Abstract
Bacterial infections and other pathologic conditions induce complex dysfunctions of the hypothalamic–pituitary–thyroid axis, collectively known as nonthyroidal illness (NTI). To explore the pathogenesis of bacterial NTI, we injected Mycobacterium tuberculosis extracts or Escherichia coli LPS in mice lacking key components of the Toll-like receptor or crystallizable fragment (Fc) receptor pathways. In wild-type mice, the bacterial components induced a hypothyroidism characterized by elements of both hypothalamic and thyroidal dysfunction. This NTI hypothyroidism did not develop in mice lacking the MyD88 adaptor or in those with a reduced number of mast cells. The hypothyroid responsiveness to LPS, however, was restored upon reconstitution with mast cells derived from the bone marrow of wild-type donors. In addition to bacterial components, whole immunoglobulins induced NTI hypothyroidism in wild-type mice, but not in those lacking activating Fc receptors or mast cells. The study demonstrates a link between Toll-like and Fc receptor signaling and thyroid gland function, uncovering a role of mast cells in murine NTI.
Keywords: innate immunity, euthyroid sick syndrome, mastocyte, hypothyroidism
Nonthyroidal illness (NTI) is a common clinical condition defined as the biochemical changes of the hypothalamic–pituitary–thyroid axis that occur in patients suffering from illnesses not primarily originating in the thyroid (1, 2). These illnesses include bacterial infections, burns, myocardial infarction, respiratory distress syndrome, cirrhosis, end-stage renal disease, psychosis, and starvation. The key serum biochemical changes in NTI, although varying with the type and severity of the initiating illness, consist of: (i) increased reverse tri-iodo-thyronine (T3), principally due to decreased activity of type I deiodinase (3), an enzyme in peripheral tissues (mainly liver) that converts reverse T3 to di-iodo-thyronine and, in decreasing order of affinity, thyroxine (T4) to T3 and T3 to di-iodo-thyronine; (ii) decreased T3, also secondary to decreased type I deiodinase activity (4); (iii) decreased T4 more likely due to a reduced binding capacity and/or affinity of serum carrier proteins for T4, given that the T4 production rate appears similar to that of healthy euthyroid individuals with low or absent T4-binding globulin (5, 6); and (iv) inappropriately normal or low levels of thyroid stimulating hormone (TSH) with respect to the decreased thyroid hormone levels. This defective TSH production is likely of hypothalamic origin in light of demonstrated reduction in TSH-releasing hormone (TRH) secretion from paraventricular nucleus neurons (7–9) and blunting of the physiological nocturnal TSH surge (10). It is unclear whether the thyroid gland is directly affected during NTI. An autopsy study showed that thyroids from deceased patients with chronic illness had lower weight, smaller follicles, and less colloid than thyroids from suddenly deceased, previously healthy controls (11).
NTI has an incompletely understood pathogenesis (9). Some scholars consider it a salutary, physiologic adaptation to illness (to conserve energy by suppressing the catabolic effects of thyroid hormones), whereas others consider it a true pathologic state. Consequently, a frequent clinical debate is whether to administer synthetic T4 (12) or not (13) to patients with NTI, although the majority opinion opposes T4 administration. To gain mechanistic insights into the pathogenesis of bacterial NTI, numerous experiments have been performed in humans, mice, and rats by using injections of LPS. In humans, LPS induces flu-like symptoms (fever, chills, general malaise, muscle ache, headache, exacerbated sensitivity to light, and nausea), monocytopenia (14), and release of proinflammatory cytokines, including IL-1, IL-6, and TNF-α (15). Serum T4, T3, and TSH decrease, reaching a nadir after 6 h, and then return to baseline by 15 h (16). In mice and rats, LPS induces similar systemic illness (decreased food intake and physical activity, piloerection, hypothermia, and chills) (17); release of proinflammatory cytokines, including TNF-α, IL-6 (17), IL-12 (18), and IL-18 (19); and decreased serum T4 and T3 (up to 30 h after injection).
All these experiments have implicated proinflammatory cytokines as the key mediators of bacterial NTI. It has remained unclear, however, what cell releases the pathogenic cytokines and through which signaling pathway. In this study, we demonstrate a pivotal role of mast cells in the pathogenesis of bacterial NTI. Using the Toll-like receptor (TLR) and crystallizable fragment (Fc) receptor pathways, mast cells initiate the elements of thyroid dysfunction typical of NTI, linking the innate immune system to thyroid pathophysiology.
Results
The Decrease in T4 Is Independent of Antigen and Strain.
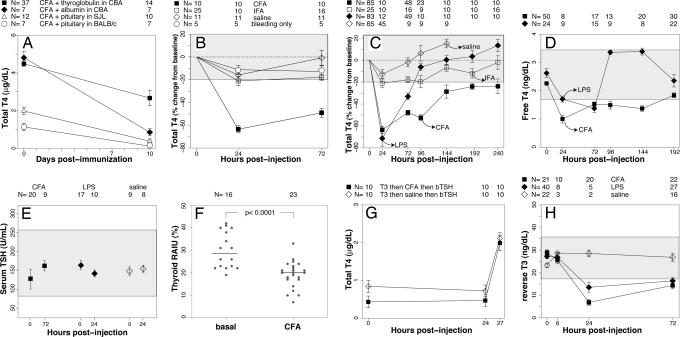
During experiments performed to induce experimental autoimmune thyroiditis [two injections of thyroglobulin emulsified in complete Freund's adjuvant (CFA) on days 0 and 7], we noticed that serum T4 was significantly lower on day 10 than at baseline (Fig. 1A, P < 0.0001 for all four groups). This day-10 decrease occurred at a time when no pathological lesions of the thyroid were present and could not, therefore, be explained by the autoimmune damage to thyroid follicular cells. The decrease in T4 was independent of the antigen used because it occurred when CFA was mixed with thyroglobulin, BSA, or pituitary proteins. It was also independent of the strain because it occurred in CBA, SJL, and BALB/c mice.
