Abstract
The interaction between FasL on tumor cells and Fas on lymphocytes may represent a tumor immune escape mechanism. We explored FasL expression and function in human urinary bladder transitional cell carcinomas (TCCs). FasL expression was observed in situ in 45% of TCCs (n = 45) and was absent in normal urothelium (n = 20). A correlation existed between FasL expression and high tumor grade (0% in G1, 14% in G2, and 75% in G3; P < 0.0001) and stage (13% in superficial Ta-T1 versus 81% in invasive T2-T4; P < 0.0001). FasL function was shown by the ability of two FasL-positive primary culture TCC cell lines (established from two FasL-positive invasive TCCs) to induce Fas-mediated killing not only of conventional Fas-sensitive targets (such as Jurkat cells or phytohemagglutinin-lymphoblasts), but also of autologous T lymphocytes generated in a mixed lymphocyte tumor-cell culture. In addition, an association between FasL expression by TCC cells and activated caspase-8, -9, and -3 expression by interferon-γ-producing CD8-positive tumor-infiltrating lymphocytes was observed in situ. Our results show a functional expression of TCC-expressed FasL that correlates with tumor progression. These results suggest that TCC-expressed FasL may induce apoptosis of anti-tumor T lymphocytes in vivo, providing new insights on the mechanisms involved in bladder TCC progression.
Urinary bladder cancer is the fifth leading cause of cancer death among males in Western countries. 1 Transitional cell carcinoma (TCC) is the most common bladder cancer, which in ∼80% of the cases occurs as superficial (confined to the urothelium, stage Ta; or the lamina propria, stage T1) and in ∼20% as invasive (penetrating the muscle, stages T2-T4) tumor. The prognosis of TCC is directly related to the stage of the tumor. In superficial tumors a 5-year-survival rate of 80 to 90% of the patients is currently described, whereas in invasive tumors less than 40% of the patients reach a 5-year survival. 1
The immune system is likely to be implicated in the control of urinary bladder TCC cell growth. 2 Indeed, TCC is sensitive to immunotherapeutic agents, such as bacillus Calmette-Guérin 3 and interleukin-2. 4 Moreover, TCCs express tumor-associated antigens, including the MAGE, BAGE, and GAGE families; 5 HOM-MEL-40/SSX-2; and LAGE 1/NY-ESO-1. 6
Neoplastic development and/or progression may result from tumor evasion of the host immune surveillance, and several potential strategies of tumor immune escape have been proposed. 7 Fas ligand (FasL, CD178), a cell-surface protein of the tumor necrosis factor family, binds to its receptor Fas, thus inducing apoptosis of Fas-bearing cells. 8 Fas-mediated apoptosis is dependent on the activation of different members of a family of cysteinyl-aspartate proteases (called caspases), such as caspase-8, -9, and -3, which are responsible for both the initiation of the apoptotic cascade and the execution of the cell damage. 9 The expression of FasL was originally thought to be restricted to the immune system (ie, activated T and natural killer cells), 8 where it plays a role in the maintenance of T-cell homeostasis. However, it is now well-known that FasL is also expressed by nonlymphoid cells 10 and a variety of nonlymphoid human tumors. 11-14 This discovery raises the possibility that tumor-expressed FasL induces apoptosis of anti-tumor-activated T lymphocytes, representing a novel mode of tumor immune escape. 15
In the present work we document the expression and function of FasL in TCCs of the urinary bladder. In particular, we investigate FasL expression in bladder TCCs as well as in normal urothelial cells in vivo and we analyze its relevance particularly with respect to the pathological characteristics of the tumor. In addition, we investigate the ability of FasL-expressing TCC cells to kill T lymphocytes, examining both Fas-mediated T-cell death induced by FasL-positive primary culture TCC cells in vitro, and apoptosis of interferon (IFN)-γ-producing CD8-positive tumor-infiltrating lymphocytes (TILs) in situ.
Materials and Methods
Urothelial Tissues
Sixty-four urothelial tissues, including 44 neoplastic and 20 normal samples, were obtained from 56 patients surgically treated at the Henri-Moudor Hospital (University Hospital Centre, Créteil, France) following a protocol approved by the local ethics committee. All tissues were immediately fixed with 4% buffered formalin and embedded in paraffin. Tumor tissue samples were collected from 44 patients (40 males and 4 females; median age, 69 years; age range, 34 to 91 years) with untreated primary TCCs of the urinary bladder at different histological stages and grades as classified according to the International Union against Cancer (UICC) 16 and the World Health Organization, 17 respectively (Table 1) ▶ . Twenty-three of the 44 TCCs were superficial (Ta, T1) TCCs. They included 6 low-grade (G1), 12 intermediate-grade (G2), and 5 high-grade (G3) tumors. Twenty-one of the 44 TCCs were invasive (T2-T4) TCCs; 8 of these were associated with a metastatic disease. Invasive TCCs included 2 G2 and 19 G3 tumors. Invasive TCCs included the 1207-T (T3;G3) and 1512-T (T2;G3) primary tumors, from which the two corresponding primary culture TCC cell lines were established. 18 The following normal tissues were analyzed: one calyx and two ureters from three patients treated for nontumor renal diseases; three bladders from patients with a prostatic disease without involvement of the bladder; 14 bladders from patients with bladder TCC, from which eight corresponding tumor tissues were also analyzed.
Table 1.
Urinary Bladder TCCs: Pathological Stage, Grade, and FasL Expression
| Tumor | Stage | Grade | FasL staining |
|---|---|---|---|
| 01 | Ta | G1 | − |
| 02 | Ta | G1 | − |
| 03 | Ta | G1 | − |
| 04 | Ta | G2 | − |
| 05 | T1 | G2 | − |
| 06 | Ta | G2 | − |
| 07 | Ta | G2 | − |
| 08 | Ta | G3 | − |
| 09 | T1 | G2 | − |
| 10 | T1 | G3 | − |
| 11 | T1 | G2 | − |
| 12 | Ta | G2 | − |
| 13 | T1 | G1 | − |
| 14 | T1 | G2 | − |
| 15 | T1 | G2 | − |
| 16 | T1 | G2 | − |
| 17 | T1 | G2 | − |
| 18 | T1 | G1 | − |
| 19 | T1 | G2 | + |
| 20 | T1 | G3 | − |
| 21 | T1 | G3 | + |
| 22 | Ta | G1 | − |
| 23 | Ta | G3 | + |
| 24 | T2;N+ | G3 | + |
| 25 | T4;N+ | G3 | + |
| 26 | T2;M+ | G3 | + |
| 27 | T2;M+ | G3 | + |
| 28 | T2 | G3 | − |
| 29 | T2;M+ | G3 | + |
| 30 | T2 | G3 | + |
| 31 | T3 | G3 | + |
| 32 | T3 | G3 | − |
| 33 | T3 | G3 | + |
| 34 | T3 | G3 | + |
| 35 | T3 | G3 | + |
| 36 | T3 | G3 | + |
| 37 | T2 | G2 | − |
| 38 | T4;M+ | G3 | + |
| 39 | T4;M+ | G3 | + |
| 40 | T2 | G3 | + |
| 41 | T3 | G3 | − |
| 42 | T3;M+ | G3 | + |
| 43 | T2 | G2 | + |
| 44 | T2 | G3 | + |
Cells
The 1207 and 1512 human bladder TCC cell lines were generated in our laboratory by direct culturing of resected 1207-T and 1512-T bladder TCC tissues, respectively; 18 early-passage cell lines were used in our experiments. T24, J82, RT112, 647V, and SD48 human long-term established bladder cancer cell lines were obtained from originators’ laboratories or from American Type Culture Collection (Rockville, MD). YT-indi, a human FasL-expressing natural killer-like cell line, and Jurkat, a human Fas-sensitive T-cell leukemia cell line, were gifts from Dr. S. Chouaib (INSERM, Villjuif, France) and Dr. A. Bensussan (INSERM, Créteil, France), respectively.
