Abstract
As important as memory is to our daily functions, the ability to extract fundamental features and commonalities from various episodic experiences and to then generalize them into abstract concepts is even more crucial for both humans and animals to adapt to novel and complex situations. Here, we report the neural correlates of the abstract concept of nests or beds in mice. Specifically, we find hippocampal neurons that selectively fire or cease to fire when the mouse perceives nests or beds, regardless of their locations and environments. Parametric analyses show that responses of nest cells remain invariant over changes in the nests' physical shape, style, color, odor, or construction materials; rather, their responses are driven by conscious awareness and physical determination of the categorical features that would functionally define nests. Such functionality-based abstraction and generalization of conceptual knowledge, emerging from episodic experiences, suggests that the hippocampus is an intrinsic part of the hierarchical structure for generating concepts and knowledge in the brain.
Keywords: bed cell, episodic memory, nest cell, semantic memory, hippocampus
Formation of concepts and abstract knowledge has been traditionally considered to be exclusive hallmarks that define humans and possibly other highly intelligent nonhuman primates (1, 2). Such high levels of abstract cognition play an essential role in guiding our adaptive behaviors in everyday life (3–8). For example, when we check into a hotel, the concept of “bed” in our brains can help us identify the bed effortlessly among various furniture in the room despite the fact that the bed could be drastically distinct in terms of its physical shape or style from the ones that we have used in our homes. Currently, it is not clear how our brain actually generates and encodes such abstract concepts from daily experiences.
Just like the human's ability to recognize beds, we hypothesize that nonprimate animals such as rodents and birds should also possess the ability to recognize a refuge or object that can serve as their nests. Because a nest or bed can vary widely in its physical appearances (e.g., distinct physical shapes and styles, color, and construction materials, etc.), we further hypothesize that internal representations of those objects are likely to require the brain to encode abstract knowledge and concepts into categories beyond the specific shape or form of each item.
To search for the underlying neural correlates, we focused on the mouse hippocampus, because this structure is crucial for the formation of not only memories of events, people, and places (often known as episodic memory) (9–14) but also memories of knowledge, facts, and concepts (also known as semantic memory), as indicated by neurological studies of human patients (3, 5, 15, 16). Indeed, our recent large-scale recording suggests that some of the memory-coding units in the hippocampus seem to be intrinsically involved in extracting commonality and abstract general features from various episodic events (17, 18). Therefore, we set out to investigate the neural encoding of conceptual knowledge of nests or beds in the mouse hippocampus and studied the correlation between the neural activity and behavioral encounters of various types of nests. Our experimental analyses have revealed the existence of hippocampal cells whose firing exhibited dynamic changes when the mice encountered nests or beds.
Results
Nest-Responsive Cells and Their Firing Characteristics.
Although there are many excellent paradigms for the measurement of memory function (14, 15, 19–24), there is no paradigm that would readily permit the molecular and neural analyses of abstract knowledge and concepts in classic genetic models such as mice in which various sophisticated genetic technologies have been developed (21, 25–27). In this study, we propose to use the mouse's natural interactions with various nests as the behavioral paradigm for investigating the neural correlates underlying the representations of conceptual knowledge of objects in the brain.
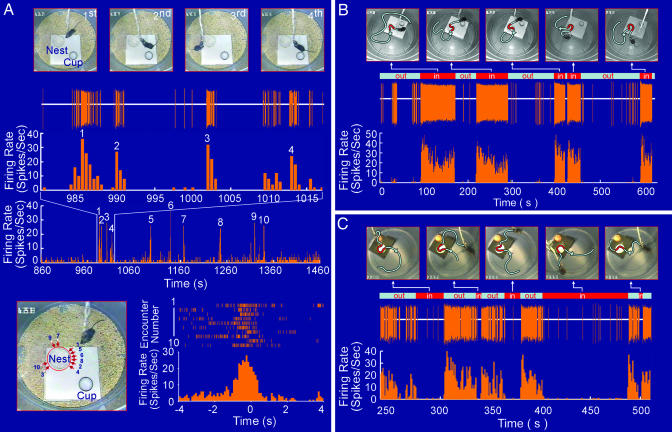
We have used large-scale ensemble recording techniques (18, 28, 29) and monitored single-unit activities in the CA1 region of the mouse hippocampus when the animals were physically interacting with their nests. We have recorded from seven mice and identified eight single units that responded significantly to nests. Based on their spike-discharge patterns [for waveforms and basic firing properties, see supporting information (SI) Fig. 6], these eight cells can be classified into three groups, namely, the transient-on type (Cells #1, #4, and #5), persistent-on type (Cells #2 and #6), and persistent-off type (Cells #3, #7, and #8). For example, Cell #1 from mouse A exhibited transient-on-type responses, that is, it would transiently, but drastically, increase its firing whenever the animal encountered the nest (Fig. 1A). If the animal faced away from the nest, this cell would remain in near silence. Top video snapshots in Fig. 1A illustrate four consecutive encounters of the home nest within a period of 35 s during which the mouse approached the nest from four different angles (the first encounter from the 1 o'clock direction, the second encounter from the 4 o'clock direction, the third