Abstract
Intestinal fibrosis and strictures frequently occur in Crohn’s disease but not ulcerative colitis. We have recently shown that, compared to myofibroblasts obtained from normal and ulcerative colitis tissue, myofibroblasts isolated from fibrotic Crohn’s disease mucosal samples express significantly lower amounts of transforming growth factor (TGF)-β3, but the expression of TGF-β2 was significantly greater. We now report that in myofibroblast cultures established from fibrotic Crohn’s disease mucosal samples there is significantly higher constitutive expression of tissue inhibitor of metalloproteinase (TIMP)-1 compared to similar cells isolated from normal or ulcerative colitis tissue. Myofibroblasts derived from normal mucosa and from mucosa affected by ulcerative colitis or Crohn’s disease also expressed matrix metalloproteinase (MMP)-1, MMP-2, and MMP-3 but did not express MMP-9. Recombinant (r) TGF-β1 and rTGF-β2, but not rTGF-β3, induced expression of TIMP-1 in normal intestinal myofibroblasts. These studies illustrate a potential mechanism by which differential expression of isoforms of TGF-β may lead to excessive deposition of extracellular matrix and stricture formation via TIMP-1-mediated inhibition of MMP activity.
Ulcerative colitis and Crohn’s disease are chronic inflammatory diseases of the intestine characterized by relapses and remission. During a clinical relapse, the intestinal mucosa is infiltrated by a large number of polymorphonuclear cells, lymphocytes, and macrophages and the mucosal architecture is often markedly disrupted, with destruction of the extracellular matrix. 1 During the process of disease remission, often after treatment, there is resolution of the inflammatory response, associated with repair and remodeling of the mucosal architecture. Repeated episodes of mucosal inflammation and repair often lead to more permanent changes in the mucosal architecture.
In contrast to ulcerative colitis, intestinal stricture formation because of excessive deposition of fibrous tissue often occurs in patients with Crohn’s disease. 2 Although they share a number of clinical, pathological, and immunological similarities, the reason for the frequent development of intestinal strictures in Crohn’s disease, but not ulcerative colitis, remains unknown. The turnover of extracellular matrix during the processes of mucosal repair and remodeling is regulated by factors that influence its synthesis and degradation.
Myofibroblasts are the predominant mucosal cells that synthesize components of the extracellular matrix and their breakdown is mediated by proteolytic enzymes derived from different cell types of which matrix metalloproteinases (MMPs) represent a large and important family. 3 MMPs are a family of zinc- and calcium-dependent proteases that are secreted as proforms and become activated via proteolytic cleavage. 4 The proteolytic activity of MMPs is controlled by tissue inhibitors of metalloproteinases (TIMPs) via noncovalent binding of the active forms of MMPs at molar equivalence. 4,5 TIMP-1 is inducible whereas TIMP-2 is mainly constitutive. An imbalance because of reduced MMP activity and/or increased expression of TIMPs may lead to the excessive deposition of extracellular matrix proteins with subsequent fibrosis and stricture formation in Crohn’s disease.
The transforming growth factor (TGF)-β family of proteins has been shown to be important in the regulation of the synthesis and breakdown of extracellular matrix proteins. 6 TGF-β1 has been studied the most and has been shown to down-regulate MMP expression and to enhance the expression of TIMP-1. 4,7 There is limited information on the role of isoforms of TGF-β in the regulation of the turnover of extracellular matrix. In addition to expressing extracellular matrix proteins 8 and MMPs, 9 myofibroblasts also express distinct isoforms of TGF-β. Thus, myofibroblasts isolated from normal intestinal mucosal samples express predominantly TGF-β3, 10 whereas those from ulcerative colitis express both TGF-β1 and TGF-β3. By contrast, in myofibroblast cultures from fibrotic Crohn’s disease tissue, there was significantly lower expression of TGF-β3 but enhanced release of TGF-β2. 11
In the present study, we have investigated the expression of MMP-1, -2, -3, and -9 and TIMP-1 and TIMP-2 by myofibroblasts isolated from normal intestinal mucosal samples and those affected by inflammatory bowel disease. TIMP-1 inhibits active forms of all MMPs and is recognized as the predominant enzyme in inhibiting matrix degradation in the intestine and therefore the effects of recombinant isoforms of TGF-β on myofibroblast expression of TIMP-1 have also been studied.