Peripheral blood mononuclear cells (PBMCs), indicated as 1207-PBMCs, were obtained from the patient with the 1207-T TCC and were isolated by Ficoll-Hypaque gradient centrifugation. Phytohemoagglutinin (PHA)-lymphoblasts were obtained by stimulating 1207-PBMCs with PHA (2.5 μg/ml) and interleukin-2 (25 IU/ml). Mixed lymphocyte tumor-cell culture (MLTC)-derived T lymphocytes were obtained by stimulating 1207-PBMCs with the autologous 1207 TCC cell line in a MLTC, performed essentially as described. 19
Immunostaining
Immunostaining on tissue sections was performed using the standard avidin-biotin-peroxidase complex method. Tissue sections (4- to 5-μm thick) were deparaffinized and heat-induced epitope retrieval was achieved by microwave treatment in 10 mmol/L of citrate buffer (pH 6.0) for 10 minutes. Endogenous peroxidase was quenched with 2.5% H2O2 in methanol and nonspecific binding was blocked with 5% nonfat dry milk in phosphate-buffered saline. The sections were then incubated overnight at 4°C with three different recommended (FASL, CD178. 2001. Available at http://www.ncbi.nlm.nih.gov/PROW/guide/333879674_g.htm.) 20 anti-FasL Alf-2.1 (2 μg/ml, IgG1; from Ancell, Bayport, MN), A11, or H11 (2.5 μg/ml, IgM and IgG2a, respectively; from Alexis Corp., Lausen, CH) monoclonal antibodies (mAbs), or the rabbit IgG affinity purified anti-activated caspase-3 (R&D Systems, Minneapolis. MN) or anti-activated caspase-9 (Alexis Corp.) polyclonal antibodies, the anti-activated caspase-8 mAb (Alexis Corp.), the anti-IFN-γ (Chemicon International Inc., Temecula, CA), the anti-CD3 polyclonal antibody (DAKO Corp., Carpinteria, CA), or the anti-CD8 mAb (Novocastra Laboratories, Newcastle, UK). Tissue sections were subsequently immunolabeled by an avidin-biotin complex immunoperoxidase technique, using the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA). A solution of 0.5 mg/ml of diaminobenzidine tetrahydrochloride and 0.02% H2O2 was used as the chromogen. Slides were counterstained with hematoxylin and examined in a light microscope. All slides were examined independently by two observers. Sections without primary antibody, as well as those with nonimmunized rabbit IgG or IgM (Sigma-Aldrich Immunochemicals), or irrelevant anti-thyroglobulin (DAKO) polyclonal antibodies were used as negative controls.
Immunostaining on cell lines was performed using the biotin-streptavidin horseradish peroxidase (LSAB kit, DAKO) method. Cytospins (5 × 105 cells/ml) were fixed in acetone for 10 minutes at room temperature and immunostained using the three different anti-FasL mAbs used in paraffin sections.
Reverse Transcriptase (RT)-Polymerase Chain Reaction (PCR)
Total RNA was obtained by the TRIzol method (Gibco BRL, Invitrogen, Milan, Italy). PCR was performed on the cDNA using the following intron-spanning primers: 21 forward, ATAGGATCCATGTTTCTGCTCTTCCACCTACAGAAGGA; and reverse, ATAGAATTCTGACCAAGAGAGAGCTCAGATACGTTGAC. These primers generated a 522-bp product from FasL mRNA and a 6.4-kb fragment from genomic DNA. Primers used to amplify β2-microglobulin were as follows: ACCCCCACTGAAAAAGATGA and ATCTTCAAACCTCCATGATG. Thirty-five cycles were performed for both FasL and β2-microglobulin PCRs. PCR products were electrophoresed on a 1.5% agarose gel and visualized by ethidium bromide staining and UV illumination.
Evaluation of Lymphocyte Death
Quantitative evaluation of lymphocyte death was measured by the 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. 22 TCC cells were seeded in 24-well tissue culture plates and allowed to adhere overnight. After washing, TCC cells were incubated with target cells, such as Jurkat cells, PHA-lymphoblasts, or MLTC-derived T lymphocytes at the indicated effector:target cell ratios. Control target cells were plated with medium alone. A FasL-negative TCC cell line was used as control for FasL-positive effector cell activity. At the indicated times, target cells were recovered, washed, and incubated for 3 hours at 37°C in a 5 mg/ml MTT solution. After removing culture supernatants, acid isopropanol was added and the absorbance was determined in a microtiter plate reader at 550 nm. In some experiments, tumor cells were preincubated with 0.75 μg/ml of the recommended anti-FasL-neutralizing NOK-1 mAb (PharMingen) (FASL, CD178. 2001. Availableat http://www.ncbi.nlm.nih.gov/PROW/guide/333879674_g.htm.) or with an isotype-matched irrelevant antibody 1 hour before adding lymphocyte target cells. The percentage of specific target cell killing was expressed as relative to that of target cells with medium alone, set arbitrarily at 100%. At least three independent experiments were performed for each TCC cell line.
Statistical Analysis
The Pearson chi-square test for contingency table was used.