encounter from 7 o'clock, and the fourth encounter from 5 o'clock). The recorded spike-discharge pattern (below the video snapshots) shows the time-locked, robust firing every time the mouse encountered the nest. A total of 10 encounters took place during the 10-min-period, and the cell drastically increased its firing at each encounter (Fig. 1A Middle). By marking the time point at which the mouse's nose tip is 1 cm away from the edge of the nest before crossing as time 0, we plotted perievent spike raster and perievent spike histograms (Fig. 1A Bottom). Both analyses show that this cell significantly increased its firing at the averaged rate of 23.7 ± 5.8 Hz (mean ± SD, by using 100-ms bin) upon encountering the nest, in comparison with the basal firing rate of 1.47 Hz. The nest encounter-induced firing was clearly independent of the approaching angles and positions [which are marked by the labeled arrows in the video snapshot (Fig. 1A Bottom Left). It is noteworthy to point out that the cell fired only when the mouse approached the nest edge from the outside and not when it moved out of the nest from inside (data not shown). Similarly, Cell #4 and Cell #5 from two different mice also exhibited low basal rates at 1.89 and 2.34 Hz, respectively but fired robustly and transiently at the averaged 32.89 ± 10.51 Hz and 35.38 ± 10.02 Hz, respectively, upon encountering their home nests (SI Fig. 7).
Fig. 1.
Three types of nest-responsive cells. (A) Transient-on type. Four video snapshots of mouse A approaching its home nest from four different angles during a period of 35 s. Firing patterns of Cell #1 (below the snapshots) show that this cell fired robustly (up to 35 Hz from its basal level of 1.47 Hz) during this 35-s epoch (four encounters). The bottom row shows the firing rate histogram of the entire 10-min period during which a total of 10 encounters took place (labeled 1–10) (an average firing rate of 23.7 ± 5.82 Hz). The perievent spike raster (Top Right) and perievent spike histogram (Bottom Right) of the 10 encounters show a significant increase in the firing of this cell. The arrows and numbers in the video snapshot (Bottom Left) illustrate the positions and angles of those 10 encounters. The time “zero” is set to the time point at which the tip of the animal's nose was 1 cm away from the edge of the nest before crossing. The bin width in the perievent spike histogram is 100 ms. (B) Persistent-on type. Five video snapshots of mouse B moving into its home nest five times during a period of 630 s (10.5 min). Firing patterns of Cell #2 (below the snapshots) show that this cell fired robustly and persistently during each of the five “inside-the-nest” episodes. The cell exhibited the persistent firing (persistent-on type) for the entire 76.8 s during the first stay, 71.9 s for the second stay, 23.0 s for the third stay, 30.1 s for the fourth stay, and 26.6 s for the fifth stay. The firing rate histogram (Bottom) shows the cell had an average firing rate of 29.96 ± 5.15 Hz when the mouse moved into the nest. (C) Persistent-off type. Five video snapshots of mouse C moving into its home nest five times during the epoch of 270 s. The firing dynamics of Cell #3 (below the snapshots) shows that this cell had a high basal firing rate (16.08 ± 3.49 Hz) when the mouse was outside the nest. But this cell would cease to fire (average firing rate at 0.56 ± 0.27 Hz) once the animal entered the nest and stayed inside (e.g., the cell remained silent during the fourth stay, which lasted for ≈85.5 seconds). The bottom row is the spike-histogram. The gray lines illustrate the animal's movement paths, and the red segments show the duration for which the mouse was inside the nest.
The second class of nest-responsive cells exhibited “persistent-on”-type firing characteristics. In other words, these cells would increase their firing rates once the animals entered the nest and would remain at the elevated firing rates as long as the mice were inside the nests. For example, Cell #2 and Cell #6 from two different mice increased their firings from the moment when the mice moved into the home nests and would maintain the heightened firings as long as the animals remained inside the nest. A 10.5-min spike-discharge pattern and five video snapshots are presented to illustrate the nest-responsiveness of Cell #2 from mouse B (Fig. 1B). During this period, the mouse entered its home nest a total of five times. The movement trajectories in the video snapshots represent the paths by which the animal moved toward the nest (gray line), stayed inside the nest (red segment), and then moved away from the nest (gray line again). The recorded spike pattern shows that this cell exhibited the time-locked, elevated spike discharge in each of those five Inside-Nest (IN) epochs. The spike histogram reveals that this cell fired persistently at the average 29.96 ± 5.15 Hz, with a peak firing rate as high as 48 Hz (Fig. 1B). On the other hand, the average firing rate of this cell during the time in which the animal was outside the nest was only ≈0.31 ± 0.25 Hz. Similarly, Cell #6 from another mouse showed a persistent increase in its firing at an average of 10.38 ± 3.24 Hz when the animal was inside the nest but had a much lower firing rate when the mouse was outside its nest (average of 0.83 ± 0.35 Hz) (also see SI Fig. 7).