Materials and Methods
Myofibroblast cultures (up to 18 in each group) were established from intestinal mucosal samples obtained from operation resection specimens. Normal colonic mucosal samples (obtained >5 cm from tumor from nine intestinal resections for cancer) and mucosal samples with active ulcerative colitis (four resection specimens) and fibrotic Crohn’s disease (five resection specimens) were studied. Myofibroblast cultures, with no other contaminating cells, were established and characterized as previously described 8 and cultured in Dulbecco’s modified Eagle’s medium (GIBCO, Paisley, UK), supplemented with 10% fetal calf serum, 1% nonessential amino acids (Gibco), penicillin (100 U/ml), and streptomycin (0.1 mg/ml). Subconfluent monolayers of myofibroblasts were studied at passages 2 to 6. Myofibroblast-conditioned medium (MFCM) was obtained after 24 hours of culture in 0.1% fetal calf serum/Dulbecco’s modified Eagle’s medium.
Subconfluent monolayers of myofibroblasts derived from normal colonic mucosal samples, seeded in 24-well plates (Nunc, Nunc, UK) at 5 × 104 per well, were also exposed for 24 hours to recombinant (r) TGF-β1, TGF-β2, and TGF-β3 (R&D Systems, UK), at a final concentration of 5 ng/ml. MFCM of control and rTGF-β-exposed cells was collected for determination of TIMP-1 concentration.
Expression of mRNA Transcripts
RNA was isolated using the Rneasy RNA extraction kit (Qiagen, Germany) and reverse transcribed using the Ready-To-Go T-Primed First Strand Reaction Kit (Pharmacia Biotech, Brussels, Belgium). Reverse transcription to cDNA was performed in buffered solution containing dATP, dCTP, dGTP, dTTP, FPLCpure murine reverse transcriptase, RNA guard (porcine), RNase/DNase-free bovine serum albumin, and NotI-d(T)18 primer [5′-d(AACTGGAAGAATTCGCGGCCGCAGGAAT18)-3′] according to the manufacturer’s instructions.
The expression of MMP-1, -2, -3, and -9; and TIMP-1 and -2 mRNA transcripts was subsequently studied by polymerase chain reaction (PCR) using the primer sequences: GAPDH, 5′-TGC CGT CTA GAA AAA CCT GC-3′ and 5′-ACC CTG TTG CTG TAG CCA AA-3′; MMP-1, 5′-AAT GTG CTA CAC GGATAC CC-3′ and 5′-CTT TGT GGC CAA TTC CAG GA-3′; MMP-2, 5′-CCG CCT TTA ACT GGA GCA AA-3′ and 5′-TTT GGT TCT CCA GCT TCA GG-3′; MMP-3, 5′-GAG GAA AAT GCA TGC AGC CA-3′ and 5′-CTC CAA CTG TGA AGA TCC AG-3′; MMP-9, 5′-GAA GAT GCT GCT GTT CAG CG-3′ and 5′-ACT TGG TCC ACC TGG TTC AA-3′; TIMP-1, 5′-CGG GGC TTC ACC AAG ACC-3′ and 5′-TCA GGC TAT CTG GGA CCG C-3′; TIMP-2, 5′-GAA GAA GAG CCT GAA CCA CA-3′ and 5′-GTC CTC GAT GTC GAG AAA CT-3′.
In subsequent quantitative analysis, the expression of TIMP-1 transcripts were related to that of the constitutive product, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), by real-time PCR using GeneAmp 5700 Sequence Detection System (Perkin Elmer, Emeryville, CA). PCR reactions contained 2.5 μl of 10× SYBR Green PCR buffer, 3 μl of 25 mmol/L MgCl2, 0.2 mmol/L of dATP, 0.2 mmol/L of dCTP, 0.2 mmol/L of dGTP, 0.4 mmol/L of dUTP, 1 μl of sense and anti-sense primers (5 pmol), 0.25 U of uracil N-glycosylase, 0.125 U Amplitaq Gold DNA polymerase, 1 μl of cDNA, and 15.125 μl of sterile water.
