TWEAK suppresses cell migration. Even though TWEAK does not induce migration in TWEAK-expressing glioma cells, reduced migration was observed in these cells in the presence of the decoy receptor. It is likely that these glioma cells are not responsive to exogenous TWEAK because endogenous expression of TWEAK occupies the Fn14 receptor. Soluble Fn14-Fc decoy receptors are able to quench the secreted TWEAK produced by these cells. Moreover, the viability assay after the migration interval indicated that neither TWEAK nor the soluble Fn14-Fc protein has cytotoxic activity on any of the four glioma cell lines tested (Figure 6 ▶ and data not shown), suggesting a positive role for TWEAK/Fn14 function on glioma behavior. Although the biological significance of Fn14 in glioblastoma is presently still unclear, it is possible that high levels of Fn14 may promote changes in adhesion to the extracellular matrices at the tumor rim that may contribute to enhanced local migration and invasion. This possibility is consistent with a previous study demonstrating that Fn14 overexpression in murine fibroblasts results in decreased cellular adhesion to ECM proteins. 21 Additional experiments are required to elucidate the biological function of Fn14 in glioblastoma invasion and/or proliferation. We speculate that Fn14 or its signaling pathways may be novel targets for modulating the malignant invasive behavior of gliomas.
Acknowledgments
We thank Greg Alberts and Heather Hanscom for technical assistance, Drs. Alf Giese and Andy Sloan for providing glioblastoma tissue samples and biopsies, Dr. Mitsutoshi Nakada for assistance with analysis of immunohistochemistry, and Dr. Tim Zheng for providing the human TWEAK cDNA clone.
Footnotes
Address reprint requests to Michael E. Berens, Ph.D., Translational Genomics Research Institute, 400 North Fifth St., Suite 1600, Phoenix, AZ 85004. E-mail: mberens@tgen.org.
Supported by the National Institutes of Health (grants NS-42262 to M. E. B. and HL-39727 to J. A. W.) and an Arthritis Investigator Award (to P. J. D.).
References
- 1.Giese A, Westphal M: Glioma invasion in the central nervous system. Neurosurgery 1996, 39:235-250 [DOI] [PubMed] [Google Scholar]
- 2.Pilkington GJ: Tumour cell migration in the central nervous system. Brain Pathol 1994, 4:157-166 [DOI] [PubMed] [Google Scholar]
- 3.Davis FG, McCarthy BJ: Epidemiology of brain tumors. Curr Opin Neurol 2000, 13:635-640 [DOI] [PubMed] [Google Scholar]
- 4.Mornex F, Nayel H, Taillandier L: Radiation therapy for malignant astrocytomas in adults. Radiother Oncol 1993, 27:181-192 [DOI] [PubMed] [Google Scholar]
- 5.Vick NA, Paleologos NA: External beam radiotherapy: hard facts and painful realities. J Neurooncol 1995, 24:93-95 [DOI] [PubMed] [Google Scholar]
- 6.Rooprai HK, Vanmeter T, Panou C, Schnull S, Trillo-Pazos G, Davies D, Pilkington GJ: The role of integrin receptors in aspects of glioma invasion in vitro. Int J Dev Neurosci 1999, 17:613-623 [DOI] [PubMed] [Google Scholar]
- 7.Bolteus AJ, Berens ME, Pilkington GJ: Migration and invasion in brain neoplasms. Curr Neurol Neurosci Rep 2001, 1:225-232 [DOI] [PubMed] [Google Scholar]
- 8.Berens ME, Rief MD, Loo MA, Giese A: The role of extracellular matrix in human astrocytoma migration and proliferation studied in a microliter scale assay. Clin Exp Metastasis 1994, 12:405-415 [DOI] [PubMed] [Google Scholar]
