Abstract
Platelet-derived growth factor (PDGF)-B and its receptor (PDGF-R) β are overexpressed in human gliomas and responsible for recruiting peri-endothelial cells to vessels. To establish the role of PDGF-B in glioma angiogenesis, we overexpressed PDGF-B in U87MG glioma cells. Although PDGF-B stimulated tyrosine phosphorylation of PDGF-Rβ in U87MG cells, treatment with recombinant PDGF-B or overexpression of PDGF-B in U87MG cells had no effect on their proliferation. However, an increase of secreted PDGF-B in conditioned media of U87MG/PDGF-B cells promoted migration of endothelial cells expressing PDGF-Rβ, whereas conditioned media from U87MG cells did not increase the cell migration. In mice, overexpression of PDGF-B in U87MG cells enhanced intracranial glioma formation by stimulating vascular endothelial growth factor (VEGF) expression in neovessels and by attracting vessel-associated pericytes. When PDGF-B and VEGF were overexpressed simultaneously by U87MG tumors, there was a marked increase of capillary-associated pericytes as seen in U87MG/VEGF165/PDGF-B gliomas. As a result of pericyte recruitment, vessels induced by VEGF in tumor vicinity migrated into the central regions of these tumors. These data suggest that PDGF-B is a paracrine factor in U87MG gliomas, and that PDGF-B enhances glioma angiogenesis, at least in part, by stimulating VEGF expression in tumor endothelia and by recruiting pericytes to neovessels.
Progressive angiogenesis is composed of two distinct processes. In response to nearby stimuli, vascular endothelial cells (ECs) migrate to the tips of capillaries where they proliferate and form lumenal structures and vacuoles, which in turn connect with each other to form new tubes. These new tubes then fuse to forming vascular networks. Pruning of the vessel networks occurs and the vasculature is optimized. Finally, cells such as smooth muscle cells (SMCs) and pericytes are recruited and form a layer that encapsulates the endothelium to confine and support the newly formed vasculature. 1 Vascular endothelial growth factor (VEGF) is a major angiogenic factor that promotes the EC proliferation, migration, vessel sprouting, vessel permeability, and the remodeling of emerging vessels. 2 Several other growth factors are responsible for recruiting SMCs/pericytes to new naked vessels or capillary walls during the second stage of angiogenesis. The growth factors include angiopoietins, 3 transforming growth factor-β, 4 and platelet-derived growth factor (PDGF). 5 PDGF is a potent mitogen and chemoattractant for mesenchymal cells and fibroblasts. PDGF is composed of A, B, C, and D polypeptide chains that form the homodimers PDGF-AA, BB, CC, and DD and the heterodimer PDGF-AB. Its biological activities are linked to two tyrosine kinase receptors, PDGF-α and -β receptors (PDGF-Rα and PDGF-Rβ). PDGF-Rα binds to PDGF isoforms AA, BB, AB, and CC, whereas PDGF-Rβ interacts at a higher affinity with PDGF isoforms BB and DD. 6
Accumulated evidence has shown that PDGF-B plays an important role in angiogenesis. Genetic studies have demonstrated that PDGF-B 7-9 and PDGF-Rβ 10 are involved in vessel maturation through the recruitment of SMCs and pericytes to growing vessels during embryonic development. Mice deficient in either PDGF-B 7 or PDGF-Rβ 10 developed hemorrhages or edemas during the later stages of embryogenesis. The vascular defects in PDGF-B- or PDGF-Rβ-deficient embryos were attributed to an inability to attract PDGF-Rβ-positive pericytes to the developing capillaries 8 and to the developing kidney glomerular capillary tuft. 11 These results suggested that PDGF-B recruits the PDGF-Rβ-positive mesenchymal cells into growing vessels. 8,9 Increased PDGF and PDGF-R expression have also been implicated in the progression of human cancers including gliomas. 12-14 In human glioblastomas, PDGF-Rα expression is increased in tumor cells, whereas PDGF-Rβ expression is elicited in neovascular ECs, indicating both autocrine and paracrine actions of PDGF in tumor growth and angiogenesis. 15 Overexpression of PDGF-B enables nontumorigenic human keratinocytes to form tumors in vivo. Exogenously expressed PDGF-B increased proliferation of stromal cells and enhanced angiogenesis in these tumors. 16 In vitro, PDGF-B acts on vascular ECs that express PDGF-Rβ promoting tube formation. 17 PDGF-B also increases the expression of several angiogenic factors that include increased VEGF expression in fibroblasts 18 and ECs. 19 However, the mechanism by which PDGF-B augments angiogenesis and tumor formation in vivo, remains unknown. In this report, we investigated the role of PDGF-B in human glioma angiogenesis by overexpressing PDGF-B in U87MG glioma cells. Our results show that PDGF-B is a paracrine growth factor for U87MG glioma tumorigenesis, and acts, at least in part, by stimulating VEGF expression in tumor endothelia that leads to increased EC proliferation and by promoting pericyte cell recruitment.
Materials and Methods
Cell Lines and Reagents
U87MG and porcine aorta endothelial (PAE) cells and their culture were described previously. 20 PAE cells lack endogenous VEGFR and PDGF-Rβ. The PAE cells that stably express wild-type PDGF-Rβ (PAE/PDGF-Rβ) were generated previously. 19 A cDNA for PDGF-B in a pcDNA3 expression vector was provided by Dr. D. Bowen-Pope at the University of Washington, Seattle, WA.
Western Blot Analyses of Receptor Tyrosine Phosphorylation
Immunoblotting of PDGF receptor tyrosine phosphorylation was performed as described previously. 19 Briefly, serum-starved U87MG or PAE cells were stimulated with a human recombinant PDGF-B protein (R&D Systems, Minneapolis, MN) or 0.1% bovine serum albumin (control). The PDGF-Rβ in the cell lysates was immunoprecipitated by a polyclonal anti-human PDGF-Rβ antibody (Upstate Biotechnology, Lake Placid, NY), separated, and transferred onto a nitrocellulose membrane. The membrane was then blocked, and probed with a monoclonal antibody against phosphotyrosine (4G10, Upstate Biotechnology). Phosphorylation of PDGF-Rβ was then detected. PDGF-Rβ proteins in the blot were identified using the anti-human PDGF-Rβ antibody.
Generation of U87MG Cell Lines that Stably Overexpress PDGF-B
Transfected U87MG cell clones that stably express PDGF-B were generated by methods as described previously. 20 Briefly, PDGF-B cDNA was transfected into U87MG- or U87MG VEGF165-expressing cells. A pool of resultant clones was then harvested and sorted using a fluorescence-activated cell sorter into 96-well flat-bottom plates with 200 μl of Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen/Life Technologies, Inc., Grand Island, NY) supplemented with 15% heat-inactivated cosmic calf serum (Hyclone, Salt Lake City, UT). The clones that expressed exogenous biologically active PDGF-B were identified by Northern blot, PDGF-B enzyme-linked immunosorbent assay (ELISA), and EC migration analyses.
Northern Blot, VEGF ELISA, and PDGF-B ELISA Assays
RNA purification and Northern blot analysis were performed as previously described. 21 Hybridization was performed using a 32P-labeled PDGF-B cDNA probe (2.2 kb).
To generate conditioned media (CM), the cells were seeded at 3.0 × 105 cells/well in 12-well plates. Twenty-four hours later, the media was changed to DMEM/0.5% bovine serum albumin/1% dialyzed fetal bovine serum (Sigma, St. Louis, MO). After an additional 24 hours, the media was replaced and the cells were then allowed to grow for an additional 48 hours. The resultant CM was collected and subsequently used for ELISA and EC migration assay analyses. PDGF-B ELISA analysis (R&D Systems) was performed according to the manufacturer’s instruction with minor modifications. The PDGF-B concentrations in the CM were calculated from a standard curve generated by a recombinant human PDGF-B protein. VEGF ELISAs were done using collected CM as described previously. 20
The amounts of VEGF in various tumor lysates were measured as reported previously. 22,23 Briefly, established brain tumors (parental U87MG- or PDGF-B-expressing) were removed and immediately frozen on dry ice. Lysates were made by homogenizing 100 mg of frozen tumor tissues per ml of lysis buffer (10.0 mmol/L Tris-HCl, pH 7.5, supplemented with 1% Nonidet P-40, 1.0 mmol/L phenylmethyl sulfonyl fluoride, 1.0 mmol/L leupeptin, and 0.2 trypsin-inhibiting U/ml aprotinin). The homogenate was passed three times through a 25-gauge needle and then centrifuged at 2000 rpm at 4°C for 15 minutes to remove debris. Proteins in the clarified lysates were then quantified. The amounts of VEGF in the lysates were measured using a human VEGF ELISA kit according to the manufacturer’s instruction (Quantikine human VEGF, R&D Systems). The VEGF amounts were recorded as pg of VEGF per μg of total proteins in the lysates.