The following primer pairs were used: 5′-CTT CGG GAA AAG TCT CGG AA-3′ (sense) and 5′-ACA AGG GTG AGG GTA GAA AG-3′ (anti-sense) to amplify TIMP-1 transcripts and 5′-GGT GAA GGT CGG AGT CAA CGG A-3′ (sense) and 5′-GAG GGA TCT CGC TCC TGG AAG A-3′ (anti-sense) to amplify GAPDH transcripts. The reactions were incubated at 50°C for 2 minutes and 95°C for 10 minutes. Amplification was performed by 40 cycles consisting of denaturation at 95°C for 15 seconds and annealing at 60°C for 1 minute. Amplification of the expected single PCR products was confirmed by gel electrophoresis. Expression of TIMP-1 transcripts, as a ratio of GAPDH transcripts, was determined using the sequence detection system software. In control reactions, reverse transcriptase enzyme or cDNA were omitted.
Gelatin Zymography
Gelatin zymography was used to assess the presence and bioactivity of MMP-2 and MMP-9. MFCM was collected as described above and diluted 1:1 with nonreducing sample buffer (Novex, San Diego, CA). Twenty-five μl of this mixture was applied per well and separated on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis gel containing 0.1% gelatin (Novex) immersed in running buffer (Tris base, 29 g; glycine, 144g; SDS, 10g, and dH2O to a volume of 1 L) (Novex) for 150 minutes at 125 V/gel. SDS was removed by incubation with renaturing buffer [Triton X-100, 2.5% (v/v) in dH2O] (Novex) for 30 minutes at room temperature. The gels were then washed for 30 minutes in developing buffer [50 mmol/L Tris, 0.2 mol/L NaCl, 5 mmol/L CaCl2, 0.02% Brij 35 (w/v), pH 7.6] (Novex) and then incubated overnight at 37°C in developing buffer. The gels were then stained with Coomassie brilliant blue R-250 and destained with 30% methanol (v/v) and 10% acetic acid (v/v) in dH2O for 10 minutes. The gels were finally immersed in Gel-Dry solution (Novex) and mounted between cellophane sheets and allowed to air dry.
Western Blot Analysis and Enzyme-Linked Immunosorbent Assay (ELISA)
After the concentration of MFCM was obtained from normal, ulcerative colitis, and Crohn’s disease myofibroblast cultures, equal amounts of protein were separated by SDS-polyacrylamide gel electrophoresis using 12.5% acrylamide-resolving gel (Bio-Rad Protean II Slab Gel Electrophoresis equipment). After transfer onto polyvinylidene difluoride membranes, immunostaining was performed using antibodies to MMP-1, MMP-3, TIMP-1, and TIMP-2 (obtained from Calbiochem-Novabiochem International) and the Vectastain Elite ABC kit (Vector Laboratories).
TIMP-1 concentrations in MFCM samples were assessed using specific ELISA (Amersham Pharmacia Biotech, UK). Protein concentration in each MFCM sample was determined using the Bradford-Lowry assay and samples with equivalent protein concentrations were used in the TIMP-1 ELISAs.
Statistical Analyses
For data obtained from real-time PCR, the Mann-Whitney U-test for unmatched samples was used. For data obtained from the ELISAs, the differences between the paired sample data were not normally distributed therefore the Wilcoxon signed sank test was used, P values <0.05 were taken as statistically significant.
