Abstract
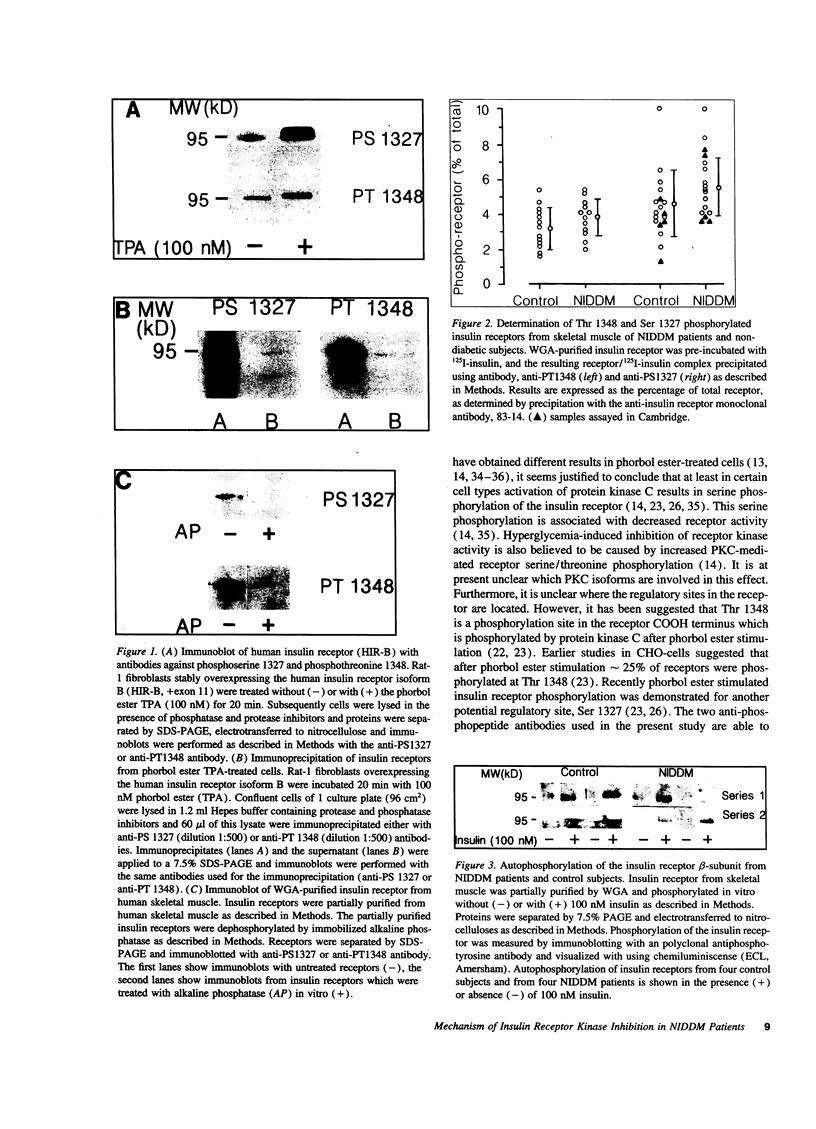
The tyrosine kinase activity of insulin receptor isolated from the skeletal muscle of NIDDM patients has previously been found to be decreased compared with the activity of receptor from nondiabetic subjects but the mechanism underlying this defect is unknown. Phosphorylation of receptor serine/threonine residues has been proposed to exert an inhibitory influence on receptor tyrosine kinase activity and Ser 1327 and Thr 1348 have been identified as specific sites of phosphorylation in the insulin receptor COOH terminal domain. To address the potential negative regulatory role of phosphorylation of these residues in vivo, we assessed the extent of phosphorylation of each site in insulin receptor isolated from the skeletal muscle of 12 NIDDM patients and 13 nondiabetic, control subjects. Phosphorylation of Ser 1327 and Thr 1348 was determined using antibodies that specifically recognize insulin receptor phosphorylated at these sites. In addition, a phosphotyrosine-specific antibody was used to monitor receptor tyrosine phosphorylation. The extent of insulin-induced tyrosine autophosphorylation was decreased in receptor isolated from diabetic versus nondiabetic muscle, thus confirming earlier reports. In contrast, there was no significant difference in the extent of phosphorylation of either Ser 1327 or Thr 1348 in receptor isolated from diabetic or nondiabetic muscle as assessed by immunoprecipitation (Ser 1327: 5.6 +/- 1.6% diabetics vs. 4.7 +/- 2.0% control; Thr 1348: 3.8 +/- 1.0% diabetics vs. 3.2 +/- 1.2% control). Moreover, within each group there was no correlation between the level of tyrosine kinase activity and the extent of serine/threonine phosphorylation. It is concluded that the stoichiometry of serine/threonine phosphorylation of insulin receptor in vivo is low, and that increased phosphorylation of Ser 1327 or Thr 1348 is not responsible for the decreased insulin receptor tyrosine kinase activity observed in the skeletal muscle of NIDDM patients.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. M., Olefsky J. M. Phorbol ester-mediated protein kinase C interaction with wild-type and COOH-terminal truncated insulin receptors. J Biol Chem. 1991 Nov 15;266(32):21760–21764. [PubMed] [Google Scholar]
- Arner P., Pollare T., Lithell H., Livingston J. N. Defective insulin receptor tyrosine kinase in human skeletal muscle in obesity and type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1987 Jun;30(6):437–440. doi: 10.1007/BF00292549. [DOI] [PubMed] [Google Scholar]
- Beck-Nielsen H., Groop L. C. Metabolic and genetic characterization of prediabetic states. Sequence of events leading to non-insulin-dependent diabetes mellitus. J Clin Invest. 1994 Nov;94(5):1714–1721. doi: 10.1172/JCI117518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom R. W., Newell-Morris L. L., Leonetti D. L., Shuman W. P., Wahl P. W., Fujimoto W. Y. Association of elevated fasting C-peptide level and increased intra-abdominal fat distribution with development of NIDDM in Japanese-American men. Diabetes. 1990 Jan;39(1):104–111. doi: 10.2337/diacare.39.1.104. [DOI] [PubMed] [Google Scholar]