EC Migration Assay
EC migration assays were performed using PAE cells that express exogenous PDGF-Rβ. 19 After serum starvation, 50 μl of the cell suspension of 1 × 106 cells/ml was placed in the top wells of a modified Boyden chamber. The lower wells contained either 25 μl of CM of various cells or plain media containing 10 ng/ml of human recombinant PDGF-B proteins. The lower wells also included either 50 μg/ml of a neutralizing anti-PDGF-B antibody (AB-220-NA) or a purified goat IgG protein (R&D Systems). Human recombinant VEGF proteins (10 ng/ml) or a neutralizing anti-VEGF antibody (MAB-293; R&D Systems) were also added into some samples. After a 5-hour incubation, the filters were fixed, stained, and mounted. Nonmigrated cells on the upper surface were carefully wiped off with a cotton swab. Migrated cells were counted in 10 randomly chosen high-power fields (×400 total magnification) per filter. 20,24
Proliferation of U87MG Cells that Stably Express PDGF-B
The parental and U87MG PDGF-B-expressing cells were seeded at 2.0 × 104 cells/well in 24-well plates. On the next day, the media was changed to DMEM/0.5% bovine serum albumin/1% dialyzed fetal bovine serum. After a 16-hour incubation, the media was again replaced with fresh media. To the PDGF-B-overexpressing cells, the neutralizing anti-PDGF-B antibody or its isotype IgG (50 μg/ml) was added to the culture media. To the parental U87MG cells, recombinant PDGF-B proteins (50 ng/ml) were included. The cells were harvested and counted at indicated times. The CM from these cells was collected at the same time points as indicated above. The amount of VEGF in the CM was assessed by the VEGF ELISAs. 20
Intracerebral Stereotactic Implantation, Mouse Brain Tissue Management, BrdUrd Incorporation, and Immunohistochemical Analyses
Intracerebral stereotactic implantation and preparation of frozen brain samples was performed as described. 20 To estimate tumor volumes, thin cryostat sections (5 μm) were stained with hematoxylin and tumor dimensions were captured by a SPOT digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI) by a ×2 objective equipped on an Olympus BX51 microscope. Tumor dimensions were measured in mm2 using an image analysis program (Image Pro Plus, Version 4.1; Media Cybernetics, L.P., Silver Spring, MD). The areas were then multiplied by the thickness of the section (5 μm) to yield a section volume measurement. The volumes of all of the sections were added to arrive at the total volume for each tumor. 20,24,25 To evaluate the proliferative activities of glioma and ECs in vivo, 300 μl of BrdUrd solution (Amersham, Piscataway, NJ) was injected intraperitoneally into one group of mice 30 minutes before the animals were sacrificed. The brains of these mice were fixed and embedded as described. 25,26 Mouse brain sections were analyzed using the following primary antibodies: rat anti-mouse CD31 antibody (MEC13.3; BD-PharMingen, San Diego, CA), rabbit polyclonal anti-VEGF antibody (A-20; Santa Cruz Biotechnologies, Santa Cruz, CA), goat anti-human PDGF-B antibody (AB-220-NA; R&D Systems), rabbit anti-desmin antibody (Chemicon, Temecula, CA), rabbit anti-von Willebrand factor (DAKO, Carpinteria, CA), and an anti-BrdUrd antibody (Amersham). The immunohistochemistry analyses were performed as described previously. 20,24,25
Quantitative Analysis of Immunohistochemistry Data
Quantitative analysis of the blood vessel or pericyte densities of tumor samples was done on serial-cut brain sections that were stained by the anti-CD31 antibody or the anti-desmin antibody without counter staining. 20,24,25 Five to seven serial-cut brain sections from each individual mouse were analyzed. Captured images (10 to 15 random areas per slide) were imported into the Image Pro Plus program. The mean values of calculated densities from serial sections of separate mouse brains (five or more individual mouse) in each group were used for the quantitative analysis. The blood vessel or pericyte densities were calculated as the ratio of positively stained areas to the total area of the image or field. 20,24,25
The frequency of tumor-associated hemorrhage was determined by examining the existence of clusters of red blood cells within the tumor areas or at peripheries of the tumors under light microscopy after the brain sections were stained with hematoxylin and eosin. 20
The proliferative index of tumor cells or ECs in various gliomas was assessed by double staining of the paraffin-embedded tumor samples that were in vivo labeled by BrdUrd. Immunofluorescent staining was performed using the anti-BrdUrd antibody (red fluorescent color) and the anti-von Willebrand factor antibody (green fluorescent color) on these tumor sections. After the photographs were taken, nuclei in these tissue sections were stained by a Hoescht dye 33258 (blue fluorescent color. Sigma). Five serial-cut brain sections from each individual mouse were analyzed. More than 2000 cells were examined in each section. The mean values of the serial sections from six separate mouse brains in each group were used for the quantitative analysis. The proliferation index of BrdUrd incorporation in tumor cells or ECs was calculated as a percentage of positive nuclei (red fluorescent color) to total numbers of nucleus (blue color) or to the total vessel densities (green color) within the same areas of the section under fluorescent microscopy. 25,26
Results
PDGF-B Stimulated the Phosphorylation of Endogenous PDGF-Rβ in U87MG Cells
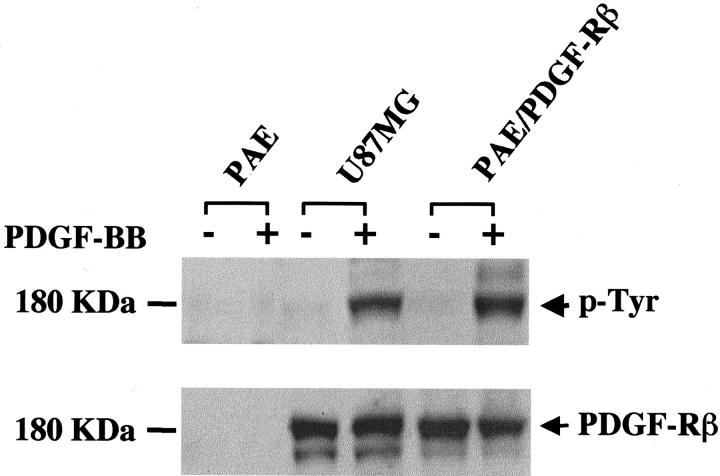
PDGF-B binds to PDGF-Rβ with high affinity. 27 Therefore, we first examined whether the phosphorylation of endogenous PDGF-Rβ could be stimulated by PDGF-B in U87MG cells. Protein analyses shows that U87MG cells express the endogenous PDGF-Rβ at 180 kd (Figure 1 ▶ , bottom). On treatment of U87MG cells with a human recombinant PDGF-B, the tyrosine residues on the PDGF-Rβ were phosphorylated (Figure 1 ▶ , top). In contrast, low levels of endogenous PDGF-B [Figure 2A ▶ (top, 3.8-kb-sized mRNA messages) and B] were not sufficient to cause tyrosine phosphorylation of endogenous PDGF-Rβ in U87MG cells (Figure 1 ▶ , top, untreated lane in U87MG sample). In addition, low levels of PDGF-Rα and PDGF-AA expression were also found in U87MG cells (data not shown).
Figure 1.
PDGF-B activates endogenous PDGF-Rβ in U87MG glioma cells. PAE, PAE-PDGF-Rβ, and U87MG cells were serum-starved overnight. The cells were then treated with 50 ng/ml of a human recombinant PDGF-B protein at 37°C for 15 minutes. The cell lysates were subjected to immunoblotting for detection of tyrosine phosphorylation on PDGF-Rβ. The membrane was then reprobed with an anti-human PDGF-Rβ antibody. The PDGF-Rβ protein runs as a 180-kd band in a 7.5% SDS-PAGE gel (bottom). Duplicate experiments yielded identical results.
