Abstract
Environmental contamination by endocrine-disrupting chemicals (EDC) can have epigenetic effects (by DNA methylation) on the germ line and promote disease across subsequent generations. In natural populations, both sexes may encounter affected as well as unaffected individuals during the breeding season, and any diminution in attractiveness could compromise reproductive success. Here we examine mate preference in male and female rats whose progenitors had been treated with the antiandrogenic fungicide vinclozolin. This effect is sex-specific, and we demonstrate that females three generations removed from the exposure discriminate and prefer males who do not have a history of exposure, whereas similarly epigenetically imprinted males do not exhibit such a preference. The observations suggest that the consequences of EDCs are not just transgenerational but can be “transpopulational”, because in many mammalian species, males are the dispersing sex. This result indicates that epigenetic transgenerational inheritance of EDC action represents an unappreciated force in sexual selection. Our observations provide direct experimental evidence for a role of epigenetics as a determinant factor in evolution.
Keywords: endocrine disruption, vinclozolin, sexual selection, odor salience
Some chemicals released into the environment interfere with reproductive development and can render individuals exposed in utero or early in life functionally sterile (1). The shared quality of reproductive dysfunction has led many to speculate that endocrine-disrupting chemical (EDC) exposure may have evolutionary significance, but exactly how this may occur is open to question. In persistently contaminated environments, it is common for a population to decline and even go extinct unless there is immigration of uncompromised individuals. In such instances, affected individuals have no direct evolutionary impact unless, by mating with a fertile individual, they diminish or deprive their mates of an opportunity to reproduce. The discovery that EDCs can induce an epigenetic transgenerational phenotype through reprogramming the germ-line in a sex-specific manner (2–4) presents another dimension to this widespread problem (5). This phenomenon, in which exposure of pregnant animals results in several generations of progeny exhibiting a disease phenotype without exposure of any but the first generation to the original event, has been documented in animals and humans (2–4, 6). For example, exposure of pregnant female rats to the pesticide methoxychlor or the fungicide vinclozolin during the period of embryonic sex determination and gonadal differentiation results in the male offspring exhibiting progressive spermatogenic cell apoptosis, decreased sperm count and motility and, later in adulthood, the development of cancer, prostate disease, kidney disease, and immune abnormalities (2, 3). Remarkably, these effects can still be detected over four subsequent generations of males without diminution (2, 3). Further analysis of the sperm epigenome has demonstrated that vinclozolin exposure induces the appearance of a series of new imprinted-like genes that transgenerationally transmits this altered epigenome to promote disease phenotypes (7). Thus, in this case, it appears that EDC exposure may not involve DNA mutations but rather alters the epigenome. EDCs that exert transgenerational epigenetic changes without altering the DNA itself represent an element of nongenomic inheritance whose role has not previously been evaluated in the context of sexual selection.
If evolution is predicated on reproductive success, then it is in the individual's interest to select the best mate. Mate preferences can evolve because of their genetic effects on offspring due to enhanced genetic complementarity or the inheritance of “good genes” that enhance survival. Embryonic exposure to a variety of EDCs results either in altered or dysfunctional sociosexual behavior or a decline in an individual's attractiveness to potential mates (8, 9). However, previous studies used as their test animals those individuals that were exposed in utero or early in life, and that thus had a body burden of the contaminant, together with untreated stimulus animals. These studies provided conclusions about the direct effect of the EDC on the reproductive performance of the exposed animal but not about transgenerational effects.
In nature, animals are often free to select their mates (10). Even if both sexes are compromised, they may encounter unaffected as well as affected individuals during the breeding season. Should affected individuals choose to mate with affected conspecifics, there may be no evolutionary impact. However, if there were asymmetry in mate preference (e.g., affected males mating with unaffected females or vice versa), then the impact on the population would be significant. For these reasons, it would be useful to know whether an individual's stimulus qualities and/or perception of suitable mates are modified by their progenitors' exposure to environmental contaminants.
The ability of environmental factors to influence evolutionary processes has led to the speculation that epigenetic mechanisms are a significant determinant factor in evolution (5). A combination of DNA sequence mutation (i.e., classic genetic processes) and epigenetic processes are postulated to be important for evolutionary adaptation events. The current study provides an experimental observation that directly supports the role of epigenetics in the regulation of a major determinant factor for evolution (i.e., sexual selection). We demonstrate a transgenerational imprint on both attractiveness (of the selected individual) and perception (of the selecting individual) three generations removed after the exposure.
