Abstract
The arachidonic acid-generating enzyme cytosolic phospholipase A2 alpha (cPLA2α) has been implicated in the progression of excitotoxic neuronal injury. However, the mechanisms of cPLA2α toxicity have yet to be determined. Here, we used a model system exposing mouse hippocampal slices to NMDA as an excitotoxic injury, in combination with simultaneous patch-clamp recording and confocal Ca2+ imaging of CA1 pyramidal neurons. NMDA treatment caused significantly greater injury in wild-type (WT) than in cPLA2α null CA1 neurons. Bath application of NMDA evoked a slow inward current in voltage-clamped neurons (composed of both NMDA receptor-mediated and other conductances) that was smaller in cPLA2α null than in WT slices. This was not due to down-regulation of NMDA receptor function because NMDA receptor-mediated currents were equivalent in each genotype following brief photolysis of caged glutamate. Current-clamp recordings were made during and following NMDA exposure by eliciting a single action potential with a brief current injection. After NMDA exposure, WT CA1 neurons developed a spike-evoked plateau potential and an increased spike-evoked dendritic Ca2+ transient. These effects were absent in CA1 neurons from cPLA2α null mice and WT neurons treated with a cPLA2α inhibitor. The Ca-sensitive K-channel toxins, apamin and paxilline, caused spike broadening and Ca2+ enhancement in WT and cPLA2α null slices. NMDA application in WT and arachidonate applied to cPLA2α null cells occluded the effects of apamin/paxilline. These results indicate that cPLA2α activity is required for development of aberrant electrophysiologic events triggered by NMDA receptor activation, in part through attenuation of K-channel function.
Keywords: action potential, arachidonic acid, calcium, excitotoxicity, inhibition
The phospholipase A2 (PLA2) enzymes catalyze ester hydrolysis of fatty acids from the second position of membrane glycerophospholipids. In mammalian cells, because this sn-2 position is highly enriched with arachidonic acid (AA), the PLA2s are considered the major regulated source of cellular AA. Once liberated by PLA2s, AA can be metabolized by a number of enzymes to create the eicosanoids. These products and AA have important functions in regulating cellular homeostasis and inflammation and have been implicated in a number of neurologic injuries (1, 2).
Of the PLA2s expressed in the brain, the enzyme cytosolic phospholipase A2 alpha (cPLA2α) has a unique set of biochemical properties that implicate it in AA signaling in response to neuronal activity. In response to micromolar increases in intracellular Ca2+, cPLA2α translocates from the cytosol to cellular membranes, where its phospholipid substrate resides (3). In addition, the enzymatic activity of cPLA2α depends upon phosphorylation by p38 MAPK (4) and ERK-2 (5). cPLA2α has a marked preference for phospholipid substrates, with AA in the second position (6). Macrophages and mast cells derived from cPLA2α-deficient mice (cPLA2α−/−) are unable to express prostaglandins and leukotrienes in response to inflammatory stimuli (7, 8).
Several lines of investigation implicate cPLA2α in the potentiation of neurotoxicity. Transient global cerebral ischemia was shown to persistently activate cPLA2α (9), and cPLA2α−/− mice were significantly protected from transient focal cerebral ischemia (1). A broader role for cPLA2α in neurologic injury was demonstrated in cPLA2α−/− mice that suffered significantly less destruction of dopaminergic neurons following exposure to the toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in an experimental model of Parkinsonism (10). In rat organotypic hippocampal cultures, the cPLA2α inhibitor arachidonyltrifluoromethyl ketone (AACOCF3) protected pyramidal neurons of the CA1 region from oxygen and glucose deprivation (11). In addition, mice treated with AACOCF3 suffered less neuronal death and had dramatically reduced induction of key cytokines and chemokines in autoimmune encephalomyelitis (2).
Following cerebral ischemia and reperfusion, increased glutamate release activates NMDA receptors to increase neuronal Ca2+ flux, resulting in excitotoxicity. Application of exogenous AA potentiates neuronal NMDA receptor-mediated currents (12) and NMDA-evoked Ca2+ transients (13). The roles of PLA2s in this process have not been clearly defined. We hypothesized that NMDA-triggered cPLA2α activation up-regulates both NMDA receptor-associated cation influx and its delayed electrophysiologic sequelae. To test this hypothesis, we measured both the neuronal toxicity and the Ca2+ and electrophysiologic responses to NMDA in hippocampal neurons.
Results
The toxicity of NMDA on hippocampal CA1 neurons was determined in acute hippocampal slices derived from littermate wild-type (WT) and cPLA2α−/− mice. Acute hippocampal slices were recovered for 1 h in artificial cerebral spinal fluid (aCSF) 2. Following this recovery period, they were then switched to Mg2+-free aCSF2 supplemented with the GABAA receptor antagonist GABAzine (5 μM) for 10 min, exposed to 10 μM NMDA or vehicle for 6 min, and finally replaced in aCSF for an additional 20 min. Fluorescent Nissl and propidium iodide (PI) stains were used as markers of neuronal integrity and irreversible injury. In WT hippocampi, a small amount of PI uptake was observed in untreated pyramidal neurons, presumably reflecting damage from slice preparation. This uptake was decreased in cPLA2α−/− neurons. However, NMDA treatment caused significant injury, demonstrated by an ≈20-fold increase in the neurotoxic index [the ratio of PI to Nissl staining in comparable sections; see supporting information (SI) Fig. 6]. In contrast, injury to cPLA2α−/− hippocampal neurons following NMDA treatment was 23-fold less than that in the WT (P < 0.01). NMDA did increase neuronal injury in cPLA2α−/− slices 7.5-fold, as compared with untreated cPLA2α−/−, indicating that deletion of cPLA2α does not completely eliminate NMDA toxicity. Pretreatment of WT hippocampi with the cPLA2α inhibitor AACOCF3 (10 μM) before NMDA exposure also resulted in a significant reduction in the ratio of PI to Nissl staining fluorescence intensity (P < 0.05). These results indicate that cPLA2α enzymatic activity significantly amplifies the neurotoxicity of NMDA in acute hippocampal slices.