- Berti L., Mosthaf L., Kroder G., Kellerer M., Tippmer S., Mushack J., Seffer E., Seedorf K., Häring H. Glucose-induced translocation of protein kinase C isoforms in rat-1 fibroblasts is paralleled by inhibition of the insulin receptor tyrosine kinase. J Biol Chem. 1994 Feb 4;269(5):3381–3386. [PubMed] [Google Scholar]
- Caro J. F., Sinha M. K., Raju S. M., Ittoop O., Pories W. J., Flickinger E. G., Meelheim D., Dohm G. L. Insulin receptor kinase in human skeletal muscle from obese subjects with and without noninsulin dependent diabetes. J Clin Invest. 1987 May;79(5):1330–1337. doi: 10.1172/JCI112958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan M. P., Pillay T. S., Tavaré J. M., Siddle K. Site-specific anti-phosphopeptide antibodies: use in assessing insulin receptor serine/threonine phosphorylation state and identification of serine-1327 as a novel site of phorbol ester-induced phosphorylation. Biochem J. 1994 Nov 1;303(Pt 3):893–899. doi: 10.1042/bj3030893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan M. P., Siddle K. Phorbol esters induce insulin receptor phosphorylation in transfected fibroblasts without affecting tyrosine kinase activity. Biochem Biophys Res Commun. 1993 May 28;193(1):371–377. doi: 10.1006/bbrc.1993.1633. [DOI] [PubMed] [Google Scholar]
- Cox N. J., Epstein P. A., Spielman R. S. Linkage studies on NIDDM and the insulin and insulin-receptor genes. Diabetes. 1989 May;38(5):653–658. doi: 10.2337/diab.38.5.653. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Bonadonna R. C., Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 1992 Mar;15(3):318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- Endre T., Mattiasson I., Hulthén U. L., Lindgärde F., Berglund G. Insulin resistance is coupled to low physical fitness in normotensive men with a family history of hypertension. J Hypertens. 1994 Jan;12(1):81–88. [PubMed] [Google Scholar]
- Freidenberg G. R., Reichart D., Olefsky J. M., Henry R. R. Reversibility of defective adipocyte insulin receptor kinase activity in non-insulin-dependent diabetes mellitus. Effect of weight loss. J Clin Invest. 1988 Oct;82(4):1398–1406. doi: 10.1172/JCI113744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häring H. U. The insulin receptor: signalling mechanism and contribution to the pathogenesis of insulin resistance. Diabetologia. 1991 Dec;34(12):848–861. doi: 10.1007/BF00400192. [DOI] [PubMed] [Google Scholar]