Results
The study of transgenerational epigenetic transmission of an altered phenotype due to reprogramming of the germ-line (2, 3, 7) is complicated in wild mammals because of a high degree of DNA polymorphisms. Therefore, the current study used male and female Sprague–Dawley rats that were the F3 generation descendants of F0 females that had been injected daily on embryonic days 8–14 with vinclozolin (EDC lineage) or dimethyl sulfoxide buffer alone (control lineage) (2, 3). Importantly, the disease phenotypes do not occur until after postnatal day (PND) 90–120 for the testis and PND 180–365 for others organs, well after the sexes become sexually mature (around PND 40). The ages of the rats used in the current study are before the development of disease.
Males were tested first and presented with a pair of stimulus females, one from the control lineage and the other from the EDC lineage (Fig. 1). One month later, these roles were reversed and the females tested, choosing between two stimulus males representing each treatment group. In this manner, the mate preference and stimulus quality of both males and females from the control and EDC lineages were assessed. We found that all females preferred males from the control lineage, whereas males from both lineages exhibited no preferences for female type (Fig. 2).
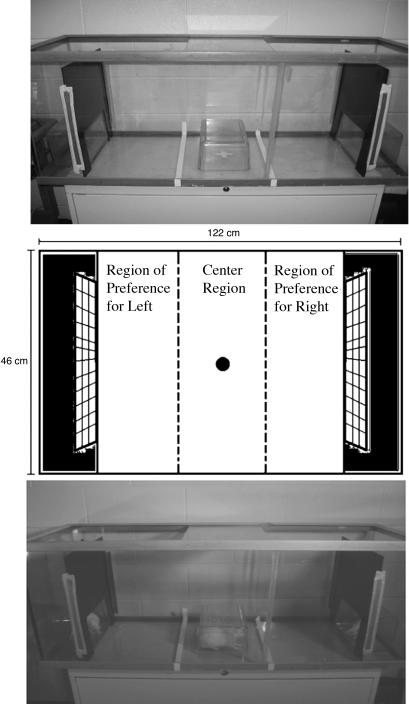
Fig. 1.
Testing apparatus for mate-preference tests. The schematic (Middle) illustrates the regions of interest. Experimental animals were considered to have exhibited a preference if all four feet had crossed the dotted line into the right or left third of the chamber. While in these areas, the time spent at the glass sides or engaged in the following behaviors (Still, Standing/Sniffing, Walking, and Grooming) was quantified. Time spent in the left or right third of the chamber was also calculated for three additional behaviors (Plexiglas, Facial Investigation, and Wire Mesh). Finally, time in the center third was calculated. Also illustrated is the testing chamber with Plexiglas release box in place under fluorescent illumination (Top) or under dim red light with animals in place (Bottom).
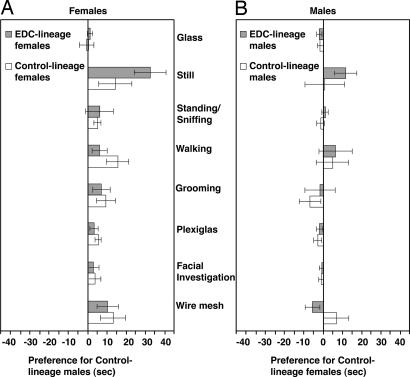
Fig. 2.
Epigenetically altered third-generation female rats (EDC lineage, shown in gray bar) whose progenitors were exposed to vinclozolin, a common-use fungicide with endocrine-disrupting (EDC) properties, prefer males from the unexposed control lineage (A Left). Females from an unexposed lineage (control lineage, shown in open bar) show a similar preference for control-lineage males. A score of 0 on the x axis indicates no preference. Data in the positive direction indicate time spent in the third of the chamber by control-lineage males. Data in the negative direction indicate a preference for the EDC-lineage male. A significant preference of all females, regardless of their lineage, was found for the behaviors Still, Walking, Grooming, Plexiglas, and Wire Mesh, as well as in Total preference behaviors (see text). Males, whether from EDC lineage (gray bar) or control lineage (open bar), do not show any significant preferences (B Right). A score of 0 on the x axis indicates no preference, and there was no significant positive (toward control lineage) or negative (toward EDC lineage) preference by males, regardless of their lineage, for either lineage of females. Presented are the mean (± 1 SEM) differences in the time spent in each behavior. The various behavioral measures and test are described in Methods.