Moreover, we have found three cells (Cells #3, #7, and #8) that exhibited the “persistent-off”-type firing patterns when the mice entered and stayed inside the nests. For example, Fig. 2C Top shows the video snapshots of five epochs during a 5-min period during which the mouse encountered its nest. The spike-discharge patterns of Cell #3 (from mouse C) show that it had a very high basal firing level (16.08 ± 3.49 Hz) when the mouse was outside of its home nest but ceased to fire once the animal's head got inside the nest (with an average firing rate of 0.56 ± 0.27 Hz) and continued to stay in near silence as long as the mouse remained inside the nest (Fig. 1C Middle). We also noted that the cell tended to fire at a higher level (up to 40 Hz) immediately upon exiting the nest and then tapered off somewhat to ≈10–20 Hz range. Similarly, Cells #7 and #8 from two different mice also exhibited persistent reductions in their firings once the animals entered their nests (1.08 ± 0.59 Hz inside the nest, and 10.66 ± 3.15 Hz outside the nest for Cell #7; 0.22 ± 0.15 Hz inside the nest, and 9.98 ± 2.59 Hz outside the nest for Cell #8) (also see SI Fig. 7).
Fig. 2.
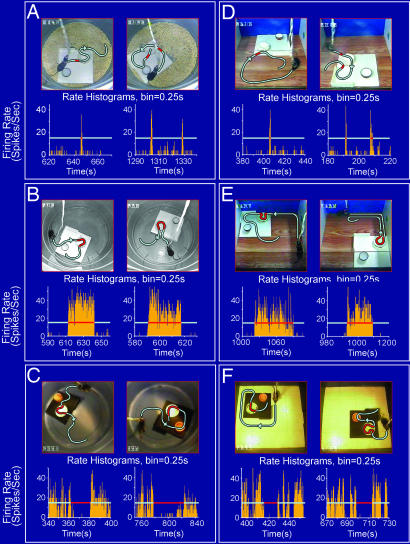
Firing changes of nest-responsive cells are independent of the nest's spatial locations and environments. (A) Cell #1 of mouse A fired robustly upon encountering the nest regardless of the nest's locations as indicated by the firing-rate histograms beneath each video snapshot. The gray lines illustrate the animal's movement path; the red segments show the near contact points where the mouse crossed the edge of the nests. (B) Cell #2 from mouse B showed a persistent increase in its firing upon entry into the nest regardless of the fact that the nest was placed at different locations in the home environment. (C) Cell #3 of mouse C ceased to fire once the mouse entered the nest regardless of the fact that the nest was placed at different locations. (D) Cell #1 preserved its robust responsiveness to the nest after it was relocated to two different locations in this new environment. The gray lines illustrate the animal's movement paths, and the red segments show the near contact points right before the mouse's nose crossed the edge of the nests. (E) Cell #2 continued to exhibit persistent-on-type firing even after the nest was relocated to two different locations in this new environment. (F) Cell #3 ceased to fire once the mouse entered the nest, which was placed in two different locations in a new environment. For B, C, E, and F, the gray lines illustrate the animal's movement paths, and the red segments indicate the time duration in which the mouse was inside the nest. The time bin used in the firing-rate histograms is 250 ms.
Responses of Nest Cells Are Independent of the Nests' Locations and Environments.
Because it is well known that some of the hippocampal cells exhibit place-related firing changes (9, 13), it is necessary to determine whether the observed nest-related firing changes are influenced by the spatial locations of the nest. To address this question, we placed the home nests in various locations in their home environments. We found that all of those nest cells maintained their nest-responses regardless of the spatial locations of the nests. For instance, Cell #1 fired robustly when the mouse encountered the nest which was moved from its original place to two different locations with different orientations (Fig. 2A). Cell #2 also showed a persistent increase in its firing during the period when the mouse was inside the nest whether the nest was placed at one location or another (even with rotated orientation) (Fig. 2B). Similarly, Cell #3 ceased to fire whenever the mouse stayed inside of the nest regardless of its location (Fig. 2C). Therefore, the nest cell responses are independent of the nest's spatial locations and orientations.