Figure 2.
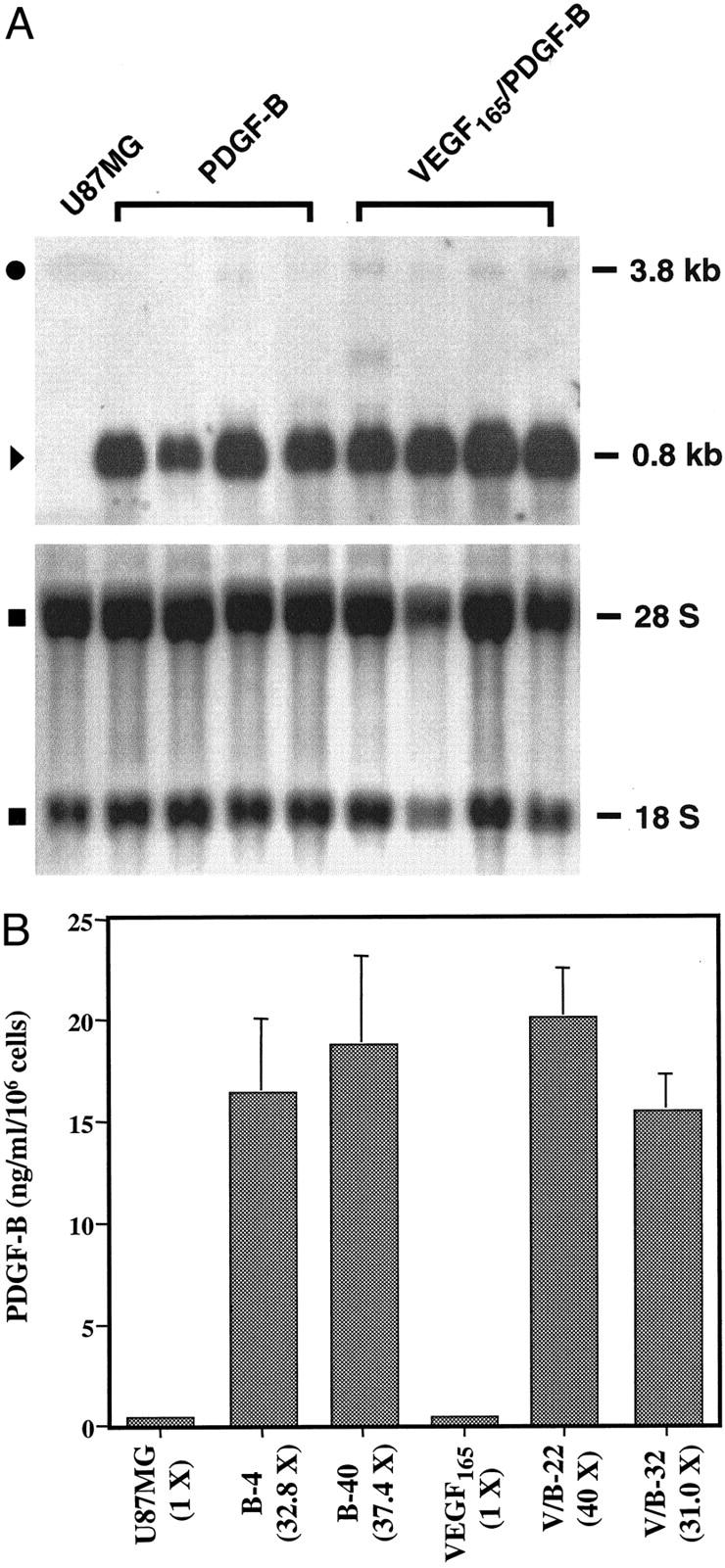
Expression of exogenous PDGF-B in U87MG glioma cells. A: Northern blot analysis. Top: Samples of the parental U87MG and two types of PDGF-B-overexpressing cells are shown. In each class, four individual PDGF-B-expressing clones are presented. Endogenous (•, 3.8 kb) and exogenous (▸, 0.8 kb) PDGF-B transcripts and their descriptions are in the text. Bottom: Methylene blue staining of the membrane after RNA was transferred into the membrane. ▪: 18 S and 28 S ribosomal RNA species. Duplicate experiments yielded similar results. B: PDGF-B ELISA analyses. Cells (3 × 105) of the parental U87MG- or PDGF-B-expressing cells or VEGF165- or VEGF165/PDGF-B-expressing cells were seeded onto 12-well plates in triplicate. On the next day, the media was replaced with DMEM/0.5% bovine serum albumin/1% dialyzed fetal bovine serum for another 24 hours. CM was collected after the cells were cultured for an additional 48 hours. Each bar represents the mean ± SEM of three triplicates. Identical experiments were also performed using CM of other PDGF-B-overexpressing clones, B-26, B-34, V/B-11, and V/B-33. The assays were performed two additional times using cells of various passage numbers with similar results.
Stable Overexpression of PDGF-BB in U87MG and U87MG/VEGF165 Cells
We stably transfected U87MG and U87MG/VEGF165 cells with PDGF-B cDNA and isolated single neomycin-resistant cells using a fluorescence-activated cell sorter. Fifteen U87MG and nine U87MG/VEGF165 clones expressing abundant exogenous PDGF-B messages (Figure 2A ▶ ; top, ▸, 0.8 kb) were identified by Northern blot analysis. Low-level expression of the 3.8-kb endogenous PDGF-B transcript in the parental U87MG- or PDGF-B-overexpressing cells was also detected (Figure 2A ▶ ; •, 3.8 kb). Methylene blue staining of 18 S and 28 S ribosomal RNA on the nylon membrane after the RNA was transferred demonstrates that the amounts of RNA of each clone in the assays were approximately equal (Figure 2A ▶ , bottom).
We next performed PDGF-B ELISAs to analyze the amount of secreted PDGF-B protein in the CM collected after a 48-hour culture. Clones of both types of U87MG- PDGF-B-overexpressing cells secreted PDGF-B at concentrations of 15.5 to 20.1 ng/ml/106 cells (Figure 2B) ▶ . This is consistent with increased levels of exogenous PDGF-B mRNA in U87MG PDGF-B-expressing cells (Figure 2A) ▶ . In contrast, the parental U87MG or U87MG/VEGF165 cells only secreted 0.5 ng/ml/106 cells into the CM after a 48-hour cell culture (Figure 2B) ▶ . Among the positive clones that we identified, four clones that overexpressed PDGF-B at high levels were selected for subsequent studies [Figure 2 ▶ , A (top) and B]. These clones are U87MG/PDGF-B/4, -B/26, -B/34, and -B/40 (referred to as B-4, B-26, B-34, and B-40, respectively); and U87MG/VEGF165/PDGF-B/11, PDGF-B/22, PDGF-B/32, and PDGF-B/33 (referred to as V/B-11, V/B-22, V/B-32, and V/B-33, respectively). U87MG/VEGF165 will be referred to as V. Thus, we have obtained several clones of the two types of U87MG cells that are stably expressing high levels of PDGF-B proteins.
Secreted PDGF-B Elicited PAE/PDGF-Rβ Cell Migration
We next sought to determine the biological activity of the overexpressed PDGF-B in U87MG cells. We measured EC migration stimulated by PDGF-B in vitro using a PAE/PDGF-Rβ cell line that previously has been used for assessing the activities of PDGF-B. 19 The levels of migration are reflective of the activity of PDGF-B secreted into the CM by different PDGF-B-overexpressing cells. Two PDGF-B-overexpressing cloned cell lines of each type, B-4, B-40, V/B-22, and V/B-32, had strong effects in promoting PAE/PDGF-Rβ cell migration (Table 1) ▶ . Next, we determined whether PDGF-B was the factor that stimulated PAE/PDGF-Rβ cell migration by including a neutralizing anti-PDGF-B antibody in the assay (Table 1) ▶ . The neutralizing anti-PDGF-B antibody completely blocked the stimulation of cell migration elicited by 10 ng/ml of purified human recombinant PDGF-B or the CM of the PDGF-B-overexpressing cells. The specificity of this inhibition was demonstrated by showing that purified normal goat IgG (a control) had no affect on cell migration. The low levels of endogenous PDGF-B in the parental U87MG or U87MG/VEGF165 cells (Figure 2B) ▶ also did not have any observable effect on the migration of the PAE/PDGF-Rβ cells. When a neutralizing monoclonal anti-VEGF antibody was included in the assay, suppression of VEGF activity 20 in the CM had no effect on the promotion of EC migration (Table 1) ▶ . In addition, a recombinant human VEGF165 protein did not cause any observable migration of PAE/PDGF-Rβ cells (data not shown). Thus, these data demonstrate that PDGF-B was the component in the CM that stimulated PAE/PDGF-Rβ cell migration in vitro.