- 9.Friedlander DR, Zagzag D, Shiff B, Cohen H, Allen JC, Kelly PJ, Grumet M: Migration of brain tumor cells on extracellular matrix proteins in vitro correlates with tumor type and grade and involves alphaV and beta1 integrins. Cancer Res 1996, 56:1939-1947 [PubMed] [Google Scholar]
- 10.Giese A, Rief MD, Loo MA, Berens ME: Determinants of human astrocytoma migration. Cancer Res 1994, 54:3897-3904 [PubMed] [Google Scholar]
- 11.VanMeter TE, Rooprai HK, Kibble MM, Fillmore HL, Broaddus WC, Pilkington GJ: The role of matrix metalloproteinase genes in glioma invasion: co-dependent and interactive proteolysis. J Neurooncol 2001, 53:213-235 [DOI] [PubMed] [Google Scholar]
- 12.Tysnes BB, Mahesparan R: Biological mechanisms of glioma invasion and potential therapeutic targets. J Neurooncol 2001, 53:129-147 [DOI] [PubMed] [Google Scholar]
- 13.Gladson CL: The extracellular matrix of gliomas: modulation of cell function. J Neuropathol Exp Neurol 1999, 58:1029-1040 [DOI] [PubMed] [Google Scholar]
- 14.Liotta LA, Steeg PS, Stetler-Stevenson WG: Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 1991, 64:327-336 [DOI] [PubMed] [Google Scholar]
- 15.Rutka JT, Apodaca G, Stern R, Rosenblum M: The extracellular matrix of the central and peripheral nervous systems: structure and function. J Neurosurg 1988, 69:155-170 [DOI] [PubMed] [Google Scholar]
- 16.Uhm JH, Gladson CL, Rao JS: The role of integrins in the malignant phenotype of gliomas. Front Biosci 1999, 4:D188-D199 [DOI] [PubMed] [Google Scholar]
- 17.Koochekpour S, Merzak A, Pilkington GJ: Extracellular matrix proteins inhibit proliferation, upregulate migration and induce morphological changes in human glioma cell lines. Eur J Cancer 1995, 31A:375-380 [DOI] [PubMed] [Google Scholar]
- 18.Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M, Radmacher M, Simon R, Yakhini Z, Ben Dor A, Sampas N, Dougherty E, Wang E, Marincola F, Gooden C, Lueders J, Glatfelter A, Pollock P, Carpten J, Gillanders E, Leja D, Dietrich K, Beaudry C, Berens M, Alberts D, Sondak V: Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature 2000, 406:536-540 [DOI] [PubMed] [Google Scholar]
- 19.Chicoine MR, Silbergeld DL: The in vitro motility of human gliomas increases with increasing grade of malignancy. Cancer 1995, 75:2904-2909 [DOI] [PubMed] [Google Scholar]
- 20.Mariani L, Beaudry C, McDonough WS, Hoelzinger DB, Demuth T, Ross KR, Berens T, Coons SW, Watts G, Trent JM, Wei JS, Giese A, Berens ME: Glioma cell motility is associated with reduced transcription of proapoptotic and proliferation genes: a cDNA microarray analysis. J Neurooncol 2001, 53:161-176 [DOI] [PubMed] [Google Scholar]
- 21.Meighan-Mantha RL, Hsu DK, Guo Y, Brown SA, Feng SL, Peifley KA, Alberts GF, Copeland NG, Gilbert DJ, Jenkins NA, Richards CM, Winkles JA: The mitogen-inducible Fn14 gene encodes a type I transmembrane protein that modulates fibroblast adhesion and migration. J Biol Chem 1999, 274:33166-33176 [DOI] [PubMed] [Google Scholar]
- 22.Feng SL, Guo Y, Factor VM, Thorgeirsson SS, Bell DW, Testa JR, Peifley KA, Winkles JA: The Fn14 immediate-early response gene is induced during liver regeneration and highly expressed in both human and murine hepatocellular carcinomas. Am J Pathol 2000, 156:1253-1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiley SR, Cassiano L, Lofton T, Davis-Smith T, Winkles JA, Lindner V, Liu H, Daniel TO, Smith CA, Fanslow WC: A novel TNF receptor family member binds TWEAK and is implicated in angiogenesis. Immunity 2001, 15:837-846 [DOI] [PubMed] [Google Scholar]