Results
Expression of MMP and TIMP mRNA Transcripts by Intestinal Myofibroblasts
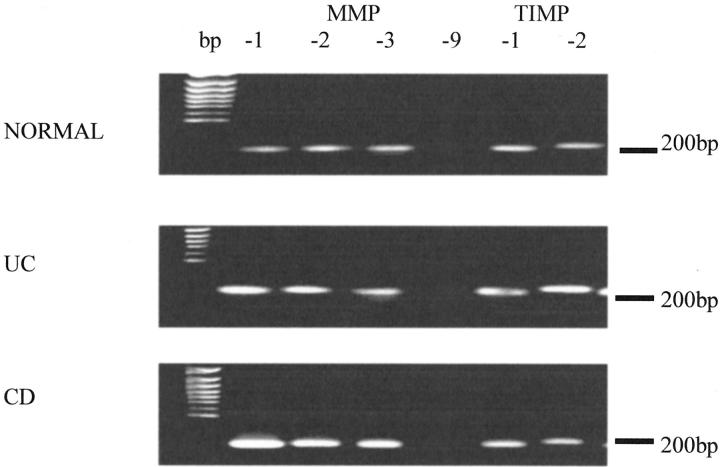
Normal, ulcerative colitis and Crohn’s disease myofibroblasts expressed mRNA transcripts for MMP-1, -2, and -3 and TIMP-1 and -2. No MMP-9 mRNA was expressed by any of the myofibroblast cultures (Figure 1) ▶ .
Figure 1.
Normal, ulcerative colitis (UC), and Crohn’s disease (CD) intestinal myofibroblasts express mRNA transcripts for MMP-1, MMP-2, and MMP-3 (lanes 2 to 4, respectively) and TIMP-1 and TIMP-2 (lanes 6 and 7, respectively). No MMP-9 mRNA was detected in any of the myofibroblasts (lane 5). Specificity of the PCR products was confirmed by DNA sequence analysis. One hundred-bp markers are shown in the left lane of the figure. The figure shown is representative of six separate experiments.
Expression of TIMP-1 by Intestinal Myofibroblasts
In quantitative analysis by real-time PCR, expression of TIMP-1 transcripts was significantly higher in myofibroblasts isolated from fibrotic Crohn’s disease tissue compared to those obtained from normal mucosal samples (Table 1) ▶ .
Table 1.
Ratios of TIMP-1:GAPDH mRNA Transcripts in Myofibroblast Cultures Established from Normal, Ulcerative Colitis, and Crohn’s Disease Mucosal Samples
| Normal | Ulcerative colitis | Crohn’s disease | |
|---|---|---|---|
| TIMP-1:GAPDH | 1.46 (2.99) | 3.53 (2.31) | 7.31 (59.7)* |
TIMP-1 and GAPDH transcripts were determined by real-time PCR and data expressed as median (interquartile range; n = 10;
*, P < 0.05 versus normal and ulcerative colitis).
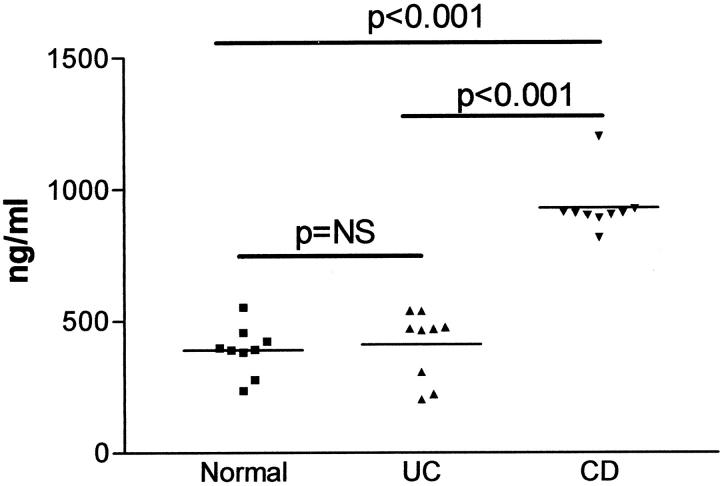
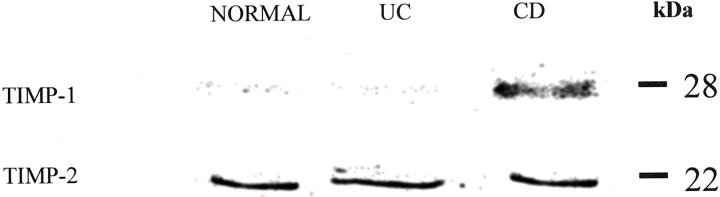
Studies by ELISA showed that MFCM from fibrotic Crohn’s disease myofibroblasts (median, 909.4 ng/ml; interquartile range, 24.6 ng/ml) contained significantly higher concentrations of TIMP-1 protein than conditioned media of myofibroblast cultures established from normal (median, 391.9 ng/ml; interquartile range, 110.6 ng/ml) and ulcerative colitis (median, 470.3 ng/ml; interquartile range, 242.4 ng/ml) mucosal samples (Figure 2) ▶ . TIMP-1 concentrations in conditioned media of myofibroblasts obtained from ulcerative colitis samples were similar to those of normal mucosal myofibroblast cultures (Figure 2) ▶ . The above findings using ELISA were confirmed by Western blot analysis of MFCM (Figure 3) ▶ . In these studies, TIMP-2 release was similar in myofibroblast cultures established from normal, ulcerative colitis, and fibrotic Crohn’s disease mucosal samples. By contrast, there was markedly increased expression of TIMP-1 in conditioned media of fibrotic Crohn’s disease myofibroblasts.