Table 1.
Cells Overexpressing PDGF-B Have Increased Abilities to Elicit PAE/PDGF-Rβ Cell Migration
| CM | No addition* | + PDGF-neutralizing antibody* | + Normal goat IgG* | + VEGF-neutralizing antibody* |
|---|---|---|---|---|
| Media | 608.7 ± 30.8 | ND | ND | ND |
| rhPDGF | 1861.0 ± 46.7 | 721.0 ± 83.2 | 1858.0 ± 8.8 | ND |
| U87MG | 652.1 ± 36.7 | 559.7 ± 33.2 | 752.8 ± 18.8 | 694.4 ± 34.5 |
| B-4 | 1253.0 ± 29.7 | 492.3 ± 47.1 | 1200.0 ± 64.5 | 1379.7 ± 29.3 |
| B-40 | 1998.0 ± 72.1 | 817.7 ± 15.1 | 2024.0 ± 74.1 | 2081.3 ± 93.4 |
| V | 687.3 ± 40.3 | 634.4 ± 36.1 | 690.2 ± 37.4 | 703.3 ± 51.7 |
| V/B-22 | 1939.0 ± 79.8 | 548.7 ± 38.7 | 2122.3 ± 93.6 | 2199.0 ± 48.2 |
| V/B-32 | 1273.7 ± 28.5 | 597.7 ± 9.6 | 1385.7 ± 37.3 | 1385.3 ± 3.0 |
*, n = 3.
ND, not done.
Overexpression of PDGF-B in U87MG Cells Did Not Affect Proliferation or VEGF Secretion of These Cells in Vitro
PDGF-B stimulates cell proliferation of fibroblasts and mesenchymal cells 27 by binding to PDGF-Rβs that are expressed by these cells. PDGF-B also increases VEGF secretion in PAE/PDGF-Rβ cells. 19 Because U87MG cells express endogenous PDGF-Rβ (Figure 2) ▶ , we sought to determine whether PDGF-B acts through autocrine growth stimulation by affecting the growth rate and levels of VEGF expression in U87MG glioma cells. We found that treatment of the parental U87MG cells by the recombinant human PDGF-B protein did not affect cell growth. Similarly, the proliferation of parental U87MG cells or the U87MG/VEGF165 cells was not altered by overexpression of PDGF-B in U87MG cells. In both cases, inclusion of neutralizing anti-PDGF-B antibody or an isotype-matched IgG in the cell cultures did not affect the proliferation rates of the parental U87MG- or PDGF-B-expressing cells (data not shown).
Next, we examined whether the levels of VEGF secretion in the parental U87MG, or PDGF-B, or V/B cells were modified by overexpression of PDGF-B in these cells. We collected CM from the same sets of cultured cells as described above and assessed the VEGF production using a VEGF ELISA in the presence or absence of the neutralizing anti-PDGF-B antibody or an isotype-matched IgG. We found that treatment with the recombinant human PDGF-B protein or overexpression of PDGF-B by U87MG cells did not stimulate VEGF expression in these cells. There were minimal variations in the level of VEGF secretion among the parental U87MG and the various clone cell lines (data not shown). We also measured the levels of secreted VEGF from U87MG cells and its transfectant derivatives after 48 hours in cell culture before injection into mouse brains (see below). In a total of five separate intracranial inoculation experiments, the average amounts of VEGF secreted from the cells into the CM (expressed as ng/ml/106 cells ± SD) were: U87MG, 45.2 ± 5.62; B-4, 45.4 ± 4.74; B-40, 50.1 ± 11.0; VEGF165, 588.5 ± 85.1; V/B-22, 565.1 ± 86.7; and V/B-32, 493.6 ± 74.8, respectively. The growth rates and the VEGF secretion of the other two clones in each type (B-26, B-34, V/B-11, and V/B-33) were also similar (data not shown). In addition, when a PAE cell line that expresses exogenous VEGF receptor-2 (PAE/KDR) 20 was used in identical assays, no enhancement of the PAE/KDR migration elicited by the recombinant PDGF-B or the CM of the PDGF-B-expressing cells was found (data not shown). Thus, overexpression of PDGF-B in U87MG cells did not alter the cell proliferation or VEGF secretion in vitro. Thus, in human U87MG glioma cells (Figure 1) ▶ , PDGF-B does not stimulate VEGF secretion or the cell growth rate in vitro by an autocrine mechanism.
Overexpression of PDGF-B Increased the Tumorigenicity of U87MG Gliomas in Mouse Brains
We then determined whether overexpression of PDGF-B by U87MG cells would enhance glioma tumorigenesis and angiogenesis. 8 We stereotactically implanted the parental U87MG or B-4 or B-40 cells into mouse brains. In general, mice that received 5 × 105 of U87MG cells developed intracranial gliomas of a volume of 56.00 ± 2.3 mm3 or larger such that mice succumbed in 40.2 ± 2.2 days. 20,21,24,25 However, as shown in Table 2 ▶ , 20 all mice that received U87MG PDGF-B-overexpressing cells developed tumors of a volume of 51.2 ± 3.2 mm3 or larger (n = 12, P < 0.001) in 22.4 ± 1.4 days after implantation. In contrast, all of the mice that were implanted with parental U87MG cells grew much smaller sized brain tumors (16.2 ± 4.2 mm3, n = 8, P < 0.001) in a similar period of time (21.5 ± 4.2 days). Thus, overexpression of PDGF-B by U87MG gliomas increased intracranial tumor growth.
Table 2.
Overexpression of PDGF-B in U87MG Cells Promoted Tumorigenicity and Angiogenicity in Vivo
| Cells | Secretion of VEGF (ng/106/cells) | Tumor volumes (mm3, mean ± SEM) | Hemorrhage | Days after implantation |
|---|---|---|---|---|
| U87MG | 45.0 | 16.2 ± 4.2 (n = 8) | 0/8 (0.0%) | 21.5 ± 4.2 |
| PDGF-B | 45.8 | 51.2 ± 3.2 (n = 12) (P < 0.001) | 0/12 (0.0%) | 22.4 ± 1.4 |
| V + U87MG | 150.0 | 9.02 ± 0.7 (n = 14) | 14/14 (100%) | 5.14 ± 0.2 |
| V/B + PDGF-B | 150.0 | 8.60 ± 1.2 (n = 14) | 1 /14 (7.1%) (P < 0.001) | 5.32 ± 0.07 |
PDGF-B Promoted U87MG Glioma Angiogenesis by Increasing VEGF Expression in Tumor ECs and Enhances Proliferation of Both Endothelial and Tumor Cells
It has been demonstrated that PDGF-B stimulates vascular ECs that express PDGF-Rβ to form tube-like networks in vitro. 28 Overexpression of PDGF-B in tumor cells has also been shown to increase stromal and EC proliferation in vivo. 16 When we examined angiogenesis in the parental U87MG and PDGF-B-expressing tumors, we found a 3.47-fold increase of vessel densities in the PDGF-B-overexpressing tumors in comparison to that in the tumors formed by the parental U87MG cells [Figure 3A ▶ (compare a with b) and B]. We have previously shown that PDGF-B stimulates VEGF expression in PAE/PDGF-Rβ cells in cell culture. 19 We hypothesized that the increased tumor angiogenesis in the U87MG PDGF-B-overexpressing tumors could be because of an up-regulation of VEGF levels occurring in the tumor endothelium. To test our hypothesis, we analyzed the various U87MG tumors by double-fluorescent immunostaining using a polyclonal anti-VEGF antibody and a rat anti-CD31 antibody. We found increased levels of VEGF expression in tumor vessels in U87MG PDGF-B-overexpressing tumors. In contrast, little or no VEGF expression could be found in the neovasculature in the parental U87MG gliomas (Figure 3A ▶ , arrows, compare e with f). In addition, comparable levels of VEGF proteins were detected in tumor cells in both parental U87MG- (Figure 3A, e) ▶ and PDGF-B-expressing tumors (Figure 3A, f) ▶ .