Next, we asked whether the overall environmental context has any significant influence on the responses of these nest-responsive cells. We examined this question by placing the home nest in a completely new environment and recording the responses of those cells (Fig. 2 D–F). We found that the nest cells continued to exhibit nest encounter-induced responses even when the nests were placed in the drastically different recording environments. Therefore, those experiments show that the responses of the nest cells are not affected by either the nest's spatial locations or the surrounding environments in which the nests are located.
Selectivity of Nest-Responsive Cells.
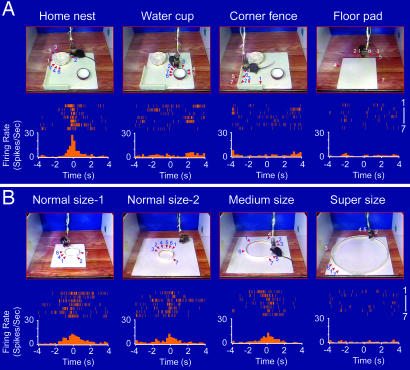
To investigate the response selectivity of these cells, we measured their responses to various objects that existed in the nest environments. The first question we asked was whether the nest-responsive cells reacted to a similar shaped object (e.g., the drinking water cup) or to other differently shaped objects (e.g., the corner fence, floor pads, or food pellets) in the home environments. Our perievent spike raster and spike histograms revealed no significant change in the firings of those nest cells during the encounters of nonnest objects, indicating their nest-response selectivity. For example, although Cell #1 exhibited strong responses to the encounter of the home nest (7.5 cm in diameter, with 2-cm low wall) (Fig. 3A, the first image on the left), it did not respond to the encounter of the edge of its water cup, which has a similar geometric shape (Fig. 3A, the second image from the left). Moreover, this cell was not responsive upon encountering other objects in the environment such as the corner fence which had a similar height (2-cm height) to the nest wall (Fig. 3A, the third image from the left) or the edge of a floor pad (Fig. 3A, the image on the right).
Fig. 3.
The selectivity of the nest-responsive cells. (A) Although Cell #1 responded robustly to the home nest (7.5 cm in diameter and 2 cm wall height) (the first column on the left), it did not respond to other objects such as the water cup (the second column from the left), the corner fence (the third column from the left), and the edges of a floor pad (right column). Seven trials are shown for each spike histogram. (B) The responses of the same nest cell were sensitive to the size of the nest. Two normal-size nests (7.5 cm in diameter and 1.5 cm wall height) placed on two different-sized floor pads, a medium-size nest (15 cm in diameter and 1.5 cm in wall height), and a super-sized nest (30 cm in diameter and 1.5 cm in wall height) were tested. The arrows indicate the position at which the mouse encountered the nests or other objects. The time “zero” was set to the time point at which the tip of the mouse's nose crossed the edge of the nest. The perievent spike (top of each histogram) and perievent spike histograms (bottom of each histogram) are shown. The bin width in the perievent spike histogram is 250 ms.
To further define the encoding features of the nest cells, we investigated whether the size of the nest plays any role in defining the responses of these cells. We constructed several versions of the circular nests of various sizes, ranging from the normal size (7.5 cm in diameter, 1.5-cm low wall) to medium size (15 cm in diameter, 1.5-cm low wall) and super-size (30 cm in diameter, 1.5-cm low wall) and recorded the nest cell responses. As shown in Fig. 3B, when two new circular nests (which had the identical diameter in comparison with the home nest but made with a 1.5-cm wall by using different cardboard materials) were presented to the mouse, Cell #1 exhibited a robust increase in its firing regardless of whether the nests were placed on a small or large floor pad (the left two images in Fig. 3B). Because the nests were newly constructed, this also allowed us to exclude the possibility that nest encounter-induced responses depended on odors associated with home nests. To further control the odor effects, we introduced these new nests to mice in novel environments, outside of their home environments. The fact that the cell continued to exhibit robust nest encounter-induced firing suggests that odor does not play any significant role. Moreover, this cell also exhibited significant firings when the animal encountered a medium-sized nest that had twice the diameter of the normal nest (the third image from the left in Fig. 3B). However, when the diameter of the nest was increased to approximately four times the size of the original, the cell no longer responded to this super-sized circular structure (Fig. 3B Right). Our metric analyses suggest that the size of the nest plays an important role in defining the nest cell's response.
Because the hippocampal cells are known to respond strongly to the startling episodes such as sudden air blows to the back of the animals or earthquake-like shakes of the mouse cages (17, 18), we also measured the effects of those episodic stimuli on the firing of those nest-responsive cells. Our analyses revealed that those nest-responsive cells did not change their firings in response to those startling events (SI Fig. 8A), whereas other simultaneously recorded CA1 cells in the same animals changed their firings (17, 18) (SI Fig. 8B).