The behavior of the experimental animal in 10-min mate preference tests with six pairs of stimulus animals from the control and EDC lineages were quantified. There was a main effect of male treatment in most of the behaviors, whereas the main effect of female treatment and Interaction (male treatment × female treatment) for these behaviors were not statistically significant. There was an overall preference exhibited by both control- and EDC-lineage females for control-lineage males over EDC-lineage males in time spent at the wire mesh (F1,10 = 7.06, P = 0.024) and Plexiglas (F1,10 = 9.34, P = 0.012) (Fig. 2A). There was also a main effect of male treatment for other female behaviors: grooming (F1,10 = 5.55, P = 0.040), walking (F1,10 = 9.20, P = 0.013), and still (F1,10 = 15.28, P = 0.003) (Fig. 2A). The main effect of male treatment was not statistically significant for facial investigation, standing/sniffing, and glass. The main effect of male treatment on preference behaviors, or the total time spent in behaviors directed toward the stimulus males (wire mesh, facial investigation, and Plexiglas) was significant (F1,10 = 6.81, P = 0.026) (Fig. 2A). Control-lineage females spent more time in the center compartment than did the EDC-lineage females (t = −2.50, P = 0.032). The ratio of center time relative to time spent in preference behaviors was not significant (t = 0.86, P = 0.438), indicating this was not a factor in the mate preference exhibited.
The main effects of male and female treatment, as well as their interaction, were not significantly different for any of the male behaviors (Fig. 2B). The total time spent in preference behaviors was also not significant. There was no statistical difference in the time spent in center between control- and EDC-lineage males (t = −1.33, P = 0.212) or the ratio of center time to time spent in the preference behaviors (t = −0.10, P = 0.919).
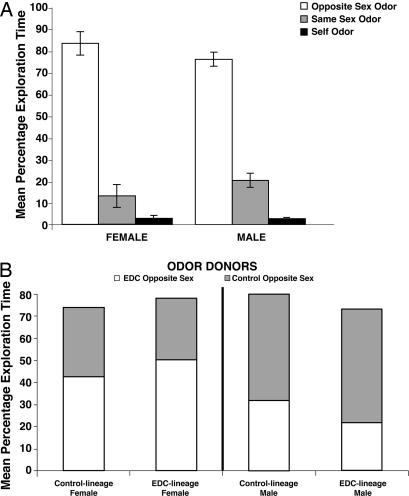
It seemed possible that the preference for control-lineage males in the EDC- and control-lineage females was perhaps because of nonodor cues (e.g., differences in ultrasound calls or motor behavior by the EDC- vs. control-lineage males during the preference test), or that females are generally better in odor discrimination. To explore these possibilities, all individuals were tested in the home cage for salience of same- vs. opposite-sex odors and same-sex novel vs. self odors and for EDC- vs. control-lineage odors. A new social-odor discrimination test was used. Odor salience and discrimination were determined by relative exploration of the odors. This test eliminated any variable linked to phonation, motor behavior, or other cue from the odor donor, because only the different odors were introduced. The sexes and exposure types did not differ in their detection and exploration of odors [opposite sex, t = −1.11, P = 0.27; same sex, t = 1.13, P = 0.27; familiar odor, t = 0.003, P = 0.98 (Fig. 3)]. Among females, there was a significant main effect of odor, resulting from significant differences in individual odor exploration time within all females (F2,59 = 278.36, P = 0.0001). Post hoc tests revealed that females spent significantly more time exploring male odors than novel female odors (P < 0.0001); females spent significantly more time exploring male odor than their own (familiar) odor (P < 0.0001) and exploring novel female odors than their own familiar odor (P < 0.023). Finally, EDC-lineage females spent significantly more time exploring the odor of a novel EDC-lineage male than of a novel control-lineage male (P < 0.01), whereas control-lineage females did not spend significantly more time exploring EDC-lineage male odors. Regarding males, there was a significant main effect of odor, which resulted from significant differences in individual odor exploration time within males (F2,71 = 75.84, P < 0.0001). Post hoc tests further revealed that males spent significantly more time exploring female than novel male odors (P < 0.0001). Males also spent more time exploring female odors than their own familiar odor (P < 0.0001) and novel male odors than their own familiar odor (P < 0.018) (Fig. 3). Finally, EDC-lineage males spent significantly more time exploring control-lineage female odors than EDC-lineage female odors (P < 0.05), whereas control-lineage males did not spend significantly more time exploring control-lineage female odors. Hence, the inability to discriminate among social odors (male from female, novel same sex from self, EDC lineage from control lineage) cannot account for the observed differences in the mate-preference tests.