- Häring H., Kirsch D., Obermaier B., Ermel B., Machicao F. Decreased tyrosine kinase activity of insulin receptor isolated from rat adipocytes rendered insulin-resistant by catecholamine treatment in vitro. Biochem J. 1986 Feb 15;234(1):59–66. doi: 10.1042/bj2340059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häring H., Kirsch D., Obermaier B., Ermel B., Machicao F. Tumor-promoting phorbol esters increase the Km of the ATP-binding site of the insulin receptor kinase from rat adipocytes. J Biol Chem. 1986 Mar 15;261(8):3869–3875. [PubMed] [Google Scholar]
- Häring H., Obermaier B., Ermel B., Su Z., Mushack J., Rattenhuber E., Hölzl J., Kirsch D., Machicao F., Herberg L. Insulin receptor kinase defects as a possible cause of cellular insulin resistance. Diabete Metab. 1987 Jul;13(3 Pt 2):284–293. [PubMed] [Google Scholar]
- Karasik A., Rothenberg P. L., Yamada K., White M. F., Kahn C. R. Increased protein kinase C activity is linked to reduced insulin receptor autophosphorylation in liver of starved rats. J Biol Chem. 1990 Jun 25;265(18):10226–10231. [PubMed] [Google Scholar]
- Kellerer M., Obermaier-Kusser B., Pröfrock A., Schleicher E., Seffer E., Mushack J., Ermel B., Häring H. U. Insulin activates GTP binding to a 40 kDa protein in fat cells. Biochem J. 1991 May 15;276(Pt 1):103–108. doi: 10.1042/bj2760103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. E., Cao L., Perregaux D., Czech M. P. Threonine 1336 of the human insulin receptor is a major target for phosphorylation by protein kinase C. Biochemistry. 1990 Feb 20;29(7):1807–1813. doi: 10.1021/bi00459a020. [DOI] [PubMed] [Google Scholar]
- Lewis R. E., Wu G. P., MacDonald R. G., Czech M. P. Insulin-sensitive phosphorylation of serine 1293/1294 on the human insulin receptor by a tightly associated serine kinase. J Biol Chem. 1990 Jan 15;265(2):947–954. [PubMed] [Google Scholar]
- Maegawa H., Shigeta Y., Egawa K., Kobayashi M. Impaired autophosphorylation of insulin receptors from abdominal skeletal muscles in nonobese subjects with NIDDM. Diabetes. 1991 Jul;40(7):815–819. doi: 10.2337/diab.40.7.815. [DOI] [PubMed] [Google Scholar]
- Mårin P., Krotkiewski M., Holm G., Gustafsson C., Björntorp P. Effects of acute exercise on insulin and non-insulin-dependent glucose uptake in normal and moderately obese women. Eur J Med. 1993 Apr;2(4):199–204. [PubMed] [Google Scholar]
- Nolan J. J., Freidenberg G., Henry R., Reichart D., Olefsky J. M. Role of human skeletal muscle insulin receptor kinase in the in vivo insulin resistance of noninsulin-dependent diabetes mellitus and obesity. J Clin Endocrinol Metab. 1994 Feb;78(2):471–477. doi: 10.1210/jcem.78.2.8106637. [DOI] [PubMed] [Google Scholar]
- Nyomba B. L., Ossowski V. M., Bogardus C., Mott D. M. Insulin-sensitive tyrosine kinase: relationship with in vivo insulin action in humans. Am J Physiol. 1990 Jun;258(6 Pt 1):E964–E974. doi: 10.1152/ajpendo.1990.258.6.E964. [DOI] [PubMed] [Google Scholar]
