Abstract
t(11;18)(q21;q21) is associated with mucosa-associated lymphoid tissue (MALT)-type lymphoma and results in API2-MALT1 fusion. However, its clinicopathologic significance remains unclarified. API2-MALT1 fusion is detected most frequently in MALT lymphomas primarily involving the lung. We therefore screened 51 cases of pulmonary MALT lymphoma for API2-MALT1 fusion, and studied its relationship with clinicopathologic factors including the immunohistochemical expression of BCL10, another MALT lymphoma-associated molecule. The API2-MALT1 fusion transcript was detected in 21 of 51 (41%) cases, and was correlated with the absence of any underlying autoimmune disease, and with a normal serum lactate dehydrogenase, a “typical” histology without marked plasmacytic differentiation or an increased number of large cells, and aberrant nuclear BCL10 expression. However, its prognostic impact was not identified in the limited follow-up (6 to 187 months, median 27). These data suggest that the API2-MALT1 fusion may be a causative gene abnormality unrelated to autoimmune disease. In addition, this alteration may define a homogeneous MALT lymphoma subtype that is clinically more indolent and histologically more “typical.” Aberrant nuclear BCL10 expression may have a possible role as a tool to screen for this API2-MALT1 fusion. A large-scale study with a long follow-up is necessary to establish the prognostic significance of API2-MALT1 fusion.
Extranodal marginal zone B-cell lymphoma (MZBL) of mucosa-associated lymphoid tissue (MALT) is listed as a distinct clinicopathologic entity by the World Health Organization (WHO) Classification of Tumors of the Hematopoietic and Lymphoid Tissues. 1 Since first described, the clinical, histological, immunophenotypic, and genetic features of this lymphoma have been highlighted by many authors. In general, MALT lymphomas run an indolent clinical course, preferably involve extranodal sites, and exhibit varied histological spectra consisting of lymphoepithelial lesions, follicular colonization, and prototypically centrocyte-like (CCL) cells. 1 Interestingly, a pre-existing chronic inflammation, eg, Helicobacter pylori (H. pylori) gastritis, Hashimoto’s thyroiditis, or Sjogren’s syndrome, may make an important contribution to its development. 2-4 However, MALT lymphomas are found in some patients with no evidence of such precursor lesions or conditions.
The chromosome translocation t(11;18)(q21;q21) has been identified as a recurring cytogenetic abnormality of MALT lymphomas. 5 Our group as well as others revealed that API2 at 11q21 and a novel gene, MALT1 at 18q21, are fused as a result of this translocation. 6,7 Subsequently, API2-MALT1 fusion has been demonstrated exclusively in MALT lymphomas with varying frequency depending on the anatomical site. 9,12,13 On the other hand, its involvement was rare in nodal and splenic MZBLs and in diffuse large B-cell lymphomas. 8-13 API2is a member of the IAP (inhibitor of apoptosis) genefamily, 14,15 and is essential for the suppression of apoptosis. 16 MALT1 is identical to a novel caspase-like protein termed paracaspase, 17 although its biological function remains unclear. Our previous data suggest that API2-MALT1 chimeric transcripts may lead to an increased inhibition of apoptosis and thereby help MALT lymphomas to survive. 10 BCL10 was cloned from another translocation of t(1;14)(p22;q32) in MALT lymphomas; through this rare translocation, the entire BCL10 is juxtaposed to the IgH enhancer region, and activates nuclear factor kappa B (NF-κB), a transcription factor for several survival-related genes, via interaction with MALT1 protein. 18-20 Moreover, the API2-MALT1 fusion protein also strongly activates NF-κB through a common signaling pathway. 20 Recently, several researchers have shown that these two genetic alterations, t(1;14) and t(11;18), which both result in NK-κB activation, are associated with an aberrant nuclear expression of BCL10 protein on paraffin section immunohistochemistry of gastric MALT lymphoma. 21-23
The clinicopathologic and biological significance of API2-MALT1 fusion has not fully been appreciated. In the stomach, approximately 60–80% of MALT lymphomas eventually regress by H. pylori eradication therapy alone. 24-27 However, it has been indicated that the API2-MALT1 fusion transcript serves as a molecular marker for those not responding to H. pylori eradication. 28-31 Nakamura et al recently demonstrated that gastric MALT lymphomas that were negative for H. pylori, as determined from serological and culture findings, possessed API2-MALT1 fusion, suggesting that this genetic alteration has pathogenetic significance independent of precursor lesions. 32
Interestingly, MALT lymphomas primarily involving the lung have a higher frequency of this fusion gene than those located anywhere else. 8-12 In this study, we examined 51 cases of pulmonary MALT lymphoma from the viewpoint of API2-MALT1 fusion, which is now detectable by a multiplex reverse transcription (RT)-polymerase chain reaction (PCR) using formalin-fixed, paraffin-embedded sections, 10 to verify this finding in a large cohort and to further clarify the clinicopathologic significance of this genetic abnormality. We also investigated the possible relationship between the aberrant expression of BCL10 protein and the detection of the API2-MALT1 fusion transcript.
Materials and Methods
Case Selection
From the pathology files of Nagoya City University Medical School, Aichi Cancer Center Hospital, Fukuoka University Medical School, Okayama University Medical School, and other collaborating institutions, 84 cases of MALT lymphoma involving the lung were retrieved. Specimens were obtained at the initial presentation of the patients, fixed in formalin, and embedded in paraffin. All cases were carefully reviewed by three independent pathologists (H. I., T. E., and S. N.), and the diagnosis of MALT lymphoma was made according to the criteria of the WHO Classification for Tumors of Hematopoietic and Lymphoid Tissues. 1 The immunophenotype of the tumor cells was CD20+, CD79a+, cyclin D1-, CD5-, CD10-, CD3-, CD45RO-, CD23-, and CD56-. Monoclonality was examined by molecular and/or immunophenotypic techniques. Briefly, DNA extracted from paraffin sections was amplified by PCR with primers (FR2A, FR3A, LJH, and VLJH) to detect rearrangement occurring between framework regions and the joining region of the immunoglobulin heavy chain gene. 33,34 Amplification of a β-globin DNA fragment (268 bp) was used to indicate satisfactory preservation of DNA in the sample. Monotypic immunoglobulin light chain was detected by immunohistochemistry for κ and λ light chains. In addition to verifying the tumor monoclonality, we carried out a preliminary examination of the extent of RNA preservation for detection of the API2-MALT1 fusion transcript in all cases by means of RT-PCR amplification of β-actin mRNA. 10 Excluded were those cases whose monoclonality was not established by either molecular or immunohistochemical means and those where the RNA was not of satisfactory quality. Finally, 51 cases of the present series were selected for this study.