Fig. 3.
Performance of animals in odor-salience test. Female and male rats have the ability to discriminate odors regardless of their lineage. (A) Both sexes show similar patterns, exploring odors of the opposite sex more than odors of unfamiliar individuals of the same sex or their own familiar odor. Data are expressed as mean percentages of total exploration time (± 1 SEM). (B) EDC-lineage males spend significantly more time exploring control- than EDC-lineage female odors. EDC-lineage females spend significantly more time exploring the odor of a novel EDC- than of a novel control-lineage male.
Discussion
Normally, fertilization is possible only by mutual consent, with the interacting individuals being chosen by, as well as choosing, their partners (10, 11). This consent is based not only on the internal milieu that motivates each individual to seek a partner but also on the satisfactory nature of the phenotypic traits the potential mate displays. The importance of self selection of mates has long been appreciated in animal husbandry, but the scientific study of this phenomenon has lagged, particularly in relation to mate choice. Yet experiments with flies (12), birds (13), and rodents (14, 15) have the common result that those individuals who are allowed to select and be selected by their mate enjoy greater reproductive success than force-paired animals. This Complementarity Principle (11), in which each partner participates in the mate selection process, has broad implications for all animals, regardless of their mode of reproduction. This principle has been extended to the genetic and now to the epigenetic levels.
The epigenetic transgenerational phenotype of multiorgan disease that has been described (2, 3, 7) involves the ability of an environmental factor (e.g., vinclozolin) to promote an epigenetic reprogramming of the germ line, induction of imprinted-like genes, and subsequent transmission to progeny. This permanent modification of the epigenome alters the activity of the genome and a large number of physiological processes (7), including gene expression in the brain and in reproduction (6). This epigenetic transgenerational phenotype is proposed as the primary mechanism involved in the altered mate preference observed, but several specific mechanisms are described below.
Allelic differences in the highly polymorphic genes within the MHC participate in mate choice in vertebrates, including humans (16–20). MHC genes are central to both adaptive and innate immune responses, and individuals heterozygous at MHC loci withstand pathogens better than do homozygous individuals. MHC genes contain CpG islands that are hotspots for methylation (21, 22). Analysis of epigenetic transgenerational changes in the brain and testis transcriptomes indicates changes in expression of the MHC genes in three generations of vinclozolin animals (M.K.S., unpublished data). Altering the MHC complex in both laboratory and wild animal populations (23, 24) influences mating behavior, and epigenetic regulation may be a factor in the current observations.
Many vertebrates, including rodents, use odor cues to distinguish and assess sex and reproductive condition, individual identity (25), and parental origin (26). Some of these chemical cues are hormone-dependent, whereas others depend on the social status of the individual. These socially relevant odors are detected and processed in rodents primarily by the vomeronasal organ (VNO), and both sexes exhibit increased Fos activation in the VNO after exposure to the odor of breeding conspecifics (27–32). It has recently been discovered that male rodents (both mouse and rat) secrete from the eyes a male-specific exocrine gland-secreting peptide (ESP1) contained in the extraorbital lachrymal gland, that is deposited on the facial hair, and that is critical to the female for assessing the stimulus qualities of males; a second peptide (ESP34) is similarly produced by females and serves a complementary function in males (30). ESP1 is detected in the VNO by V2Rp-positive neurons, whereas ESP34 is detected by V2Ro-positive neurons. The ESP gene family is proximate to MHC class I loci, and MHC class I molecules are coexpressed with the V2R receptors (33). Thus, facial investigation is a critical aspect of the assessment process and may underlie our finding that males investigate females equally, whereas females show a greater amount of time investigating males from the control lineage (Fig. 2). Other pheromones potentially involved are contained in the urine, and methylation analysis reveals that the Major Urinary Protein 4 (MUP4) is one of the candidate imprinted-like genes induced in the vinclozolin generation males (7). This MUP group of gene products binds and releases male-specific pheromones in rodents (33, 34). Of course, other cues such as ultrasounds could also play a role in rodent mate choice, but this was not measured.