Results
Expression of FasL by Urinary Bladder TCCs
To investigate the expression of FasL by urinary bladder TCCs, immunohistochemical staining using three different recommended anti-FasL mAbs (Alf-2.1, A11, or H11) (FASL, CD178. 2001. Available at http://www.ncbi.nlm.nih.gov/PROW/guide/333879674_g.htm.) 20 was performed on 44 bladder TCCs (Table 1) ▶ . We observed that 20 of 44 (45%) bladder TCCs were positive for FasL immunostaining (as exemplified in Figure 1, C and D ▶ ). In FasL-positive TCCs, specific immunostaining was limited to neoplastic urothelial cells, as well as a stromal leukocyte population and vascular endothelial cells (Figure 1, C and D) ▶ . Although FasL staining was usually uniform throughout the tumor (Figure 1C) ▶ , FasL-negative single cells or small tumor areas (never exceeding 5 to 10% of the tumor extension) were also found (Figure 1D) ▶ . At a cellular level, FasL staining was observed in the cytoplasm and showed a cell surface reinforcement. In FasL-positive TCCs, the staining pattern was similar among the different tumors, independently from the tumor grade and stage. In FasL-negative TCCs, neoplastic urothelial cells were completely negative, and, in a minority of the cases, a positive staining was found in few (<10%) scattered single or clustered neoplastic cells (Figure 1E) ▶ ; FasL-positive leukocytes and endothelial cells were found in the stroma (Figure 1F) ▶ .
Figure 1.
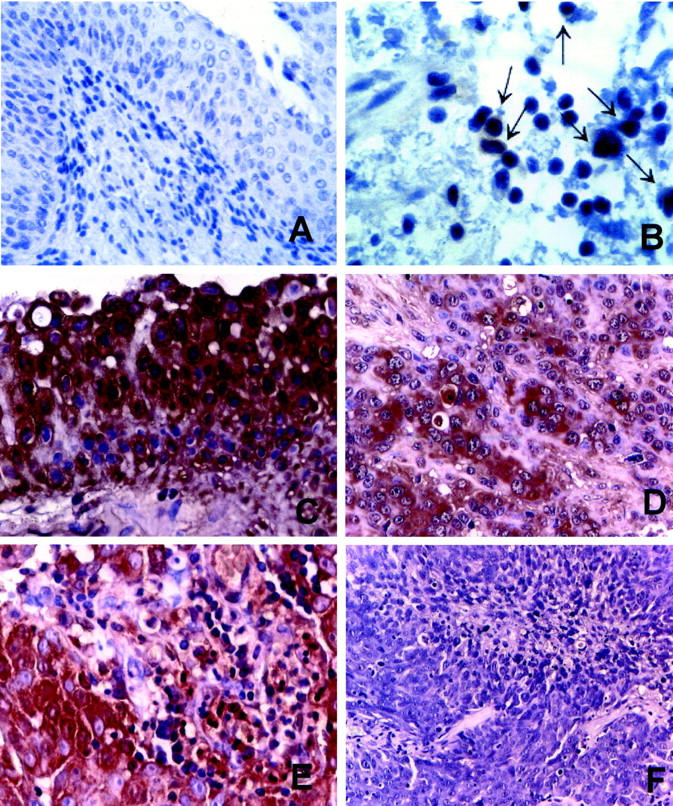
FasL expression in normal bladder urothelium and TCCs. Representative immunohistochemical stainings using the anti-FasL A11 mAb. Normal bladder: A: Normal urothelial cells are negative for FasL immunostaining. B: A higher magnification of A shows occasional stromal-positive lymphoid cells (arrows). Bladder TCC: C: FasL is expressed by all of the tumor cells that show evident membrane reinforcements. D: An infiltrating TCC, showing negative scattered tumor cells. E: Stromal FasL-positive lymphoid cells in a FasL-positive tumor. F: A FasL-negative high-grade TCC. Immunostaining is in brown. Counterstain with hematoxylin. Original magnifications: ×20 (A, F); ×40 (C, D, E); ×100 (B).
Normal urothelial biopsies from 20 patients, including five samples matching with five FasL-positive TCCs, were also analyzed for FasL expression. In all tissues, normal urothelial cells were consistently negative for FasL immunostaining (as exemplified in Figure 1, A and B ▶ ), whereas a specific staining was found in occasional stromal lymphoid cells (Figure 1B ▶ , arrows). Comparable results were observed using the three different anti-FasL mAbs.
Correlation Analysis of FasL Expression by TCC Cells and the Pathological Tumor Grade and Stage
To understand the in vivo significance of bladder TCC-expressed FasL, we investigated whether a correlation existed between FasL expression by TCCs and the pathological grade and stage of the tumor. A strong correlation between FasL expression and the worsening of the tumor grade (P < 0.0001) was observed (Table 2) ▶ . In addition, 3 of 23 (13%) superficial (Ta, T1) versus 17 of 21 (81%) invasive (T2-T4) TCCs were positive for FasL immunostaining (Table 3) ▶ , showing that a strong correlation also existed between FasL expression and invasive TCCs when compared to superficial tumors (P < 0.0001). Interestingly, all of the invasive TCCs associated with a metastatic disease (n = 8) were positive for FasL immunostaining (Table 1) ▶ . It should be also noted that the three FasL-positive superficial TCCs consisted of G2 and G3 tumors, whereas no expression of FasL was observed in G1 tumors (Table 1) ▶ .
Table 2.
Correlation between FasL Expression and Pathological Tumor Grade
| Grade | n | %* |
|---|---|---|
| G1 | 6 | 0 |
| G2 | 14 | 14 |
| G3 | 24 | 75 |
*Percentage of tissue samples positive for FasL by immunostaining; P < 0.0001.
Table 3.
Correlation between FasL Expression and Pathological Tumor Stage
| Stage | n | %* |
|---|---|---|
| Ta, T1† | 23 | 13 |
| T2 to T4‡ | 21 | 81 |
*Percentage of tissue samples positive for FasL by immunostaining.
†Ta, T1 are superficial TCCs.
‡T2 to T4 are invasive TCCs; P < 0.0001.
Induction of Fas-Mediated T-Lymphocyte Killing by Urinary Bladder TCC Cells
To direct assess and quantify the ability of FasL-expressing TCC cells to induce T-cell death, two primary culture bladder TCC cell lines, 1207 and 1512, were established from two FasL-positive invasive bladder TCC tissues, 1207-T and 1512-T respectively, and their FasL expression and function were investigated.