Clinical Data
The following clinical factors were analyzed: age, sex, chief complaints, autoimmune disease, lactate dehydrogenase (LDH), paraproteinemia, radiographical tumor distribution, regional lymph node involvement, clinical stage, B-symptoms, treatment, and follow–up. Staging of the disease was performed according to the Ann Arbor Classification System for Extranodal Lymphomas 35 by examining the clinical status, chest X-ray, CT scan of the mediastinum and the abdomen, 67Ga scintigraphy, and a bone marrow aspiration.
Multiplex RT-PCR for the API2-MALT1 Fusion Transcript
The API2-MALT1 fusion transcript was detected using archival paraffin sections according to the method we recently reported. 10 All eight variant fusion transcripts that have been reported can be detected with this assay (Figure 1 ▶ , accession number L49432 for API2 and accession number AF130356 for MALT1). Briefly, total RNA was extracted from the paraffin sections by proteinase K (Roche Diagnostics, Mannheim, Germany) digestion. RNA was subjected to first-round multiplex one-tube RT-PCR, then to second-round nested multiplex PCRs (three parallel; Second PCR-A, Second PCR-B, and Second PCR-C). The final PCR products were run on 8% polyacrylamide gels and stained with ethidium bromide. The band size ranged from 80 to 179 bp. RNA samples known to possess API2-MALT1 fusion were used as positive controls. As an internal control for RNA quality, the ubiquitously expressed β-actin mRNA fragment (190 bp) was amplified. In all cases positive for API2-MALT1 fusion transcripts, the breakpoints were confirmed by direct sequencing. In short, fragments obtained in the second round PCR were separated and purified. They were then directly sequenced by means of cycle sequencing with dye-labeled terminators (BigDye Terminators, Applied Biosystems, Foster City, CA) and analyzed on a DNA sequencer (Model 310, Applied Biosystems). API2 primers required for the second round PCR were used as sequencing primers.
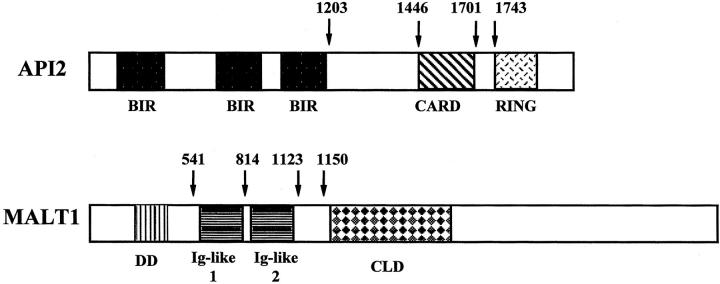
Figure 1.
API2 and MAL1 gene structure and locations of known breakpoints. Numbering is accordance with accession number L49432 for API2 and accession number AF130356 for MALT1. BIR, baculovirus IAP repeat; IAP, inhibitor of apoptosis; CARD, caspase recruit domain; RING, RING finger domain; DD, death domain; Ig, immunoglobulin; CLD, caspase-like domain.
Efficiency of Multiplex RT-PCR for Detection of the API2-MALT1 Fusion Transcript from Paraffin-Embedded Tissues
Both freshly frozen samples and formalin-fixed, paraffin-embedded samples were obtained from 23 MALT lymphomas of various anatomical sites (pulmonary origin, 13; gastric, 9; and salivary, 1). These cases were examined by RT-PCR for API2-MALT1 fusion using high quality RNA extracted from frozen lymphoma materials as described previously; 12 16 cases (pulmonary, 8; gastric, 7; and salivary, 1) were positive for the fusion and the remaining seven were negative. RNA extracted from paraffin-embedded tissues of these 23 lymphomas was analyzed for API2-MALT1 fusion using multiplex RT-PCR as described above. When analyzed using paraffin sections as a source of RNA, 15 (pulmonary, 7; gastric, 7; and salivary, 1) of 16 positive controls had the API2-MALT1 fusion transcript (sensitivity, 94%) and all of the seven negative controls did not (specificity 100%), indicating the high efficacy of multiplex RT-PCR assay using paraffin tissues. Direct sequencing confirmed identical breakpoints of API2-MALT1 fusion products between frozen and paraffin materials.
Immunohistochemistry for BCL10
Tissue sections cut at a thickness of three micrometers were deparaffinized and rehydrated. After antigen retrieval by heat treatment, endogenous peroxidase activity was blocked by 3% H2O2. Immunohistochemistry was performed by an automated immunostainer (OptiMax Plus, BioGenex, San Ramon, CA) using monoclonal antibody against BCL10 (clone 151, 22 Zymed, South San Francisco, CA). MALT lymphomas harboring BCL10 gene rearrangement, as detected by Southern blot analysis, were used as positive controls, and normal lymph nodes and spleens, as normal controls. When most lymphoma cells of a patient showed nuclear BCL10 expression, the case was considered positive.
Statistical Analysis
Statistical evaluation of data from two groups was performed using the Fischer’s exact test, Mann-Whitney U-test, and Student’s t-test with the statistical package StatView (Abacus Concepts, Berkley, CA). All analyses were two-tailed. A value of P < 0.05 for each test was regarded as statistically significant, and 0.05 < P < 0.1 as marginally significant.
Results
API2-MALT1 Fusion Transcript in Pulmonary MALT Lymphomas
Among the 51 pulmonary MALT lymphoma cases with a sufficient quality of RNA, 21 (41%) were positive for the API2-MALT1 fusion transcript with RT-PCR products varying in size depending on the breakpoints and primer sets used (Figure 2) ▶ . The fusion types were estimated with 8% polyacrylamide electrophoresis and confirmed by direct sequencing. All but one case (20 of 21 cases, 95%) had an API2 breakpoint at 1446, and the one exception had a breakpoint at 1701 (accession number L49432). MALT1 breakpoints (accession number AF130356) varied as follows: seven cases (33%) at 814, 10 cases (48%) at 1123, and four cases (19%) at 1150 (Figure 1) ▶ . All fusion transcripts were fused in-frame, and none of the positive cases showed an atypical transcript such as an insertion or deletion. All API2-MALT1 fusion variants were in keeping with those that have been reported previously. 10
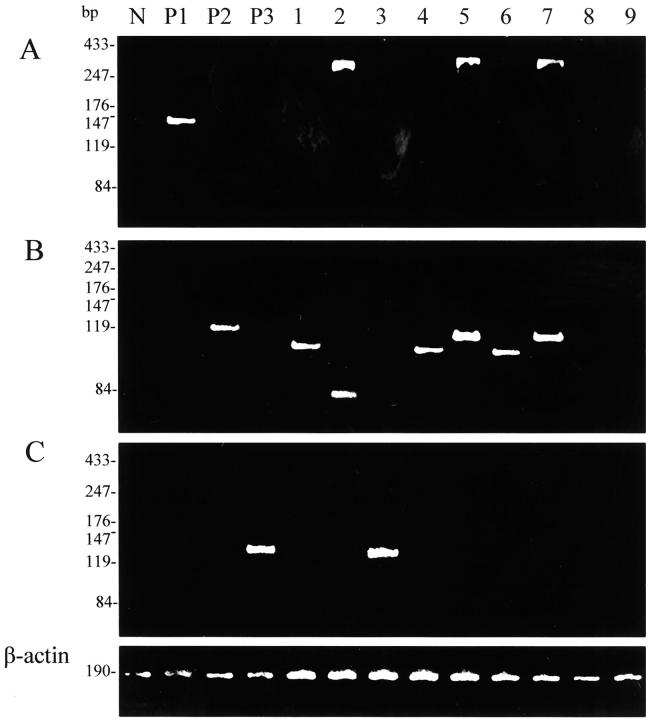
Figure 2.