Figure 2.
TIMP-1 concentrations in conditioned media of myofibroblast cultures established from normal, ulcerative colitis (UC), and Crohn’s disease (CD) mucosal samples. The samples contained equivalent total protein concentrations and TIMP-1 levels were assessed using specific ELISA.
Figure 3.
Expression by Western blot analysis of TIMP-1 and TIMP-2 in conditioned media of normal, ulcerative colitis (UC), and Crohn’s disease (CD) intestinal myofibroblast cultures. Samples containing equal amounts of protein were applied to each lane and separated by SDS-polyacrylamide gel electrophoresis. After transfer to polyvinylidene difluoride membranes, immunostaining was performed using antibodies specific to TIMP-1 and TIMP-2. In contrast to TIMP-2, this representative Western blot shows greater expression of TIMP-1 in conditioned medium of Crohn’s disease intestinal myofibroblasts.
Expression of MMP Protein by Normal, Ulcerative Colitis, and Crohn’s Disease Myofibroblasts
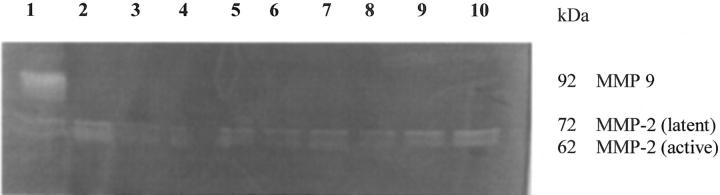
Studies by Western blot analyses showed that MMP-1 and MMP-3 were expressed by all myofibroblast cultures but were expressed only in their inactive forms (Figure 4) ▶ . Gelatin zymography showed that MMP-2 was expressed in both active and inactive forms, however, MMP-9 was not expressed by any myofibroblast cultures (Figure 5) ▶ .
Figure 4.
Representative Western blot showing expression of only the latent forms of MMP-1 (55 kd) and MMP-3 (66 kd) by normal myofibroblasts. Similar patterns were seen for myofibroblast cultures derived form ulcerative colitis and Crohn’s disease mucosa.
Figure 5.
Representative gelatin zymogram showing expression of both active and latent forms of MMP-2, but no expression of MMP-9 by normal, ulcerative colitis, and Crohn’s disease myofibroblasts. Lane 1 shows a positive control, lanes 2 to 4 show normal MFCM, lanes 5 to 7 show UC MFCM, and lanes 8 to 10 show Crohn’s disease MFCM. No bands corresponding to the 92-kd MMP-9 are seen, however in all cultures, both 72-kd (latent) and 62-kd (active) bands of MMP-2 can be seen.
Recombinant TGF-β1 and TGF-β2, but Not TGF-β3, Induce Expression of TIMP-1 in Normal Intestinal Myofibroblasts
Incubation of isolated normal human intestinal myofibroblasts with rTGF-β1 and rTGF-β2 led to a significant increase in the release of TIMP-1 when compared to control cultures (Table 2) ▶ . However, concentrations of TIMP-1 in MFCM of rTGF-β3-exposed myofibroblasts did not differ from those of cells cultured in control medium.
Table 2.