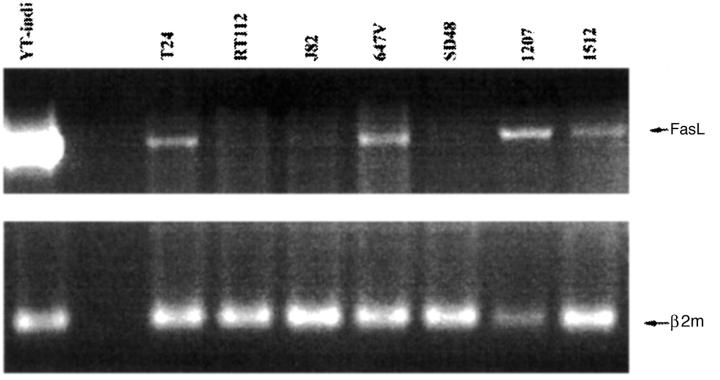
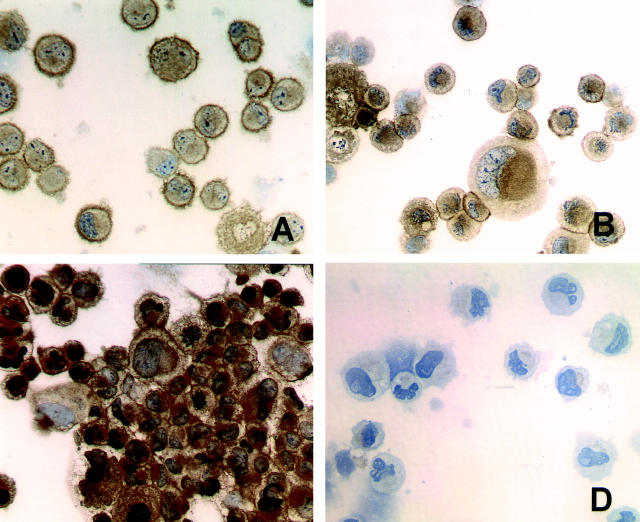
FasL expression was analyzed at both the mRNA and the protein level. FasL mRNA was assessed by RT-PCR using intron-spanning primers to avoid amplification of contaminating genomic cDNA. 21 As shown in Figure 2 ▶ , a cDNA signal of the expected size (522 bp) was amplified in both 1207 and 1512 primary culture TCC cells and four of five long-term established bladder cancer cell lines. YT-indi, a natural killer-like cell line, served as positive control. FasL protein expression was examined by immunocytochemical staining with the same three mAbs (Alf-2.1, A11, or H11) used in paraffin sections (FASL, CD178. 2001. Available at http://www.ncbi.nlm.nih.gov/PROW/guide/333879674_g.htm.). 20 In agreement with the RT-PCR results, a FasL-positive staining was observed in 1207, 1512, and T24 TCC cells, but not in the RT112 TCC cell line (Figure 3) ▶ . Interestingly, a positive staining was prevalent on the cell surface of the two 1207 and 1512 primary culture cell lines, whereas a prevalent cytoplasmic staining was observed in the T24 long-term established TCC cell line (Figure 3) ▶ .
Figure 2.
FasL mRNA expression by bladder TCC cell lines. RT-PCR analysis of FasL mRNA expression was performed on urinary bladder TCC cell lines using intron-spanning primers. 1207 and 1512 are primary culture TCC cell lines, established from two FasL-positive invasive TCC tissues. YT-indi, a natural killer-like cell line, served as positive control. β2-microglobulin (β2m) served as control for equal loading. The amplified products were electrophoresed on a 1.5% agarose gel and stained with ethidium bromide. Amplification product bands for FasL (522 bp) and β2m (120 bp) are indicated.
Figure 3.
FasL protein expression by bladder TCC cell lines. Immunocytochemical staining was performed using the anti-FasL A11 mAb. The 1207 (A) and 1512 (B) primary culture TCC cell lines, obtained from two FasL-expressing tumors, show a membrane immunostaining. C: T24 bladder cell line is characterized by a predominant granular and cytoplasmatic immunostaining. D: RT112 bladder cell line is negative. Immunostaining is in brown. Counterstain with hematoxylin. Original magnifications, ×40 (A–D).
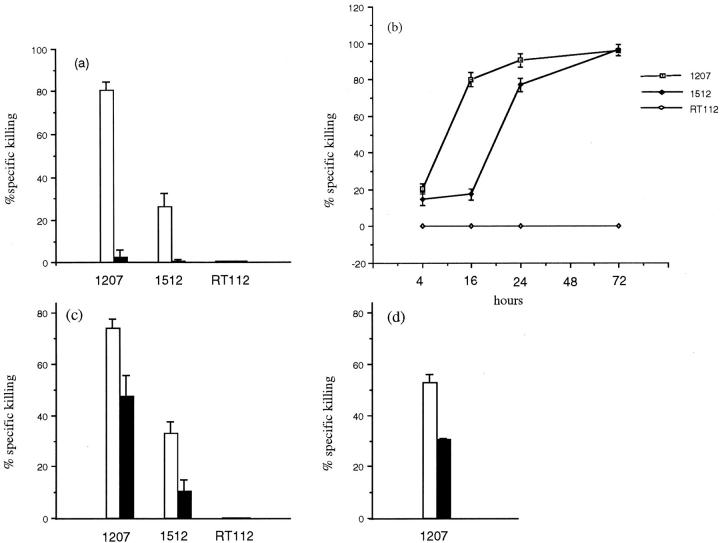
To evaluate the function of TCC cell-expressed FasL, early-passage 1207 and 1512 cell lines were co-cultured with conventional Fas-sensitive T lymphocyte targets, such as Jurkat cells or PHA lymphoblasts, and T-cell death was measured by MTT assay. The FasL-negative RT112 bladder TCC cell line was used as control for FasL-positive effector cell activity. In contrast to the RT112 cell line, FasL-positive 1207 and 1512 bladder TCC cells killed Jurkat cells (Figure 4, a and b) ▶ as well as autologous or allogeneic PHA lymphoblasts (Figure 4c) ▶ . Jurkat cell killing increased in a time-dependent manner, reaching high levels between 16 and 24 hours, depending on the tumor cell line (Figure 4b) ▶ . Moreover, increased killing paralleled the increased effector:target cell ratio (data not shown). To verify whether T lymphocyte death was mediated by tumor cell-associated FasL, bladder TCC effector cells were preincubated with the recommended anti-FasL neutralizing NOK-1 mAb (FASL, CD178. 2001. Available at http://www.ncbi.nlm. nih.gov/PROW/guide/333879674_g.htm.) 1 hour before adding lymphocyte target cells, and lymphocyte death was subsequently analyzed. As shown in Figure 4a ▶ , Jurkat cell killing was almost completely inhibited when TCC cells were preincubated with the anti-FasL mAb, and a partial inhibition of killing was observed when PHA lymphoblasts were used as target cells (Figure 4c) ▶ . These results show that primary culture bladder TCC cell lines express a functional FasL, capable of killing conventional Fas-sensitive T cells.
Figure 4.
Fas-mediated T lymphocyte killing induced by bladder TCC cell lines. Primary culture TCC cell lines, as effector, pretreated with (closed bars) or without (open bars) 0.75 μg/ml of the anti-FasL-neutralizing NOK-1, were co-cultured with lymphocytes as target cells for 18 hours. a: A Fas-positive Jurkat cell line was killed by the 1207 and 1512 FasL-positive bladder TCC cells but not by the RT112 FasL-negative bladder TCC cell line. b: Kinetics of Jurkat cell killing mediated by bladder TCC cell lines. c: PHA lymphoblasts were killed by autologous and allogeneic FasL-positive TCC cell lines. d: MLTC-derived T lymphocytes were killed by the autologous FasL-positive TCC cell line. All experiments were performed at a 10:1 E:T ratio. Target cell killing was measured by MTT assay and was expressed as relative to that of target cells with medium alone, set arbitrarily at 100%. The mean ±SD of triplicate co-culture is indicated.