Detection of API2-MALT1 fusion transcript by multiplex RT-PCR. A: Second PCR-A, B: Second PCR-B, C: Second PCR-C; lane N, negative control (normal lymph node); P1, P2, P3, positive controls for Second PCR-A, -B, and -C, respectively; lanes 1–7, pulmonary MALT lymphomas positive for API2-MALT1 fusion transcript; lanes 8 and 9, pulmonary MALT lymphomas negative for the fusion transcript. Note that the fusion transcript is detected in both Second PCR-A and Second PCR-B (lanes 2, 5, and 7). β-actin mRNA is amplified in all cases.
Clinical Features, Stage, Treatment, and Survival
There were 22 males and 29 females with ages ranging from 31 to 81 years (mean, 62 years and median, 64 years). Radiological detection of a pulmonary lesion in an asymptomatic patient was the most common route of discovery (38 of 51 cases). The remaining 13 patients presented with chest-related symptoms such as cough, chest pain, fever, and hemoptysis. Six of the 51 patients had an underlying autoimmune disorder: three with Sjogren’s syndrome, two with rheumatoid arthritis, and one with progressive systemic sclerosis. An elevated serum LDH was observed in 5 of 51 patients and paraproteinemia in 11 of 30. Multiple pulmonary lesions were recognized in 16 of 51 patients, both lungs were involved in 7 of 51, and regional lymph node involvement was detected in 11 of 51.
Twenty-nine patients had Stage I disease, four had Stage II, and 18 had Stage IV. Five patients showed B-symptoms including fever, weight loss, and night sweats. In the 29 patients with Stage I disease, 21 were treated by surgery alone, four by surgery with additional chemotherapy, two by doxorubicin-based multi-agent chemotherapy, and the remaining two were observed without treatment. These 27 treated patients achieved complete remission. In the 22 patients whose disease had progressed past Stage I, 11 were treated by doxorubicin-based multi-agent chemotherapy alone, three by chemoradiotherapy, two by chemoradiotherapy after surgical burden reduction, and the remaining six were observed without treatment. Among the 16 treated patients with a disease stage > Stage I, complete remission was obtained in seven, partial response in eight, and no change in one. Patients with pulmonary MALT lymphomas including those who were not treated showed a favorable clinical course with no tumor-related death in the median follow-up of 27 months. Tumor recurrence occurred in two of 34 complete remission cases 29 and 60 months after the treatment, respectively. Five patients died of other causes, and the other 46 were alive with (n = 17) or without (n = 29) disease at the last follow-up.
Histological Features of Pulmonary MALT Lymphoma
MALT lymphomas showed a characteristic diffuse and/or marginal zone-based infiltration of small lymphoid cells with a lymphangitic pattern along bronchovascular bundles, interlobular septa, and visceral pleura (Figure 3, A and B) ▶ . The tumor cells were composed of a spectrum of cell types, which included CCL cells, small lymphocytes, plasma cells, and occasional large transformed cells. The most prominent population was CCL cells forming broad pale-stained bands and sheets (Figure 3C) ▶ . The tumor cells had frequently infiltrated the epithelial lining and glands, forming lymphoepithelial lesions (Figure 3D) ▶ . An increased number of germinal centers, and a foreign body-type giant cell reaction, probably due to destruction of the epithelium by lymphoma cells, were occasionally observed in 12 of 51 and 9 of 51 cases, respectively. All of the cases enrolled in the present study were within the morphological boundaries of MALT lymphoma. A precise microscopic observation revealed that 39 of 51 cases were morphologically “typical” showing characteristic histology for MALT lymphoma as described above. However, seven cases showed striking plasmacytic differentiation with sheets of plasma cells (Figure 3E) ▶ , 1,36 and five cases exhibited an increased number of large cells without solid or sheet-like proliferation of large cells (Figure 3F) ▶ . 1 In the present study, these 12 cases were categorized morphologically as “atypical” MALT lymphomas.
Figure 3.
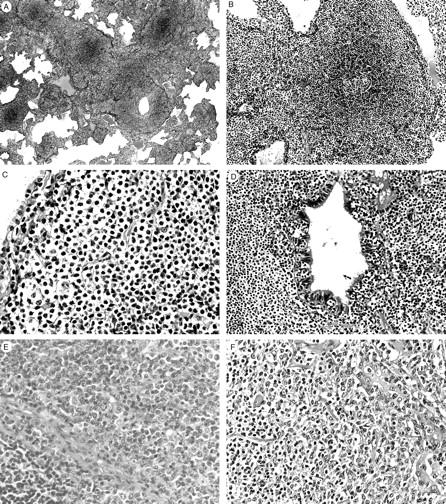
Pulmonary MALT lymphomas showing “typical” (A–D) or “atypical” (E and F) histology. A: The neoplastic lymphocytic infiltrate extends into the pulmonary parenchyma along bronchovascular septa. B: A lymphoid follicle with intact mantle zone is surrounded by a diffuse small lymphoid infiltrate. C: Tumor cells show indented or folded nuclei and a moderate amount of pale cytoplasm. D: Tumor cells infiltrate in the bronchial mucosa forming a lymphoepithelial lesion. E: A pulmonary MALT lymphoma with “atypical” histology showing marked plasmacytic differentiation. A non-neoplastic lymphoid follicle is seen in the left lower corner. F: A MALT lymphoma with “atypical” histology showing an increased number of large cells involving the bronchial glands (right). H&E: original magnifications; ×8 (A), ×33 (B), ×50 (C), ×100 (D), ×100 (E), ×66 (F).