Expression of TIMP-1 Protein by Normal Human Intestinal Myofibroblasts after Incubation with rTGF-β Isoforms (5 ng/ml)
| TIMP-1 (ng/ml) | |
|---|---|
| Control | 132.39 (43.55) |
| rTGF-β1 | 158.06 (23.09)* |
| rTGF-β2 | 159.40 (38.09)† |
| rTGF-β3 | 133.74 (18.05) |
Data expressed as median (interquartile range; n = 10; *, P = 0.028 and †, P = 0.04; versus control).
Discussion
Intestinal myofibroblasts are increasingly recognized as an important cell population that makes a major contribution to the maintenance of normal mucosal homeostasis, 12 tissue repair and remodeling, 10,13 and intestinal fibrosis. 11,14 Myofibroblasts derived form normal and inflammatory bowel disease mucosal samples maintain their phenotype after isolation and prolonged culture. 8,11 Similarly, myofibroblasts derived from different parts of the intestine 15 and normal and diseased skin 16 have been shown to maintain their distinct characteristics implying that these cells have significant influence on their milieu in vivo and are not simply mesenchymal cells influenced phenotypically by local regulatory peptides. 17
We have shown that myofibroblasts isolated from fibrotic Crohn’s disease mucosal samples have an enhanced capacity to constitutively express higher levels of TIMP-1 than similar cells isolated from normal and ulcerative colitis mucosal samples. The resulting increase in inhibition of the activity of MMPs would be expected to inhibit degradation of the extracellular matrix, leading to its excessive deposition and subsequent stricture formation in Crohn’s disease. Our studies have also shown, for the first time, that recombinant isoforms of TGF-β differ in their ability to regulate the expression of TIMP-1 by normal intestinal myofibroblasts. Thus, rTGF-β1 and rTGF-β2 induce the expression of TIMP-1 but rTGF-β3 had no effect. These studies illustrate a potential mechanism by which TGF-β isoforms may play an important role in the regulation of extracellular matrix proteins in tissue repair and remodeling.
In recent studies we have shown that, compared to normal intestinal cells, myofibroblast cultures established from fibrotic Crohn’s disease mucosal samples expressed significantly higher levels of bioactive TGF-β1 and TGF-β2. However, the expression of TGF-β3 bioactivity by the Crohn’s disease intestinal myofibroblasts was significantly lower than by normal intestinal myofibroblasts. 11 Our current studies suggest that the enhanced expression of TGF-β1 and TGF-β2 by Crohn’s disease myofibroblasts may lead to the increased expression of TIMP-1 by these cells. The expression of TIMP-1 by myofibroblasts from ulcerative colitis mucosal samples was similar to that expressed by normal intestinal myofibroblasts. Because the former also express TGF-β1 at significantly higher levels than the latter, it is likely that factors other than TGF-β1 are responsible for the enhanced expression of TIMP-1 by fibrotic Crohn’s disease myofibroblasts. These include TGF-β2, which is released in significant amounts by Crohn’s disease intestinal myofibroblasts but not by myofibroblasts from normal or ulcerative colitis mucosal samples. 11 By contrast, the expression of TGF-β3 by Crohn’s disease myofibroblasts is significantly lower compared to myofibroblasts from normal or ulcerative colitis tissue. An important role for TGF-β3 in tissue repair without scarring has been demonstrated in a model of rat cutaneous wound repair. 18
TGF-β1 and TFG-β2 have been shown to promote repair of rat cutaneous wounds with excessive deposition of fibrous tissue. 19 By contrast, TGF-β3 induced wound repair without fibrosis and was also able to inhibit the profibrogenic effects of TGF-β1 and TGF-β2. 18 Our previous studies 11 suggest that the different isoforms of TGF-β may also play an important role in the regulation of mucosal repair and remodeling.
A number of recent studies suggest that MMPs are the most important group of proteolytic enzymes responsible for the breakdown of extracellular matrix in inflammatory bowel disease. 20-25 Studies of mRNA transcripts by competitive PCR suggest that MMP-1 and MMP-3 are the predominant MMPs in mucosal samples of patients with inflammatory bowel disease, 23,24 but these studies have not consistently found significant differences between ulcerative colitis and Crohn’s disease in MMP expression in mucosal samples. Recent in vitro studies have demonstrated increased expression of MMPs and down-regulation of TIMP expression in an ex vivo model in which T cells in explants of second trimester human fetal intestine were activated with pokeweed mitogen or anti-CD3 plus interleukin 12. 25 This model of acute injury, in which extracellular matrix degradation and ulceration predominate, may not reflect changes that occur in the chronically inflamed intestinal mucosa in ulcerative colitis and Crohn’s disease. We believe that our studies using myofibroblasts isolated from diseased tissue reflect the dysregulation of chronic intestinal mucosal repair and regeneration that leads to fibrosis and extracellular matrix deposition in Crohn’s disease, in contrast to ulcerative colitis.