Figure 3.
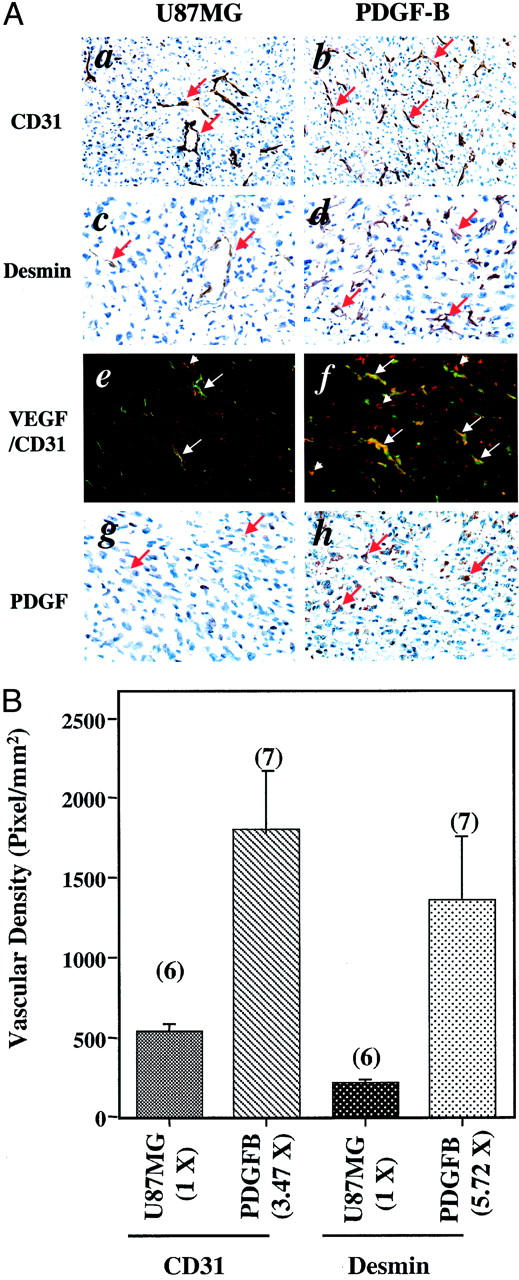
PDGF-B enhances U87MG glioma angiogenesis by increasing VEGF expression in tumor ECs and by recruiting vessel-associated pericytes. A: Immunohistochemical analyses of gliomas formed by the parental U87MG- and PDGF-B-expressing cells. The analyses were performed with a rat monoclonal anti-CD31 antibody (a and b), a polyclonal anti-desmin antibody (c and d), a polyclonal anti-VEGF antibody (red color) together with the anti-CD31 antibody (green color, e and f) and a polyclonal anti-PDGF-B antibody (g and h). Arrows show positive staining for blood vessels (a and b) or pericytes (c and d). In e and f, arrows indicate blood vessels in the tumors. Arrowheads indicate VEGF staining. In f, most of the vessels were stained by both the anti-VEGF and the anti-CD31 (vessels) antibodies (orange color). a, c, b, and d: Immunohistochemical staining in identical areasof serial sections from the same individual mouse brains. Six or more individual tumor samples of each type were analyzed. Experiments were repeated two additional times with similar results. B: Increased vessel densities and recruitment of vessel-associated pericytes in U87MG PDGF-B-expressing gliomas. Quantitative analyses of immunohistochemistry data that are shown in a, b, c, and d were done as described in Materials and Methods. The representative immunohistochemical stains from the parental U87MG- and PDGF-B-expressing gliomas are shown in a and b (vessel stains) or c and d (desmin stains). In each analysis, 10 to 15 random areas within the same tissue section were examined. The mean values of five to seven serial sections from six to seven separate mouse brains in each group were used for the quantitative analyses. Data are presented as means ± SD. Numbers above each column are the numbers of mice analyzed in each group. Numbers in the parentheses under the x axis are the fold difference of the densities found in U87MG- and PDGF-B-expressing gliomas compared with that in parental U87MG tumors. Original magnifications in A: ×200 (a, b); ×400 (c, d, e, f, g, and h).
To further support our observation, we assessed the levels of VEGF expression in the parental U87MG- or PDGF-B-expressing tumors using VEGF ELISA analyses. Because VEGF ELISAs require clarifying tumor lysates and the measured VEGF levels have to be normalized by determining the total proteins in the tumor lysates that are subjected to the VEGF ELISAs, a microdissection approach on the frozen or paraffin brain sections was not successful. We then performed VEGF ELISA analyses as described in the Materials and Methods. 22,23 Five individual parental U87MG- or PDGF-B-expressing gliomas were analyzed. The mean value of VEGF detected in the tumor lysates of the parental U87MG tumors was 317.3 ± 40.1 pg of VEGF per μg of total protein (n = 5). In contrast, VEGF levels detected in the tumor lysates of the PDGF-B-expressing tumors were 1453 ± 137.4 pg of VEGF per μg of total protein (n = 5, P < 0001). A 4.58-fold increase of VEGF expression in the PDGF-B-expressing gliomas is sufficient to cause the elicited neovessel growth in the PDGF-B-expressing tumors (Figure 3) ▶ . These results corroborate with our previous observation of a correlated increase of vessel densities and VEGF amounts detected in human primary glioma tissue specimens 22 or in several human glioma mouse models including the U87MG intracranial tumors. 23
To establish whether overexpression of PDGF-B increased cell proliferation in the U87MG PDGF-B overexpression tumors, we measured the levels of cell proliferation in the U87MG gliomas by in vivo BrdUrd labeling. Cells that had incorporated BrdUrd were then visualized by using immunostaining of the tumors with an anti- BrdUrd antibody. 25,29 To demonstrate whether the elevated VEGF expression in ECs in the PDGF-B-expressing tumors could promote vessel growth, we performed double-immunofluorescent staining using the anti-BrdUrd antibody and an anti-von Willebrand factor antibody in various paraffin-embedded U87MG gliomas that were labeled by BrdUrd. As shown in Figure 4 ▶ , the BrdUrd incorporation was increased by 1.89-fold in total labeled cells (both tumor cells and ECs) and by 2.08-fold in proliferative ECs in the U87MG PDGF-B-overexpressing tumors relative to the parental U87MG tumors [Figure 4, A (a and b) and B ▶ ]. The significant elevated EC proliferation in the PDGF-B tumors support the observation that the PDGF-B-expressing gliomas had increased VEGF expression in the tumor endothelia (Figure 3A, f) ▶ and increased blood vessel densities [Figure 3, A (b) and B ▶ ]. These results suggest that PDGF-B overexpressed by the U87MG tumor cells acted in a paracrine pathway to stimulate VEGF expression in tumor endothelia, thus increasing EC proliferation that led to increased neovascularization.
Figure 4.