Aberrant Nuclear BCL10 Expression
Immunohistochemistry for BCL10 expression in normal spleen and lymph node tissue sections showed that the molecule was expressed in the cytoplasm of follicle center cells and marginal zone cells whereas it was absent or weakly expressed in the small lymphocytes of the mantle zone. No nuclear staining was observed in any of the normal tissue sections. Aberrant BCL10 was expressed in the nuclei of the tumor cells in 24 of 51 pulmonary MALT lymphoma cases (Figure 4) ▶ . In these 24 positive cases, strong nuclear staining was found in four cases, two of which were positive for API2-MALT1 fusion.
Figure 4.
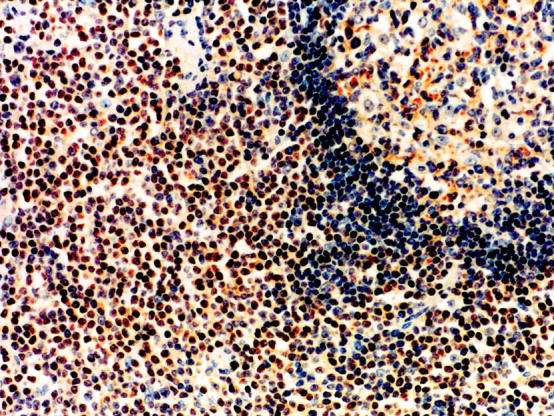
Immunohistochemical study for BCL10 expression. Lymphoma cells surrounding a reactive lymphoid follicle show aberrant nuclear BCL10 expression. BCL10 expression is displayed in the cytoplasm of follicular center cells (right upper corner) while the expression is not detectable in small lymphocytes in the mantle zone. Hematoxylin counterstain; original magnification, ×80.
Impact of API2-MALT1 Fusion on Clinicopathologic Characteristics of the Patients with Pulmonary MALT Lymphomas
Table 1 ▶ shows correlation of the API2-MALT1 fusion transcript with clinicopathologic factors in pulmonary MALT lymphoma cases. Clinically, all of the six patients with autoimmune disease were found in the API2-MALT1-negative group, which contrasted significantly with the absence of such patients in the positive group (P = 0.036). An elevated serum LDH level was found more frequently in the fusion-negative cases with marginal significance (P = 0.069). No differences were observed which were related to the following factors: age, sex, chief complaints, paraproteinemia, bilateral lung involvement, multiple tumors, nodal involvement, clinical stage, and B-symptoms. The prognostic impact of API2-MALT1 fusion on survival was not identified in our series in which the patients pursued an excellent clinical course with tumor recurrence in two cases and tumor-related death in none.
Table 1.
Clinicopathologic Characteristics of 51 Patients with Pulmonary MALT Lymphoma Positive or Negative for the API2-MALT1 Fusion Transcript
| Variables | API2-MALT1 fusion transcripts | |||
|---|---|---|---|---|
| Positive (n = 21) | Negative (n = 30) | P | ||
| Clinical findings | ||||
| Age (yr) | Mean | 63.2 | 61.3 | N.S. |
| Median | 65 | 61 | ||
| >60 | 15 | 18 | N.S. | |
| <60 | 6 | 12 | N.S. | |
| Sex | Male | 9 | 13 | |
| Female | 12 | 17 | N.S. | |
| Chief complaints | Present | 4 | 9 | |
| Absent | 17 | 21 | N.S. | |
| Autoimmune disease† | Positive | 0 | 6 | |
| Negative | 21 | 24 | 0.036 | |
| Serum LDH† | Elevated | 0 | 5 | |
| Normal | 21 | 25 | 0.069 | |
| Paraproteinemia (n = 30) | Positive | 4 | 7 | |
| Negative | 6 | 13 | N.S. | |
| Radiographic findings | Single | 16 | 19 | |
| Multiple | 5 | 11 | N.S. | |
| Unilateral | 18 | 26 | ||
| Bilateral | 3 | 4 | N.S. | |
| Regional node involvement | Yes | 4 | 7 | |
| No | 17 | 23 | N.S. | |
| Stage | I, II | 16 | 17 | |
| III, IV | 5 | 13 | N.S. | |
| B symptoms | Present | 2 | 3 | |
| Absent | 19 | 27 | N.S. | |
| Histologic and immunohistochemical findings | ||||
| Histologic subgroup† | “Typical” | 20 | 19 | |
| “Atypical”* | 1 | 11 | 0.0088 | |
| Marked plasma cell differentiation | Yes | 1 | 6 | |
| No | 20 | 24 | N.S. | |
| Increased number of large cells | Yes | 0 | 5 | |
| No | 21 | 25 | N.S. | |
| Increased number of lymphoid follicles | Yes | 5 | 7 | |
| No | 16 | 23 | N.S. | |
| Granulomas | Yes | 6 | 3 | |
| No | 15 | 27 | N.S. | |
| Nuclear BCL-10 expression† | Positive | 17 | 7 | |
| Negative | 4 | 23 | <0.0001 | |
*, “Atypical” histology includes marked plasma cell differentiation and an increased number of large cells; †, among the four variables correlated with API2-MALT1 fusion (autoimmune disease, serum LDH, histologic subgroup, and nuclear BCL10 expression) a significant correlation was found between autoimmune disease and the histologic subgroup (P = 0.0018).
N.S., not significant.
A “typical” histology was regarded as one of the features of pulmonary MALT lymphomas possessing API2-MALT1 fusion (P = 0.0088); while the fusion was detected in 20 of 39 cases with “typical” histology, it was found in only one of 12 cases with “atypical” histology showing either marked plasmacytic differentiation or an increased number of large cells (Table 2) ▶ . This gene abnormality was not associated with increased number of germinal centers or presence of granulomas. Aberrant nuclear BCL10 expression, which was detected immunohistochemically in 24 of 51 cases, was highly correlated with detection of API2-MALT1 fusion (P < 0.0001): 17 of 21 fusion-positive cases exhibited nuclear BCL10 expression whereas only 7 of 30 fusion-negative cases did. When nuclear BCL10 was considered as a surrogate marker for API2-MALT1 fusion, the sensitivity and specificity were calculated to be 81% and 77%, respectively. The impact of nuclear BCL10 expression on clinicopathologic factors was not evident: in contrast to API2-MALT1 fusion, nuclear BCL10 expression was associated with none of the clinicopathologic factors examined, except with API2-MALT1 fusion.
Table 2.