MMPs and TIMPs are expressed by other cells within the intestinal lamina propria including macrophages and epithelial cells. 25 Undoubtedly, MMP and TIMP secretion from these cells is likely to influence tissue remodeling, however, further studies are required to determine the relative contribution of the nonmyofibroblast cell types to this process in inflammatory bowel disease. Previous studies have also demonstrated an important contribution of smooth muscle cells in stricture formation in Crohn’s disease 26 via proliferation and laying down extracellular matrix.
In conclusion, we have shown that myofibroblasts derived from normal and inflammatory bowel disease tissue express MMP-1 and MMP-3 (in their inactive forms) and MMP-2 in both active and latent forms. No MMP-9 expression was observed. Because TIMP-1 is capable of inhibiting all MMPs, we postulate that its enhanced expression by myofibroblasts in Crohn’s disease is a major determinant in the development of strictures in Crohn’s disease but not ulcerative colitis.
Footnotes
Address reprint requests Dr. Brian C. McKaig, Consultant Gastroenterologist, Royal Wolverhampton Hospitals NHS Trust, New Cross Hospital, Wolverhampton WV10 0QP, UK. E-mail: dr.mckaig@rwh-tr.nhs.uk.
Supported by a Programme Grant from the Medical Research Council (UK) and a project grant from the National Association for Colitis and Crohn’s Disease.
References
- 1.McAlindon ME, Gray T, Galvin A, Sewell HF, Podolsky DK, Mahida YR: Differential lamina propria cell migration via basement membrane pores of inflammatory bowel disease mucosa. Gastroenterology 1998, 115:841-848 [DOI] [PubMed] [Google Scholar]
- 2.Graham MF: Pathogenesis of intestinal strictures in CD—an update. Inflamm Bowel Dis 1995, 1:220-227 [PubMed] [Google Scholar]
- 3.Nagase H, Woessner JF, Jr: Matrix metalloproteinases. J Biol Chem 1999, 274:21491-21494 [DOI] [PubMed] [Google Scholar]
- 4.Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP: Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol 1997, 74:111-122 [PubMed] [Google Scholar]
- 5.Brew K, Dinakarpandian D, Nagase H: Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta 2000, 1477:267-283 [DOI] [PubMed] [Google Scholar]
- 6.Border WA, Noble NA: Transforming growth factor beta in tissue fibrosis. N Engl J Med 1994, 331:1286-1292 [DOI] [PubMed] [Google Scholar]
- 7.Overall CM, Wrana JL, Sodek J: Transcriptional and post-transcriptional regulation of 72-kDa gelatinase/type IV collagenase by transforming growth factor-beta 1 in human fibroblasts. Comparisons with collagenase and tissue inhibitor of matrix metalloproteinase gene expression. J Biol Chem 1991, 266:14064-14071 [PubMed] [Google Scholar]
- 8.Mahida YR, Beltinger J, Makh S, Goke M, Gray T, Podolsky DK, Hawkey CJ: Adult human colonic subepithelial myofibroblasts express extracellular matrix proteins and cyclooxygenase-1 and -2. Am J Physiol 1997, 273:G1341-G1348 [DOI] [PubMed] [Google Scholar]
- 9.Pender SL, Tickle SP, Docherty AJ, Howie D, Wathen NC, MacDonald TT: A major role for matrix metalloproteinases in T cell injury in the gut. J Immunol 1997, 158:1582-1590 [PubMed] [Google Scholar]
- 10.McKaig BC, Makh SS, Hawkey CJ, Podolsky DK, Mahida YR: Normal human colonic subepithelial myofibroblasts enhance epithelial migration (restitution) via TGF-beta3. Am J Physiol 1999, 276:G1087-G1093 [DOI] [PubMed] [Google Scholar]
- 11.McKaig BC, Hughes K, Tighe PJ, Mahida YR: Differential expression of TGF-isoforms by normal and inflammatory bowel disease intestinal myofibroblasts. Am J Physiol 2002, 282:C172-C182 [DOI] [PubMed] [Google Scholar]
