Abstract
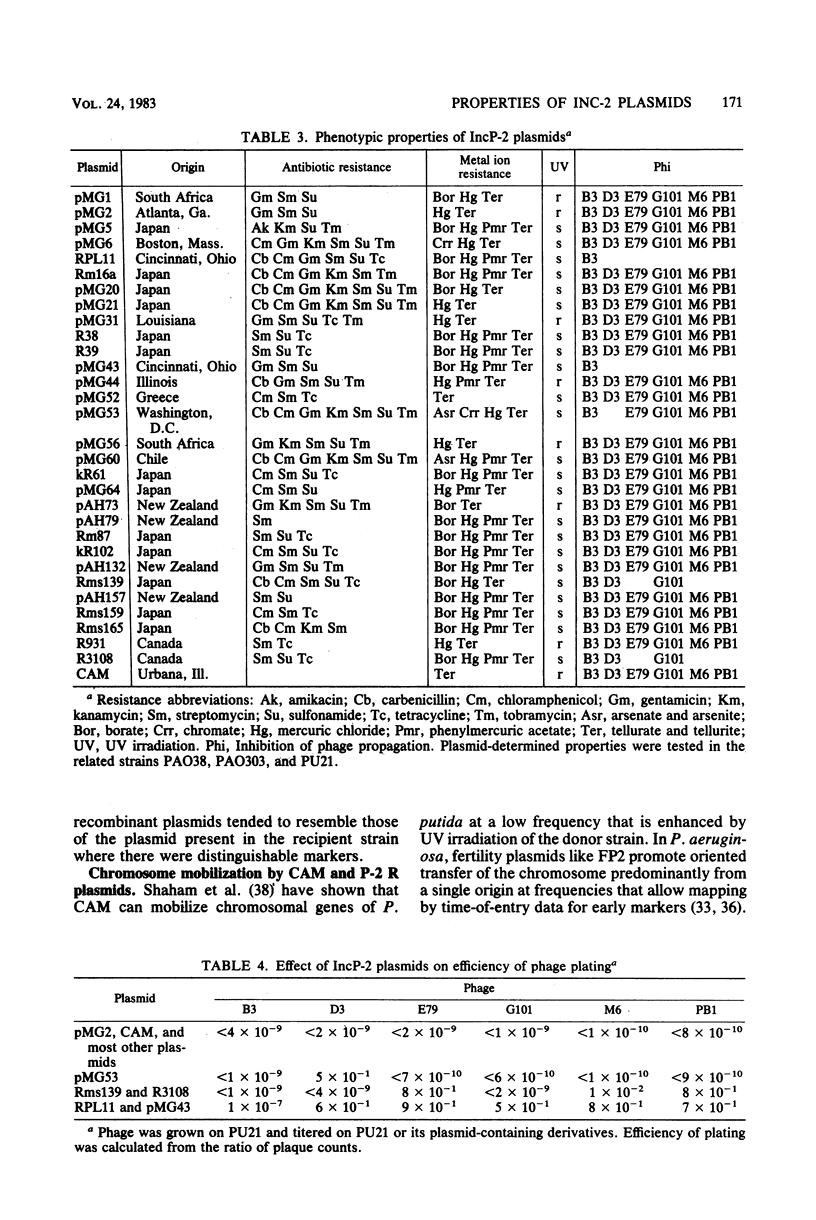
Thirty IncP-2 R plasmids from isolates of Pseudomonas spp. of diverse geographical origins were examined for the production of resistance properties. All the plasmids determined resistance to tellurite and all inhibited the propagation of certain DNA phages, although several patterns of phage inhibition were detected. Of the 30 plasmids, 29 determined resistance to streptomycin, 28 determined resistance to mercuric ion, and 24 determined resistance to sulfonamide. Resistance to other antibiotics, to compounds of arsenic, boron, or chromium, and to UV irradiation was less common. The degradative plasmid CAM also belonged to this group. When CAM was introduced into recipients carrying an IncP-2 R plasmid, recombinant plasmids were often formed in which antibiotic resistance and the ability to grow on camphor were transferred together to further recipients or were lost together in a strain in which IncP-2 plasmids were unstable. Such hybrid plasmid formation was rec dependent. CAM and other IncP-2 plasmids that determine UV light resistance demonstrated UV-enhanced, nonpolarized transfer of the Pseudomonas aeruginosa chromosome. By agarose gel electrophoresis, all IncP-2 R plasmids and CAM were ca. 300 X 10(6) in molecular weight.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bremner D. A. Transfer of plasmid-mediated antibiotic resistance in strains of Pseudomonas aeruginosa isolated in Auckland. J Med Microbiol. 1979 Aug;12(3):303–310. doi: 10.1099/00222615-12-3-303. [DOI] [PubMed] [Google Scholar]
- Bryan L. E., Semaka S. D., Van den Elzen H. M., Kinnear J. E., Whitehouse R. L. Characteristics of R931 and other Pseudomonas aeruginosa R factors. Antimicrob Agents Chemother. 1973 May;3(5):625–637. doi: 10.1128/aac.3.5.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Shahrabadi M. S., van den Elzen H. M. Gentamicin resistance in Pseudomonas aeruginosa: R-factor-mediated resistance. Antimicrob Agents Chemother. 1974 Aug;6(2):191–199. doi: 10.1128/aac.6.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A. M., Chou G., Gunsalus I. C. Genetic regulation of octane dissimilation plasmid in Pseudomonas. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1137–1140. doi: 10.1073/pnas.70.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B. J., Holloway B. W. Bacterial mutation affecting plasmid maintenance in Pseudomonas aeruginosa. J Bacteriol. 1977 May;130(2):943–945. doi: 10.1128/jb.130.2.943-945.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Evenchik Z., Stacey K. A., Hayes W. Ultraviolet induction of chromosome transfer by autonomous sex factors in Escherichia coli. J Gen Microbiol. 1969 Apr;56(1):1–14. doi: 10.1099/00221287-56-1-1. [DOI] [PubMed] [Google Scholar]