Fig. 1.
Thyroid axis changes observed during bacterial NTI. (A) The decrease in serum T4 is independent of antigen and strain. After induction of experimental autoimmune thyroiditis with thyroglobulin (filled squares), T4 decreased significantly on day 10. A similar decrease in T4 was observed when CFA was mixed with BSA (filled diamonds) or pituitary proteins (open symbols). The decrease in T4 occurred in CBA (filled symbols), SJL (open triangles), and BALB/c (open circles) mice (P < 0.0001 vs. day 0 in all groups). Data represent the mean ± SE. The numbers above the graph indicate how many mice were used in each group at each time point. (B) The decrease in T4 is caused by the mycobacterial component present in CFA. Injection of CFA alone (filled squares) reproduced the decrease in T4. IFA (open squares) induced a milder decrease in T4 (−20%), as did saline injection (−16%, open diamonds) or the retrobulbar bleeding only (−13%, open circles). Therefore, a range of 0 ± 20% (the gray-shaded area) was considered as the maximum variation in serum T4 observable in experiments by using controls. (C) The decrease in T4 induced by CFA is longer than that induced by LPS. The decrease in T4 induced by LPS (filled diamonds) reached a nadir on day 1 and disappeared on day 4. In contrast, the decrease induced by CFA (filled squares) was maximal between days 1 and 4, and then ameliorated but never returned to the normal range up to 10 days after injection. Open diamonds and open squares indicate the T4 kinetics after saline or IFA injection, respectively. (D) The decrease in total T4 is accompanied by a decrease in free T4. Free T4 followed a trend similar to that described for total T4 after LPS (filled diamonds) or CFA (filled squares) injection. The gray-shaded area represents the normal free T4 range (5th to 95th percentile) in C57BL/6 mice at day 0. (E) Serum TSH levels are inappropriately normal for the decreased thyroid hormone levels. Serum TSH levels did not change significantly 1 day after LPS injection or 3 days after CFA injection, suggesting a central origin for the thyroid hormone alterations. (F) The RAIU is decreased in response to CFA injection. The thyroidal uptake of radioactive iodine was significantly lower in CFA-injected mice than in normal littermates. Bars indicate the median. (G) Injection of bovine TSH overcomes the impaired thyroid function. After suppressing thyroid function by T3 injections (total T4 lower than 1 μg/dl at time 0), administration of bovine TSH induced a similar T4 secretion in mice with ongoing NTI (CFA group, filled squares) and in mice without NTI (saline controls, open diamonds). (H) Reverse T3 decreases during bacterial NTI. Upon induction of NTI by CFA (filled squares) or LPS (filled diamonds), reverse T3 significantly decreased at day 1 after injection and remained lower than baseline at day 3. No significant changes in serum-reverse T3 were observed in mice injected with saline (open diamonds). These results indicate an adequate activity of type I deiodinase. The gray-shaded area represents the normal reverse T3 range (5th to 95th percentile) in C57BL/6 mice at day 0.
The Decrease in T4 Is Caused by the Mycobacterial Component of CFA.
We then tested whether the effect could be due to the dried M. tuberculosis present in CFA by comparing a single injection of CFA to a single injection of incomplete Freund's adjuvant (IFA). CFA alone reproduced the T4 decline, whereas IFA did not (Fig. 1B). The CFA effect was most remarkable 1 day after the injection, when the T4 was 63 ± 9% lower than baseline (P < 0.0001), but persisted also at day 3 (−49 ± 12%, P < 0.0001). IFA induced a milder decrease in T4 (≈−20%), which was likely due to the experimental procedure (injection and retrobulbar bleedings), given that a similar decrease was also observed during saline injection (−16% at day 1) or bleedings only (−13% at day 1). Consequently, in subsequent experiments, we considered a range of 0 ± 20% (gray-shaded area) as the maximum variation in serum T4 observable in all controls.
The Decrease in T4 Induced by CFA Lasts Longer than That Induced by LPS.
The drop in T4 induced by LPS (Fig. 1C) reached a nadir at day 1 (−71 ± 24%, P < 0.0001), persisted (although milder) at day 3 (−33 ± 21%, P = 0.001), and disappeared by day 4 after injection. In contrast, the drop induced by CFA was more prolonged, never returning to the normal range even at day 10 after injection. Specifically, it was maximal at day 1 (−63 ± 9%), but not significantly different from that present at day 4 (−52 ± 13%, P = 0.25 vs. day 1). After day 4, T4 increased but still remained beneath the lower limit of the normal range (at days 8 and 10, T4 was 24 ± 9% < day 0, P = 0.001). Overall, LPS and CFA induced similar changes during the early phases (up to 3 days after injection) of NTI and were therefore used interchangeably when studying early time points. Starting on day 4, however, thyroid function normalized in the soluble LPS model, whereas it remained decreased in the depot CFA model. These results suggest that CFA induces a chronic form of bacterial NTI that more closely resembles the human counterpart.