Voltage-clamp recordings were made from Cs+-loaded CA1 pyramidal cells in hippocampal slices prepared from 3- to 5-week-old littermate WT and cPLA2α−/− mice. A command potential of −70 mV was applied to the soma during a period of baseline recording in the presence of a GABAA receptor antagonist. This baseline recording was characterized by a small holding current (WT, −2.4 ± 4.2 pA, n = 7; cPLA2α−/−, −5.1 ± 4.0 pA, n = 7), which was occasionally punctuated by a spontaneous excitatory postsynaptic current. At t = 10 min, the perfusion was switched to aCSF supplemented with 10 μM NMDA and continued until t = 16 min, when washout commenced (Fig. 1). NMDA evoked an inward current that reached a peak value of 152.3 ± 24.8 pA in WT mice, but only 64.9 ± 11.2 pA in cPLA2α−/− mice (P < 0.01 by unpaired t test). Although the kinetics of the NMDA-evoked current varied, the current generally had a slow onset (reflecting the speed of bath solution exchange) and an inflection in the onset period. The current peaked before washout commenced in both genotypes. The total charge transfer from NMDA application was also significantly greater in pyramidal cells of WT mice (30 ± 7.8 nC), as compared with cPLA2α−/− mice (13 ± 2.8 nC, P < 0.05). However, no significant differences were observed in the recovery phase of the NMDA response, as indexed by the 50% decay time. Transmembrane current returned to baseline values in both groups by t = 25 min (WT, −0.7 ± 9.3 pA; cPLA2α−/−, –2.9 ± 3.5 pA). These results indicate that the total and peak cation flux into CA1 pyramidal cells evoked by neurotoxic NMDA stimulation is significantly attenuated in cPLA2α−/− mice.
Fig. 1.
The slow inward current evoked by neurotoxic bath application of NMDA is attenuated under conditions of cPLA2α inhibition. (A) Cells were bathed in a GABAA receptor antagonist (GABAzine, 5 μM) and held at −70 mV. At t = 10 min, NMDA (10 μM) was applied to the bath for a duration of 6 min (as indicated by the horizontal bar), after which recording continued until t = 60 min. Representative, unaveraged traces from single cells in slices are shown. The rapid inward currents, which are superimposed on the slow responses, are spontaneous excitatory postsynaptic currents. (B) Population measures for the experiment shown in A. Bars indicate the means ± SEM. cPLA2α−/−, n = 7; WT, n = 7; WT, AACOCF3, n = 6. ∗, P < 0.05; ∗∗, P < 0.01 for comparisons to the +/+ condition.
Does the altered electrophysiologic response of cPLA2α−/− mice to NMDA stem from the loss of cPLA2α enzymatic activity or from compensation for chronic cPLA2α loss? To address this question, we repeated these experiments in WT slices, but added 10 μM AACOCF3 to the bath at least 30 min before the beginning of patch-clamp recording. Essentially, AACOCF3 pretreatment completely replicated the effects of cPLA2α deletion. The peak current (86.7 ± 9.7 pA, P < 0.05) and charge transfer (16.9 ± 1.5 nC, P < 0.05) evoked by bath NMDA application were significantly attenuated, compared with untreated WT neurons. These results indicate that the slow inward current evoked by bath application of NMDA is attenuated under conditions of either chronic or acute cPLA2α deficiency.