API2-MALT1 Fusion, Histological Subtype, and Nuclear BCL10 Expression in Pulmonary MALT Lymphomas
| Case | Age (yr) | Sex | API2-MALT1 fusion transcripts | Histologic subgroup | Nuclear BCL10 | |
|---|---|---|---|---|---|---|
| API2* | MALT1† | |||||
| 1 | 36 | M | 1446 | 1150 | T | ++ |
| 2 | 64 | F | 1446 | 1150 | T | ++ |
| 3 | 63 | F | 1446 | 814 | T | + |
| 4 | 68 | F | 1446 | 814 | T | + |
| 5 | 69 | M | 1446 | 814 | T | + |
| 6 | 74 | M | 1446 | 814 | T | + |
| 7 | 76 | M | 1446 | 814 | T | + |
| 8 | 81 | F | 1446 | 814 | T | + |
| 9 | 52 | F | 1446 | 1123 | T | + |
| 10 | 53 | F | 1446 | 1123 | T | + |
| 11 | 64 | M | 1446 | 1123 | T | + |
| 12 | 65 | M | 1446 | 1123 | T | + |
| 13 | 68 | M | 1446 | 1123 | T | + |
| 14 | 69 | F | 1446 | 1123 | T | + |
| 15 | 43 | F | 1446 | 1150 | T | + |
| 16 | 51 | M | 1446 | 1150 | T | + |
| 17 | 66 | F | 1446 | 814 | T | − |
| 18 | 57 | F | 1446 | 1123 | T | − |
| 19 | 68 | F | 1446 | 1123 | T | − |
| 20 | 63 | F | 1701 | 1123 | T | − |
| 21 | 77 | M | 1446 | 1123 | A/P | + |
| 22 | 68 | M | – | – | T | ++ |
| 23 | 74 | F | – | – | T | ++ |
| 24 | 55 | M | – | – | T | + |
| 25 | 73 | M | – | – | T | + |
| 26 | 31 | F | – | – | T | − |
| 27 | 40 | M | – | – | T | − |
| 28 | 47 | M | – | – | T | − |
| 29 | 49 | F | – | – | T | − |
| 30 | 54 | F | – | – | T | − |
| 31 | 57 | F | – | – | T | − |
| 32 | 57 | F | – | – | T | − |
| 33 | 61 | M | – | – | T | − |
| 34 | 61 | M | – | – | T | − |
| 35 | 61 | M | – | – | T | − |
| 36 | 65 | F | – | – | T | − |
| 37 | 67 | M | – | – | T | − |
| 38 | 69 | M | – | – | T | − |
| 39 | 73 | F | – | – | T | − |
| 40 | 77 | F | – | – | T | − |
| 41 | 56 | F | – | – | A/P | + |
| 42 | 67 | F | – | – | A/P | + |
| 43 | 57 | F | – | – | A/P | − |
| 44 | 61 | M | – | – | A/P | − |
| 45 | 66 | F | – | – | A/P | − |
| 46 | 68 | M | – | – | A/P | − |
| 47 | 54 | F | – | – | A/L | + |
| 48 | 56 | M | – | – | A/L | − |
| 49 | 66 | F | – | – | A/L | − |
| 50 | 71 | F | – | – | A/L | − |
| 51 | 78 | F | – | – | A/L | − |
*, API2 breakpoint (accession number L49432); †, MALT1 breakpoint (accession number AF130356); T, “typical” histology; A/P, atypical histology with increased plasma cells; A/L, atypical histology with increased large cells; ++, strongly positive; +, positive; and −, negative.
API2-MALT1 fusion correlated significantly with the absence of autoimmune disease, and with a normal LDH level, “typical” histology, and nuclear BCL10 expression. We further analyzed the statistical relationship between any two of these four factors. A significant correlation was found between “typical” histology and the absence of autoimmune disease only (P = 0.0018), and any other combination did not achieve statistical significance.
Discussion
In the present study, we analyzed 51 cases of pulmonary MALT lymphoma for the clinicopathologic and biological significance of the API2-MALT1 fusion transcript. In all cases, the clonal nature of the infiltrating lymphoid cells was confirmed by a PCR immunoglobulin heavy chain gene rearrangement assay and/or immunohistochemistry for immunoglobulin light chains. The API2-MALT1 fusion transcript was examined with paraffin sections by a multiplex RT-PCR, 10 which is capable of 93% of the sensitivity and 100% of the specificity obtained with RT-PCR using frozen materials. 12 This gene alteration was detected in 41% of pulmonary MALT lymphoma cases. This positive rate was somewhat lower than the 55 to 75% previously described for a short series of pulmonary MALT lymphoma cases, 9-12 but similar to that (41%) of a recent large series. 37 API2-MALT1 fusion-positive cases were significantly associated with the absence of autoimmune disease, and with a normal level of serum LDH, a “typical” tumor histology, and aberrant nuclear BCL10 expression. These data suggest that API2-MALT1 fusion may define a distinctive clinicopathologic subtype of pulmonary MALT lymphoma.
Although primary pulmonary lymphoma is rare, representing 1 to 3.6% of extranodal lymphomas, 70 to 90% of these cases correspond to MALT lymphoma. 38-40 Thieblemont et al 41 analyzed 108 MALT lymphomas at various anatomical sites and reported that 11 cases (10%) originated in the lung, 55 cases in the stomach (51%), and 13 cases in the orbit (12%). These observations indicate that the lung is one of the preferential sites for extranodal MALT lymphoma. The most important question is whether precursor lesions or conditions are present in normal human lung that facilitate the development of MALT lymphoma. Indeed, a high frequency of pre-existing autoimmune disorders or predisposing inflammatory stimuli have been observed in patients with extranodal MALT lymphomas, including Sjogren’s syndrome, Hashimoto’s thyroiditis, and H. pylori gastritis, and a pre-existing chronic inflammation has been established as a critical risk factor for developing malignant lymphoma. 3,42,43 It is worth noting that a low frequency (less than 4%) of API2-MALT1 fusion has been reported for MALT lymphomas of the salivary glands and thyroid, 9,13,37 where autoimmune disease plays an important role in their development. In addition, we recently reported the absence of API2-MALT1 fusion in thymic MALT lymphoma that is closely associated with autoimmune disease. 34 A few groups including ours have indicated that API2-MALT1-positive gastric MALT lymphomas do not respond to H. pylori eradication. 28-31 Nakamura et al 32 also recently found that all MALT lymphomas negative for H. pylori by both culture and serology possessed the API2-MALT1 fusion. These findings suggest that this genetic alteration may arise independently of autoimmune diseases and H. pylori infection. The present study clearly provided additional evidence for this assertion because none of our six patients with an autoimmune disease that pre-dated the diagnosis of lymphoma had the API2-MALT1 fusion transcript. In this line, at least in a subset of gastric MALT lymphomas possessing API2-MALT1 fusion, H. pylori may not play an important role for their development.