- Fennewald M., Prevatt W., Meyer R., Shapiro J. Isolation of inc P-2 plasmid DNA from Pseudomonas aeruginosa. Plasmid. 1978 Feb;1(2):164–173. doi: 10.1016/0147-619x(78)90036-7. [DOI] [PubMed] [Google Scholar]
- Govan J. R., Gillies R. R. Further studies in the pyocine typing of Pseudomonas pyocyanea. J Med Microbiol. 1969 Feb;2(1):17–25. doi: 10.1099/00222615-2-1-17. [DOI] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. IncP2 group of Pseudomonas, a class of uniquely large plasmids. Nature. 1978 Aug 17;274(5672):715–717. doi: 10.1038/274715a0. [DOI] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., Sublett R., Hedges R. W., Jacob A., Falkow S. Origin of the TEM-beta-lactamase gene found on plasmids. J Bacteriol. 1975 Apr;122(1):250–256. doi: 10.1128/jb.122.1.250-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby G. A., Jacob A. E., Hedges R. W. Recombination between plasmids of incompatibility groups P-1 and P-2. J Bacteriol. 1976 Sep;127(3):1278–1285. doi: 10.1128/jb.127.3.1278-1285.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby G. A., Matthew M. The distribution of beta-lactamase genes on plasmids found in Pseudomonas. Plasmid. 1979 Jan;2(1):41–47. doi: 10.1016/0147-619x(79)90004-0. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A. Properties of R plasmids determining gentamicin resistance by acetylation in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1974 Sep;6(3):239–252. doi: 10.1128/aac.6.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby G. A. Properties of an R plasmid in Pseudomonas aeruginosa producing amikacin (BB-K8), butirosin, kanamycin, tobramycin, and sisomicin resistance. Antimicrob Agents Chemother. 1974 Dec;6(6):807–810. doi: 10.1128/aac.6.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnapillai V. The use of bacteriophages for differentiating plasmids of Pseudomonas aeruginosa. Genet Res. 1974 Jun;23(3):327–334. doi: 10.1017/s0016672300014968. [DOI] [PubMed] [Google Scholar]
- LOW B., WOOD T. H. A QUICK AND EFFICIENT METHOD FOR INTERRUPTION OF BACTERIAL CONJUGATION. Genet Res. 1965 Jul;6:300–303. doi: 10.1017/s001667230000416x. [DOI] [PubMed] [Google Scholar]
- Lehrbach P., Kung A. H., Lee B. T., Jacoby G. A. Plasmid modification of radiation and chemical-mutagen sensitivity in Pseudomonas aeruginosa. J Gen Microbiol. 1977 Jan;98(1):167–176. doi: 10.1099/00221287-98-1-167. [DOI] [PubMed] [Google Scholar]
- Loutit J. S., Marinus M. G. Investigation of the mating system of Pseudomonas aeruginosa strain 1. II. Mapping of a number of early markers. Genet Res. 1968 Aug;12(1):37–44. doi: 10.1017/s0016672300011599. [DOI] [PubMed] [Google Scholar]
- Loutit J. S., Pearce L. E., Marinus M. G. Investigation of the mating system of Pseudomonas aeruginosa strain 1. I. Kinetic studies. Genet Res. 1968 Aug;12(1):29–36. doi: 10.1017/s0016672300011587. [DOI] [PubMed] [Google Scholar]