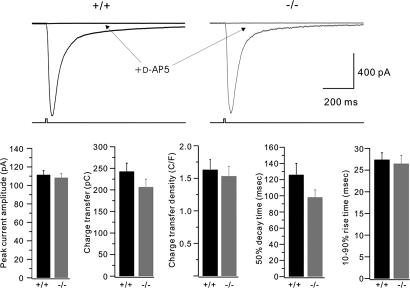
Does cPLA2α inhibition directly attenuate NMDA receptor function? We addressed this question in two ways. First, cPLA2α deletion may down-regulate expression of NMDA receptors. Therefore, we performed Western analysis of the NR1 subunit of the NMDA receptor in posterior hippocampi protein from WT and cPLA2α−/− mice and found that the relative amounts of NR1 protein were similar in both (+/+, 0.85 ± 0.55, n = 3; −/−, 1.0 ± 0.5, n = 4; arbitrary units normalized to actin; P = NS). Second, to determine the functional status of NMDA receptors, we loaded slices with caged glutamate, 100 μM 4-methoxy-7-nitroindolinyl (MNI)-glutamate, and performed local photolysis with 10-msec-long flashes of UV light. This tests the responses of all NMDA receptors and not just those activated by synaptically released glutamate. To restrict activation to NMDA receptors on the recorded cell, slices were bathed in tetrodotoxin (to suppress Na+ spiking), together with antagonists of AMPA, kainate, GABAA, and mGluR1/5 receptors. Test flashes delivered at 60- or 120-sec intervals evoked large, reasonably stable responses that were completely blocked by the NMDA receptor antagonist D-AP5 (Fig. 2). In between cell population comparisons, flash-evoked NMDA currents in cPLA2α−/− and WT cells were similar as shown by the peak current amplitude (1083.5 ± 42.5 and 1114.2 ± 45.1 pA, respectively; n = 32 and 29, respectively), charge transfer (206.6 ± 18.2 and 242.6 ± 19.4 pC, respectively), or kinetics. Following baseline recording, WT cells were exposed to 10 μM AACOCF3 for >30 min (to mimic the pretreatment regimen used in Fig. 1), and flash-evoked NMDA currents were recorded and compared with baseline values (SI Fig. 7). AACOCF3 treatment produced a small attenuation of peak NMDA current (77.4 ± 1.4% of baseline, P < 0.05, compared with baseline values, n = 5), but also a small slowing of the decay phase. Thus, no significant change was observed in NMDA receptor-mediated charge transfer (82.2 ± 5.9% of baseline, P > 0.05). Peak NMDA current following photolysis was not attenuated in cPLA2α−/−. Thus, the small effect of AACOCF3 is unlikely to underlie the significant attenuation of slow currents evoked by NMDA bath application in cPLA2α−/−- and AACOCF3-treated WT neurons (Fig. 1).
Fig. 2.
Rapid NMDA currents evoked by glutamate photolysis are normal in CA1 pyramidal cells derived from cPLA2α−/− mice. Hippocampal slices were bathed in Mg2+-free aCSF supplemented with MNI-glutamate and antagonists of GABAA, AMPA, kainate, and mGluR1/5 receptors. In these conditions, 10-msec-long flashes of UV light produced glutamate photolysis, which evoked an NMDA receptor-mediated inward current. The current traces shown are the averages of five consecutive responses. The lower trace illustrates the duration of the UV flash. These responses were completely blocked by the NMDA receptor antagonist D-AP5. Bar graphs show population comparisons. No significant differences were observed between +/+ and −/− groups (P > 0.10 for all). n = 29 and 32 cells, respectively.
Voltage-clamp recordings allow resolution of NMDA-evoked currents, but they do not illustrate the behavior of neurons under more natural conditions. To this end, we performed current-clamp recordings by using a K+-based internal saline. CA1 neurons rarely exhibited spontaneous action potentials in this configuration. A stimulating pulse, delivered every 2 min, consisted of a constant 50-msec-long injection of depolarizing current (range 0.3–0.6 nA), with the amplitude adjusted at the outset to reliably evoke a single spike. This was preceded by a small hyperpolarizing current injection to monitor Rinput (Fig. 3A). The responses to this pulse could be stably recorded for >36 min (Fig. 3 B and C) in WT neurons. When a separate group of these neurons was challenged with a 6-min-long bath application of NMDA, a small, transient depolarization was produced, but no significant alterations were observed in the evoked spike during NMDA application. However, following a delay of ≈15 min, the properties of the evoked spike began to change: the falling phase slowed, and a plateau potential developed at ≈−20–0 mV for 4–30 msec, after which a falling phase returned the membrane voltage to the resting potential. The size of this plateau potential, as indexed by measures of spike width (at 60% height) and the time-integrated depolarization, continued to increase for the duration of the recording. The development of the plateau potential was not associated with significant changes in the resting membrane potential, Rinput, or the peak amplitude of the spike. In cPLA2α−/− neurons, exposure to NMDA produced a smaller transient depolarization (Fig. 3C), consistent with the reduced NMDA-evoked inward current seen in voltage-clamped cells (Fig. 1). Most importantly, cPLA2α−/− neurons never developed spike-evoked plateau potentials (spike 60% width: WT, 13.4 ± 2.8 msec at t = 36 min, n = 10; cPLA2α−/−, 2.8 ± 0.3 msec, n = 9; P < 0.002).
Fig. 3.
Development of a spike-evoked plateau potential triggered by NMDA treatment is blocked in CA1 pyramidal cells from cPLA2α−/− mice. (A) A sample trace illustrates the stimulation and recording protocol. Current-clamp recordings were combined with dendritic Ca2+ imaging; the latter data are shown in Fig. 4. Initially, a brief injection of hyperpolarizing current was delivered to allow for the measurement of Rinput; 500 msec later, a 50-msec-long injection of depolarizing current was given. This current was calibrated at the outset of the experiment to reliably evoke a single spike and was held constant thereafter. Current-clamp recording continued until t = 0.8 sec to capture the evoked spike, and image acquisition continued until t = 1.2 sec to capture the spike-evoked Ca2+ transient. (B) Representative single traces of evoked spikes taken at the points indicated on the time-course graph in C. Time a (dark blue line) is at the start of the recording period. Time b (green line) occurs 12 min from the start of the experiment, which is after 4 min of NMDA exposure. Time c (light blue line) is 36 min after the start of recording and is the last time point in the experiment. The +/+ group was a control, in which cells from WT mice were recorded without NMDA challenge. (Scale bars: 10 msec.) (C) Time-course graphs showing population measures of electrophysiologic parameters. +/+ NMDA, n = 10; +/+, n = 5; −/− NMDA, n = 9. AP, action potential.