- 24.Westphal M, Haensel M, Mueller D, Laas R, Kunzmann R, Rohde E, Koenig A, Hoelzel F, Herrmann HD: Biological and karyotypic characterization of a new cell line derived from human gliosarcoma. Cancer Res 1988, 48:731-740 [PubMed] [Google Scholar]
- 25.Berens ME, Weisman AS, Spencer DR, Dougherty DV, Ellinger SS, Rosenblum ML: Growth properties and oncogene expression in 2 newly derived human glioma cell lines: assessment of growth determinants. Proc AACR 1990, 31:273 [Google Scholar]
- 26.Vlodavsky I, Levi A, Lax I, Fuks Z, Schlessinger J: Induction of cell attachment and morphological differentiation in a pheochromocytoma cell line and embryonal sensory cells by the extracellular matrix. Dev Biol 1982, 93:285-300 [DOI] [PubMed] [Google Scholar]
- 27.Mariani L, McDonough WS, Hoelzinger DB, Beaudry C, Kaczmarek E, Coons SW, Giese A, Moghaddam M, Seiler RW, Berens ME: Identification and validation of P311 as a glioblastoma invasion gene using laser capture microdissection. Cancer Res 2001, 61:4190-4196 [PubMed] [Google Scholar]
- 28.Lehmann U, Glockner S, Kleeberger W, von Wasielewski HF, Kreipe H: Detection of gene amplification in archival breast cancer specimens by laser-assisted microdissection and quantitative real-time polymerase chain reaction. Am J Pathol 2000, 156:1855-1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrison TB, Weis JJ, Wittwer CT: Quantification of low-copy transcripts by continuous SYBR Green I monitoring during amplification. Biotechniques , 24:954-960 [PubMed] [Google Scholar]
- 30.Ririe KM, Rasmussen RP, Wittwer CT: Product differentiation by analysis of DNA melting curves during the polymerase chain reaction. Anal Biochem 1997, 245:154-160 [DOI] [PubMed] [Google Scholar]
- 31.St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW: Genes expressed in human tumor endothelium. Science 2000, 289:1197-1202 [DOI] [PubMed] [Google Scholar]
- 32.Donohue PJ, Richards CM, Brown AN, Hanscom HN, Buschmann J, Thangada S, Hla T, Williams MS, Winkles JA: TWEAK is an endothelial cell growth and chemotactic factor that also potentiates FGF-2 and VEGF-A mitogenic activity. Arterioscler Thromb Vasc Biol 2003, in press [DOI] [PubMed]
- 33.Giese A, Loo MA, Rief MD, Tran N, Berens ME: Substrates for astrocytoma invasion. Neurosurgery 1995, 37:294-301 [DOI] [PubMed] [Google Scholar]
- 34.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA: Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 1995, 3:673-682 [DOI] [PubMed] [Google Scholar]
- 35.Saas P, Boucraut J, Walker PR, Quiquerez AL, Billot M, Desplat-Jego S, Chicheportiche Y, Dietrich PY: TWEAK stimulation of astrocytes and the proinflammatory consequences. Glia 2000, 32:102-107 [PubMed] [Google Scholar]
- 36.Chicheportiche Y, Bourdon PR, Xu H, Hsu YM, Scott H, Hession C, Garcia I, Browning JL: TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem 1997, 272:32401-32410 [DOI] [PubMed] [Google Scholar]
- 37.Marsters SA, Sheridan JP, Pitti RM, Brush J, Goddard A, Ashkenazi A: Identification of a ligand for the death-domain-containing receptor Apo3. Curr Biol 1998, 8:525-528 [DOI] [PubMed] [Google Scholar]
- 38.Lynch CN, Wang YC, Lund JK, Chen YW, Leal JA, Wiley SR: TWEAK induces angiogenesis and proliferation of endothelial cells. J Biol Chem 1999, 274:8455-8459 [DOI] [PubMed] [Google Scholar]