The Decrease in Total T4 Is Accompanied by a Reduction in Free T4.
Free T4 followed a trend similar to that described for total T4, indicating a true hypothyroidism and not a reflection of reduced binding capacity and/or affinity of the serum carrier proteins. After LPS injection (Fig. 1D), free T4 began to decrease on day 1 (P = 0.023 vs. day 0), reached a nadir on day 3 (P < 0.0001), and normalized by day 4. After CFA injection, free T4 was significantly lower on day 1 (0.99 ng/dl vs. 2.26 ng/dl on day 0, P < 0.0001) and remained lower up to 8 days after injection, confirming the earlier total T4 findings. These free T4 results, obtained by using a competitive RIA kit, were confirmed in a smaller subset of mice by the direct equilibrium dialysis method (data not shown). The hypothyroidism and kinetics of T4 were also validated by measuring total T3 [supporting information (SI) Fig. 3A].
The Hypothyroidism in Bacterial NTI Is Mainly Due to a Hypothalamic Dysfunction and Can Be Overcome by TRH Administration.
Serum TSH levels did not change significantly 1 day after LPS injection (Fig. 1E) or 3 days after CFA injection despite the clearly reduced levels of total T4, free T4, and T3 described above. These inappropriately normal TSH levels suggested a central (i.e., hypothalamic-pituitary) origin of the hypothyroidism. To distinguish between pituitary and hypothalamic involvement, we injected human TRH (or saline) 5.25 h after LPS (or saline) injection and then measured TSH and total T4 45 min thereafter.
TSH markedly increased in both mice with ongoing NTI (SI Fig. 4A) and, to similar levels, in saline controls. Despite the criticisms that can be raised about the TRH test, the results suggested a normal pituitary reserve and a hypothalamic origin of the central hypothyroidism.
Total T4 decreased as expected in response to LPS, but this decrease was markedly attenuated by TRH (SI Fig. 4B), whereas it progressed in saline controls (T4 was −53% at 5.25 h and −75% at 6 h). The attenuated T4 decline suggested that the thyroid gland retained the ability to respond to an increased pituitary release of TSH. The experiment also suggested that TSH has a normal biological activity during bacterial NTI because TRH induced a similar increase in thyroidal T4 output both in mice that received LPS at baseline (filled diamonds) and in those that did not (open circles).
The Hypothyroidism in Bacterial NTI Could Also Be Due to an Impairment of Thyroid Gland Function, Which Is Overcome by TSH Administration.
To evaluate whether the thyroid gland is intrinsically dysfunctional during bacterial NTI, we measured the thyroid radioiodine uptake 3 days after CFA injection. Thyroids from CFA-injected mice showed a significantly lower radioiodine uptake (19.1 ± 5.8%) than those from control littermates (31.7 ± 6.6%, P < 0.0001, Fig. 1F). The reduced uptake, in light of TSH levels within the normal range, could reflect a direct inhibition of thyroid function by inflammatory products. However, thyroid secretion of T4 in response to bovine TSH stimulation increased similarly in mice with (Fig. 1G, CFA) or without (saline controls) NTI, suggesting that if a direct block of the thyroid is present during bacterial NTI it can be overcome by supraphysiological doses of exogenous TSH.
The Hypothyroidism in Bacterial NTI Is Not Due to an Impaired Peripheral Tissue Type I Deiodination Because Reverse T3 Levels Are Decreased.
To assess the contribution of peripheral tissue type I deiodination, we measured serum-reverse T3 at 6, 24, and 72 h after CFA or LPS injection. Reverse T3 significantly decreased at 24 h after injection in both the CFA (Fig. 1H) and LPS group (6.85 ± 3.99 ng/dl for CFA and 13.52 ± 4.84 for LPS vs. average baseline values of 26.51 ± 6.78, P < 0.0001). Reverse T3 increased slightly on day 3, but still remained significantly lower than baseline. No significant changes in reverse T3 were seen upon saline injection. These results suggest that peripheral tissue type I deiodination is not defective in murine NTI, and that the decrease in reverse T3 chiefly reflects decreased thyroidal output of its precursor T4. Reverse T3 levels, in fact, correlated strongly with T4 levels (SI Fig. 3B).
The Hypothyroidism in Bacterial NTI Depends upon the Activation of the TLR–MyD88 Pathway.
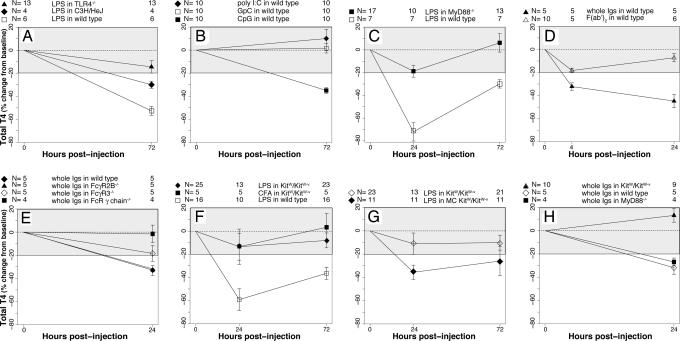
To assess the contribution of the innate immune system in the previously described thyroid dysfunctions, we tested specific activators and adaptors of the TLR pathway. TLR4-deficient mice failed to develop NTI hypothyroidism in response to LPS injection (Fig. 2A). In addition, C3H/HeJ mice, which harbor a spontaneous mutation of the TLR4 gene that makes them hyporesponsive to LPS, developed milder NTI changes than wild-type littermates.
