Abstract
Spinal stability is related to both the intrinsic stiffness of active muscle as well as neuromuscular reflex response. However, existing analyses of spinal stability ignore the role of the reflex response, focusing solely on the intrinsic muscle stiffness associated with voluntary activation patterns in the torso musculature. The goal of this study was to empirically characterize the role of reflex components of spinal stability during voluntary trunk extension exertions. Pseudorandom position perturbations of the torso and associated driving forces were recorded in 11 healthy adults. Nonlinear systems-identification analyses of the measured data provided an estimate of total systems dynamics that explained 81% of the movement variability. Proportional intrinsic response was less than zero in more than 60% of the trials, e.g. mean value of PINT during the 20% maximum voluntary exertion trunk extension exertions 415±354 N/m. The negative value indicated that the intrinsic muscle stiffness was not sufficient to stabilize the spine without reflex response. Reflexes accounted for 42% of the total stabilizing trunk stiffness. Both intrinsic and reflex components of stiffness increased significantly with trunk extension effort. Results reveal that reflex dynamics are a necessary component in the stabilizing control of spinal stability.
Keywords: Low-back, Spine, Stability, Reflex, Stiffness
1. Introduction
Reflex response may play an important role in the control of spinal stability. Stability describes the ability to maintain spinal equilibrium despite the manifest presence of kinematic disturbances and motor-control errors. Panjabi (1992a) describes three sub-systems that contribute to stability. One is the passive sub-system including the spinal ligaments, discs and bone; the second is the active sub-system attributed to steady-state muscle recruitment; the third is the neural feedback that includes reflex and voluntary responses. The mechanical contribution of passive tissues is considered minimal in neutral spinal postures (Panjabi, 1992b) but they may contribute sensory information to the neural feedback loop (Solomonow et al., 1998). Torso stiffness during active trunk flexion or extension exertions is dominated by active muscles (McGill et al., 1994; Moorhouse and Granata, 2005). Therefore, existing models focus on the stiffness of active muscles as the primary control mechanism for spinal stability (Bergmark, 1989; Cholewicki and McGill, 1996).
Stiffness of active muscles includes intrinsic and reflex components (Nichols and Houk, 1976). The intrinsic component is associated with viscoelastic change of force when a muscle is stretched in the absence of reflex or voluntary change in recruitment. Steady-state muscle recruitment influences intrinsic stiffness of active paraspinal muscles thereby contributing to torso stiffness and spinal stability (Lee et al., 2006; Gardner-Morse and Stokes, 2001). However, to achieve spinal stability, the stiffness of the muscles must be sufficient to compensate for the gradient in gravitational moment at each vertebra, i.e. Hessian matrix of the potential energy must be positive definite (Stokes and Gardner-Morse, 1995; Granata and Wilson, 2001). It is unclear whether this is true, i.e. whether the intrinsic stiffness of active muscles without reflex response is sufficient to achieve stability of the spine.
Reflex response also contributes to spinal stability by means of active feedback control (Granata et al., 2004). Reflexes provide restorative forces similar to intrinsic stiffness but are time-delayed by the response latency of the reflex loop. Existing biomechanical models of spinal stability ignore the role of reflexes despite evidence that reflex response may play a role in the mechanics of low-back pain. For example, patients with low-back pain have been found to demonstrate abnormal reflex response including reduced reflex gain and slowed latency (Hodges and Richardson, 1996;Luoto et al., 1996). Data from Radebold et al. (2001) suggest that neuromuscular response dynamics are impaired in patients with low-back pain. However, we are unaware of any study to quantify the reflex contribution to trunk stiffness and spinal stability. In the ankle joint, up to 55% of the total joint stiffness may be attributed to reflexes (Mirbagheri et al., 2000). Likewise, the reflex response in the torso and paraspinal muscles may contribute significantly to the stiffness of the torso and stability of the spine. Therefore, the goal of this study was to apply nonlinear system identification techniques to separate and quantify the intrinsic and reflexive components of trunk stiffness from measured data recorded during voluntary isometric extension exertions. Results will confirm that the reflex response in the torso musculature is a necessary component of spinal stability.