In addition, we examined the response selectivity of those nest cells by introducing additional novel objects (e.g., toys) to the mice. We found that those cells did not alter their firing patterns when encountering those toys (SI Fig. 8C). Moreover, those cells also did not change their firings upon encountering food pellets, during food consumption and/or drinking-water behaviors (data not shown). Together, these experiments show that the nest-responsive cells do not respond to other external events or any other objects (e.g., the floor pads, toys, or corner fence).
Invariant Responses over Physical Shapes, Appearances, and Construction Materials.
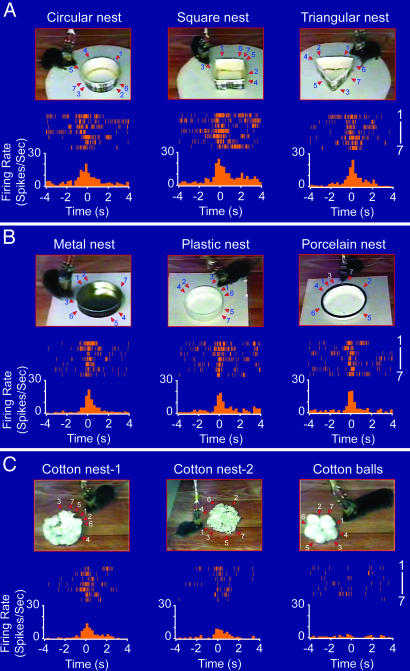
The fundamental feature of abstract concepts and generalized knowledge is that it should no longer ascribe to a specific type of shape and physical appearance of the objects. Therefore, we conducted a parametric analysis by constructing a set of new nests that were made from cardboard in several different geometric shapes. As exemplified by the perievent spike raster and perievent spike histograms shown in Fig. 4A, Cell #1 fired robustly during encounters with a circular nest (Fig. 4A Left), a square nest (Fig. 4A Center), and a triangular nest (Fig. 4A Right), suggesting that the cell's response is invariant over the geometric shapes of nests.
Fig. 4.
Invariant responses over the geometric shapes, physical appearances, colors, construction materials, etc. (A) Invariant responses of Cell #1 to the geometric shapes of nests. As shown by both the perievent spike rasters and perievent spike histograms, Cell #1 exhibited a significant firing increase in response to a new circular nest made out of the top part of a cardboard coffee cup with a wall height of 2.5 cm and a diameter of 7.5 cm (Left), a square cardboard nest (Center), and a triangular cardboard nest (Right). (B) Invariant responses of Cell #1 to nests made from different materials. A metal nest (Left), plastic nest (Center), and porcelain nest (Right) were tested. (C) The cell also increased its firing when the mouse encountered natural cotton nests (Left and Center) but not to five cotton balls that were simply lumped together (Right). The bin width in the perievent spike histogram is 250 ms.
Furthermore, we introduced the mice to additional new nests that were made from distinct materials including tin can caps, plastic bottle caps, porcelain caps, and cotton. This cell still exhibited highly significant changes in its firing when the animal encountered a metal nest which has distinct contexture and color (Fig. 4B Left). Similarly, encounters with a plastic nest also triggered marked changes in its firing (Fig. 4B Center). In addition, the cell further exhibited strong responses to a porcelain nest (Fig. 4B Right). Interestingly, two kinds of natural cotton bedding, made from cotton balls by other mice, also triggered robust responses of the cell in this animal (Fig. 4C Left and Center). However, five cotton balls that were simply placed together in the similar geometric shape were incapable of eliciting the responses from the nest cell (Fig. 4C, Right). Together, the above experiments suggest that the responses of the nest cell remained invariant over the physical appearances, geometric shapes, design styles, colors, odors, and construction materials, thereby encoding highly abstract information about nests. The invariant responses over the shapes, styles, and materials were also observed in other nest cells. Several examples of invariant responses of Cell #2 (persistent-on type) and Cell #3 (persistent-off type) over shapes, appearances, color, and materials are shown in the SI Fig. 9 A and B, respectively.
Functionality-Based Abstraction.
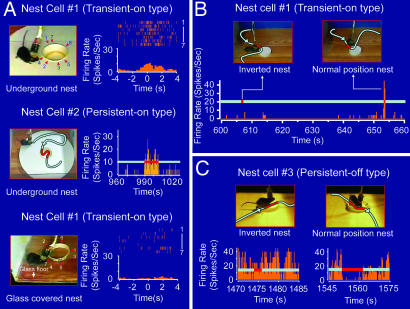
To investigate whether nest cells might tune toward the fundamental features that would categorically define the functionality of nests, we built a nest that was buried in the ground rather than above ground (as in previous experiments). Interestingly, we found that the nest cells also exhibit significant responses to the underground nests. For example, both Cell #1 and Cell #2 increased their firing in response to the underground nests (Fig. 5A Top and Middle; also see SI Movie 1).