A followup study showed that the capacity to detect or discriminate odors (male from female, self from novel same-sex, EDC from control lineage) could not account for the findings. Although the EDC-lineage males indeed spent more time exploring control- than EDC-lineage female odors, this effect was modest, suggesting that, in contrast to their nonpreference for EDC-lineage females in the novel environment in the mate preference study, in the home cage, male rats could distinguish, and found more salient, control- than EDC-lineage female odor. The relative salience of odors in a familiar environment such as the home cage does not always match up with preference, and sometimes the reverse is the case. For example, predator odors in a familiar environment do not elicit avoidance behavior, although the predator odor itself is avoided in a novel open-field situation (35). The EDC-lineage, and to a nonsignificant degree the control-lineage, male rats spent more time exploring control-lineage female odors in the home cage. This observation is interesting because it suggests they can distinguish between the odors of EDC- and control-lineage females, and thus ruling out odor discrimination capacity as a potential explanation for their failure to show a preference in the mate preference test. All rats, male and female, explored odors of the opposite sex much more than familiar (self) or novel odors of the same sex, and all animals explored novel odors of the same sex more than self odors (Fig. 3A).
In most mammals, sexually maturing males disperse at an age before the consequence of a compromised epigenome is evident. Such males would have a significant negative impact on the population unless females chose to avoid mating with these males, thereby potentially mitigating the propagation of the trait. Alternatively, such a mating may generate a novel phenotype for subsequent propagation. In birds, females are the heterogametic sex and often disperse first and further than do males (36). Should EDCs exert epigenetic transgenerational effects in the female germ line in birds, the opposite situation pertains, with a significant impact of the dispersing females on the population unless males were able to detect and preferentially breed with unaffected females. In the case of species with environmental sex determination (e.g., temperature), it is predicted that the consequences of transgenerational epigenetic effects of EDCs would compromise the epigenome of both sexes, making populations more vulnerable. The current study demonstrates that an environmental factor can promote a transgenerational alteration in the epigenome that influences sexual selection and could impact the viability of a population and evolution of the species.
Methods
Transgenerational Animals.
Sprague–Dawley male and female rats (n = 12 each) obtained from Washington State University [Institutional Animal Care and Use Committee (IACUC) approval no. 2568] were used. F3 generation animals were then shipped and marked on receipt at the University of Texas at Austin (IACUC approval no. A4107-01) by using a double-blind procedure for behavioral studies. All were the F3 descendents of F0 females treated during gestation between embryonic day (E)8 and E14 with daily i.p. injections of the dimethyl sulfoxide buffer alone (control lineage) or 100 mg/kg dose of vinclozolin (EDC lineage) (2). Control- and EDC-lineage males and females were from two litters each (n = three from each of the four litters, all having an ≈0.5 sex ratio). All animals were between PND 90–120 when tested in the mate preference trials; each battery of mate preference tests was performed in a 12-day period. At this age, there are no differences in body weight within each sex. The animals show normal tissue development, and fertility is not impaired. With age, disease manifests, such that by PND 180–420, 85% of the EDC-lineage males have at least one of the following phenotypes: prostate and kidney lesions, immune system abnormalities, testis abnormalities and azoospermia, or tumors; these conditions normally do not manifest except in aged (24 months) untreated rats (2, 3). The odor-salience trials were performed when males were PND 403 (11 months) and the females PND 458 (15 months). At that time, the EDC-lineage males had the expected reduced testis size and tumors.
