Abstract
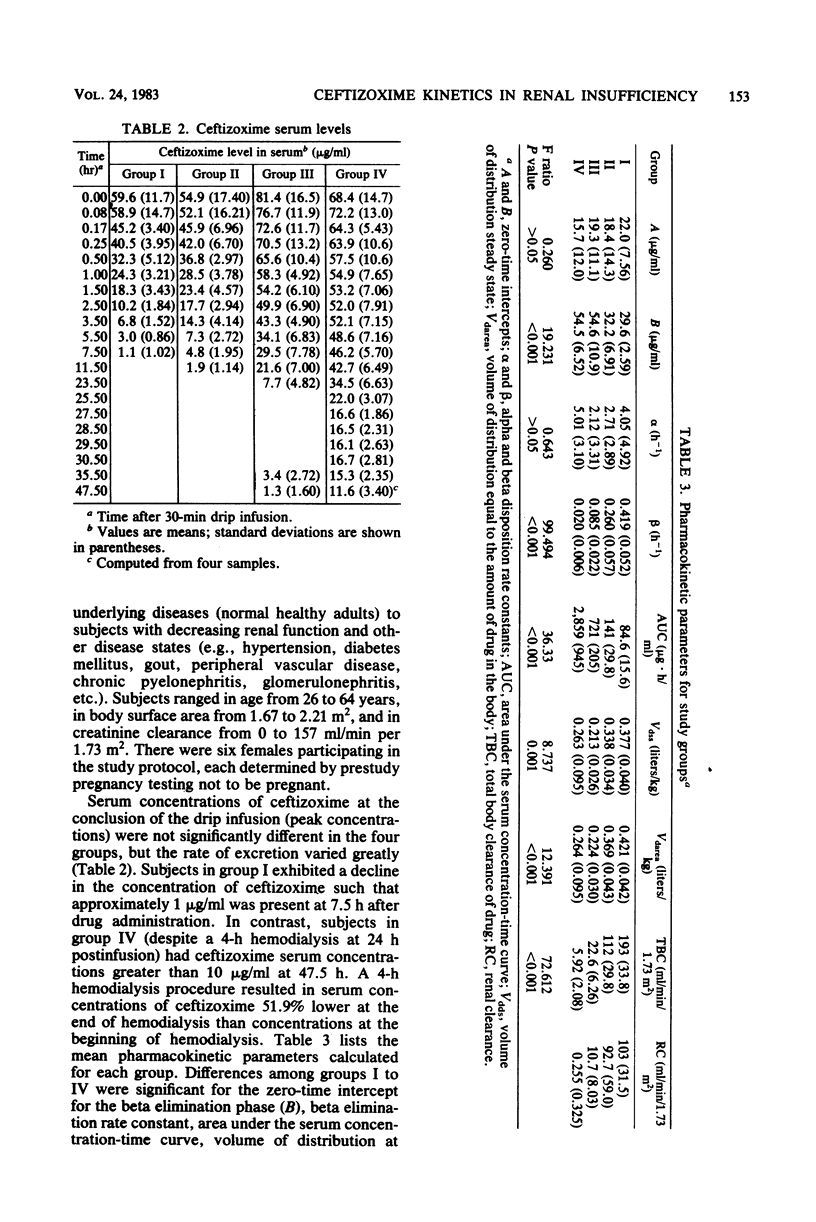
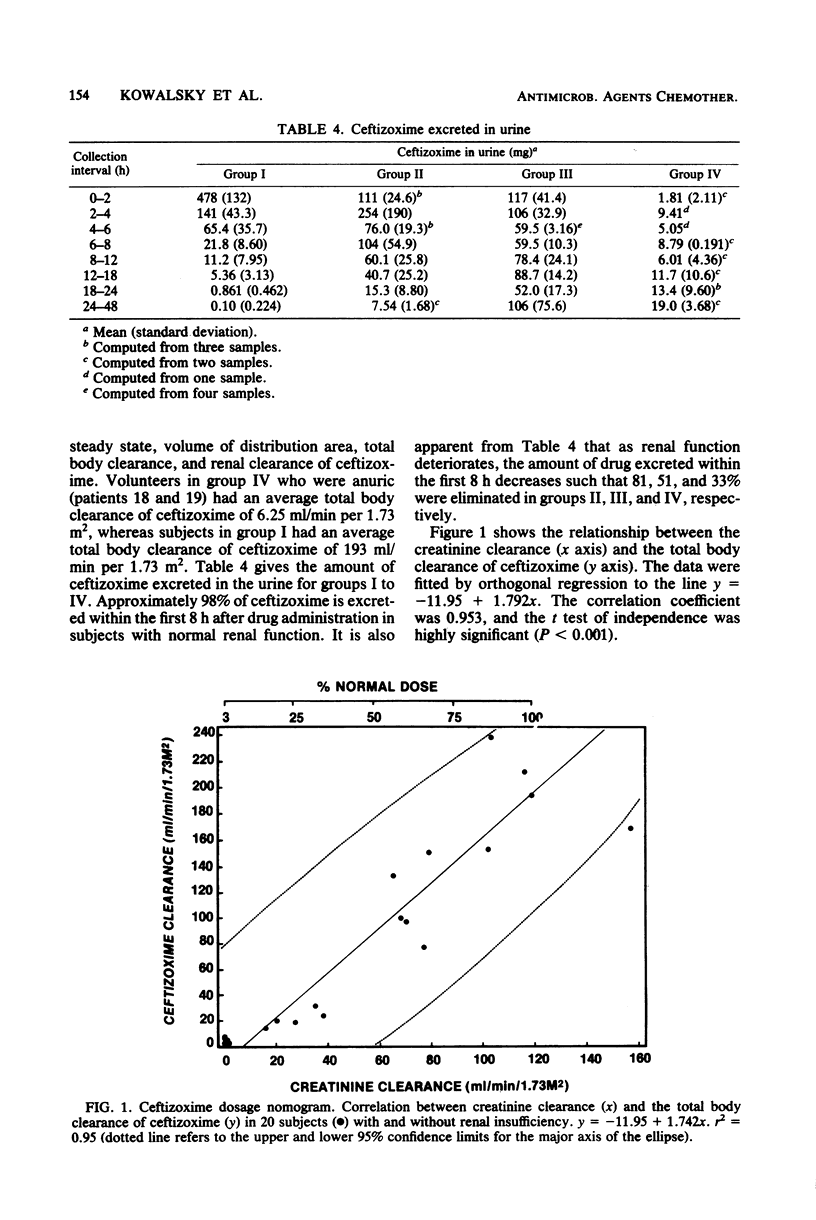
The pharmacokinetics of ceftizoxime (FK-749) were studied in 20 volunteers with various degrees of renal function. Creatinine clearances ranged from zero to 157 ml/min per 1.73 m2. One gram of ceftizoxime was administered by a 30-min drip infusion, and blood and urine samples were collected for up to 48 h after drug administration. For volunteers with a creatinine clearance of greater than or equal to 80 ml/min per 1.73 m2 (group I), the mean half-life was 1.65 h, whereas for volunteers with a creatinine clearance of less than 10 ml/min per 1.73 m2 (group IV), the half-life was 34.7 h. The volume of distribution at steady state (Vdss) and the volume of distribution area (Vdarea) were calculated for each group and ranged from 0.377 to 0.263 and 0.421 to 0.264 liters/kg for groups I and IV, respectively. Total body clearance of ceftizoxime correlated with creatinine clearance (r = 0.953), and the mean urinary recovery of unchanged drug in normal volunteers was 72.4%. A 4-h hemodialysis procedure reduced serum ceftizoxime concentrations by approximately 52%; however, serum concentrations at 48 h after drug administration were still greater than 10 micrograms/ml in dialysis subjects. By using the relationship between total body clearance of ceftizoxime and creatinine clearance, a nomogram was developed to assist in the administration of ceftizoxime to patients with renal dysfunction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Levison M. E., Levison S. P., Ries K., Kaye D. Pharmacology of cefazolin in patients with normal and abnormal renal function. J Infect Dis. 1973 Oct;128(Suppl):S354–S357. doi: 10.1093/infdis/128.supplement_2.s354. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Srinivasan S. Pharmacology of ceftizoxime compared with that of cefamandole. Antimicrob Agents Chemother. 1981 Sep;20(3):366–369. doi: 10.1128/aac.20.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. G. Linear pharmacokinetic equations allowing direct calculation of many needed pharmacokinetic parameters from the coefficients and exponents of polyexponential equations which have been fitted to the data. J Pharmacokinet Biopharm. 1976 Oct;4(5):443–467. doi: 10.1007/BF01062831. [DOI] [PubMed] [Google Scholar]
- Wagner J. G. Pharmacokinetic data. Pharmacokinetic parameters estimated from intravenous data by uniform methods and some of their uses. J Pharmacokinet Biopharm. 1977 Apr;5(2):161–182. doi: 10.1007/BF01066219. [DOI] [PubMed] [Google Scholar]