2. Methods
2.1. Experimental protocol
Eleven healthy male subjects with no self-reported history of low-back pain participated after signing informed consent approved by the Virginia Tech institutional review board. Mean age (±standard deviation), height and body mass were 26±3 years (range 23-29), 181±8 cm, and 82±13 kg, respectively. Subjects stood upright in a pelvic restraint structure designed to restrict the motion of the pelvis and lower body (Fig. 1). A harness and rod system attached the subject to a DC servomotor (Pacific Scientific, Rock-ford, IL, USA). Subjects generated isometric trunk extension exertions at pre-specified levels of 20%, 35% and 50% of maximum voluntary exertion (MVE) by observing a real-time video display of the applied force. They were instructed to relax their trunk flexor muscles during the extension exertions so as to minimize antagonistic co-contraction. Once steady-state exertion was achieved, a pseudorandom binary sequence (PRBS) of trunk position perturbations was applied to the T10 level of the subject. Practice trials were provided before each trial until the subjects stated that they were comfortable with each experimental condition.
Fig. 1.

Experimental setup. A servomotor applied a pseudorandom binary sequence (PRBS) of position perturbations to the T10 level of the trunk and the resulting force was measured. Subjects were securely strapped into a rigid structure to isolate movement to the trunk. Figure not to scale.
Unlike our previous studies wherein disturbance forces were applied to the subject, in the current study, the motor accurately controlled position so as to apply small postural displacements of the torso. Perturbation sequences forced the trunk ±2 mm in the anterior posture direction measured with respect to the pelvis with perturbation switching-rate of 150 ms. This means that the trunk was moved rapidly between two positions at random multiples of pulse-width of 150, 300, 450, 600 or 750 ms with 50 pulses per 30-s trial (Fig. 2A). Pilot studies indicated that position perturbations with amplitude of ±2 mm (trunk flexion/extension angle of ±11) were sufficient to (1) allow the motor to achieve motion perturbations with a peak-to-peak rise time of 36.0±1.6 ms, i.e. less than the reflex delay (Granata et al., 2004), (2) had an average velocity low enough to avoid attenuating reflex responses, (3) contained power over a wide enough bandwidth to identify the dynamics (Moorhouse and Granata, 2005) and (4) permitted the subject to maintain voluntary trunk extension exertion throughout the perturbation sequence.
Fig. 2.
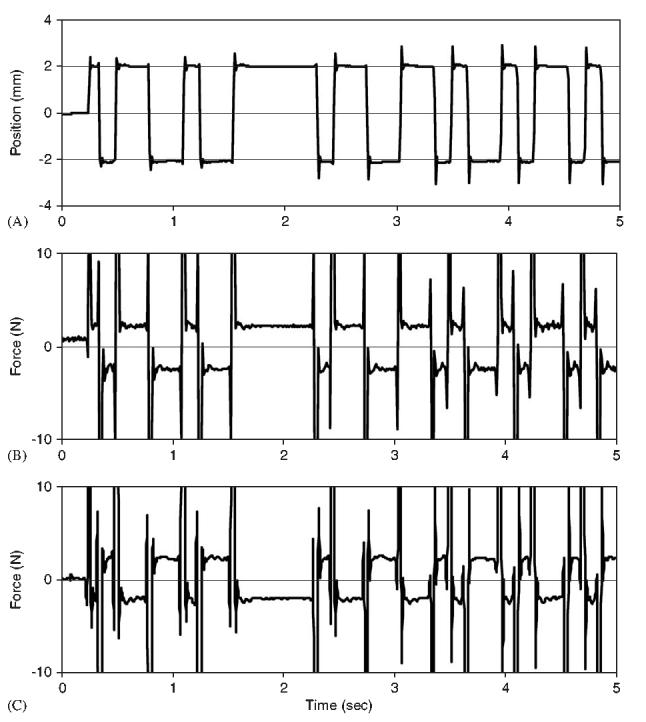
Typical experimental record for a subject maintaining an extension exertion level of 35% MVE. (A) Pseudorandom binary sequence of trunk position. (B) Measured force for a subject demonstrating a positive value of PINT. (C) Measured force for a subject demonstrating a negative value of PINT.