To further investigate the function of bladder TCC-expressed FasL in an experimental condition that might more closely reflect the in vivo tumor-lymphocyte interaction, we analyzed whether the 1207 bladder TCC cell line was capable of inducing Fas-mediated killing of autologous lymphocytes, generated on stimulation of 1207 PBMCs with the autologous tumor cell line in a MLTC. The MLTC-derived effector cells consisted of ∼90% CD3-, 65% CD4-, and 26% CD8-positive T lymphocytes, and exerted a weak but reproducible specific cytotoxic activity against autologous tumor cells (data not shown). As shown in Figure 4d ▶ , 1207 bladder TCC cells killed MLTC-derived effector T lymphocytes and the killing was partially mediated by tumor cell-associated FasL, because it was partially inhibited by preincubation of the tumor cell with the anti-FasL mAb (Figure 4d) ▶ . Nevertheless, the high level of MLTC-derived T-cell death in the MLTC (data not shown) made us unable to further investigate the function of tumor-expressed FasL toward anti-tumor T-cell subpopulations. Overall, these results show that FasL-positive bladder TCC cells are capable of inducing Fas-mediated killing of autologous T lymphocytes in vitro generated on a specific stimulus, such as the tumor cell.
Association of FasL Expression by TCC Cells with Apoptosis of IFN-γ-Producing CD8-Positive TILs
To investigate whether FasL-positive bladder TCC cells were able to induce apoptosis of tumor-infiltrating T lymphocytes in vivo, we analyzed in situ T-cell expression of caspase-8-, -9-, and -3-activated specific proteases, known to be responsible for Fas-mediated apoptosis. 9 We immunostained parallel sections of FasL-positive and -negative tumors with highly specific and sensitive antibodies directed against caspase-8, -9, or -3 active forms 23 and CD3 or CD8 T-cell antigens. Caspase-8-positive cells were usually stained very weakly and were clearly found only in FasL-positive bladder TCCs (Figure 5B) ▶ . In contrast, caspase-9 (Figure 5C) ▶ - and caspase-3 (Figure 5D) ▶ -positive cell staining was more intense and was observed both in FasL-positive (Figure 5 ▶ ; A to D) and FasL-negative tumors (data not shown). The correlation analysis between FasL and caspase-3 cell expression showed that the caspase-3-positive cell number was higher in the FasL-positive (196 ± 22/mm2) versus the FasL-negative (30 ± 3/mm2 tissue) TCCs (P < 0.002). Similar results were observed when tissue sections were immunostained for caspase-9. In FasL-positive TCCs, caspase-8-, -9-, and -3-positive cells were often located within the areas of necrosis, at the center of the tumor nests. Although some of these cells reminded urothelial neoplastic cells, caspase-8-, -9, and -3-positive cells, with a morphology of lymphoid cells, were frequently scattered throughout the tumor solid areas in close contact to tumor cells, or clustered at the tumor stromal edges in close contact each other (Figure 5 ▶ ; B to D). When parallel tissue sections were stained with the anti-CD3 or anti-CD8 antibodies, most of the caspase-8-, -9-, and -3-positive cells co-localized with CD3 (Figure 5E) ▶ - and CD8 (Figure 5F) ▶ -positive T lymphocytes. In addition, CD3- and CD8-positive T cells, located in close contact to either FasL-positive TCC cells or other T cells, showed coarctate nuclei, very likely resulting from the triggering of the apoptosis signaling (Figure 6A) ▶ . In FasL-negative TCCs, caspase-9- and -3-positive cell distribution did not correspond to the distribution of CD3- and CD8-positive T cells (data not shown). In fact caspase-9- and -3-positive cells, rare and mostly with a morphology of epithelial-like cells, were located within the tumor nest, whereas CD3- and CD8- positive T lymphocytes were mostly found in the peritumoral stromal infiltrate (data not shown). Overall, these results show that an association exists between FasL expression by bladder TCC cells and the apoptosis of CD3- and CD8-positive TILs, indicating that FasL-positive TCC cells are able to induce apoptosis of tumor-infiltrating T lymphocytes in vivo.
Figure 5.
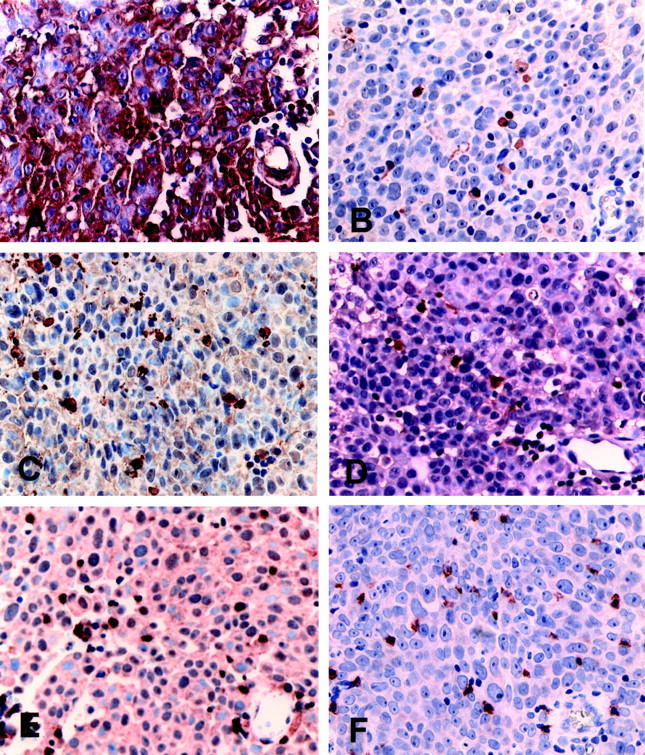
Caspase-8, -9, and -3 expression by both CD3- and CD8-positive TILs in FasL-positive TCCs. Representative immunohistochemical stainings using the anti-FasL A11 mAb (A), the anti-activated caspase-8 mAb (B), the anti-activated caspase-9 (C), anti-activated caspase-3 (D), and anti-CD3 (E) polyclonal antisera, and the anti-CD8 mAb (F) on parallel tissue sections. A: FasL staining showing both positive tumor and stromal lymphoid cells. Caspase-8 (B), caspase-9 (C), and caspase-3 (D) active forms were expressed by small cells scattered throughout the tumor. The tissue distribution of caspase-8-, -9-, and -3-positive cells was similar. CD3-positive (E) and CD8-positive (F) TILs infiltrate the tumor mass. The CD3- and CD8-positive TIL distribution results were comparable to that of the caspase-8-, -9-, and -3-positive cells. Immunostaining is in brown. Counterstain with hematoxylin. Original magnifications, ×20 (A–F).