Fig. 5.
The responses of nest cells are tuned toward the functionality of nests. (A) Cell #1 of mouse A responded significantly to an underground nest (Top), as did Cell #2 of mouse B (Middle). The nest cell would no longer respond to a nest covered by a glass floor, indicating the importance of physical interactions (Bottom). (B) Cell #1 did not respond to the plastic nest that was placed in an inverted manner (as a small stage) (Left). Interestingly, once the plastic nest was reverted back to its normal position (Right), the cell exhibited robust firing up to 40 Hz. The recorded spike discharge pattern during this 1-min “nest manipulation” period is shown. (C) The nest cell #3 of mouse C did not reduce its firing when mouse C crossed over the inverted plastic nest (functions as a small stage). However, once the plastic cap was flipped back into a nest position, the cell ceased to fire (average of 0.72 Hz) from an average basal level of 19.06 Hz when the mouse crossed over the plastic cap. The gray lines in B and C illustrate the animal's movement paths, and the red segments indicate the duration in which mouse A was approaching the nest (B) or mouse C moved inside the nest (C). Also, please see representative movie clips of A–C in SI Movies 1–3.
In addition, we placed a glass floor over a typical circular nest so that the functionality, but not the visual image, of the nest was blocked (Fig. 5A Bottom). Interestingly, under this glass floor scenario, the cell no longer changed its firing during the multiple crossings over the nest, as evident from both the perievent spike raster and perievent spike histogram. Thus, those experiments suggest that the cell's responses are tuned to the behaviorally determined nest function rather than viewing the mere visual images of nests through the glass floor. In another word, this form of conscious awareness of the presence of a nest is achieved by episodic, physical explorations.
Functionality-based responsiveness of the nest cells were further analyzed by our additional sets of experiments in which we used the same plastic bottle cap as the nest but presented it to the mice in two different ways: either in an inverted manner (so the cap would function as a small stage) or in the normal nest position (flipped on its back). Because it was the same cap and placed in the same location, this experimental design also provided stringent controls for the shape, color, location, odor, etc. We found that when the mouse encountered the inverted nest (in the form of a small stage), Cell #1 of mouse A had no significant change in its firing (Fig. 5B Left). However, when the plastic cap was flipped back to the nest position, the cell fired robustly (Fig. 5B Right. Also see SI Movie 2). In another example, Cell #3 (persistent-off type) recorded from mouse C also had no significant changes when the animal crossed over the inverted plastic cap (Fig. 5C Left). On the contrary, once the plastic cap was flipped back to the nest position, the cell would cease to fire when the animal moved inside the nest (Fig. 5C Right. Also see SI Movie 3). Therefore, our above experiments demonstrate that the nest cells are tuned toward the fundamental features that would functionally define the nest as long as it can serve as a refuge for the animal to stay in a cozy and safe manner in a given environment.
Discussion
Our identification of nest cells reveals a neural mechanism by which the brain encodes generalized knowledge and the abstract concept of nest or bed. This high-level abstraction enables the animals not only to avoid the burden of remembering and storing each mnemonic detail of various objects or experiences but also to adapt to a complex and ever-changing world with great efficiency. It is interesting to note that only a tiny percentage of the recorded hippocampal cells responded to nests, as supposed to much larger percentages of cells engaged in encoding spatial locations (9, 13) or episodic memories (17, 18). This is consistent with the notion that nest cells are grandmother cells committed to the processing of the concept of nest.
Our systematic analyses have further revealed that the neural encoding of the abstract concept of nests is tuned toward the functional features of nests. This fits well with our intuition, because our conceptual knowledge of many objects such as beds, tables, chairs, or other tools is indeed defined by the functionality of these items, and not by the physical forms which can vary widely (e.g., even a piece of flat rock can function as either a chair or table, depending upon its height or whether it is viewed from the perspective of a small child or an adult). This functionality-based conceptual abstraction differs from the previously reported shape-based perceptual categorization of faces in monkey cortex (5–8, 30–35). In contrast to the shape constancy of face cell responses, nest cell's responses are independent of geometric shape, design style, color, odor, or construction materials.
Our elucidation of the functionality-based conceptualization of nests at the level of the hippocampus also provides experimental evidence for the existence of network-level encoding units capable of simultaneously extracting commonality and abstract features from various episodic encounters (17, 18). Unlike the face cells in the inferior temporal cortex which continue to respond to faces even under anesthesia (8, 36), the responses of the hippocampal nest cells depend on conscious awareness, because the nest cells would lose their firing selectivity once the mice were anesthetized or in sleep (data not shown). In addition, the fact that the mere visualization of the nest did not trigger nest cell responses, as shown by the glass-floor experiment (Fig. 5A Bottom), further shows that the hippocampal encoding of the abstract concept of nests requires episodic experiences and physical determination of the functionality of the objects. Currently, it is unclear as to the existence of nest-selective cells in higher cortical areas that might be capable of responding to the visual images of nests alone.