Postural displacement and force were recorded during each trial. Displacement was measured with an optical encoder (resolution 0.0351) attached to the servomotor shaft. Forces applied during the perturbations were measured from an in-line force transducer (resolution 0.725 N/mV, Omega, Manchester, UK). Measured force and position were processed with a similar low-pass filter to avoid phase differences in the data. EMG signals were normalized relative to the signal levels collected during maximum voluntary isometric exertions in flexion and extension tasks.
2.2. Analysis
Intrinsic and reflex dynamics were quantified by nonlinear parallel-cascade system-identification procedures (Westwick and Kearney, 2003). Briefly, the total measured force response associated with the position disturbance, FTOTAL, was considered to be the sum of forces attributed to intrinsic muscle stiffness, FINT,and reflex force, FREF (Fig. 3). In the intrinsic pathway, HINT described the viscoelastic response to small position disturbances. The reflex pathway was modeled as a reflex delay in series with differentiator, a static nonlinear element, NREF(.), and a dynamic linear element, HREF. Pilot analyses revealed that the static nonlinearity behaved as a half-wave rectifier thereby indicating that reflexes were elicited only by eccentric stretch (Mirbagheri et al., 2000). In the reflex pathway, HREF represented the transfer function relating rectified velocity to reflex force.
Fig. 3.
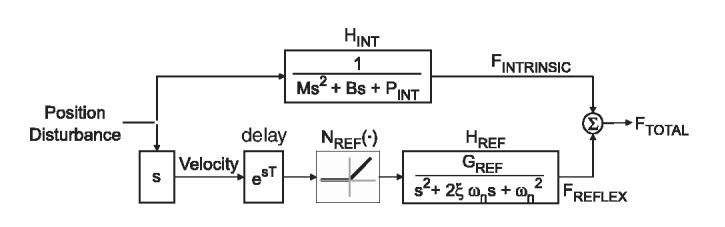
Block diagram showing the parallel-cascade structure comprised of the intrinsic and reflexive contributions to overall trunk force.
The nonlinear parallel-cascade system-identification technique used to identify the dynamics of the intrinsic and reflex pathways proceeded as follows (Hunter and Korenberg, 1986; Westwick and Kearney, 2003):
Intrinsic dynamics were estimated in terms of a linear impulse response function (IRF) HINT, relating position and force. The length of this impulse response function was fixed at a value less than the reflex delay to ensure that forces due to reflex mechanisms would not influence the estimated HINT. Granata et al. (2004) observed a reflex peak at 67 ms in the erector spinae muscles with response onset at approximately 50 ms. To assure that all forces predicted by HINT arose from intrinsic properties, an IRF length of 40 ms was chosen for this analysis.
HINT was convolved with the input position to generate an estimate of the intrinsic force, .By subtracting from the measured force, FTOTAL, an estimate of the reflex force, , was computed.
The reflex pathway was investigated by treating delayed velocity as the input and as the output. A Hammerstein identification procedure (Hunter and Korenberg, 1986) was used to simultaneously identify both the static nonlinear, NREF(.), and linear dynamic elements, HREF.
This nonlinear model was used to generate a new estimate of the reflex force, , by applying the static nonlinearity to the measured velocity signal and convolving HREF with the transformed velocity. By subtraction from the measured force, FTOTAL, a revised estimate of the component of force attributed to intrinsic force, was computed. The estimation procedure was repeated starting at the first step using the revised for the estimation of HINT.