- 12.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB: Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol 1999, 277:C183-C201 [DOI] [PubMed] [Google Scholar]
- 13.Moore R, Carlson S, Madara JL: Villus contraction aids repair of intestinal epithelium after injury. Am J Physiol 1989, 257:G274-G283 [DOI] [PubMed] [Google Scholar]
- 14.van Tol EA, Holt L, Li FL, Kong FM, Rippe R, Yamauchi M, Pucilowska J, Lund PK, Sartor RB: Bacterial cell wall polymers promote intestinal fibrosis by direct stimulation of myofibroblasts. Am J Physiol 1999, 277:G245-G255 [DOI] [PubMed] [Google Scholar]
- 15.Plateroti M, Rubin DC, Duluc I, Singh R, Foltzer-Jourdainne C, Freund J, Kedinger M: Subepithelial fibroblast cell lines from different levels of gut axis display regional characteristics. Am J Physiol 1998, 274:G945-G954 [DOI] [PubMed] [Google Scholar]
- 16.Ichiki Y, Smith E, LeRoy EC, Trojanowska M: Different effects of basic fibroblast growth factor and transforming growth factor-beta on the two platelet-derived growth factor receptors’ expression in scleroderma and healthy human dermal fibroblasts. J Invest Dermatol 1995, 104:124-127 [DOI] [PubMed] [Google Scholar]
- 17.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saadi JI, West AB: Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol 1999, 277:C1-C19 [DOI] [PubMed] [Google Scholar]
- 18.Shah M, Foreman DM, Ferguson MW: Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci 1995, 108:985-1002 [DOI] [PubMed] [Google Scholar]
- 19.Shah M, Foreman DM, Ferguson MW: Neutralising antibody to TGF-beta 1, 2 reduces cutaneous scarring in adult rodents. J Cell Sci 1994, 107:1137-1157 [DOI] [PubMed] [Google Scholar]
- 20.Bailey CJ, Hembry RM, Alexander A, Irving MH, Grant ME, Shuttleworth CA: Distribution of the matrix metalloproteinases stromelysin, gelatinases A and B, and collagenase in Crohn’s disease and normal intestine. J Clin Pathol 1994, 47:113-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaalamo M, Karjalainen-Lindsberg ML, Puolakkainen P, Kere J, Saarialho-Kere U: Distinct expression profiles of stromelysin-2 (MMP-10), collagenase-3 (MMP-13), macrophage metalloelastase (MMP-12), and tissue inhibitor of metalloproteinases-3 (TIMP-3) in intestinal ulcerations. Am J Pathol 1998, 152:1005-1014 [PMC free article] [PubMed] [Google Scholar]
- 22.Baugh MD, Perry MJ, Hollander AP, Davies DR, Cross SS, Lobo AJ, Taylor CJ, Evans GS: Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology 1999, 117:814-822 [DOI] [PubMed] [Google Scholar]
- 23.von Lampe B, Barthel B, Coupland SE, Riecken EO, Rosewicz S: Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut 2000, 47:63-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heuschkel RB, MacDonald TT, Monteleone G, Bajaj-Elliott M, Smith JA, Pender SL: Imbalance of stromelysin-1 and TIMP-1 in the mucosal lesions of children with inflammatory bowel disease. Gut 2000, 47:57-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salmela MT, MacDonald TT, Black D, Irvine B, Zhuma T, Saarialho-Kere U, Pender SL: Upregulation of matrix metalloproteinases in a model of T cell mediated tissue injury in the gut: analysis by gene array and in situ hybridisation. Gut 2002, 51:540-547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graham MF: Pathogenesis of intestinal strictures in Crohn’s disease—an update. Inflamm Bowel Dis 1995, 1:220-227 [PubMed] [Google Scholar]