Figure 6.
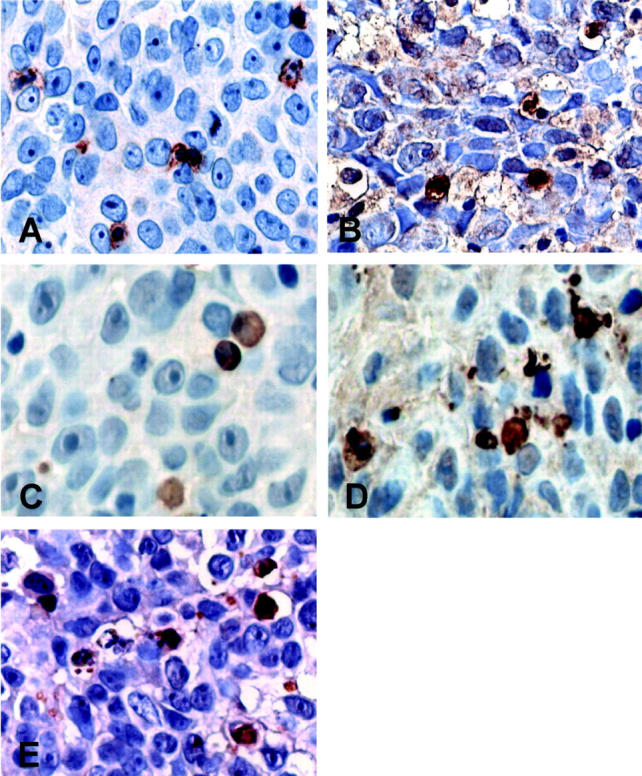
Caspase-8, -9, and -3 expression by IFN-γ-producing CD8-positive TILs in a FasL-positive TCC. Larger magnifications of central tumor areas depicted in Figure 5 ▶ are shown. A: Surface CD8-positive cell staining appeared clump-like. CD8-positive cells had large cytoplasms and coarctate nuclei; B: Some of the CD8-positive T lymphocytes, located in close contact with the tumor cells, were strongly positive for IFN-γ immunostaining. Caspase-8 (C)-, caspase-9 (D)-, and caspase-3 (E)-positive cells showed a tissue distribution and morphology comparable to that of IFN-γ- and CD8-positive T lymphocytes. Immunostaining is in brown. Counterstain with hematoxylin. Original magnifications, ×800 (A–E).
To further investigate the function of bladder TCC-expressed FasL in vivo, we analyzed whether the CD8-positive TILs, which underwent apoptosis in FasL-positive TCCs, consisted in functionally activated effector T cells in situ. We immunostained parallel sections of FasL-positive bladder TCCs with antibodies against the caspase-8, -3, and -9 active forms, the IFN-γ cytokine or the CD8 antigen. We observed that some of the caspase-expressing CD8-positive T cells (Figure 6 ▶ ; A, C, D, E), located in close contact with the tumor cells, were also strongly positive for IFN-γ immunostaining (Figure 6B) ▶ . This result indicates that FasL-positive TCC cells are able to induce apoptosis of functional effector CD8-positive TILs in vivo.
Discussion
The discovery of FasL expression by a variety of solid human tumors 11-14 raises the possibility that tumor-associated FasL, inducing apoptosis of anti-tumor Fas-bearing T lymphocytes, represents a novel mode of tumor immune escape. 15 Recently, it has been reported that FasL is expressed by poorly differentiated long-term established human bladder cancer cell lines. 24 However, no clear information is available on FasL expression by bladder TCC cells in vivo, and its relevance particularly with respect to the pathological characteristics of the tumor. 25 Moreover, little information is currently available on the functional activity of bladder TCC cell-associated FasL. 25
In the present work we investigate the expression and function of FasL in urinary bladder TCCs of different grade and stage. Using immunohistochemical staining performed with three different recommended anti-FasL mAbs (FASL, CD178. 2001. Available at http://www.ncbi.nlm.nih.gov/PROW/guide/333879674_g.htm.), 20 we show that FasL is expressed by bladder TCC cells in vivo. We also provide the first evidence that FasL expression is absent in normal urothelial tissues and that a strong correlation exists between FasL expression by bladder TCCs and the pathological grade and stage of the tumor. These results indicate that FasL expression is acquired by TCC cells in vivo and that its acquisition occurs during both increase in tumor grade and tumor progression. These data might be consistent with the observations by Mitzutani and colleagues 26 showing that higher levels of serum-soluble FasL (sFasL) were found both in patients with invasive TCCs compared with patients with superficial tumors and in patients with G3 bladder TCCscompared with patients carrying G1-2 carcinomas. In addition, the authors showed that elevated serum sFasL levels predicted early recurrence in patients with superficial TCCs in the 5-year follow-up. 26
We also show that a correlation exists in situ between FasL expression by TCC cells and tumor-infiltrating T lymphocytes expressing caspase-8-, -9-, and -3-activated specific proteases, known to be responsible for Fas-mediated apoptosis. 9 Indeed, in FasL-positive TCCs, caspase-8-, -9-, and -3-positive cells showed a distribution comparable to that of tumor-infiltrating T lymphocytes. In addition, we observed both T lymphocytes with condensed nuclei, and caspase-8-, -9-, and -3-positive cells with lymphoid morphology in close contact with FasL-positive tumor cells. These results indicate that bladder TCC cells are capable of triggering Fas-mediated apoptosis in tumor-infiltrating T lymphocytes in vivo.
To directly assess and quantify the function of TCC cell-associated FasL, two FasL-positive primary culture bladder TCC cell lines, established from the two FasL-positive invasive bladder TCC tissues, were co-cultured with T lymphocytes, and their ability to mediate T-cell death via the Fas/FasL pathway was analyzed. The function of tumor-expressed FasL has usually been demonstrated in vitro by the ability of tumor cell lines to induce apoptosis of either Fas-sensitive conventional target cell lines (eg, Fas-transfected P815, and Jurkat cells) or PBMCs 24,27 and TILs 28 in vitro stimulated with nonspecific stimuli (eg, PHA, and PMA-ionomycin). In agreement with these data, we show that FasL-expressing bladder TCC cells induce killing of Jurkat cells, as well as, autologous or allogeneic PHA lymphoblasts. Performing blocking experiments with a neutralizing anti-FasL mAb, we also show that lymphocyte death is completely or partially mediated by tumor-expressed FasL. However, considering the expression of both FasL and caspases by TILs in situ, together with the partial inhibition of TCC cell-mediated T-cell killing by pretreatment of tumor cells with the anti-FasL mAb in vitro, we cannot rule out the possibility that other mechanisms, such as activation-induced cell death, 29 are also involved in the Fas-mediated apoptosis of T cells.