- The net predicted force, was formed from the sum of the estimated intrinsic and reflex components. The percentage variance accounted for, VAFTOTAL, was computed as the normalized error between the actual and estimated total force:
(1)
The procedure was continued until successive iterations failed to improve VAFTOTAL. The procedure always converged to a solution in less than eight iterations. Thus, an impulse response function HINT and HREF were computed from each trial of 50 displacement perturbations. Intrinsic and reflex parameters were computed from these impulse response functions.
Intrinsic compliance, , was parametrically modeled as a second-order behavior (Gardner-Morse and Stokes, 2001):
| (2a) |
| (2b) |
where M is the effective trunk mass, B is the intrinsic muscle damping and s is the Laplace variable. PINT was operationally defined as the proportional intrinsic response and included intrinsic stiffness, kINT, and the linearized anterior-posterior force attributed to gravitational moment of the trunk about the neutral posture. The coefficient g represents the acceleration due to gravity and h is the height of the trunk center of mass. The large gravitational mass of the trunk resulted in values of PINT that were negative or near zero and resulted an unbounded compliance function. Therefore, PINT was parameterization by regression of trunk position versus recorded during the isometric phase of each perturbation (Fig. 2). Note that this permits calculation of PINT but fails to provide empirical measures of effective trunk mass or intrinsic damping. PINT provided an assessment of intrinsic stability because it represents the antagonistic effects of the stabilizing muscle stiffness and the destabilizing gravitational moment about the upright equilibrium posture.
The reflex impulse response function, HREF, was parameterized using a model comprising a standard second-order low-pass system in series with a reflex conduction delay, T,
| (3) |
where GR is the static reflex gain, z is the damping parameter, on is the undamped natural frequency and s is the Laplace variable. Reflex conduction delay, T = 40 ms, was determined from visual inspection of HREF. Once T was established, remaining parameters were computed by Levenberg-Marquardt algorithm.
Statistical repeated measures analyses (ANOVA) were performed to determine the effect of independent variables of trunk extension exertion (20% MVE, 35% MVE, 50% MVE) on the proportional intrinsic response, PINT, and the reflexive stiffness parameters (GR, z, on). Analyses were performed using commercial statistical analysis software (Statsoft Inc., Tulsa, OK) with a significance level of ao0:05 for all tests. Trends insignificant variables were investigated using Tukey honest significant difference (HSD) post-hoc analyses.
3. Results
The nonlinear parallel-cascade system-identification procedure was able to accurately predict the total force response to the pseudorandom position perturbations with an accuracy of VAFTOTAL = 80.9±3.6%. VAFTOTAL was not significantly influenced by exertion level (p = 0:74). This suggests represented the analyses accurately the torso dynamics regardless of the experimental condition.
Typical intrinsic force response, , to a PRBS of small position perturbations exhibited large inertial force at the onset, i.e. acceleration phase, of the movement perturbation. This was followed by a steady-state intrinsic force that tracked the perturbation sequence (Fig. 2). The intrinsic response function, HINT, was able to predict 87.7±4.6% of the intrinsic force variability and was not statistically influenced by exertion effort (p = 0:52). Positive value of the proportional intrinsic response, PINT, was noted when the shift of the steady-state force was in the same direction as the position change (Fig. 2B), e.g. was positive when position was also positive. Negative value of PINT was noted when the shift of this steady-state force was in the direction opposite of the position change (Fig. 2C), e.g. was negative when position was positive. Mean value of PINT during the 20% MVE exertions was negative, 415±354 N/m. A one-sample t-test demonstrates that this value is significantly less than zero indicating an intrinsically unstable system. It increased significantly (p <0:001)with voluntary trunk extension exertion with a positive mean value 421±796 N/m during 50% MVE exertions (Table 1). However, a one-sample t-test analyses rejects the conclusion that these are greater than zero indicating at most a neutrally stable system under intrinsic control.
Table 1.