In conclusion, we report the identification of nest cells that encode functionality-based conceptual knowledge of nests or beds. Our study suggests that the hippocampus is an intrinsic part of the hierarchical architectures engaged in extraction, processing, and representation of abstract concepts and knowledge from daily behavioral experiences.
Materials and Methods
Construction of Recording Microdrives and Animal Surgery.
The 96-channel electrodes, consisting of two independently movable bundles of 48 stereotrodes or 24 tetrodes (48-channel on each side of the hippocampi), were constructed as described (18, 28). Two or 3 d before surgery, wild-type B6BCA/J mice were removed from the standard mouse cage and placed to the customized recording home environment (plastic bucket, 50 cm in diameter) in which a cardboard circular nest, a water cup, and food pellets were provided. On the surgery day, the electrode array was inserted at 2.0 mm lateral to the bregma and 2.3 mm posterior to the bregma on both right and left sides. The electrode bundles were advanced slowly toward the hippocampal CA1 region at least 2 or 3 d after surgery, in daily increments of ≈0.07 mm until the tips of the electrodes had reached the CA1, as deduced from an assessment of high-frequency ripples, field potential, and neuronal activity patterns (18, 28). For details, see SI Methods.
On-Line Search for Nest-Responsive Cells and Off-Line Spike Sorting.
Overall off-line spike sorting was carried out by using the MClust 3.0 and KlustaKwik 1.5 programs (36), and the details are the same as previously described in our recent publications (18, 28). We used the Boxes sorting method of Sort Client to achieve initial on-line sorting and classification and then off-line sorting again by using the MClust 3.0 and KlustaKwik 1.5 programs as described (18, 28, 29, 36). Histological staining, with (1% cresyl echt violet) was used to confirm the electrode positions. A total of 255 single units from seven mice were recorded and carefully examined for nest responsiveness. Of them, eight single units were identified to respond significantly to nests. The examples of waveforms and basic firing characteristics of the units are shown in the SI Fig. 6).
Construction of Nests.
A variety of nests in different geometric shapes and sizes were made (see SI Methods). Behavioral explorations of nests were completely voluntary. All nests, other than the home nests as shown in the text (SI Fig. 10, the top two left images in the photos of nest construction), were new to mice and had not been represented to mice until the recording experiments. The functional categorization of these new objects as a nest was also confirmed by the observations that mice indeed slept inside those objects (data not shown).
Supplementary Material
Acknowledgments
We thank Erika Langer for proofreading the manuscript and Kun Xie and Shuqing Zhang for technical assistance. This work was supported by funds from the National Institute of Mental Health, the National Institute of Aging, the East China Normal University Award, and the W. M. Keck Foundations (to J.Z.T.) National Basic Research Program of China (No. 2003 CB716602, No. 2003 CB716606), National Natural Science Foundation of China (No. 30570584), Shanghai Pujiang Program (No. 05PJ14043), and Shanghai Basic Research Program (No. 05DJ 14007).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701106104/DC1.