Fig. 2.
Contribution of innate immunity and mast cells to bacterial NTI. (A) Ligation of TLR4 induces NTI. LPS failed to induce NTI in TLR4-deficient mice (filled triangles). In C3H/HeJ mice (filled diamonds), LPS induced a milder hypothyroidism than in wild-type controls (open squares). (B) Ligation of TLR9 induces NTI. Injection of CpG oligodeoxynucleotide (a ligand for TLR9) caused hypothyroidism (filled squares), whereas the control GpC nucleotide (open squares) did not. In addition, injection of poly I:C (a ligand for TLR3, filled diamonds) had no effect. (C) The TLR–MyD88 pathway is required to mediate bacterial NTI. The NTI hypothyroidism induced by LPS disappeared in MyD88-deficient mice (filled squares), whereas it was present in wild-type littermates (open squares). (D) Injection of immunoglobulins also induces NTI. Injection of normal rabbit immunoglobulins (filled triangles) significantly decreased T4, whereas injection of F(ab′)2 fragments (open triangles) did not. The hypothyroxinemia was milder than that induced by bacterial pathogens. (E) Ligation of specific Fc receptors mediates the NTI induced by Ig injection. The hypothyroidism induced by Ig was abrogated in mice lacking the common γ chain of Fc receptors (filled squares), attenuated in those lacking the FcγR3 (open diamonds), and similar to wild type (filled diamonds) in those lacking the FcγR2B (filled triangles). (F) Mast cells are key players in NTI induction. Mast cell-deficient mice did not develop NTI hypothyroidism in response to either LPS (filled diamonds) or CFA (filled squares) injection. Wild-type littermates (open squares) are shown as control. (G) Mast cell-deficient mice reconstituted with bone marrow-derived wild-type mast cells regained the response to NTI induction. Bone marrow-derived mast cell reconstitution restored the ability of KitW/KitW−v mutant mice to develop hypothyroidism upon NTI induction (filled diamonds), whereas sham reconstitution did not (open diamonds). (H) TLR and Fc receptor pathways acted independently during NTI induction. Ig injection decreased T4 in MyD88-deficient mice (filled squares) and wild-type controls (open diamonds) but not in mast cell-deficient mice (filled triangles), indicating that TLR and Fc receptor pathways responded independently to the different NTI stimuli.
Injection of CpG oligodeoxynucleotide induced the characteristic NTI hypothyroidism (Fig. 2B), whereas injection of the control GpC nucleotide did not, indicating that ligation of TLR9 was capable of initiating NTI. Of the 10 well characterized murine TLRs, 9 (except TLR3) use the cytosolic protein MyD88 as an adaptor to carry the signal initiated by receptor ligation. Therefore, we tested MyD88-deficient mice for their ability to develop bacterial NTI. The decrease in serum T4 disappeared when bacterial NTI was induced in MyD88-deficient mice (Fig. 2C). These results show that the hypothyroidism of bacterial NTI requires the activation of the TLR–MyD88 pathway, which ultimately leads to production of proinflammatory cytokines.
To address the specificity of TLR recognition, we studied the synthetic polyinosine-polycytidylic (poly I:C) nucleotide that, as a mime of viral infection, activates TLR3. Injection of poly I:C was not capable of inducing NTI (Fig. 2B), indicating that the TLR3 pathway, which uses TRIF as an adaptor and leads to the expression of interferons and the IFN-induced genes, plays little role in microbial NTI.
The Hypothyroidism in Bacterial NTI Also Depends upon the Activation of the Fc Receptor Pathway.
We serendipitously found that injection of normal rabbit serum induced a decrease in serum T4 that was similar, although less severe, to that induced by bacterial stimuli. We thus tested the contribution of the Fc receptor pathway during NTI. Normal rabbit immunoglobulins significantly decreased T4 (Fig. 2D, filled triangles), whereas Ig fragments lacking the Fc portion [F(ab′)2, open triangles] did not. The hypothyroidism induced by Ig injection was abrogated when the experiment was performed in mice that lack the common γ chain of Fc receptors (Fig. 2E), attenuated in mice that lack the FcγR3, and similar to wild-type mice lacking the inhibitory FcγR2B. These results are in keeping with the known activating (FcγR3) or inhibitory (FcγR2B) functions of Fc receptors and demonstrate that Ig injection markedly, although briefly, suppresses serum T4 levels.
Mast Cells Are Key Players in Bacterial and Ig-Induced NTI.
We next sought to identify the cells that, via activation of the TLR–MyD88 and Fc receptor pathways, mediated the described thyroid dysfunctions. Lymphocytes proved not to be involved in the NTI that follows LPS injection (SI Fig. 5). In fact, the decrease in T4 developed equally well in mice lacking natural killer lymphocytes (filled squares), γ-δ T lymphocytes (open circles), or mature T and B lymphocytes (open diamonds), as in wild-type controls. On the contrary, mast cells, known to respond promptly to ligation of their TLR and Fc receptors (20, 21), proved to be key players in NTI. The NTI hypothyroidism, in fact, could not be induced in KitW/KitW−v mutant mice (which have a decreased number of mast cells) in response to LPS (Fig. 2F) or CFA, whereas it developed promptly in control mice. Specifically, after LPS injection, T4 decreased only 13% on day 1 and 8% on day 3 (P values not significant vs. day 0 and between day 1 and day 3); similar decreases were seen after CFA injection. Furthermore, analysis of skin mast cells in C57BL/6 wild-type mice showed that the percentage of clearly degranulating mast cells (SI Fig. 6) was significantly higher in response to CFA than to saline injection (51% vs. 21%, P < 0.0001).