Another important finding of this study is that API2-MALT1 fusion-positive tumors may be associated with a clinically more indolent behavior pattern and a more morphologically “typical” histology. The serum LDH level was normal in all fusion-positive cases, and monotonous proliferation of CCL cells without marked plasmacytic differentiation or an increased number of large cells was a feature of fusion-positive tumors. It should be noted that the API2-MALT1 fusion has not been demonstrated in thyroid MALT lymphomas or in immunoproliferative small intestinal diseases 37 where plasmacytic differentiation is a constant and often striking feature. 1 In addition, this gene alteration is rare in extranodal diffuse large B-cell lymphomas of various sites 8,9,10,12,13 and gastric MALT lymphomas with a large cell component. 10,13 Recently, some groups reported that there may be two distinct pathways in the development of MALT lymphoma; Starostik et al 44 showed that while fusion-positive cases only rarely displayed secondary genetic aberrations, fusion-negative cases showed numerous allelic imbalances. Remstein et al 45 also reported that fusion-negative cases showed a much higher frequency of aneuploidy including trisomy of chromosomes 18 and 3, and are considered to have more chance of high-grade transformation. Highly stable genetic status of API2-MALT1 fusion-positive MALT lymphomas is in line with our finding. Although two recurrent cases were categorized in fusion-negative group, the presence or absence of API2-MALT1 fusion in pulmonary MALT lymphomas did not affect the clinical course in the short period of follow-up (range, 6 to 187 months and median, 27 months) during which no tumor-related deaths were recorded. Pulmonary MALT lymphoma is generally considered as an indolent tumor. 39,46 The overall survival rate was reported to be 100% at 3 years but to gradually decrease to approximately 60% by 10 years. 46 A longer follow-up is needed to clarify the prognostic significance of API2-MALT1 fusion in pulmonary MALT lymphoma.
In our series, aberrant nuclear expression of BCL10 protein was immunohistochemically detected in 24 of 51 cases, and was highly correlated with the presence of API2-MALT1 fusion (P < 0.0001). This finding supports data recently reported for gastric MALT lymphomas. 22,23 Detection of nuclear BCL10 expression seems to provide a useful means of estimating the API2-MALT1 fusion status in pulmonary as well as gastric MALT lymphomas. However, nuclear BCL10 expression should be carefully interpreted because its sensitivity (81%) and specificity (77%) do not seem sufficiently high as a surrogate marker, and there has been no evidence reported to date supporting the direct interaction between BCL10 and API2-MALT1 fusion proteins. 23 Our recent observation suggests that nuclear BCL10 expression may often be detected in certain MALT lymphomas in the absence of API2-MALT1 fusion. 47 Strong nuclear BCL10 expression may be associated with t(1;14)(p22;q32) involving BCL10 translocation, 22 and was detected in this series in 4 of 24 cases positive for nuclear BCL10 expression. Sufficient frozen tumor materials were not available to test whether these four cases harbored t(1;14)(p22;q32). Interestingly, API2-MALT1 fusion was detected in two of four cases showing strong nuclear BCL10 expression.
There are some clinicopathologic similarities between pulmonary and gastric MALT lymphomas with respect to the API2-MALT1 fusion: fusion-positive tumors possibly independent of underlying chronic inflammation, ie, autoimmune disease or H. pylori gastritis; 28-32 frequent detection of the fusion in cases showing “typical” histology without increased large cell component; 10,13 and a strong correlation between API2-MALT1 fusion and nuclear BCL10 expression. 22,23 Further study is needed to clarify whether MALT lymphomas positive for API2-MALT1 fusion constitutes a distinct clinicopathologic entity.
In conclusion, we have provided evidence that the API2-MALT1 fusion may be a causative genetic factor independent of autoimmune disease and may define a distinct subtype of pulmonary MALT lymphoma that is clinically more indolent and histologically more “typical.” Aberrant nuclear BCL10 expression was highly correlated with API2-MALT1 fusion, suggesting that it may have a possible role as a screening tool for this gene fusion in pulmonary MALT lymphomas. In our short follow-up, we failed to identify the impact of API2-MALT1 fusion on patient survival. To establish its prognostic significance, a large-scale study with a long follow-up is needed.
Acknowledgments
We thank Dr. K. Ono, Tosei Hospital, Seto, and Dr. S. Ichihara, National Nagoya Hospital, Nagoya, for providing lymphoma samples, and Mr. S. Nagaya, Mr. H. Takino, and Ms. C. Ando for their excellent technical assistance.
Footnotes
Address reprint requests to Hiroshi Inagaki, M.D., Department of Pathology, Nagoya City University Medical School, Kawasumi, Mizuho-ku, Nagoya 467-0841, Japan. E-mail: hinagaki@med.nagoya-cu.ac.jp.
Supported in part by a Grant-in-Aid from the Ministry of Education, Science, Sports, and Culture of Japan (Grant number 13670185) for H. Inagaki.
M. O. and H. I. contributed equally to this work.
References
- 1. Jaffe E Harris NL Stein H Vardiman JW eds. Tumours of Haematopoietic and Lymphoid Tissues. World Health Organization Classification of Tumors, Pathology, and Genetics. 2001. IARC Press Lyon
- 2.Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG: Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 1991, 338:1175-1176 [DOI] [PubMed] [Google Scholar]
- 3.Royer B, Cazals-Hatem D, Sibilia J, Agbalika F, Cayuela JM, Soussi T, Maloisel F, Clauvel JP, Brouet JC, Mariette X: Lymphomas in patients with Sjogren’s syndrome are marginal zone B-cell neoplasms, arise in diverse extranodal and nodal sites, and are not associated with viruses. Blood 1997, 90:766-775 [PubMed] [Google Scholar]
- 4.Isaacson PG: Mucosa-associated lymphoid tissue lymphoma. Semin Hematol 1999, 36:139-147 [PubMed] [Google Scholar]