All individuals were gonadally intact, and both males and females from both lineages have normal circulating levels of sex steroid hormones (6). However, because the females did not exhibit coordinated estrous cycles, they were hormone-treated before the test with a s.c. injection of 50 μg of estradiol benzoate (EB) at 0800 h, followed by an injection of 500 μg of progesterone 48 h after the EB injection; behavioral tests were administered 4 h later. The rats were housed in the same room in same-sex pairs in standard translucent polycarbonate rat cages (46 × 24 × 20.5 cm), except for testing done in another room. Animals were maintained on a reversed 12:12-h light:dark cycle (lights off at 1100 h) at constant room temperature (23–25°C).
Mate-Preference Behavioral Analysis.
After habituation to the testing arena, each individual was tested individually (when used as an experimental subject) or in pairs (when used as stimulus animals) with all individuals; the order of the testing was rotated during the course of both the male and female trials. All tests were conducted during the dark phase of the light cycle, beginning at 1200 h, 4 h after the progesterone injection, in a room illuminated with low levels of red light. Before trials, to confirm that females were receptive, each female was placed with a sexually experienced but otherwise experimentally naïve male; all females exhibited robust lordosis (arched back and lifted head posture) in response to mounting by the male.
Partner preference tests consisted of placing an individual (male or female) in the center of a large three-chamber glass-testing arena (122 × 46 × 54 cm). At either end was a small compartment (28 × 28 × 12.5 cm) containing the stimulus rats separated by a wire-mesh barrier to allow exchange of olfactory, visual, and tactile cues. The area directly in front of the stimulus cage was marked by tape. Tests were conducted 2 h after the onset of the dark cycle under red-light illumination and lasted 10 min; all tests were videotaped for further review and analysis. At the end of each test, all animals were removed, and the entire testing arena was washed with a household cleaner and then wiped down with 70% ethanol to remove scent marks and residual odors. All males were tested with both types of females as stimulus animals (72 trials), and all females were tested with both types of males as stimulus animals (72 trials).
The videotaped trials were analyzed by using JWatcher v1.0 (www.jwatcher.ucla.edu) computer software to quantify the behavior of each experimental animal. Time spent with a stimulus animal was recorded as soon as all four paws of the experimental animal crossed over the line of tape marking the boundary of that stimulus animal's compartment. As soon as one paw crossed over the tape back into the center compartment, the time recorded with the experimental animal was stopped. Preference behaviors were defined as those directed to the stimulus animal and included time spent in contact with the wire mesh separating the experimental and stimulus animal (Wire Mesh), during which the animals often touched noses through the wire mesh engaging in facial investigation (Facial Investigation) and contacted the Plexiglas surface surrounding the front of the stimulus cage; the cumulative total time in these preference behaviors toward each stimulus animal was also calculated (Total). Other activity measures included time spent: grooming (Grooming), undirected walking and sniffing (Walking), standing on hind paws and sniffing with nose pointed upward (Standing/Sniffing), still with minimal head movement (Still), contacting the walls of the test cage (Glass), and time in the center compartment (Center). Videos demonstrating the test can be viewed as supporting information (SI) Movie 1.
Odor-Salience Analysis.
All behavioral testing occurred over a 2-day period, during the dark cycle, in the laboratory colony room where the animals were housed by using a modification of a novel-odor recognition test developed from a method described by Tillerson et al. (37). For the familiarization phase of the task, animals were removed from group-housing cages, weighed, and rehoused singly in identical translucent cages with removable wire tops. Once singly housed, animals remained in these test cages for the duration of the experiment. During the initial 24-h familiarization period, four 1-inch-round wooden beads with a small hole through the center (CraftWorks, Seattle, WA) were introduced into the test cages to acquire the odor of the animal and serve as familiar odors for subsequent use in the experiment. Beads were also introduced into the cages of odor donors removed from their group housing, one EDC lineage and one control lineage, whose cages had not been changed for 1 week to allow for a buildup of odor. Wood beads incubated in these odor-donor cages provided novel odors for the upcoming task.
The day after the familiarization phase, the novel-odor test was conducted. For this phase of the task, rats explored two unfamiliar novel odor beads taken from different opposite-sex odor-donor cages, one EDC and one control lineage, two unfamiliar novel odor beads taken from different same-sex odor donor cages, one EDC and one control lineage, and one familiar (own-cage) odor. The beads were placed near the front of the testing cage, and the rats were allowed 1 min to actively approach and smell a bead. The first approach made during this period initiated the timing of a 1-min trial. Exploration time for each of the five beads was recorded as the amount of time spent actively smelling and contacting individual beads.