- Obermaier-Kusser B., White M. F., Pongratz D. E., Su Z., Ermel B., Muhlbacher C., Haring H. U. A defective intramolecular autoactivation cascade may cause the reduced kinase activity of the skeletal muscle insulin receptor from patients with non-insulin-dependent diabetes mellitus. J Biol Chem. 1989 Jun 5;264(16):9497–9504. [PubMed] [Google Scholar]
- Pillay T. S., Whittaker J., Lammers R., Ullrich A., Siddle K. Multisite serine phosphorylation of the insulin and IGF-I receptors in transfected cells. FEBS Lett. 1991 Aug 19;288(1-2):206–211. doi: 10.1016/0014-5793(91)81035-7. [DOI] [PubMed] [Google Scholar]
- Rosholt M. N., King P. A., Horton E. S. High-fat diet reduces glucose transporter responses to both insulin and exercise. Am J Physiol. 1994 Jan;266(1 Pt 2):R95–101. doi: 10.1152/ajpregu.1994.266.1.R95. [DOI] [PubMed] [Google Scholar]
- Shmueli E., Alberti K. G., Record C. O. Diacylglycerol/protein kinase C signalling: a mechanism for insulin resistance? J Intern Med. 1993 Oct;234(4):397–400. doi: 10.1111/j.1365-2796.1993.tb00761.x. [DOI] [PubMed] [Google Scholar]
- Sinacore D. R., Gulve E. A. The role of skeletal muscle in glucose transport, glucose homeostasis, and insulin resistance: implications for physical therapy. Phys Ther. 1993 Dec;73(12):878–891. doi: 10.1093/ptj/73.12.878. [DOI] [PubMed] [Google Scholar]
- Soos M. A., Siddle K., Baron M. D., Heward J. M., Luzio J. P., Bellatin J., Lennox E. S. Monoclonal antibodies reacting with multiple epitopes on the human insulin receptor. Biochem J. 1986 Apr 1;235(1):199–208. doi: 10.1042/bj2350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter S. L., Nolan J. J., Wallace P., Gumbiner B., Olefsky J. M. Metabolic effects of new oral hypoglycemic agent CS-045 in NIDDM subjects. Diabetes Care. 1992 Feb;15(2):193–203. doi: 10.2337/diacare.15.2.193. [DOI] [PubMed] [Google Scholar]
- Takayama S., White M. F., Kahn C. R. Phorbol ester-induced serine phosphorylation of the insulin receptor decreases its tyrosine kinase activity. J Biol Chem. 1988 Mar 5;263(7):3440–3447. [PubMed] [Google Scholar]
- Takayama S., White M. F., Lauris V., Kahn C. R. Phorbol esters modulate insulin receptor phosphorylation and insulin action in cultured hepatoma cells. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7797–7801. doi: 10.1073/pnas.81.24.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavaré J. M., Zhang B., Ellis L., Roth R. A. Insulin-stimulated serine and threonine phosphorylation of the human insulin receptor. An assessment of the role of serines 1305/1306 and threonine 1348 by their replacement with neutral or negatively charged amino acids. J Biol Chem. 1991 Nov 15;266(32):21804–21809. [PubMed] [Google Scholar]
- Zachayus J. L., Cherqui G., Plas C. Protein kinase C and insulin receptor beta-subunit serine phosphorylation in cultured foetal rat hepatocytes. Mol Cell Endocrinol. 1994 Oct;105(1):11–20. doi: 10.1016/0303-7207(94)90030-2. [DOI] [PubMed] [Google Scholar]