To confirm the role of mast cells in bacterial NTI, we reconstituted KitW/KitW−v mutant mice with mast cells derived from the bone marrow of wild-type C57BL/6 littermates. The bone marrow-derived mast cells were CD45 (100%), c-kit (96%), and FcεR1 (96%) positive (data not shown); they also expressed, in keeping with previous reports (22), low levels of TLR4 (data not shown). When injected into KitW/KitW−v mutant mice, the bone marrow-derived mast cells restored the ability to develop NTI in response to LPS (Fig. 2G, filled diamonds), whereas sham reconstitution (open diamonds) had no effect. The decrease in serum T4 in mast cell-reconstituted mice (−35% at day 1, P = 0.022 vs. day 0; −26% at day 3, P = 0.09 vs. day 0; and not significant vs. day 1) was milder than the decrease observed in the other strains. This amplitude difference likely reflects the notion that injection of bone marrow-derived mast cells reconstitutes mast cells only in certain organs and tissues (lungs, stomach, liver, and spleen), but not in the entire animal, including not in skin, which is the site where NTI-inducing stimuli are injected (23). It might also reflect the low TLR4 expression levels displayed by in vitro-derived mast cells.
LPS-induced NTI was associated with the release of numerous cytokines and chemokines. Deletion of a single cytokine, however, did not prevent the development of NTI (SI Fig. 7).
We finally assessed the response of KitW/KitW−v mutant mice to the injection of rabbit immunoglobulins and compared it to the response of MyD88-deficient mice. Ig injection decreased T4 in MyD88-deficient mice (Fig. 2H) and in wild-type controls, but not in KitW/KitW−v mutant mice, indicating that the TLR–MyD88 and Fc receptor pathways act independently in response to the stimuli capable of inducing NTI.
Discussion
This paper provides a fresh look at the pathogenesis of NTI by revealing that it occurs through activation of the TLR–MyD88 and Fc receptor pathways, and by highlighting a role for mast cells. Mast cells, mainly scattered in skin and mucosae, sample the environment through their TLR and Fc receptors. They have been classically considered the effectors of allergic reactions, capable of releasing into the bloodstream a vast array of cytokines and chemokines, lipid mediators, and granule-associated mediators (20, 21). More recently, mast cells were proven important orchestrators during the early phases of antibody-mediated autoimmune diseases (24). Our study identifies a new role for mast cells as a sensor capable of controlling the homeostatic responsiveness of the hypothalamus–pituitary–thyroid axis. We developed a murine model of bacterial NTI based on injection of CFA. CFA induced a marked and prolonged hypothyroidism (up to 10 days after injection), which disappeared, however, in mast cell-deficient mice. The hypothyroid effect was partially restored upon reconstitution of mast cell-deficient mice with wild-type mast cells. Molecularly, the hypothyroidism occurred via activation of TLRs that use the cytosolic MyD88 adaptor, such as TLR2 (CFA), TLR4 (LPS), and TLR9 (CpG). Interestingly, activation of TLR3 by poly I:C did not induce the typical hypothyroid changes, in keeping with the clinical observation that, with a few exceptions, NTI is rarely seen during acute viral infections, and with the recent report that poly I:C inhibits mast cell adhesion and IgE-mediated degranulation (25).
In addition to bacterial stimuli and the TLR pathway, injection of normal immunoglobulins significantly reduced the thyroid hormone levels. This effect was mediated by mast cells through the activation of specific Fc receptors. In fact, it disappeared in mice lacking stimulatory Fc receptors (FcγR3 and common γ chain), but persisted in those lacking the inhibitory FcγR2B. The two signaling pathways acted independently given that immunoglobulins were still capable of reducing T4 in MyD88-deficient mice. Both pathways, however, required the presence of mast cells, as indicated by the fact that mast cell-deficient mice did not respond to the injection of either bacterial products or immunoglobulins. The notion that injection of immunoglobulins transiently decreases thyroid function is of clinical value given the growing number of conditions that are treated with i.v. immunoglobulins (26).
Other cells of the innate immune system, such as natural killer and γ-δ T cells, played no role in NTI. Although we cannot exclude that other cells, such as dendritic cells and neutrophils not tested in this or previous (27) studies, contribute to the pathogenesis of NTI, mast cells appear to be the prime effectors of the condition due to their rich diversity of TLR and Fc receptors and the variety and abundance of mediators they evoke.