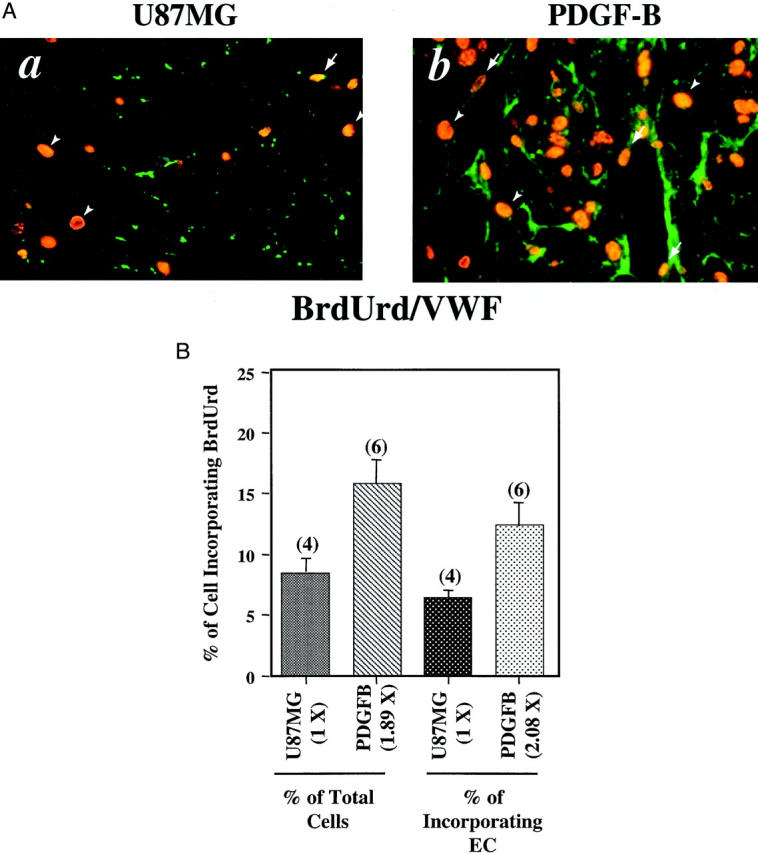
Overexpression of PDGF-B increased both tumor and EC proliferation. A: Immunohistochemical analyses of gliomas formed by parental U87MG- and PDGF-B-expressing cells. The analyses were performed with a monoclonal anti-BrdUrd antibody together with a polyclonal anti-von Willebrand factor antibody (a and b). Arrows show positive staining of proliferative nuclei in blood vessels. Arrowheads indicate BrdUrd staining only in the nuclei of tumor cells. Three to five serial sections from four to six individual tumor samples of each type were analyzed independently. Experiments were repeated two additional times with similar results. B: Increased proliferative index in both total cell (left columns) and ECs (right columns) in the PDGF-B-expressing gliomas. Quantitative analyses of immunohistochemistry data were done as described in Materials and Methods. Original magnification: ×400 (A).
PDGF-B Promoted U87MG Glioma Angiogenesis by Recruiting Capillary-Associated Pericytes into Growing Neovessels
We next examined the overexpression of PDGF-B in the gliomas by immunostaining with an anti-PDGF-B antibody. Strong cytoplasmic expression of PDGF-B was detected in both the PDGF-B (Figure 3A, h) ▶ and V/B tumors (Figure 5A, h) ▶ . Only low levels of endogenous PDGF-B were found in parental U87MG (Figure 3A, g) ▶ and the VEGF165-expressing gliomas (Figure 5A, g) ▶ . During angiogenesis, in addition to stimulating vascular EC proliferation by increasing VEGF expression in ECs 19 and fibroblasts, 18 PDGF-B augments vessel growth by recruiting SMCs/pericytes into developing vessels. 8,9,11 To establish that the increased recruitment of SMCs/pericytes was associated with enhanced neovascularization in the PDGF-B-expressing tumors, we analyzed the brain tissues using a monoclonal anti-α smooth muscle actin antibody. 9 No detectable immunopositive SMCs were present in the tumor vessels of either the parental U87MG- or the PDGF-B-expressing tumors (data not shown). Moreover, it has been demonstrated that desmin is a specific cellular marker that is expressed in pericytes and SMC-negative mesenchymal cells. 8,9,11 Therefore, we examined the expression of desmin in the parental U87MG- or PDGF-B-expressing tumors using an anti-desmin antibody. We found a 5.72-fold increase of desmin-positive vessels in the PDGF-B-expressing tumors relative to the parental U87MG tumors [Figure 3, A (c and d) and B ▶ ]. Interestingly, the increased amount of recruited pericytes in these tumor vessels (5.72-fold) is in parallel to the elevated vessel densities (3.47-fold) detected in these PDGF-B tumors [Figure 3, A (b) and B ▶ ].
Figure 5.
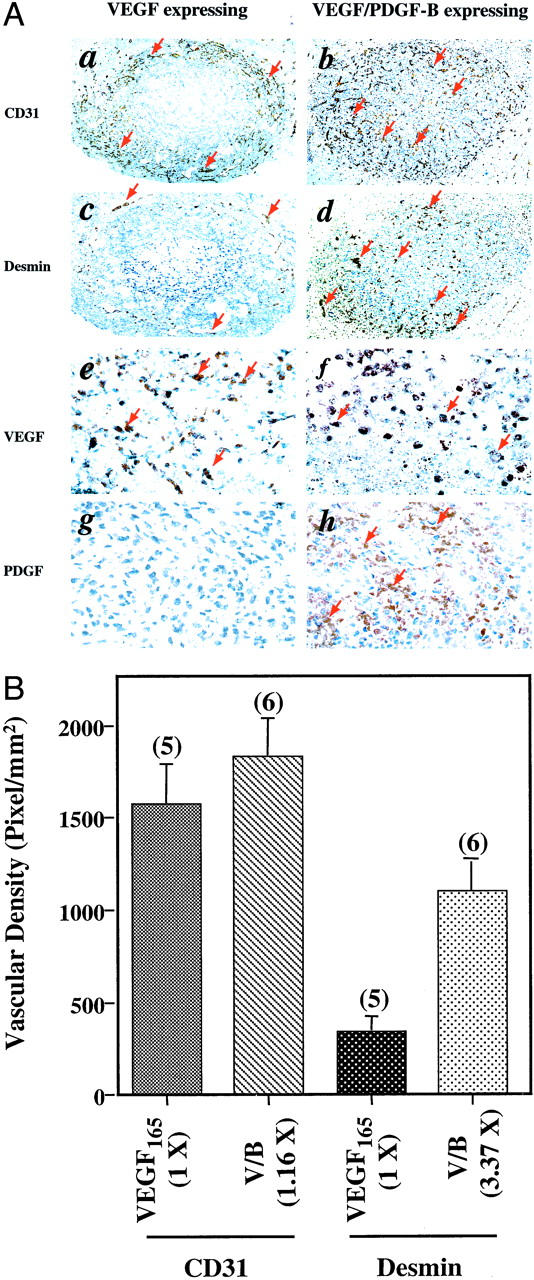
PDGF-B augments U87MG glioma angiogenesis by promoting vessel migration and recruiting vessel-associated pericytes. A: Immunohistochemical analyses of gliomas formed by either VEGF165- or VEGF165/PDGF-B-expressing cells. The analyses were performed with the anti-CD31 antibody (a and b), the polyclonal anti-desmin antibody (c and d), the polyclonal anti-VEGF antibody (e and f), and the polyclonal anti-PDGF-B antibody (g and h). Arrows show positive staining for blood vessels (a and b) or pericytes (c and d) or VEGF (e and f) or PDGF-B (g and h). Areas shown in a, c, and b, d or e, g, and f, h are immunohistochemical staining in identical areas from the same individual mouse brains. Five serial sections from five or six individual tumor samples of each type were analyzed independently. Experiments were repeated two independent times with similar results. B: Increased recruitment of vessel-associated pericytes in U87MG/VEGF/PDGF-B-expressing gliomas. Quantitative analyses of immunohistochemistry data were performed as described in Materials and Methods. Original magnifications in A: ×40 (a, b, c, and d); ×400 (e, f, g, and h).
Recruitment of Capillary-Associated Pericytes by Co-Expressing PDGF-B in U87MG/VEGF165 Gliomas Promoted Vessel Maturation and Inhibited VEGF-Induced Intracranial Hemorrhage
To further demonstrate that PDGF-B could recruit pericytes into rapidly growing vessels and enhance vessel maturation, we overexpressed PDGF-B in U87MG VEGF165-overexpressing tumors. We have previously reported that overexpression of VEGF165 by U87MG cells at levels of 150 ng/ml/106 cells or higher caused tumor-associated intracranial hemorrhage. 20 We postulated that co-overexpression of PDGF-B in the U87MG/ VEGF165 gliomas would facilitate vessel maturation by attracting pericytes into rapidly growing vessels, thus inhibiting VEGF-induced intracranial hemorrhage. To test our hypothesis, we implanted the following types of cells into mouse brains: an equal admixture of U87MG and VEGF165 cells or equal admixtures of U87MG/B (B-4) with U87MG/V/B (V/B-22) clone cells. Both admixtures had a final level of VEGF165 expression that was 150 ng/106 cells per 48-hour culture period in vitro. This level of VEGF secretion was sufficient to promote vigorous vessel growth without causing death of the mice. 20,24 The purpose of mixing U87MG/B and U87MG V/B cells was to assure that 100% of U87MG cells would overexpress PDGF-B. As we reported previously, mice that received the admixture of parental and VEGF-expressing cells developed intracranial hemorrhages in 5.14 ± 0.2 days (14 of 14 mice). Moreover, implantation of the admixture of PDGF-B and V/B cells caused no observable or punctuated hemorrhage in mouse brains in 5.32 ± 0.07 days (13 of 14 mice, P < 0.001; Table 2 ▶ ). We analyzed these U87MG tumors by immunohistochemical staining using the anti-CD31 and the anti-desmin antibodies. As shown in Figure 5 ▶ , when PDGF-B is overexpressed in the U87MG/VEGF165 gliomas, the neovessels that rapidly formed in the vicinity of VEGF165-overexpressing tumors (Figure 5A, a) ▶ 20 migrated into the central region of V/B tumors, but few or no hemorrhages were found [Figure 5A (b) ▶ and Table 2 ▶ ]. Quantitative analysis on the densities of the tumor vessels showed that there was only a 1.16-fold increase of total vessel densities in the V/B gliomas than that in the VEGF165 tumors (Figure 5B) ▶ . However, a 3.37-fold increase of desmin-positive vessel densities (more than 80% of the total vessels) were found in the V/B tumors than that in the vicinity of U87MG/VEGF165 tumors [Figure 5, A (d) and B ▶ ]. These results suggest that overexpression of PDGF-B in U87MG/VEGF165 tumors recruited significant numbers of pericytes into vigorously growing vessels, leading to promote neovessel growth and migration. The facilitated vessel encapsulation protected these otherwise naked vessels from rupture.