- Medeiros A. A., Hedges R. W., Jacoby G. A. Spread of a "Pseudomonas-specific" beta-lactamase to plasmids of enterobacteria. J Bacteriol. 1982 Feb;149(2):700–707. doi: 10.1128/jb.149.2.700-707.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton J. M., Clark A. J. Detection and characterization of plasmids in Pseudomonas aeruginosa strain PAO. J Bacteriol. 1973 Apr;114(1):424–433. doi: 10.1128/jb.114.1.424-433.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton J. M., Holloway B. W. Chromosome mapping in Pseudomonas aeruginosa. Genet Res. 1972 Jun;19(3):251–260. doi: 10.1017/s0016672300014518. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Chakrabarty A. M., Gunsalus I. C. A transmissible plasmid controlling camphor oxidation in Pseudomonas putida. Proc Natl Acad Sci U S A. 1973 Mar;70(3):885–889. doi: 10.1073/pnas.70.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe B., Holloway B. W. Alterations in host specificity of bacterial deoxyribonucleic acid after an increase in growth temperature of Pseudomonas aeruginosa. J Bacteriol. 1966 Jul;92(1):43–48. doi: 10.1128/jb.92.1.43-48.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle P. L., Matsumoto H., Holloway B. W. Genetic circularity of the Pseudomonas aeruginosa PAO chromosome. J Bacteriol. 1981 Jan;145(1):145–155. doi: 10.1128/jb.145.1.145-155.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagai H., Hasuda K., Iyobe S., Bryan L. E., Holloway B. W., Mitsuhashi S. Classification of R plasmids by incompatibility in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1976 Oct;10(4):573–578. doi: 10.1128/aac.10.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham M., Chakrabarty A. M., Gunsalus I. C. Camphor plasmid-mediated chromosomal transfer in Pseudomonas putida. J Bacteriol. 1973 Nov;116(2):944–949. doi: 10.1128/jb.116.2.944-949.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O., Jacoby G. A. Plasmid-determined resistance to boron and chromium compounds in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1978 Apr;13(4):637–640. doi: 10.1128/aac.13.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O., Jacoby G. A. Plasmid-determined resistance to tellurium compounds. J Bacteriol. 1977 Jan;129(1):276–281. doi: 10.1128/jb.129.1.276-281.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton L., Jacoby G. A. Plasmid-determined resistance to hexachlorophene in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1978 Apr;13(4):634–636. doi: 10.1128/aac.13.4.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., Summers A. O. Association of tellurium resistance and bacteriophage inhibition conferred by R plasmids. J Bacteriol. 1979 Mar;137(3):1430–1433. doi: 10.1128/jb.137.3.1430-1433.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