All of the hormone-treated female rats, six EDC and six control lineage, explored the odors of two novel males, one EDC lineage and one control lineage, in the presence of two novel female odor beads each from an EDC- and a control-lineage female, and a bead that had their own (familiar) odor. All of the male rats, six EDC lineage and six control lineage, explored the novel odor beads of hormone-treated EDC- and control-lineage females, in the presence of two novel male odor beads each from an EDC- and a control-lineage male, and a bead that had their own (familiar) odor, following the procedure outlined above. Percentages of total exploration time were calculated by dividing the mean exploration times on each individual odor by the total time spent exploring any or all of the odors during the 1-min trial.
Statistical Analysis.
For each data set (male or female as the experimental individual), a 2 × 2 ANOVA with repeated measures was conducted to assess the main effect of male and female treatment and the interaction between male and female treatment on the behavior of the experimental animals with the two groups of stimulus animals (SPSS, Ver. 13.0; SPSS, Chicago, IL). Data for each experimental animal toward each opposite-sex pair of stimulus animals were averaged into a single score, resulting in n = 6 for comparisons. Two-tailed t tests were performed for differences in the total time experimental animals spent in center with the two groups of stimulus animal and for the proportion of center time the experimental animal spent exhibiting preference behaviors.
Supplementary Material
Acknowledgments
We thank Jim Bull and Michael Ryan for commenting on the manuscript and Jill Griffin for assistance in preparation of the manuscript. The work was supported in part by Grants MH 068273 (to D.C.), ES 12272 and ES 07784 (to A.C.G.), and ES 012974 (to M.K.S.).
Abbreviations
- EDC
endocrine-disrupting chemicals
- PND
postnatal day.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610410104/DC1.
References
- 1.McLachlan JA. Endocr Rev. 2001;22:319–341. doi: 10.1210/edrv.22.3.0432. [DOI] [PubMed] [Google Scholar]
- 2.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anway MD, Leathers C, Skinner MK. Endocrinology. 2006;147:5524–5541. doi: 10.1210/en.2006-0987. [DOI] [PubMed] [Google Scholar]
- 4.Newbold RR, Padilla-Banks E, Jefferson WN. Endocrinology. 2006;147:S11–S17. doi: 10.1210/en.2005-1164. [DOI] [PubMed] [Google Scholar]
- 5.Crews D, McLachlan JA. Endocrinology. 2006;147:S4–S10. doi: 10.1210/en.2005-1122. [DOI] [PubMed] [Google Scholar]
- 6.Anway MD, Skinner MK. Endocrinology. 2006;147:S43–S49. doi: 10.1210/en.2005-1058. [DOI] [PubMed] [Google Scholar]
- 7.Chang HS, Anway MD, Rekow SS, Skinner MK. Endocrinology. 2006;147:5515–5523. doi: 10.1210/en.2006-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guerrero-Bosagna C, Sabat P, Valladares L. Evol Dev. 2005;7:341–350. doi: 10.1111/j.1525-142X.2005.05033.x. [DOI] [PubMed] [Google Scholar]
- 9.Zala MS, Penn DJ. Anim Behav. 2004;68:649–664. [Google Scholar]
- 10.Carson HL. Proc Natl Acad Sci USA. 2003;100:6584–6587. doi: 10.1073/pnas.0732174100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crews D. In: Introduction to Behavioral Endocrinology. Becker J, Breedlove M, Crews D, editors. Cambridge, MA: MIT Press/Bradford Books; 1992. [Google Scholar]