In addition, we evaluated the function of TCC-expressed FasL in an experimental condition that might more closely reflect the in vivo tumor-lymphocyte interaction. We demonstrated the ability of a FasL-positive primary culture bladder TCC cell line to induce Fas-mediated killing of autologous T lymphocytes, generated on stimulation of PBMCs with a specific stimulus, such as the tumor cell, in a MLTC. Nevertheless, the high level of effector T-cell death in the MLTC made us unable to directly assess a possible role for the TCC-expressed FasL in the killing of autologous tumor-specific T lymphocytes. This finding could explain the difficulties reported in the characterizing of cytotoxic T lymphocytes (CTLs) against bladder carcinomas. 30,31 Indeed, it has been reported that the isolation of bladder tumor-specific CTL clones could be obtained only when PBMCs were stimulated with tumor cells transfected with the B7.1 cDNA. 30,31 Co-stimulatory signals, through B7.1 and its CD28 receptor, known to prevent apoptosis of activated T lymphocytes during tumor cell killing, 32 might also prevent apoptosis of tumor-specific CTLs induced by tumor-expressed FasL. This, together with our results, might indicate a possible role for the TCC-expressed FasL in the killing of autologous tumor-specific T lymphocytes.
Functionally activated T cells generally secrete a number of effector cytokines in a highly regulated manner. Detection of IFN-γ- or tumor necrosis factor-α-secreting CD8-positive T cells has been used to detect tumor-reactive T cells, including tumor-specific CTLs, in vitro. 33-35 To characterize the functional activity of CD8-positive TILs undergoing apoptosis in FasL-positive TCCs in vivo, we analyzed their in situ expression of the IFN-γ-cytokine. The approach we used has the advantage of not requiring any in vitro expansion of T cells, thus avoiding the selection and amplification of T-cell populations with a minor representation in vivo. We showed that some of the CD8-positive TILs, undergoing apoptosis and located in close contact with FasL-positive TCC cells, expressed the IFN-γ effector cytokine in situ. This result indicates that bladder TCC cells are capable of triggering Fas-mediated apoptosis in functionally activated CD8-positive effector T cells in vivo. This data strongly supports a possible role for tumor-expressed FasL on the apoptosis of tumor-reactive T cells, such as tumor-specific CTLs.
In conclusion, our results documenting FasL expression by urinary bladder TCCs in vivo and the ability of FasL-expressing TCC cells to induce Fas-mediated killing of autologous T lymphocytes both in vitro and in vivo, suggest a role for bladder TCC-associated FasL in tumor immune escape and provide new insights into potential mechanisms underlying the progression and the aggressiveness of the disease.
Acknowledgments
We thank Drs. W. I. De Boer, S. Chouaib, and A. Bensusson for providing cells and useful reagents; and Prof. A. Santoni, Prof. L. Ruco, Prof. G. Palmieri, Prof. A. Tubaro, and Mrs. P. I. Franke for helpful comments.
Footnotes
Address reprint requests to Prof. Francesca Velotti, Department of Environmental Sciences, Largo dell’Università, Tuscia University, 01100 Viterbo, Italy. E-mail: velotti@unitus.it.
Supported by a grant from the Ligue Contre le Cancer, Comité du Val de Marne (Créteil), Université Paris XII, Association Claude Bernard, Association de la Recherche Contre le Cancer, Délégation à la Recherche Clinique and Assistance Publique-Hôpitaux de Paris (AP-HP) (Program Hôpitalier Recherche Clinique no. AOA94015), Ministero dell’Istruxione dell’Università e della Ricerca, the Ministero della Sanità, and European Community (QLK-CT-1999-00064).
R. B.-M. and P. M. contributed equally to this work.
References
- 1.Hudson MA, Catalona WJ: Urothelial tumors of the bladder, upper tracts and prostate. Gillenwater JY Grayhack JT Howards SS Duckett JW eds. Adult and Pediatric Urology. 1996:pp 643-694 Mosby, St. Louis
- 2.Velotti F, Chopin D, Gil-Diez S, Maillé P, Abbou CC, Kourilsky P, Even J: Clonality of tumor-infiltrating lymphocytes in human urinary bladder carcinoma. Immunotherapy 1997, 20:470-478 [DOI] [PubMed] [Google Scholar]
- 3.Herr HW, Schwalb DM, Zhang Z-F, Sogani PC, Fair WR, Whitmore WF, Jr, Oettgen HF: Intravesical bacillus Calmette-Guerin therapy prevents tumor progression and death from superficial bladder cancer: ten-year follow-up of a prospective randomized trial. J Clin Oncol 1995, 13:1404-1408 [DOI] [PubMed] [Google Scholar]
- 4.Velotti F, Cippitelli M, Giuffrida AM, Punturieri A, Moretti F, Stoppacciaro A, Tubaro A, De Maria R, Santoni A: Local immunotherapy of human bladder cancer with recombinant IL-2. Forni G Foa R Santoni A Frati L eds. Cytokine-Induced Tumor Immunogenicity. 1994:pp 429-442 Academic Press, New York
- 5.Patard J-J, Brasseur F, Gil-Diez S, Radvanyi F, Marchand M, François P, Abi-Aad A, Van Caugh P, Abbou CC, Chopin D, Boon T: Expression of MAGE genes in transitional-cell carcinomas of the urinary bladder. Int J Cancer 1995, 64:60-64 [DOI] [PubMed] [Google Scholar]
- 6.Tureci O, Chen Y-T, Sahin U, Gure AO, Zwick C, Villena C, Tsang S, Seitz G, Old LJ, Pfreundschuh M: Expression of SSX genes in human tumors. Int J Cancer 1998, 77:19-23 [DOI] [PubMed] [Google Scholar]
- 7.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S: Escape of human solid tumors from T-cell recognition: molecular mechanisms and significance. Adv Immunol 2000, 74:181-273 [DOI] [PubMed] [Google Scholar]
- 8.Nagata S, Golstein P: The Fas death factor. Science 1995, 267:1449-1456 [DOI] [PubMed] [Google Scholar]
- 9.Barry M, Bleakley RC: Cytotoxic T lymphocytes: all roads lead to death. Nature 2002, 2:401-409 [DOI] [PubMed] [Google Scholar]
- 10.Grifith ST, Fergusson TA: The role of FasL-induced apoptosis in immune privilege. Immunol Today 1997, 18:240-244 [DOI] [PubMed] [Google Scholar]