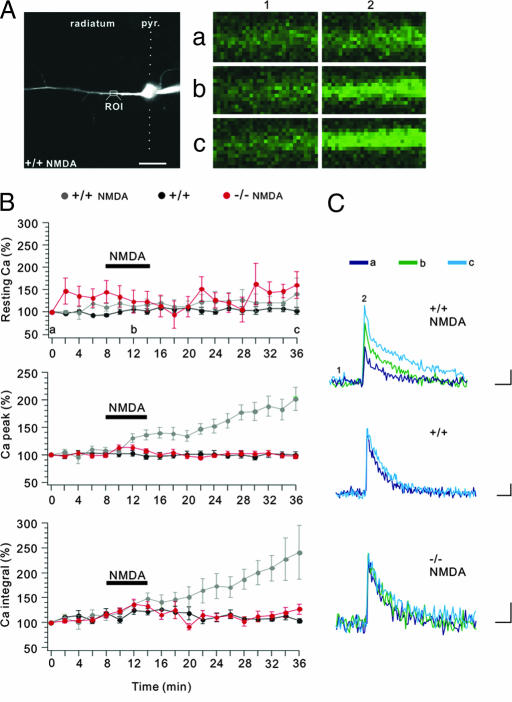
Simultaneous confocal Ca2+ imaging was performed on the CA1 pyramidal neurons that were challenged with NMDA. A region of interest was defined in the proximal dendrite (≈25 μm from the soma). Following a 20-min waiting period to allow dye diffusion in the dendrite, both resting and spike-evoked dendritic Ca2+ were stably recorded in WT neurons over the 36-min recording period (Fig. 4). NMDA treatment produced a small increase in the spike-evoked Ca2+ transient that was stable for ≈5 min. Following NMDA washout, the spike-evoked Ca2+ transient increased further, reaching a level of 240 ± 54% of baseline integrated Ca2+ at t = 36 min, the last time point recorded. Interestingly, application of NMDA did not produce a measurable change in resting Ca2+ concentration, likely reflecting that small changes in Ca2+ are not easily detected by Fluo-4 (Kd for Ca2+ of ≈500 nM) in neurons. When these measurements were repeated by using cPLA2α−/− neurons, spike-evoked Ca2+ slightly increased during NMDA application. Following washout, the spike-evoked Ca2+ transients returned to pre-NMDA levels and did not change during the recording (cPLA2α−/−, 127 ± 10% of baseline integrated Ca2+ at t = 36 min; P < 0.05). Similar changes were seen when the peak of the Ca2+ transient was measured (WT, 201 ± 21% of baseline; cPLA2α−/−, 97 ± 4% of baseline at t = 36 min; P < 0.0005). The delayed spike-evoked plateau potentials and the associated increases in spike-evoked Ca2+ transients are likely to be early manifestations of NMDA-induced excitotoxic injury that occur in WT, but not in cPLA2α−/− mice.
Fig. 4.
Development of enhanced spike-evoked Ca2+ transients triggered by NMDA treatment is blocked in CA1 pyramidal cells from cPLA2α−/− mice. (A) (Left) Typical dye-filled CA1 pyramidal cell with an attached patch pipette protruding to the right of the region of interest (ROI) in the proximal apical dendrite. This is a projected z-stack of confocal images. The excited fluorophore is Alexa-Fluor 594 hydrazide. (Scale bar: 20 μm.) (Right) Raw Fluo-4 fluorescence images from the ROI. The letters correspond to times in the course of the experiment, as shown in B; and the numbers correspond to time points in individual spike-evoked Ca2+ transients, as shown in C. (B) Time-course graphs showing normalized population measures of spike-evoked Ca2+ transient parameters. +/+ NMDA, n = 10; +/+, n = 5; −/− NMDA, n = 9. (C) Representative single traces of spike-evoked Ca2+ transients taken at the points indicated on the time-course graph in B. No filtering or averaging was applied to these traces. The frame acquisition speed was 50 Hz. (Scale bars: 250 msec, 25% ΔF/F.)
Simultaneous current-clamp and Ca2+ imaging experiments were repeated by using WT mice pretreated with 10 μM AACOCF3 or its inactive analog, AACOCH3 (Fig. 5 and SI Fig. 8). Remarkably, AACOCF3 pretreatment completely prevented the development of the spike-evoked plateau potential following NMDA challenge (60% spike width, 3.0 ± 0.6 msec; n = 10, t = 36 min; Fig. 5), thereby replicating the effect of cPLA2α genetic deletion. When WT neurons were pretreated with the control compound AACOCH3, they developed spike-evoked plateau potentials following NMDA challenge in a manner similar to untreated WT neurons (25.9 ± 14.0 msec; n = 5). AACOCF3, but not AACOCH3, prevented the delayed increase in spike-evoked Ca2+ transients following NMDA challenge, which was reflected in measures of both the Ca2+ transient peak (AACOCH3, 176 ± 30% of baseline at t = 36 min; AACOCF3, 98 ± 5% of baseline; P < 0.01) and the Ca2+ transient integral (AACOCH3, 175 ± 21% of baseline; AACOCF3, 115 ± 5% of baseline; P < 0.01). These results suggest that either chronic cPLA2α gene deletion or acute inhibition protects CA1 pyramidal neurons from the electrophysiologic sequelae of neurotoxic NMDA challenge.