Discussion
In the present study, we demonstrate that PDGF-B acts through a paracrine mechanism in human U87MG glioma cells. In vitro, treatment by recombinant PDGF-B protein on U87MG cells caused tyrosine phosphorylation in endogenous PDGF-Rβ in these cells. However, U87MG cells that were stimulated by recombinant PDGF-B or exposed chronically to overexpressed PDGF-B by U87MG cells did not increase the secretion of VEGF or promote cell proliferation in both U87MG parental and PDGF-B-overexpressing cells. In vivo, overexpression of PDGF-B by U87MG gliomas augmented VEGF expression in tumor endothelia and recruitment of pericytes to growing neovessels. Increased tumor angiogenesis and elicited proliferation of ECs and tumor cells in U87MG PDGF-B-expressing tumors resulted in an increase of tumorigenicity in mouse brains. In addition, co-overexpression of PDGF-B with VEGF by U87MG gliomas greatly facilitated the recruitment of pericytes into vigorously growing neovessels and promoted vessel migration.
PDGF-B plays a major role in human gliomagenesis. 14 Expression profiles of PDGF and PDGF-R in primary human glioma tissues suggest that PDGF acts both in autocrine and paracrine pathways during glioma progression. 12,14,15 Several studies indicate autocrine signaling of PDGF in gliomas. PDGF-B stimulated cell growth and tumor formation of human T98G glioma cells that express endogenous PDGF-Rβ. 30 Somatic cell type-specific expression of PDGF-B in either nestin-expressing neural progenitors or glial fibrillary acidic protein-expressing astrocytes induced oligodendrogliomas and oligoastrocytomas from the neural progenitors and astrocytes in mouse brains, suggesting autocrine stimulation by PDGF-B in both somatic cell types is potentially sufficient to promote gliomagenesis. 31 A survey of 11 brain tumor cell lines and 5 primary glioma specimens showed that all four PDGF ligands (-A, -B, -C, and -D) except PDGF-B, PDGF-Rα, and PDGF-Rβ are expressed in the glioma cell lines and tissues. A synthetic inhibitor, CT52923, that specifically inhibits the tyrosine kinase activity of PDGF-R, blocked PDGF autocrine-mediated phosphorylation of PDGF-R, Akt, and mitogen-activated protein kinase. CT52923 also inhibited soft agar colony formation in cell culture and reduced tumor formation by a glioma cell line in mice. 32 Moreover, lack of expression of PDGF-B in 6 of the 11 glioma cell lines including U87MG cells examined in this study implicates that a paracrine pathway by PDGF-B/PDGFR-β may exist in the glioma cells. 32 In addition to genetic evidence of the existence of a paracrine signaling by PDGF-B/PDGFR-β in gliomas, 12,15 other reports support that PDGF-B may also act by a paracrine mechanism in promoting tumor growth and angiogenesis. In vitro, PDGF-B stimulated cell proliferation and cord/tube formation of vascular ECs that express PDGF-Rβ, but had no effect on nonangiogenic and PDGF-Rβ-negative ECs. 28 Stimulation of fibroblasts and ECs by PDGF-B increased the expression of VEGF through the Akt signaling pathway in these cells. 18,19 In addition, EC-derived PDGF-B and heterotypic cell-cell interactions of ECs and mural cell precursors mediated EC-induced recruitment and proliferation of mesenchymal 10T1/2 cells. 4 In vivo, in WM9 melanoma cells 33 and immortalized HaCat keratinocytes that lack endogenous PDGF-Rβ, 16 overexpression of PDGF-B by these cells stimulated tumor formation, neovascularization, and stromal cell proliferation. Our data corroborates and extends these observations that PDGF-B stimulated U87MG glioma angiogenesis and tumor growth by a paracrine mechanism. In U87MG glioma cells, although PDGF-B caused phosphorylation of the endogenous PDGF-Rβ, no effects by PDGF-B on cell proliferation or VEGF expression were found in U87MG cells. These results suggest that the PDGF-B/PDGF-Rβ autocrine pathway may not be intact in U87MG cells, at least in mediating the stimulation of VEGF expression or cell mitogenesis. On the other hand, no study had demonstrated a tumor model system in which the paracrine pathway of PDGF-B/PDGF-Rβ enhances tumor angiogenesis by recruiting SMCs/pericytes into developing tumor vessels. Our results here show that in addition to increasing VEGF expression in tumor endothelium, overexpressed PDGF-B promoted vessel assembly by attracting more pericytes into growing tumor vessels.
We have previously shown that overexpression of specific VEGF isoforms in human U87MG glioma cells caused vigorous growth of neovessels in tumors leading to intracranial hemorrhage. 20 We hypothesized that rapid EC proliferation induced by VEGF led to form naked tumor capillaries. The absence of pericytes surrounding these vessels could result in the development of structurally defective vessels capable of causing hemorrhage. Our data supported this hypothesis. When PDGF-B and VEGF were overexpressed simultaneously in U87MG gliomas, VEGF-induced hemorrhage was inhibited. The VEGF-induced neovessels migrated into the central regions of the tumor concurrent with VEGF-induced hemorrhage. The absence of hemorrhagic neovessels in the V/B-expressing tumors was probably because of an increase in the number of capillary-associated pericytes that enhanced maturation of the rapidly growing vessels, thereby, preventing vessel rupture. Thus, overexpression of PDGF-B in the VEGF-induced tumor-associated hemorrhage model further demonstrated another important role of PDGF-B in angiogenesis that PDGF-B recruits pericytes into growing neovessels during glioma angiogenesis.
In summary, our data show that PDGF-B stimulates human U87MG tumor angiogenesis through a paracrine pathway. PDGF-B enhances glioma angiogenesis and growth by stimulating VEGF expression in tumor endothelium that increases EC mitogenesis, and by promoting recruitment of pericytes into growing vessels, thus facilitating vessel assembly. Clearly, our results have clinical relevance to the process of glioma progression. At early stages of human glioma development (grades I, II, and III astrocytomas), there is little or no angiogenesis. There are no hypoxic conditions in these tumors that would stimulate the expression of VEGF. We hypothesize that in low-grade gliomas, overexpressions of PDGF-B in tumor cells and PDGF-Rβ in ECs 12,15 might be part of an angiogenic switch that up-regulates VEGF and enhances vessel growth, thus promoting glioma progression. During the later stages of glioma development, hypoxia and increased expression of other growth factors or genes in the tumors augments the expression of VEGF, which in turn results in vigorous growth of blood vessels. Continuous high levels of expression of PDGF-B and PDGF-Rβ in high-grade gliomas further up-regulate VEGF in the tumor endothelium leading to recruitment of pericytes. These events would allow rapid neovascularization in malignant gliomas to occur. Thus, demonstration of the roles of PDGF-B in glioma progression would provide pivotal information for targeting the PDGF-B/PDGF-Rβ pathway, along with inhibition of the VEGF/VEGF-R pathway in the clinical treatment of human gliomas.