- 11.O’Connell J, O’Sullivan GC, Collins JK, Shanahan F: The Fas counterattack: fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med 1996, 184:1075-1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahne M, Rimoldi D, Schröter M, Romer P, Schreier M, French LE, Schneider P, Bornand T, Fontana A, Lienard D, Cerottini J, Tschopp J: Melanoma cell expression of Fas (Apo-1/CD95) ligand: implications for tumor immune escape. Science 1996, 274:1363-1366 [DOI] [PubMed] [Google Scholar]
- 13.Strand S, Hofmann WJ, Hug H, Müller M, Otto G, Strand D, Mariani SM, Stremmel W, Krammer PH, Galle PR: Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells—a mechanism of immune evasions? Nat Med 1996, 2:1361-1366 [DOI] [PubMed] [Google Scholar]
- 14.O’Connell J, Bennett MW, O’Sullivan GC, Collins JK, Shanahan F: The Fas counterattack: cancer as a site of immune privilege. Immunol Today 1999, 1:46-53 [DOI] [PubMed] [Google Scholar]
- 15.O’Connell J, Houston A, Bennett MW, O’Sullivan GC, Shanahan F: Immune privilege or inflammation? Insights into the Fas ligand enigma. Nat Med 2001, 7:271-274 [DOI] [PubMed] [Google Scholar]
- 16.Spiessl B, Beahrs OH, Hermanek P, Hutter RVP, Scheibe O, Sobin LH, Wagner G: Urinary bladder. TNM Atlas, Illustrated Guide to the TNM/pTNM Classification of Malignant Tumors. Spiessl B Beahrs OH Hermanek P Hutter RVP Scheibe O Sobin LH Wagner G eds. International Union Against Cancer (Union International Contre le Cancer; UICC) 1992:pp 214-279 Springer-Verlag, Berlin
- 17.Mostofi FK, Sobin LH, Torloni H: Histological typing of urinary bladder tumors. World Health Organization eds. International Histological Classification of Tumors. 1973:pp 400-415 World Health Organization, Geneva
- 18.De Boer WI, Houtsmuller AB, Izadifar V, Muscatelli-Groux B, van der Kwast T, Chopin DK: Expression and functions of EGF, FGF, and TGFbeta-growth-factor family members and their receptors in invasive human transitional-cell-carcinoma cells. Int J Cancer 1997, 71:284-291 [DOI] [PubMed] [Google Scholar]
- 19.Herin M, Lemoine C, Weynants P, Vessiere F, Van Pel A, Knuth A, Devos R, Boon T: Production of stable cytolytic T-cell clones directed against autologous human melanoma. Int J Cancer 1987, 39:390-396 [DOI] [PubMed] [Google Scholar]
- 20.Smith D, Sieg S, Kaplan D: Technical note: aberrant detection of cell surface Fas ligand with anti-peptide antibodies. J Immunol 1998, 160:4159-4160 [PubMed] [Google Scholar]
- 21.Asselin-Paturel C, Pardoux C, Gay F, Chouaib S: Failure of TGFbeta1 and IL-12 to regulate human FasL and mTNF alloreactive cytotoxic T-cell pathways. Tissue Antigens 1998, 51:242-249 [DOI] [PubMed] [Google Scholar]
- 22.Mosmann T: Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983, 65:55-63 [DOI] [PubMed] [Google Scholar]
- 23.Gown AM, Willingham MC: Improved detection of apoptotic cells in archival paraffin sections: immunohistochemistry using antibodies to cleaved caspase 3. J Histochem Cytochem 2002, 50:449-454 [DOI] [PubMed] [Google Scholar]
- 24.Perabo FGE, Kamp S, Schmidt D, Lindner H, Steiner G, Mattes RH, Wirger A, Pegelow K, Albers P, Kohn EC, von Ruecker A, Mueller SC: Bladder cancer cells acquire competent mechanisms to escape Fas-mediated apoptosis and immune surveillance in the course of malignant transformation. Br J Cancer 2001, 84:1330-1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SH, Lee JY, Park WS, Kim SY, Jang JJ, Yoo NJ: Transitional cell carcinoma expresses high levels of fas ligand in vivo. BJU Int 1999, 83:698-702 [DOI] [PubMed] [Google Scholar]
- 26.Mizutani Y, Hongo F, Sato N, Ogawa O, Yoshida O, Miki T: Significance of soluble Fas ligand in patients with bladder carcinoma. Cancer 2001, 92:287-293 [DOI] [PubMed] [Google Scholar]
- 27.Rabinowich H, Reichert TE, Kashii Y, Gastman BR, Bell MC, Whiteside TL: Lymphocyte apoptosis induced by Fas ligand-expressing ovarian carcinoma cells. J Clin Invest 1998, 11:2579-2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker PR, Saas P, Dietrich P-Y: Role of Fas ligand (CD95L) in immune escape. J Immunol 1997, 158:4521-4524 [PubMed] [Google Scholar]
- 29.Lenardo MJ: The molecular regulation of lymphocyte apoptosis. Semin Immunol 1997, 9:1-5 [DOI] [PubMed] [Google Scholar]
- 30.Guéguen M, Patard J-J, Gaugler B, Brasseur F, Renauld J-C, Van Cangh PJ, Boon T, Van den Eynde BJ: An antigen recognized by autologous CTL on a human bladder carcinoma. J Immunol 1998, 160:6188-6194 [PubMed] [Google Scholar]
- 31.Heidecker L, Brasseur F, Probst-Kepper M, Guégen M, Boon T, Van den Eynde BJ: Cytolytic T lymphocytes raised against human bladder carcinoma recognize an antigen encoded by gene MAGE-A12. J Immunol 2000, 164:6041-6045 [DOI] [PubMed] [Google Scholar]
- 32.Daniel PT, Kroidl A, Cayeux S, Bargou R, Blankestein T, Dörken B: Costimulatory signals through B7.1/C28 prevent T cell apoptosis during target cell lysis. J Immunol 1997, 159:3808-3815 [PubMed] [Google Scholar]
- 33.Herr W, Schneider J, Lohse AW, Meyer zum Buschenfelde KH, Wolfel T: Detection and quantification of blood-derived CD8+ T lymphocytes secreting tumor necrosis factor alpha in response to HLA-A2.1-binding melanoma and viral peptide antigens. J Immunol Methods 1966, 191:131-142 [DOI] [PubMed] [Google Scholar]
- 34.Scheibenbogen C, Lee KH, Stevanovic S, Witzens M, Willhauck M, Waldmann V, Naeher H, Rammensee HG, Keilholz U: Analysis of the T cell response to tumor and viral peptide antigens by an IFNgamma-ELISPOT assay. Int J Cancer 1997, 71:932-936 [DOI] [PubMed] [Google Scholar]
- 35.Asemissen AM, Nagorsen D, Keilholz U, Letsch A, Schmittel A, Thiel E, Scheibenbogen C: Flow cytometric determination of intracellular or secreted IFNgamma for the quantification of antigen reactive T cells. J Immunol Methods 2001, 251:101-108 [DOI] [PubMed] [Google Scholar]