Fig. 5.
Development of a spike-evoked plateau potential triggered by NMDA treatment is blocked in CA1 pyramidal cells treated with the cPLA2α inhibitor AACOCF3. (A) Representative single traces of evoked spikes taken at the points indicated on the time-course graph in B. (Scale bars: 10 msec.) (B) Time-course graphs showing population measures of electrophysiologic parameters. +/+ NMDA AACOCF3, n = 10; +/+ NMDA AACOCH3 (a control compound that does not inhibit cPLA2α), n = 5.
Is the increase in the spike-evoked Ca2+ transient following NMDA treatment in WT neurons solely a consequence of the parallel development of spike-evoked plateau potentials? The time courses of the spike and Ca2+ responses suggest that this is not the case. For example, in WT neurons treated with NMDA, at t = 24 min, spike-evoked Ca2+ responses are augmented (Fig. 4), but the plateau potential has yet to develop (Fig. 3). To analyze this further, we created a scatter plot to compare the change in the Ca2+ integral to the change in the spike integral at t = 24 min (SI Fig. 9A). This revealed no significant correlation in either the cPLA2α-intact (+/+ and AACOCH3) or cPLA2α-deficient (−/− and AACOCF3) groups (for +/+ and AACOCH3 groups, Pearson r correlation value = 0.02; P = 0.96; for −/− and AACOCF3 groups, Pearson r correlation value = 0.08; P = 0.73). However, in a scatter plot constructed for a later time point, 36 min (SI Fig. 9B), a highly positive correlation emerged in the cPLA2α-intact groups (Pearson r correlation value = 0.95; P < 0.0001), but not in the cPLA2α-deficient groups (P = 0.17). This suggests that, following NMDA challenge, multiple factors contribute to the increase in spike-evoked Ca2+ transients. In an early phase, these increases appear to be independent of spike broadening. However, somewhat later, at t = 36 min, spike broadening is a strong predictor of the increase in the spike-evoked Ca2+ transient. It is likely that NMDA treatment triggers at least two different mechanisms that impact spike-evoked Ca2+ transients, both of which are cPLA2α-dependent.
If consequences of NMDA treatment include a cPLA2α-dependent defect in spike repolarization and a related increase in the spike-evoked Ca2+ transient, then perhaps pharmacologic disruption of spike repolarization could produce a similar increase in spike-evoked Ca2+ transients. A number of conductances contribute to spike repolarization in CA1 pyramidal neurons, including Ca2+-sensitive K channels of the BK and SK types. We simultaneously blocked BK and SK channels with 100 nM apamin and 1 μM paxilline, respectively, and found that this significantly increased both the spike integral and the associated Ca2+ integral in WT cells (spike integral, 177 ± 9% of baseline; Ca2+ integral, 202 ± 7% of baseline, n = 6). Furthermore, NMDA treatment occluded the effects of subsequent apamin/paxilline application on both measures (NMDA spike integral, 239 ± 53% of baseline; Ca2+ integral, 241 ± 54% of baseline, n = 10; NMDA plus apamin/paxilline spike integral, 278 ± 45% of baseline; Ca2+ integral, 252 ± 28% of baseline, n = 7), suggesting that NMDA treatment may act in part by attenuating SK and/or BK channels (SI Fig. 10).
Can the product of cPLA2α activity, AA, produce spike broadening and related increases in spike-evoked Ca2+ transients? We bath-applied 10 μM AA by using the same timing protocol as that used for NMDA (a 6-min-long exposure, followed by washout) in cPLA2α−/− neurons to determine whether AA mediated effects downstream from cPLA2α (SI Fig. 11). AA treatment produced a delayed spike-broadening and Ca2+-transient increase (spike integral, 124 ± 4% of baseline; Ca2+ integral, 175 ± 31% of baseline, n = 12), although this was a somewhat smaller effect than that evoked by NMDA in WT neurons. When the apamin/paxilline mixture was applied to neurons that had previously been treated with AA, there was a small but significant additional increase in the spike integral (152 ± 8% of baseline, n = 12; P < 0.05) and a small but insignificant increase in the spike-evoked Ca2+ transient (210 ± 32% of baseline; P > 0.05). When apamin/paxilline treatment was applied to a separate group of cPLA2α−/− cells, the increases in spike and Ca2+ integrals were not significantly different from those produced by AA plus apamin/paxilline (spike integral, 148 ± 13% of baseline; Ca2+ integral, 185 ± 13% of baseline, n = 6; P > 0.10 for both measures). This suggests that at least a portion of the action of AA on spike broadening and the increase in the spike-evoked Ca2+ transient is mediated by inhibition of SK and/or BK channels.