From the endocrine perspective, the present murine model contributes to furthering our understanding of NTI pathogenesis. First, it shows that the NTI hypothyroidism arises mainly from inhibition of hypothalamic TRH release. In fact, TSH values remain inappropriately normal with respect to the reduced thyroid hormone levels, but promptly increase in response to TRH. Second, it suggests that the thyroid gland appears dysfunctional during NTI in light of a reduced radioiodine uptake in the presence of normal TSH levels. This thyroid inhibition could be overcome by exogenous TSH administration after T3 suppression of the hypothalamus–pituitary axis, thus implying a mild and reversible thyroid block. Our results do not solve the controversial issue about the need for T4 therapy in patients with NTI, although they emphasize that the thyroid gland (i.e., the only source of T4) might be dysfunctional. Third, the model shows that reverse T3 decreases during bacterial NTI, in contrast to the typical increase observed in patients. Previous murine studies reported that liver type I deiodinase decreases significantly between 4 and 8 h and normalizes at 24 h after LPS injection (18, 19, 28, 29). Our reverse T3 findings suggest that type I deiodinase activity is not significantly impaired, allowing the degradation of reverse T3 to di-iodo-thyronine. Consequently, we postulate that the decrease in serum-reverse T3 in mice mainly reflects decreased output of its precursor, T4, from the thyroid. The discrepancy between human and murine-reverse T3 levels during NTI should be seen in light of several known differences in thyroid physiology between humans and mice. In mice, the serum T4 half-life is shorter [1 day vs. 1 week (30)], and the fraction of T3 arising from peripheral tissue T4 deiodination is smaller [45% vs. 80% (31)]. Accordingly, if we embrace the hypothesis that hypothyroidism observed during bacterial NTI is a physiologic adaptation to illness to save energy, we can imply that the reduction in thyroid hormones in mice is more efficiently obtained by restraining T4 and T3 secretion from the thyroid rather than by inhibiting peripheral type I deiodination.
Broadly speaking, the study reveals that thyroid hormones, traditionally considered a maintenance type of hormone, respond quickly to conditions of acute stress. In addition, for the many experimentations based on CFA immunization, the measurement of serum T4 emerges as a rapid and economic means to assess the efficacy of the immunization.
In conclusion, we demonstrated that TLR and Fc receptor pathways, through activation of mast cells, release an array of mediators that induce NTI acting at the hypothalamus and thyroid levels. The study defines a mechanism to regulate the thyroid gland, linking innate immunity to thyroid pathophysiology.
Experimental Procedures
Rodent Cohort.
The study analyzed 823 mice, including 666 wild type and 157 with a targeted (knockout) or spontaneous mutation (SI Table 1). All experiments were conducted in accordance with the standards established by the U.S. Animal Welfare Acts set forth in National Institutes of Health guidelines and the Policy and Procedures Manual of The Johns Hopkins University Animal Care and Use Committee.
NTI-Induction Protocols.
NTI was mainly induced by injection of LPS, CFA, or rabbit immunoglobulins. Twenty-five micrograms of Escherichia coli LPS, serotype 026:B6 (L-2762; Sigma–Aldrich, St. Louis, MO) was injected i.p. once to each mouse. An equal volume (100 μl) of sterile saline was injected as control. Desiccated (killed and dried) Mycobacterium tuberculosis, strain H37Ra (an avirulent strain from Difco Laboratory, Sparks, MD), was suspended in a mixture of paraffin oil and mannide monooleate (F-5506; Sigma–Aldrich) at a concentration of 5 mg/ml to form the CFA. CFA was then emulsified into an equal volume of saline (for a final Mycobacterium concentration of 2.5 mg/ml) and injected s.c. in a volume of 100 μl. In the initial experiments (Fig. 1A), CFA was mixed with an antigen (murine thyroglobulin, BSA, or murine pituitary proteins) and injected twice (on days 0 and 7). For all other experiments, CFA was injected alone and only once. An equal volume of IFA, or sterile saline, was used as control. One hundred thirty micrograms of rabbit immunoglobulins (Jackson ImmunoResearch, West Grove, PA), used as activators of Fc receptors, was injected i.p. As control, F(ab′)2 fragments were injected in the same fashion (130 μg per mouse).
A synthetic oligodeoxynucleotide containing unmethylated CpG motifs (5′-TCCATGACGTTCCTGATGCT-3′; Integrated DNA Technologies, Coralville, IA) was dissolved in sterile Tris buffer at a concentration of 0.1 mM and injected i.p. once in a volume of 100 μl. The control GpC (5′-TCGAGGCTTCTC-3′) was prepared and injected in a similar fashion.
A synthetic poly I:C nucleotide (PN: P-1038; Sigma–Aldrich) was dissolved in sterile saline at a concentration of 2 mg/ml and injected i.p. once in a volume of 100 μl.
Serum Thyroid Hormones, Serum TSH, and Radioactive Iodine Uptake (RAIU) by the Thyroid Gland.
Total T4, free T4, total T3, and reverse T3 were measured by using commercially available radioimmunoassays (Diasorin, Stillwater, MN; ALPCO Diagnostics, Salem, NH). TSH was measured with a highly sensitive double-antibody RIA developed by A. F. Parlow (32), which was capable of distinguishing low-normal from undetectable levels. RAIU was performed as described (33).
Thyroid Stimulation with TSH.
To directly evaluate thyroid gland function after NTI induction, we performed a TSH stimulation test. Mice were pretreated for 4 days with T3 (91990; Sigma–Aldrich) and injected daily i.p. at a dose of 1 μg per mouse to suppress endogenous TSH levels and secondarily reduce T4 (to <1 μg/dl at time 0). T3 was dissolved in 1 M sodium hydroxide at a concentration of 1 μg/μl, diluted in sterile saline to a final concentration of 0.01 μg/μl, and injected i.p. (1 μg T3 per mouse). Mice were then divided into two groups, 10 mice each. In one group, NTI was induced by CFA injection; in the other group, saline was used as control. Twenty-four hours later, all mice received i.p. 6 milli-international units (mIU) of bovine TSH (T8931; Sigma–Aldrich). Three hours after the TSH injection, blood was collected for T4 measurement.
Mast Cell Reconstitution.