- 5.Ott G, Katzenberger T, Greiner A, Kalla J, Rosenwald A, Heinrich U, Ott MM, Muller-Hermelink HK: The t(11;18)(q21;q21) chromosome translocation is a frequent and specific aberration in low-grade but not high-grade malignant non-Hodgkin’s lymphomas of the mucosa-associated lymphoid tissue (MALT-) type. Cancer Res 1997, 57:3944-3948 [PubMed] [Google Scholar]
- 6.Dierlamm J, Baens M, Wlodarska I, Stefanova-Ouzounova M, Hernandez JM, Hossfeld DK, De Wolf-Peeters C, Hagemeijer A, Van den Berghe H, Marynen P: The apoptosis inhibitor gene API2 and a novel 18q gene, MLT, are recurrently rearranged in the t(11;18)(q21;q21)p6ssociated with mucosa-associated lymphoid tissue lymphomas. Blood 1999, 93:3601-3609 [PubMed] [Google Scholar]
- 7.Akagi T, Motegi M, Tamura A, Suzuki R, Hosokawa Y, Suzuki H, Ota H, Nakamura S, Morishima Y, Taniwaki M, Seto M: A novel gene, MALT1 at 18q21, is involved in t(11;18) (q21;q21) found in low-grade B-cell lymphoma of mucosa-associated lymphoid tissue. Oncogene 1999, 18:5785-5794 [DOI] [PubMed] [Google Scholar]
- 8.Rosenwald A, Ott G, Stilgenbauer S, Kalla J, Bredt M, Katzenberger T, Greiner A, Ott MM, Gawin B, Dohner H, Muller-Hermelink HK: Exclusive detection of the t(11;18)(q21;q21) in extranodal marginal zone B cell lymphomas (MZBL) of MALT type in contrast to other MZBL and extranodal large B cell lymphomas. Am J Pathol 1999, 155:1817-1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Remstein ED, James CD, Kurtin PJ: Incidence and subtype specificity of API2-MALT1 fusion translocations in extranodal, nodal, and splenic marginal zone lymphomas. Am J Pathol 2000, 156:1183-1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inagaki H, Okabe M, Seto M, Nakamura S, Ueda R, Eimoto T: API2-MALT1 fusion transcripts involved in mucosa-associated lymphoid tissue lymphoma: multiplex RT-PCR detection using formalin-fixed paraffin-embedded specimens. Am J Pathol 2001, 158:699-706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yonezumi M, Suzuki R, Suzuki H, Yoshino T, Oshima K, Hosokawa Y, Asaka M, Morishima Y, Nakamura S, Seto M: Detection of API2-MALT1 chimaeric gene in extranodal and nodal marginal zone B-cell lymphoma by reverse transcription polymerase chain reaction (PCR) and genomic long and accurate PCR analyses. Br J Haematol 2001, 115:588-594 [DOI] [PubMed] [Google Scholar]
- 12.Motegi M, Yonezumi M, Suzuki H, Suzuki R, Hosokawa Y, Hosaka S, Kodera Y, Morishima Y, Nakamura S, Seto M: API2-MALT1 chimeric transcripts involved in mucosa-associated lymphoid tissue type lymphoma predict heterogeneous products. Am J Pathol 2000, 156:807-812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baens M, Maes B, Steyls A, Geboes K, Marynen P, De Wolf-Peeters C: The product of the t(11;18), an API2-MLT fusion, marks nearly half of gastric MALT type lymphomas without large cell proliferation. Am J Pathol 2000, 156:1433-1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV: The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell 1995, 83:1243-1252 [DOI] [PubMed] [Google Scholar]
- 15.Hofmann K, Bucher P, Tschopp J: The CARD domain: a new apoptotic signalling motif. Trends Biochem Sci 1997, 22:155-156 [DOI] [PubMed] [Google Scholar]
- 16.Roy N, Deveraux QL, Takahashi R, Salvesen GS, Reed JC: The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J 1997, 16:6914-6925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uren AG, O’Rourke K, Aravind LA, Pisabarro MT, Seshagiri S, Koonin EV, Dixit VM: Identification of paracaspases and metacaspases: two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma. Mol Cell 2000, 6:961-967 [DOI] [PubMed] [Google Scholar]
- 18.Willis TG, Jadayel DM, Du MQ, Peng H, Perry AR, Abdul-Rauf M, Price H, Karran L, Majekodunmi O, Wlodarska I, Pan L, Crook T, Hamoudi R, Isaacson PG, Dyer MJ: Bcl10 is involved in t(1;14)(p22;q32) of MALT B cell lymphoma and mutated in multiple tumor types. Cell 1999, 96:35-45 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Q, Siebert R, Yan M, Hinzmann B, Cui X, Xue L, Rakestraw KM, Naeve CW, Beckmann G, Weisenburger DD, Sanger WG, Nowotny H, Vesely M, Callet-Bauchu E, Salles G, Dixit VM, Rosenthal A, Schlegelberger B, Morris SW: Inactivating mutations and overexpression of BCL10, a caspase recruitment domain-containing gene, in MALT lymphoma with t(1;14)(p22;q32). Nat Genet 1999, 22:63-68 [DOI] [PubMed] [Google Scholar]
- 20.Lucas PC, Yonezumi M, Inohara N, McAllister-Lucas LM, Abazeed ME, Chen FF, Yamaoka S, Seto M, Nunez G: Bcl10 and MALT1, independent targets of chromosomal translocation in MALT lymphoma, cooperate in a novel NF-κ B signaling pathway. J Biol Chem 2001, 276:19012-19019 [DOI] [PubMed] [Google Scholar]
- 21.Ye H, Dogan A, Karran L, Willis TG, Chen L, Wlodarska I, Dyer MJ, Isaacson PG, Du MQ: BCL10 expression in normal and neoplastic lymphoid tissue: nuclear localization in MALT lymphoma. Am J Pathol 2000, 157:1147-1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H, Ye H, Dogan A, Ranaldi R, Hamoudi RA, Bearzi I, Isaacson PG, Du MQ: T(11;18)(q21;q21) is associated with advanced mucosa-associated lymphoid tissue lymphoma that expresses nuclear BCL10. Blood 2001, 98:1182-1187 [DOI] [PubMed] [Google Scholar]
- 23.Maes B, Demunter A, Peeters B, De Wolf-Peeters C: BCL10 mutation does not represent an important pathogenic mechanism in gastric MALT-type lymphoma, and the presence of the API2-MLT fusion is associated with aberrant nuclear BCL10 expression. Blood 2002, 99:1398-1404 [DOI] [PubMed] [Google Scholar]
- 24.Wotherspoon AC, Doglioni C, Diss TC, Pan L, Moschini A, de Boni M, Isaacson PG: Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet 1993, 342:575-577 [DOI] [PubMed] [Google Scholar]
- 25.Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, Orentreich N, Vogelman JH, Friedman GD: Helicobacter pylori infection and gastric lymphoma. N Engl J Med 1994, 330:1267-1271 [DOI] [PubMed] [Google Scholar]