Discussion
The main finding of these studies is that cPLA2α activity is required for the immediate electrophysiologic events that lead to neurotoxicity in hippocampal CA1 pyramidal cells. We found that expression of cPLA2α was highly correlated to the onset of irreversible CA1 pyramidal neuronal injury, as demonstrated by PI uptake (SI Fig. 6). Deletion of the cPLA2α gene or inhibition with AACOCF3 resulted in a significant reduction in the slow, inward current triggered by NMDA treatment (Fig. 1). This alteration in NMDA-triggered current was not due to changes in the conductance of NMDA receptors in the cPLA2α−/− mice because the currents produced by glutamate photolysis in the presence of inhibitors of other glutamate receptors were equivalent in the WT and cPLA2α−/− neurons (Fig. 2). Likewise, expression of the NR1 subunit of the NMDA receptor was equivalent in both genotypes. Coincident measurement of spike-evoked Ca2+ transients and action potentials demonstrated that cPLA2α expression is essential for the appearance of spike-evoked plateau potentials and increases in spike-evoked Ca2+ transients that follow NMDA exposure (Figs. 3 and 4). Taken together with the toxicity findings, these events are likely to constitute an early portion of the excitotoxic response to NMDA. Importantly, the cPLA2α inhibitor AACOCF3, but not the inactive compound AACOCH3, replicated the effect of gene deletion (Fig. 5 and SI Fig. 8). The concordance of the effect of the inhibitor with that of gene deletion indicates that the findings in cPLA2α−/− mice were not due to genetic compensation or chronic loss of cPLA2α activity. These experiments are the first to reveal electrophysiologic steps linking cPLA2α to excitotoxicity. Understanding the mechanism(s) by which cPLA2α mediates neurotoxicity is important for developing therapeutic strategies for stroke and other diseases.
We suggest that bath NMDA application evokes a Ca2+ flux in both the recorded neuron and its neighbors, and that these Ca2+ transients activate cPLA2α and initiate cPLA2α-dependent intracellular events that ultimately augment the peak and total NMDA-evoked current and lead to cPLA2α enhancement of early neuronal injury (14). AA has been shown by others to potentiate NMDA receptor-mediated currents (12) and NMDA-evoked Ca2+ transients (13) in cerebellar granule cells and hippocampal pyramidal cells, respectively.
The effect of NMDA on membrane current appears to be relatively brief: NMDA-evoked membrane current peaks during NMDA application and returns to baseline within a few minutes of washout. We believe that, during this time, NMDA treatment induces Ca2+ transients and cPLA2α-dependent AA production that set in motion at least two events that ultimately impact Ca2+ signaling. The first involves increases in spike-evoked Ca2+ signals that are independent of changes in spike shape, as seen soon after NMDA washout (t = 24 min; Figs. 3 and 4 and SI Fig. 9A). The second is a cascade that will ultimately result in the delayed appearance of spike-evoked plateau potentials (Fig. 3) and correlated increases in spike-evoked Ca2+ transients (Fig. 4 and SI Fig. 9B). The increases in spike-evoked Ca2+ transients are likely an early trigger of excitotoxic processes (15, 16). These sequelae of NMDA treatment are dependent upon cPLA2α activity and are therefore abolished in CA1 neurons of both cPLA2α−/−- and AACOCF3-treated slices.
Both NMDA treatment in WT neurons and AA treatment in cPLA2α−/− neurons produced spike broadening and increases in spike-evoked Ca2+ transients that occluded the effects of apamin/paxilline. This suggests that cPLA2α activity triggered by NMDA treatment produces AA that attenuates BK and/or SK channels that underlie spike repolarization. cPLA2α might also inhibit the voltage-gated transient K+ current (IA) mediated by Kv channels because AA suppresses this current when applied by intracellular pipette to CA1 neurons (17) and has been shown to attenuate the Kv1.4 channel (18).
Some caveats should be considered in the present studies. First, AACOCF3 is not the most potent or selective inhibitor of cPLA2α (19), but it has been used successfully in CNS models of disease (2), and it offers the availability of the negative control compound AACOCH3. At the 10-μM dose, its inhibition of cyclooxygenase is minor. Metabolism did not limit its efficacy because we continually superfused the drug during the experiment.
Second, we chose a low dose of NMDA (10 μM, 6 min) that is consistent with delayed excitotoxic injury in the hippocampus (20) and allowed reliable patch-clamp recording throughout the course of the experiment. Larger doses of NMDA or longer exposure times could potentially show a different cPLA2α dependence, but resulted in less stable recording conditions. Indeed, 30 μM NMDA applied to mixed neuronal-glial cultures resulted in sustained Ca2+ elevation that was cyclooxygenase-1-dependent (21). When acutely isolated CA1 neurons were exposed to 100 μM NMDA for 10 min, a large, progressive, postexposure Na+/Ca2+ conductance developed that was insensitive to later blockade of NMDA receptors and that appeared to trigger cell injury (22). However, in our model (whole-cell-patched CA1 neurons in an acutely prepared hippocampal slice), a 6-min exposure with 10 μM NMDA did not trigger a progressive postexposure current (Fig. 1).
We initiated these studies to determine whether cPLA2α had a direct effect on electrical and Ca2+ signaling that is believed to be part of the excitotoxic cascade. Our findings directly relate cPLA2α activity to electrophysiologic events of excitotoxicity. Future experiments are likely to demonstrate the potential of cPLA2α inhibition as a clinical therapy for prevention of early neurologic injury in a wide variety of diseases.
Materials and Methods
Mice.
All studies were conducted with the approval of The Johns Hopkins Animal Care and Use Committee. In these studies, we used WT and cPLA2α−/− mice (23) that had been backcrossed on the BALB/C strain for >10 generations. PLA2 activity was significantly reduced in cPLA2α−/− hippocampi (see SI Methods). cPLA2α mice were the gift of Joseph V. Bonventre (Brigham and Women's Hospital, Boston, MA).