Bone marrow cells were obtained from the femurs of wild-type C57BL/6 donors and cultured at 37°C in a 5% CO2 incubator. Cells were plated at a starting density of 1 × 106 cells per ml in RPMI medium 1640, supplemented with 10% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, 0.1 mM nonessential amino acids, 2 mM l-glutamine (Invitrogen, Carlsbad, CA), 50 μM 2-mercaptoethanol, 1 mM sodium pyruvate (Sigma–Aldrich), and 10 ng/ml murine IL-3 (PeproTech, Rocky Hill, NJ). Every week, nonadherent cells were transferred to fresh medium. After 5 weeks of culture, maturation of mast cells was tested by toluidine blue staining (34) and flow cytometry by using monoclonal antibodies to c-kit, FcεR1, TLR4, and CD45. A total of 1 × 107 bone marrow-derived mast cells per mouse were injected i.p. in a volume of 200 μl (same volume of sterile saline was injected as control). Three weeks after mast cell reconstitution, NTI was induced with LPS or CFA as described above.
Statistical Analysis.
The study analyzed eight outcome measures: the five thyroid hormones (total T4, free T4, total T3, reverse T3, and TSH), thyroid RAIU, percent of degranulating skin mast cells, and serum cytokine levels.
Total T4, the most commonly measured outcome, differed significantly at day 0 (i.e., before any injection) among the four strains used in the study (Fig. 1A), confirming what has been reported for other murine strains (35). Specifically, the mean ± SD values for day 0 T4 were 4.46 ± 1.08 μg/dl in 355 C57BL/6, 4.54 ± 0.95 μg/dl in 44 CBA, 1.98 ± 0.70 μg/dl in 12 SJL, and 1.14 ± 0.53 μg/dl in 7 BALB/c mice. Even within the same C57BL/6 strain, T4 differed among batches of mice despite identical supplier, age, sex, and housing conditions. T4 levels followed a bimodal distribution in C57BL/6 mice, with a first peak at 3.56 μg/dl and a second peak at 5.34 μg/dl. In light of this variation, T4 values measured longitudinally were expressed as percent change over the corresponding baseline values, rather than as absolute values. The remaining thyroid hormones (free T4, T3, reverse T3, and TSH) were reported as absolute values. All hormones were analyzed by using a multiple linear regression model with generalized estimating equations, which included as covariates experimental group, time of bleeding, genotype, sex, age, and route of injection.
Differences in thyroid RAIU, mast cell degranulation, and serum cytokines were assessed by using the Wilcoxon's rank-sum test. All analyses were performed by using Stata statistical software, release 9 (Stata Corporation, College Station, TX).
Supplementary Material
Acknowledgments
We thank Drs. Ferruccio Santini, Michele Marinò, Luca Chiovato, and Enio Martino for their insightful criticisms and suggestions. This work was supported in part by National Institutes of Health Grant DK55670 (to P.C.) and Scientific Research (B) from Ministry of Education, Science and Culture of Japan Grant-in-Aid 15390296 (to K.S.).
Abbreviations
- CFA
complete Freund's adjuvant
- Fc
crystallizable fragment
- IFA
incomplete Freund's adjuvant
- NTI
nonthyroidal illness
- RAIU
radioactive iodine uptake
- TLR
Toll-like receptor.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701319104/DC1.
References
- 1.De Groot LJ. Crit Care Clin. 2006;22:57–86. doi: 10.1016/j.ccc.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Fliers E, Alkemade A, Wiersinga WM. Best Pract Res Clin Endocrinol Metab. 2001;15:453–464. doi: 10.1053/beem.2001.0163. [DOI] [PubMed] [Google Scholar]
- 3.Faber J, Thomsen HF, Lumholtz IB, Kirkegaard C, Siersbaek-Nielsen K, Friis T. J Clin Endocrinol Metab. 1981;53:978–984. doi: 10.1210/jcem-53-5-978. [DOI] [PubMed] [Google Scholar]
- 4.Docter R, Krenning EP, de Jong M, Hennemann G. Clin Endocrinol (Oxford) 1993;39:499–518. doi: 10.1111/j.1365-2265.1993.tb02401.x. [DOI] [PubMed] [Google Scholar]
- 5.Kaptein EM, Robinson WJ, Grieb DA, Nicoloff JT. J Clin Invest. 1982;69:526–535. doi: 10.1172/JCI110478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilcox RB, Nelson JC, Tomei RT. Eur J Endocrinol. 1994;131:9–13. doi: 10.1530/eje.0.1310009. [DOI] [PubMed] [Google Scholar]
- 7.Fekete C, Gereben B, Doleschall M, Harney JW, Dora JM, Bianco AC, Sarkar S, Liposits Z, Rand W, Emerson C, et al. Endocrinology. 2004;145:1649–1655. doi: 10.1210/en.2003-1439. [DOI] [PubMed] [Google Scholar]