References
- 1.Fodor J. Psychosemantics. Cambridge, MA: MIT Press; 1987. [Google Scholar]
- 2.Warrington EK, Shallice T. Brain. 1984;107:829–853. doi: 10.1093/brain/107.3.829. [DOI] [PubMed] [Google Scholar]
- 3.Tranel D, Dammasio H, Dammasio AR. Neuropsychologia. 1997;35:1319–1327. doi: 10.1016/s0028-3932(97)00085-7. [DOI] [PubMed] [Google Scholar]
- 4.Gallese V. Philos Trans R Soc London B. 2003;358:1231–1240. doi: 10.1098/rstb.2003.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin A, Chao LL. Curr Opin Neurobiol. 2001;11:194–201. doi: 10.1016/s0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- 6.Quiroga RQ, Reddy L, Koch C, Fried I. Nature. 2005;435:1102–1107. doi: 10.1038/nature03687. [DOI] [PubMed] [Google Scholar]
- 7.Miller E, Nieder A, Freedman DJ, Wallis JD. Curr Opin Neurobiol. 2001;13:198–203. doi: 10.1016/s0959-4388(03)00037-0. [DOI] [PubMed] [Google Scholar]
- 8.Gross CG. Am Psychol. 2005;60:755–763. doi: 10.1037/0003-066X.60.8.755. [DOI] [PubMed] [Google Scholar]
- 9.Burgess N, Maguire EA, O'Keefe J. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- 10.Sara SJ. Nat Rev Neurosci. 2000;1:212–213. doi: 10.1038/35044575. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki WA, Amaral DG. Cortex. 2004;40:220–222. doi: 10.1016/s0010-9452(08)70958-4. [DOI] [PubMed] [Google Scholar]
- 12.Wittenberg GM, Tsien JZ. Trends Neurosci. 2002;25:501–505. doi: 10.1016/s0166-2236(02)02231-2. [DOI] [PubMed] [Google Scholar]
- 13.Leutgeb JK, Leutgeb S, Treves A, Meyer R, Barnes CA, McNaughton BL, Moser MB, Moser EI. Neuron. 2005;48:345–358. doi: 10.1016/j.neuron.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Cohen NJ, Eichenbaum H. Memory, Amnesia, and Hippocampal System. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- 15.Squire L. Memory and Brain. New York: Oxford Univ Press; 1987. [Google Scholar]
- 16.Tulving E, Craik FIM. The Oxford Handbook of Memory. New York: Oxford Univ Press; 2000. [Google Scholar]
- 17.Lin L, Osan R, Tsien JZ. Trends Neurosci. 2006;29:48–57. doi: 10.1016/j.tins.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Lin L, Osan R, Shoham S, Jin W, Zuo W, Tsien JZ. Proc Natl Acad Sci USA. 2005;102:6125–6130. doi: 10.1073/pnas.0408233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berger TW, Alger B, Thompson RF. Science. 1976;192:483–485. doi: 10.1126/science.1257783. [DOI] [PubMed] [Google Scholar]
- 20.Thompson RF. Annu Rev Psychol. 2005;56:1–23. doi: 10.1146/annurev.psych.56.091103.070239. [DOI] [PubMed] [Google Scholar]
- 21.Tang YP, Shimizu E, Dube GR, Rampon C, Kervhner GA, Zhuo M, Liu G, Tsien JZ. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- 22.McGuire SE, Deshazer M, Davis RL. Prog Neurobiol. 2005;76:328–347. doi: 10.1016/j.pneurobio.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Davis M, Hitchcock J, Rosen JB. Anxiety and the Amygdala: Pharmacological and Anatomical Analysis of the Fear-Potentiated Startle Paradigm. New York: Academic; 1987. [Google Scholar]
- 24.Wirth S, Yanike M, Frank LM, Smith AC, Brown EN, Suzuki WA. Science. 2003;300:1578–1581. doi: 10.1126/science.1084324. [DOI] [PubMed] [Google Scholar]
- 25.Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, Tonegawa S. Cell. 1996;87:1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Shimizu E, Tang YP, Cho M, Kyin M, Zuo W, Robinson DA, Alaimo PJ, Zhang C, Morimoto H, et al. Proc Natl Acad Sci USA. 2003;100:4287–4292. doi: 10.1073/pnas.0636870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mack V, Burnashev N, Kaiser KM, Rozov A, Jensen V, Hvalby O, Seeburg PH, Sakmann B, Sprengel R. Science. 2001;292:2501–2504. doi: 10.1126/science.1059365. [DOI] [PubMed] [Google Scholar]
- 28.Lin L, Chen G, Xie K, Zaia K, Zhang S, Tsien JZ. J Neurosci Methods. 2006;155:28–38. doi: 10.1016/j.jneumeth.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 29.Buzsaki G. Nat Neurosci. 2004;7:446–451. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- 30.Kourtzi Z, Kanwisher N. Science. 2001;293:1506–1509. doi: 10.1126/science.1061133. [DOI] [PubMed] [Google Scholar]
- 31.Fabre-Thorpe M, Richard G, Thorpe SJ. NeuroReport. 1998;9:303–308. doi: 10.1097/00001756-199801260-00023. [DOI] [PubMed] [Google Scholar]
- 32.Freedman DJ, Riesenhuber M, Poggio T, Miller EK. Science. 2001;291:312–316. doi: 10.1126/science.291.5502.312. [DOI] [PubMed] [Google Scholar]
- 33.Gross CG, Rocha-Miranda CE, Bender DB. J Neurophysiol. 1972;35:96–111. doi: 10.1152/jn.1972.35.1.96. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz EL, Desimone R, Albright TD, Gross CG. Proc Natl Acad Sci USA. 1983;80:5776–5778. doi: 10.1073/pnas.80.18.5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desimone R, Albright TD, Gross CG, Bruce C. J Neurosci. 1984;4:2051–2062. doi: 10.1523/JNEUROSCI.04-08-02051.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris KD, Henze DA, Csicsvari J, Hirase H, Buzsaki G. J Neurophysiol. 2000;84:401–414. doi: 10.1152/jn.2000.84.1.401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.