Slice Preparation.
Hippocampi were removed from 3- to 5-week-old WT and cPLA2α−/− mice after decapitation. Slices (250 μm) were cut with a vibrating tissue slicer (VT1000S; Leica, Wetzlar, Germany) in ice-cold aCSF1 (in mM) 110 NaCl, 2.5 KCl, 1.2 NaH2PO4, 25 NaHCO3, 0.5 CaCl2, 7 MgCl2, 2.4 pyruvate, 1.3 ascorbic acid, 20 glucose, adjusted to pH 7.4, and oxygenated with 95% O2/5% CO2. Slices were then kept in aCSF2 (in mM) 125 NaCl, 2.5 KCl, 1 NaH2PO4, 26.2 NaHCO3, 2.5 CaCl2, 1.3 MgCl2, 20 glucose (pH 7.4) for at least 1 h at room temperature.
Whole-Cell, Patch-Clamp Recording.
Slices were placed in a submerged chamber and perfused with aCSF2 at 1 ml/min. Mg2+ was omitted from and 5 μM GABAzine was added to the external saline. Whole-cell recordings were obtained under visual guidance from pyramidal neuronal somata by using infrared disseminated intravascular coagulation (DIC) microscopy. For voltage-clamp recordings, electrodes were filled with a saline containing (in mM) 130 Cs-methanesulfonate, 10 CsCl, 2 MgCl2, 10 Hepes, 0.2 EGTA, 4 Na2-ATP, and 0.4 Na-GTP (pH 7.3), yielding a final resistance of 3–5 megaohms. For current clamp recording, the internal saline contained 130 K-methanesulfonate, 10 NaCl, 2 MgCl2, 10 Hepes, 4 Na2-ATP, and 0.4 Na-GTP (pH 7.3). Cells were voltage-clamped at −70 mV. Current and voltage traces were filtered at 2 kHz, digitized at 10 kHz, and acquired with pCLAMP 9 software. Only cells with stable Rinput throughout the recording were used. Recordings from neurons with spontaneous burst firing, unstable resting membrane voltages, unstable resting membrane currents, and resting membrane potential more positive than −65 mV were discarded. Group data were expressed as means ± SEM. Statistical comparisons used Student's t test.
Glutamate Photolysis.
To selectively and broadly activate the NMDA receptors on a CA1 pyramidal cell, slices were perfused with Mg2+-free aCSF2 supplemented with MNI-glutamate (100 μM), GABAzine (10 μM), tetrodotoxin (0.5 μM), and Trolox C (40 μM). Glutamate photolysis was achieved by directing the output of a 100-W Hg burner lamp into the epifluorescence port of the upright microscope and reflecting light from a 400-nm dichroic mirror to the objective for full-field illumination. A mechanical shutter was used to deliver 10-msec-long test flashes at 60- to 120-sec intervals. Experiments were performed in the presence of NBQX (40 μM) and UBP-301 (70 μM) to block AMPA and kainate receptors, respectively, and in the presence of CPCCOEt (80 μM) and MPEP (1 μM) to block mGluR1 and mGluR5, respectively.
Ca2+ Imaging.
For pyramidal neuronal Ca2+ imaging, 400 μM Fluo-4 was added to internal saline, and 300 μM Alexa Fluor 594 hydrazide was included to reveal dendritic morphology. We chose fluo-4, as opposed to a higher affinity indicator, because we were concerned that a high-affinity indicator would introduce temporal distortion into the Ca2+ transients (25) and might also buffer Ca2+ transients sufficiently to attenuate excitotoxic processes. After obtaining the whole-cell configuration, the dyes were allowed to diffuse into cells for at least 20 min. Ca2+ transients were elicited by brief current injections (10–50 msec; sufficient to reliably evoke a single spike) every 2 min and were recorded by using a Zeiss (Carl Zeiss, Thornwood, NY) Pascal scanning confocal microscope equipped with a 40× water-immersion objective lens. Fluo-4 was excited with the 488-nm line of an Argon ion laser, and emitted light was collected through a 505-nm long-pass filter. Alexa Fluor 594 hydrazide was excited with the 543-nm line of an He-Ne laser, and the emitted light was collected through a 560-nm long-pass filter. Fluo-4 images were recorded in frame-scan mode with 4 × 4 binning of the defined region of interest, which allowed a frame rate of 50 Hz. For analysis, foreground pixels were determined by manually thresholding the peak spike response image, and these foreground pixels were then spatially averaged to calculate ΔF/F0 for each frame.
Supplementary Material
Acknowledgments
The authors thank Roland Bock and Sarah J. Texel for technical assistance, members of the Linden laboratory for constructive critique, and Tzipora Sofare for editorial assistance. This work was supported by the Develbiss Fund (D.J.L.), the National Natural Science Foundation of China Grant 30600168 (to Y.S.), and the Zhejiang Natural Science Foundation of China Grant R206018 (to Y.S.).
Abbreviations
- AACOCF3
arachidonyltrifluoromethyl ketone
- cPLA2α
cytosolic phospholipase A2 alpha
- PI
propidium iodide
- PLA2
phospholipase A2.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0605427104/DC1.