- 8.Fliers E, Guldenaar SE, Wiersinga WM, Swaab DF. J Clin Endocrinol Metab. 1997;82:4032–4036. doi: 10.1210/jcem.82.12.4404. [DOI] [PubMed] [Google Scholar]
- 9.Lechan RM, Fekete C. J Endocrinol Invest. 2004;27:105–119. [PubMed] [Google Scholar]
- 10.Romijn JA, Wiersinga WM. J Clin Endocrinol Metab. 1990;70:35–42. doi: 10.1210/jcem-70-1-35. [DOI] [PubMed] [Google Scholar]
- 11.De Jongh FE, Jobsis AC, Elte JW. Eur J Endocrinol. 2001;144:221–226. doi: 10.1530/eje.0.1440221. [DOI] [PubMed] [Google Scholar]
- 12.DeGroot LJ. J Endocrinol Invest. 2003;26:1163–1170. doi: 10.1007/BF03349151. [DOI] [PubMed] [Google Scholar]
- 13.Stathatos N, Levetan C, Burman KD, Wartofsky L. Best Pract Res Clin Endocrinol Metab. 2001;15:465–478. doi: 10.1053/beem.2001.0164. [DOI] [PubMed] [Google Scholar]
- 14.Richardson RP, Rhyne CD, Fong Y, Hesse DG, Tracey KJ, Marano MA, Lowry SF, Antonacci AC, Calvano SE. Ann Surg. 1989;210:239–245. doi: 10.1097/00000658-198908000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Poll T, Lowry SF. In: Modulation of Inflammatory Response in Severe Sepsis. Tellado JM, Forse RA, Solomkin JS, editors. Vol 20. New York: Karger; 1995. pp. 18–32. [Google Scholar]
- 16.van der Poll T, Van Zee KJ, Endert E, Coyle SM, Stiles DM, Pribble JP, Catalano MA, Moldawer LL, Lowry SF. J Clin Endocrinol Metab. 1995;80:1341–1346. doi: 10.1210/jcem.80.4.7714108. [DOI] [PubMed] [Google Scholar]
- 17.Boelen A, Platvoet-ter Schiphorst MC, Bakker O, Wiersinga WM. J Endocrinol. 1995;146:475–483. doi: 10.1677/joe.0.1460475. [DOI] [PubMed] [Google Scholar]
- 18.Boelen A, Kwakkel J, Platvoet-ter Schiphorst M, Baur A, Kohrle J, Wiersinga WM. Horm Metab Res. 2004;36:101–106. doi: 10.1055/s-2004-814219. [DOI] [PubMed] [Google Scholar]
- 19.Boelen A, Kwakkel J, Platvoet-ter Schiphorst M, Mentrup B, Baur A, Koehrle J, Wiersinga WM. Eur J Endocrinol. 2004;151:497–502. doi: 10.1530/eje.0.1510497. [DOI] [PubMed] [Google Scholar]
- 20.Galli SJ, Maurer M, Lantz CS. Curr Opin Immunol. 1999;11:53–59. doi: 10.1016/s0952-7915(99)80010-7. [DOI] [PubMed] [Google Scholar]
- 21.Marshall JS. Nat Rev Immunol. 2004;4:787–799. doi: 10.1038/nri1460. [DOI] [PubMed] [Google Scholar]
- 22.Matsushima H, Yamada N, Matsue H, Shimada S. J Immunol. 2004;173:531–541. doi: 10.4049/jimmunol.173.1.531. [DOI] [PubMed] [Google Scholar]
- 23.Wolters PJ, Mallen-St Clair J, Lewis CC, Villalta SA, Baluk P, Erle DJ, Caughey GH. Clin Exp Allergy. 2005;35:82–88. doi: 10.1111/j.1365-2222.2005.02136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benoist C, Mathis D. Nature. 2002;420:875–878. doi: 10.1038/nature01324. [DOI] [PubMed] [Google Scholar]
- 25.Kulka M, Metcalfe DD. Mol Immunol. 2006;43:1579–1586. doi: 10.1016/j.molimm.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 26.Jolles S, Hughes J. Int Immunopharmacol. 2006;6:579–591. doi: 10.1016/j.intimp.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Boelen A, Platvoet-ter Schiphorst MC, van Rooijen N, Wiersinga WM. Eur J Endocrinol. 1996;134:513–518. doi: 10.1530/eje.0.1340513. [DOI] [PubMed] [Google Scholar]
- 28.Boelen A, Kwakkel J, Thijssen-Timmer DC, Alkemade A, Fliers E, Wiersinga WM. J Endocrinol. 2004;182:315–323. doi: 10.1677/joe.0.1820315. [DOI] [PubMed] [Google Scholar]
- 29.Boelen A, Maas MA, Lowik CW, Platvoet MC, Wiersinga WM. Endocrinology. 1996;137:5250–5254. doi: 10.1210/endo.137.12.8940342. [DOI] [PubMed] [Google Scholar]
- 30.van Buul-Offers S, Hackeng WH, Schopman W. Acta Endocrinol. 1983;102:396–409. doi: 10.1530/acta.0.1020396. [DOI] [PubMed] [Google Scholar]
- 31.Chanoine JP, Braverman LE, Farwell AP, Safran M, Alex S, Dubord S, Leonard JL. J Clin Invest. 1993;91:2709–2713. doi: 10.1172/JCI116510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider MJ, Fiering SN, Pallud SE, Parlow AF, St Germain DL, Galton VA. Mol Endocrinol. 2001;15:2137–2148. doi: 10.1210/mend.15.12.0740. [DOI] [PubMed] [Google Scholar]
- 33.Rocchi R, Kunavisarut T, Ladenson PW, Caturegli P. Thyroid. 2006;16:705–706. doi: 10.1089/thy.2006.16.705. [DOI] [PubMed] [Google Scholar]
- 34.Schueller E, Peutsch M, Bohacek LG, Gupta RK. Can J Med Technol. 1967;29:137–138. [PubMed] [Google Scholar]
- 35.Pohlenz J, Maqueem A, Cua K, Weiss RE, Van Sande J, Refetoff S. Thyroid. 1999;9:1265–1271. doi: 10.1089/thy.1999.9.1265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.