Acknowledgments
We thank Gavin Robertson, Michael Jarzynka, Frank Cackowski, and Chris Schafer for critical reading and editing of this manuscript; and Daniel Bowen-Pope at University of Washington, Seattle, WA, for the cDNA of PDGF-B.
Footnotes
Address reprint requests to Shi-Yuan Cheng, Ph.D., Cancer Institute and Department of Pathology, University of Pittsburgh, Research Pavilion at the Hillman Cancer Center, Suite 2.26f, 5117 Centre Ave., Pittsburgh, PA 15213-1863. E-mail: chengs@msx.upmc.edu.
Supported in part by a grant from the Brain Cancer Program of the James F. McDonnell Foundation, a research grant from The Brain Tumor Society, and a start-up fund from the University of Pittsburgh Cancer Institute (to S.-Y. C.).
D. W. was a fellow of the Robert Steel Foundation for Pediatric Cancer Research.
References
- 1.Carmeliet P: Mechanisms of angiogenesis and arteriogenesis. Nat Med 2000, 6:389-395 [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N: Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol 1999, 237:1-30 [DOI] [PubMed] [Google Scholar]
- 3.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J: Vascular-specific growth factors and blood vessel formation. Nature 2000, 407:242-248 [DOI] [PubMed] [Google Scholar]
- 4.Hirschi KK, Rohovsky SA, D’Amore PA: PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate [published erratum appears in J Cell Biol 1998 Jun 1;141(5):1287]. J Cell Biol 1998, 141:805-814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C: Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 1999, 126:3047-3055 [DOI] [PubMed] [Google Scholar]
- 6.Betsholtz C, Karlsson L, Lindahl P: Developmental roles of platelet-derived growth factors. Bioessays 2001, 23:494-507 [DOI] [PubMed] [Google Scholar]
- 7.Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C: Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev 1994, 8:1875-1887 [DOI] [PubMed] [Google Scholar]
- 8.Lindahl P, Johansson BR, Leveen P, Betsholtz C: Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 1997, 277:242-245 [DOI] [PubMed] [Google Scholar]
- 9.Lindahl P, Hellstrom M, Kalen M, Betsholtz C: Endothelial-perivascular cell signaling in vascular development: lessons from knockout mice. Curr Opin Lipidol 1998, 9:407-411 [DOI] [PubMed] [Google Scholar]
- 10.Soriano P: Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev 1994, 8:1888-1896 [DOI] [PubMed] [Google Scholar]
- 11.Lindahl P, Hellstrom M, Kalen M, Karlsson L, Pekny M, Pekna M, Soriano P, Betsholtz C: Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development 1998, 125:3313-3322 [DOI] [PubMed] [Google Scholar]
- 12.Westermark B, Heldin CH, Nister M: Platelet-derived growth factor in human glioma. Glia 1995, 15:257-263 [DOI] [PubMed] [Google Scholar]
- 13.Kirsch M, Wilson JC, Black P: Platelet-derived growth factor in human brain tumors. J Neurooncol 1997, 35:289-301 [DOI] [PubMed] [Google Scholar]
- 14.Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, DePinho RA: Malignant glioma: genetics and biology of a grave matter. Genes Dev 2001, 15:1311-1333 [DOI] [PubMed] [Google Scholar]
- 15.Hermanson M, Funa K, Hartman M, Claesson-Welsh L, Heldin CH, Westermark B, Nister M: Platelet-derived growth factor and its receptors in human glioma tissue: expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res 1992, 52:3213-3219 [PubMed] [Google Scholar]
- 16.Skobe M, Fusenig NE: Tumorigenic conversion of immortal human keratinocytes through stromal cell activation. Proc Natl Acad Sci USA 1998, 95:1050-1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Battegay EJ, Rupp J, Iruela-Arispe L, Sage EH, Pech M: PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J Cell Biol 1994, 125:917-928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nauck M, Roth M, Tamm M, Eickelberg O, Wieland H, Stulz P, Perruchoud AP: Induction of vascular endothelial growth factor by platelet-activating factor and platelet-derived growth factor is downregulated by corticosteroids. Am J Respir Cell Mol Biol 1997, 16:398-406 [DOI] [PubMed] [Google Scholar]
- 19.Wang D, Huang HJ, Kazlauskas A, Cavenee WK: Induction of vascular endothelial growth factor expression in endothelial cells by platelet-derived growth factor through the activation of phosphatidylinositol 3-kinase. Cancer Res 1999, 59:1464-1472 [PubMed] [Google Scholar]
- 20.Cheng S-Y, Nagane M, Su Huang H-J, Cavenee WK: Intracerebral tumor-associated hemorrhage caused by overexpression of vascular endothelial growth factor (VEGF) Isoforms, VEGF121 and VEGF165 but not VEGF189. Proc Natl Acad Sci USA 1997, 94:12081-12087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng SY, Huang HJ, Nagane M, Ji XD, Wang D, Shih CC, Arap W, Huang CM, Cavenee WK: Suppression of glioblastoma angiogenicity and tumorigenicity by inhibition of endogenous expression of vascular endothelial growth factor. Proc Natl Acad Sci USA 1996, 93:8502-8507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishikawa R, Cheng SY, Nagashima R, Huang HJ, Cavenee WK, Matsutani M: Expression of vascular endothelial growth factor in human brain tumors. Acta Neuropathol 1998, 96:453-462 [DOI] [PubMed] [Google Scholar]
- 23.Roberts WG, Delaat J, Nagane M, Huang S, Cavenee WK, Palade GE: Host microvasculature influence on tumor vascular morphology and endothelial gene expression. Am J Pathol 1998, 153:1239-1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo P, Xu L, Pan S, Brekken RA, Yang ST, Whitaker GB, Nagane M, Thorpe PE, Rosenbaum JS, Su Huang HJ, Cavenee WK, Cheng SY: Vascular endothelial growth factor isoforms display distinct activities in promoting tumor angiogenesis at different anatomic sites. Cancer Res 2001, 61:8569-8577 [PubMed] [Google Scholar]
- 25.Ma HI, Guo P, Li J, Lin SZ, Chiang YH, Xiao X, Cheng SY: Suppression of intracranial human glioma growth after intramuscular administration of an adeno-associated viral vector expressing angiostatin. Cancer Res 2002, 62:756-763 [PubMed] [Google Scholar]
- 26.Nagane M, Pan G, Weddle JJ, Dixit VM, Cavenee WK, Huang HJ: Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factor-related apoptosis-inducing ligand in vitro and in vivo. Cancer Res 2000, 60:847-853 [PubMed] [Google Scholar]
- 27.Bornfeldt KE, Raines EW, Graves LM, Skinner MP, Krebs EG, Ross R: Platelet-derived growth factor. Distinct signal transduction pathways associated with migration versus proliferation. Ann NY Acad Sci 1995, 766:416-430 [DOI] [PubMed] [Google Scholar]
- 28.Battegay EJ, Rupp J, Iruela-Arispe L, Sage EH, Pech M: PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J Cell Biol 1994, 125:917-928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagane M, Coufal F, Lin H, Bogler O, Cavenee WK, Huang HJ: A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res 1996, 56:5079-5086 [PubMed] [Google Scholar]
- 30.Potapova O, Fakhrai H, Mercola D: Growth factor PDGF-B/v-sis confers a tumorigenic phenotype to human tumor cells bearing PDGF receptors but not to cells devoid of receptors: evidence for an autocrine, but not a paracrine, mechanism. Int J Cancer 1996, 66:669-677 [DOI] [PubMed] [Google Scholar]
- 31.Dai C, Celestino JC, Okada Y, Louis DN, Fuller GN, Holland EC: PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev 2001, 15:1913-1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lokker NA, Sullivan CM, Hollenbach SJ, Israel MA, Giese NA: Platelet-derived growth factor (PDGF) autocrine signaling regulates survival and mitogenic pathways in glioblastoma cells: evidence that the novel PDGF-C and PDGF-D ligands may play a role in the development of brain tumors. Cancer Res 2002, 62:3729-3735 [PubMed] [Google Scholar]
- 33.Forsberg K, Valyi-Nagy I, Heldin CH, Herlyn M, Westermark B: Platelet-derived growth factor (PDGF) in oncogenesis: development of a vascular connective tissue stroma in xenotransplanted human melanoma producing PDGF-BB. Proc Natl Acad Sci USA 1993, 90:393-397 [DOI] [PMC free article] [PubMed] [Google Scholar]