References
- 1.Bonventre JV, Huang Z, Taheri MR, O'Leary E, Li E, Moskowitz MA, Sapirstein A. Nature. 1997;390:622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- 2.Kalyvas A, David S. Neuron. 2004;41:323–335. doi: 10.1016/s0896-6273(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 3.Evans JH, Leslie CC. J Biol Chem. 2004;279:6005–6016. doi: 10.1074/jbc.M311246200. [DOI] [PubMed] [Google Scholar]
- 4.Kramer RM, Roberts EF, Um SL, Borsch-Haubold AG, Watson SP, Fisher MJ, Jakubowski JA. J Biol Chem. 1996;271:27723–27729. doi: 10.1074/jbc.271.44.27723. [DOI] [PubMed] [Google Scholar]
- 5.Nemenoff RA, Winitz S, Qian NX, Van Putten V, Johnson GL, Heasley LE. J Biol Chem. 1993;268:1960–1964. [PubMed] [Google Scholar]
- 6.Clark JD, Lin LL, Kriz RW, Ramesha CS, Sultzman LA, Lin AY, Milona N, Knopf JL. Cell. 1991;65:1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- 7.Sapirstein A, Spech RA, Witzgall R, Bonventre JV. J Biol Chem. 1996;271:21505–21513. doi: 10.1074/jbc.271.35.21505. [DOI] [PubMed] [Google Scholar]
- 8.Fujishima H, Sanchez Mejia RO, Bingham CO, III, Lam BK, Sapirstein A, Bonventre JV, Austen KF, Arm JP. Proc Natl Acad Sci USA. 1999;96:4803–4807. [Google Scholar]
- 9.Rordorf G, Uemura Y, Bonventre JV. J Neurosci. 1991;11:1829–1836. doi: 10.1523/JNEUROSCI.11-06-01829.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klivenyi P, Beal MF, Ferrante RJ, Andreassen OA, Wermer M, Chin MR, Bonventre JV. J Neurochem. 1998;71:2634–2637. doi: 10.1046/j.1471-4159.1998.71062634.x. [DOI] [PubMed] [Google Scholar]
- 11.Arai K, Ikegaya Y, Nakatani Y, Kudo I, Nishiyama N, Matsuki N. Eur J Neurosci. 2001;13:2319–2323. doi: 10.1046/j.0953-816x.2001.01623.x. [DOI] [PubMed] [Google Scholar]
- 12.Miller B, Sarantis M, Traynelis SF, Attwell D. Nature. 1992;355:722–725. doi: 10.1038/355722a0. [DOI] [PubMed] [Google Scholar]
- 13.Richards DA, Bliss TV, Richards CD. Eur J Neurosci. 2003;17:2323–2328. doi: 10.1046/j.1460-9568.2003.02671.x. [DOI] [PubMed] [Google Scholar]
- 14.Bazan NG. J Lipid Res. 2003;44:2221–2233. doi: 10.1194/jlr.R300013-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Tymianski M, Charlton MP, Carlen PL, Tator CH. J Neurosci. 1993;13:2085–2104. doi: 10.1523/JNEUROSCI.13-05-02085.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Randall RD, Thayer SA. J Neurosci. 1992;12:1882–1895. doi: 10.1523/JNEUROSCI.12-05-01882.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angelova P, Muller W. Eur J Neurosci. 2006;23:2375–2384. doi: 10.1111/j.1460-9568.2006.04767.x. [DOI] [PubMed] [Google Scholar]
- 18.Danthi S, Enyeart JA, Enyeart JJ. J Membr Biol. 2003;195:147–164. doi: 10.1007/s00232-003-0616-0. [DOI] [PubMed] [Google Scholar]
- 19.Ghomashchi F, Stewart A, Hefner Y, Ramanadham S, Turk J, Leslie CC, Gelb MH. Biochim Biophys Acta. 2001;1513:160–166. doi: 10.1016/s0005-2736(01)00349-2. [DOI] [PubMed] [Google Scholar]
- 20.McCullough L, Wu L, Haughey N, Liang X, Hand T, Wang Q, Breyer RM, Andreasson K. J Neurosci. 2004;24:257–268. doi: 10.1523/JNEUROSCI.4485-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawano T, Anrather J, Zhou P, Park L, Wang G, Frys KA, Kunz A, Cho S, Orio M, Iadecola C. Nat Med. 2006;12:225–229. doi: 10.1038/nm1362. [DOI] [PubMed] [Google Scholar]
- 22.Chen QX, Perkins KL, Choi DW, Wong RK. J Neurosci. 1997;17:4032–4036. doi: 10.1523/JNEUROSCI.17-11-04032.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonventre JV. J Lipid Mediat Cell Signal. 1997;16:199–208. doi: 10.1016/s0929-7855(97)00014-x. [DOI] [PubMed] [Google Scholar]
- 24.Yang HC, Mosior M, Ni B, Dennis EA. J Neurochem. 1999;73:1278–1287. doi: 10.1046/j.1471-4159.1999.0731278.x. [DOI] [PubMed] [Google Scholar]
- 25.Sabatini BL, Regehr WG. Biophys J. 1998;74:1549–1563. doi: 10.1016/S0006-3495(98)77867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark JD, Milona N, Knopf JL. Proc Natl Acad Sci USA. 1990;87:7708–7712. doi: 10.1073/pnas.87.19.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.