- 12.Gowaty PA, Steinichen R, Anderson WW. Evol Int J Org Evol. 2002;56:2537–2540. doi: 10.1111/j.0014-3820.2002.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 13.Stunden CE, Bluhm CK, Cheng KM, Rajamahendran R. Theriogenology. 1999;52:435–446. doi: 10.1016/S0093-691X(99)00141-7. [DOI] [PubMed] [Google Scholar]
- 14.Drickamer L, Gowaty PA, Wagner D. Anim Behav. 2003;65:105–114. [Google Scholar]
- 15.Erskine MS, Lehmann ML, Cameron NM, Polston EK. Behav Brain Res. 2004;153:295–315. doi: 10.1016/j.bbr.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 16.Boehm T, Zufall F. Trends Neurosci. 2006;29:100–107. doi: 10.1016/j.tins.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Jacob S, McClintock MK, Zelano B, Ober C. Nat Genet. 2002;30:175–179. doi: 10.1038/ng830. [DOI] [PubMed] [Google Scholar]
- 18.Richardson D, Komdeur J, Burke T, von Schantz T. Proc R Soc London Ser B. 2005;272:759–767. doi: 10.1098/rspb.2004.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sommer S. Front Zool. 2005;2:16. doi: 10.1186/1742-9994-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ziegler A, Kentenich H, Uchanska-Ziegler B. Trends Immunol. 2005;26:496–502. doi: 10.1016/j.it.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Gasper JS, Shiina T, Inoko H, Edwards SV. Genomics. 2001;75:26–34. doi: 10.1006/geno.2001.6596. [DOI] [PubMed] [Google Scholar]
- 22.Spies T, Blanck G, Bresnahan M, Sands J, Strominger JL. Science. 1989;243:214–217. doi: 10.1126/science.2911734. [DOI] [PubMed] [Google Scholar]
- 23.Cohen S. Mol Biol Evol. 2002;19:1870–1880. doi: 10.1093/oxfordjournals.molbev.a004011. [DOI] [PubMed] [Google Scholar]
- 24.Zheng WM, Yoshimura Y, Tamura T. J Reprod Fertil. 1998;114:45–54. doi: 10.1530/jrf.0.1140045. [DOI] [PubMed] [Google Scholar]
- 25.McClintock MK. In: Hormones, Brain and Behavior. Pfaff D, Arnold A, Etgen A, Faahrbach S, Rubin R, editors. Vol. 1. San Diego: Academic; 2002. pp. 797–870. [Google Scholar]
- 26.Isles AR, Baum MJ, Ma D, Keverne EB, Allen ND. Nature. 2001;409:783–784. doi: 10.1038/35057323. [DOI] [PubMed] [Google Scholar]
- 27.Bressler SC, Baum MJ. Neuroscience. 1996;71:1063–1072. doi: 10.1016/0306-4522(95)00493-9. [DOI] [PubMed] [Google Scholar]
- 28.Halem HA, Baum MJ, Cherry JA. J Neurosci. 2001;21:2474–2480. doi: 10.1523/JNEUROSCI.21-07-02474.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inamura K, Kashiwayanagi M, Kurihara K. Eur J Neurosci. 1999;11:2254–2260. doi: 10.1046/j.1460-9568.1999.00646.x. [DOI] [PubMed] [Google Scholar]
- 30.Kimoto H, Haga S, Sato K, Touhara K. Nature. 2005;437:898–901. doi: 10.1038/nature04033. [DOI] [PubMed] [Google Scholar]
- 31.Kumar A, Dudley CA, Moss RL. J Neurosci. 1999;19:RC32. doi: 10.1523/JNEUROSCI.19-20-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamaguchi T, Inamura K, Kashiwayanagi M. Brain Res. 2000;876:211–214. doi: 10.1016/s0006-8993(00)02651-2. [DOI] [PubMed] [Google Scholar]
- 33.Armstrong SD, Robertson DH, Cheetham SA, Hurst JL, Beynon RJ. Biochem J. 2005;391:343–350. doi: 10.1042/BJ20050404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clodfelter KH, Holloway MG, Hodor P, Park SH, Ray WJ, Waxman DJ. Mol Endocrinol. 2006;20:1333–1351. doi: 10.1210/me.2005-0489. [DOI] [PubMed] [Google Scholar]
- 35.Morrow BA, Elsworth JD, Roth RH. Synapse. 2002;46:11–18. doi: 10.1002/syn.10109. [DOI] [PubMed] [Google Scholar]
- 36.Newton I. Population Limitation in Birds. London: Academic; 1998. [Google Scholar]
- 37.Tillerson JL, Caudle WM, Parent JM, Gong C, Schallert T, Miller GW. Behav Brain Res. 2006;172:97–105. doi: 10.1016/j.bbr.2006.04.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.