- 26.Sackmann M, Morgner A, Rudolph B, Neubauer A, Thiede C, Schulz H, Kraemer W, Boersch G, Rohde P, Seifert E, Stolte M, Bayerdoerffer E: Regression of gastric MALT lymphoma after eradication of Helicobacter pylori is predicted by endosonographic staging: MALT Lymphoma Study Group. Gastroenterology 1997, 113:1087-1090 [DOI] [PubMed] [Google Scholar]
- 27.Nakamura S, Matsumoto T, Suekane H, Takeshita M, Hizawa K, Kawasaki M, Yao T, Tsuneyoshi M, Iida M, Fujishima M: Predictive value of endoscopic ultrasonography for regression of gastric low grade and high grade MALT lymphomas after eradication of Helicobacter pylori. Gut 2001, 48:454-460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura T, Nakamura S, Yonezumi M, Suzuki T, Matsuura A, Yatabe Y, Yokoi T, Ohashi K, Seto M: Helicobacter pylori and the t(11;18)(q21;q21) translocation in gastric low-grade B-cell lymphoma of mucosa-associated lymphoid tissue type. Jpn J Cancer Res 2000, 91:301-309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H, Ruskon-Fourmestraux A, Lavergne-Slove A, Ye H, Molina T, Bouhnik Y, Hamoudi RA, Diss TC, Dogan A, Megraud F, Rambaud JC, Du MQ, Isaacson PG: Resistance of t(11;18) positive gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication therapy. Lancet 2001, 357:39-40 [DOI] [PubMed] [Google Scholar]
- 30.Sugiyama T, Asaka M, Nakamura T, Nakamura S, Yonezumi S, Seto M: API2-MALT1 chimeric transcript is a predictive marker for the responsiveness of H. pylori eradication treatment in low-grade gastric MALT lymphoma. Gastroenterology 2001, 120:1884-1885 [DOI] [PubMed] [Google Scholar]
- 31.Liu H, Ye H, Ruskone-Fourmestraux A, De Jong D, Pileri S, Thiede C, Lavergne A, Boot H, Caletti G, Wundisch T, Molina T, Taal BG, Elena S, Thomas T, Zinzani PL, Neubauer A, Stolte M, Hamoudi RA, Dogan A, Isaacson PG, Du MQ: T(11;18) is a marker for all stage gastric MALT lymphomas that will not respond to H. pylori eradication. Gastroenterology 2002, 122:1286-1294 [DOI] [PubMed] [Google Scholar]
- 32.Nakamura T, Nakamura S, Yokoi T, Ohashi K, Seto M: Clinicopathologic comparison between the API2-MALT1 chimeric transcript-positive and -negative gastric low-grade B-cell lymphoma of mucosa-associated lymphoid tissue type. Jpn J Cancer Res 2002, 93:677-684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inagaki H, Nonaka M, Nagaya S, Tateyama H, Sasaki M, Eimoto T: Monoclonality in gastric lymphoma detected in formalin-fixed, paraffin-embedded endoscopic biopsy specimens using immunohistochemistry, in situ hybridization, and polymerase chain reaction. Diagn Mol Pathol 1995, 4:32-38 [DOI] [PubMed] [Google Scholar]
- 34.Inagaki H, Chan JK, Ng JW, Okabe M, Yoshino T, Okamoto M, Ogawa H, Matsushita H, Yokose T, Matsuno Y, Nakamura N, Nagasaka T, Ueda R, Eimoto T, Nakamura S: Primary thymic extranodal marginal-zone B-cell lymphoma of mucosa-associated lymphoid tissue type exhibits distinctive clinicopathological and molecular features. Am J Pathol 2002, 160:1435-1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Musshoff K: Stadieneneinteilung der nicht-Hodgkin lymphome. Strahlentherapie 1977, 153:218-221 [PubMed] [Google Scholar]
- 36.Isaacson PG, Dogan A, Price SK, Spencer J: Immunoproliferative small-intestinal disease: an immunohistochemical study. Am J Surg Pathol 1989, 13:1023-1033 [DOI] [PubMed] [Google Scholar]
- 37.Du M-Q, Liu H, Ye H, Ruskone-Fourmestraux A, Jong de D, Pileri S, Wundisch T, Lavergne A, Boot H, Caletti G, Thiede C, Wotherspoon AC, Nicholson AG, Charlotte F, Leblond V, Speight P, Dogan A, Isaacson PG: T(11;18) occurs at variable frequencies in MALT lymphoma of different sites and is a marker for gastric cases that do not respond to H. pylori eradication. Mod Pathol 2002, 15:239A (Abstract)
- 38.Fiche M, Caprons F, Berger F, Galateau F, Cordier JF, Loire R, Diebold J: Primary pulmonary non-Hodgkin’s lymphomas. Histopathology 1995, 26:529-537 [DOI] [PubMed] [Google Scholar]
- 39.Nicholson AG, Wotherspoon AC, Diss TC, Butcher DN, Sheppard MN, Isaacson PG, Corrin B: Pulmonary B-cell non-Hodgkin’s lymphomas: the value of immunohistochemistry and gene analysis in diagnosis. Histopathology 1995, 26:395-403 [DOI] [PubMed] [Google Scholar]
- 40.Kurtin PJ, Myers JL, Adlakha H, Strickler JG, Lohse C, Pankratz VS, Inwards DJ: Pathologic and clinical features of primary pulmonary extranodal marginal zone B-cell lymphoma of MALT type. Am J Surg Pathol 2001, 25:997-1008 [DOI] [PubMed] [Google Scholar]
- 41.Thieblemont C, Bastion Y, Berger F, Rieux C, Salles G, Dumontet C, Felman P, Coiffier B: Mucosa-associated lymphoid tissue gastrointestinal and non-gastrointestinal lymphoma behavior: analysis of 108 patients. J Clin Oncol 1997, 15:1624-1630 [DOI] [PubMed] [Google Scholar]
- 42.Shin SS, Sheibani K, Fishleder A, Ben-Ezra J, Bailey A, Koo CH, Burke JS, Tubbs R, Rappaport H: Monocytoid B-cell lymphoma in patients with Sjogren’s syndrome: a clinicopathologic study of 13 patients. Hum Pathol 1991, 22:422-430 [DOI] [PubMed] [Google Scholar]
- 43.Mackay IR, Rose NR: Autoimmunity and lymphoma: tribulations of B cells. Nat Immunol 2001, 2:793-795 [DOI] [PubMed] [Google Scholar]
- 44.Starostik P, Patzner J, Greiner A, Schwarz S, Kalla J, Ott G, Muller-Hermelink HK: Gastric marginal zone B-cell lymphomas of MALT type develop along 2 distinct pathogenetic pathways. Blood 2002, 99:3-9 [DOI] [PubMed] [Google Scholar]
- 45.Remstein ED, Kurtin PJ, James CD, Wang X-Y, Meyer RG, Dewald GW: Mucosa-associated lymphoid tissue lymphomas with t(11;18)(q21;q21) and mucosa-associated lymphoid tissue lymphomas with aneuploidy develop along different pathogenetic pathways. Am J Pathol 2002, 161:63-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cordier JF, Chailleux E, Lauque D, Reynaud-Gaubert M, Dietemann-Molard A, Dalphin JC, Blanc-Jouvan F, Loire R: Primary pulmonary lymphomas: a clinical study of 70 cases in nonimmunocompromised patients. Chest 1993, 103:201-208 [DOI] [PubMed] [Google Scholar]
- 47.Li C, Inagaki H, Kuo T-t, Hu S, Okabe M, Eimoto T: Primary cutaneous marginal zone B-cell lymphoma: a molecular and clinicopathologic study of 24 Asian cases. Am J Surg Pathol, in press [DOI] [PubMed]