Mean (standard deviation) values of outcome variables
| Force (N) p<0:001 | PINT (N/m) p<0:001 | kINT (N/m) p<0:001 | GR (N s/m) p<0:031 | ζ (N s2/m) p<0:014 | ωn (Hz) p<0:140 | kREFLEX (N/m) p<0:095 | |
|---|---|---|---|---|---|---|---|
| 20% MVE | 96 (20)a | 415 (354)a | 1280 (240)a | 197 (96)a | 0.66 (0.14)a | 8.69 (2.74)a | 1205 (990)a |
| 35% MVE | 155 (38)b | 43 (637)b | 1738 (600)b | 221 (79)a | 0.55 ((0.08)b | 9.54 (2.23)a | 1368 (926)a |
| 50% MVE | 208 (65)c | 421 (796)b | 2116 (710)b | 248 (77)b | 0.53 (0.12)b | 9.16 (1.43)a | 1619 (1024)a |
| Average | 153 (64) | 16 (694) | 1712 (638) | 221 (84) | 0.58 (0.12) | 9.13 (2.16) | 1398 (963) |
Values are reported for each condition of 20%, 35% and 50% maximum voluntary exertion (MVE). p-values represent ANOVA results for main effect of exertion effort. Statistical analyses illustrate significant effect of exertion effort on applied force, proportional intrinsic response (PINT), intrinsic stiffness (kINT), reflex gain (GR), reflex damping ratio (ζ). A trend was noted wherein reflex stiffness (kREFLEX) increased with exertion efforts but failed to achieve statistical significance (p = 0.095). Reflex natural frequency (ωn) was not statistically influenced by exertion effort.abcRepresent significant differences between exertion conditions (%MVE) in Tukey post-hoc analyses.
The reflexive response, HREF, accounted for VAFREFLEX = 74.1±2.0% of the reflex force variability. VAFREFLEX was not significantly influenced by exertion level (p = 0:71). The parametric model fit the reflexive stiffness (Fig. 4) with an accuracy of 74.4±2.8% and was also not influenced by exertion level (p = 0:21). Note that time-lags greater than 150 ms are dominated by random voluntary response but the reflex energy is adequately attenuated by this time so as to allow accurate parameterization of the reflex force. Mean value of reflexive stiffness gain, GR, was 221±84 N s/m and increased significantly po0:05 with exertion level (Table 1). The mean reflex (natural) frequency and damping ratio parameters were 9.13±2.16 Hz and 0.58±0.12, respectively. Reflex frequency was not significantly influenced by exertion (p = 0:14). However, the damping ratio demonstrated trends that approached statistical significance (p = 0:013)wherein reflexes decayed faster with increased exertion effort.
Fig. 4.

Reflexive stiffness impulse response function, HREF, along with the superimposed second-order least-squares fit and associated parameters for a typical trial wherein the subject maintained an extension exertion level of 20% MVE with minimal co-contraction.
The contribution of reflexes to the total stiffness behavior can be estimated from Eq. (3). Recall that HREF describes the transfer function from disturbance velocity to reflex force. Hence, the proportional response to describe reflex stiffness is
Standard first-order Pade approximation was used to account for the reflex delay (Ogata, 2002). Mean reflex stiffness was 1398±963 N/m (Table 1). A trend was noted wherein reflex stiffness increased with exertion efforts but this failed to achieve statistical significance (p = 0:095).
4. Discussion
Results revealed that the intrinsic stiffness alone was insufficient to stabilize gravitational effects of torso mass during active voluntary trunk extension exertions in more than 60% of the experimental trials. This is in contrast to theoretical simulations that conclude “muscle stiffness can stabilize the lumbar spine without the need for active feedback control.” (Gardner-Morse et al., 1995). However, those models assume that intrinsic stiffness increases at a rate of 4.5-17 times greater than associated increase in muscle force when in an upright posture (Gardner-Morse et al., 1995; Arjmand and Shirazi-Adl, 2006). We are unaware of previous empirical measurements to test this assumption. Our results demonstrate that intrinsic stiffness was insufficient for stability during 20% MVE exertions as evident from negative value of PINT. This proportional intrinsic response, PINT, was defined as position-dependant factor that contributes to overall trunk dynamics before the onset of the reflex response. It includes the destabilizing effects of gravity as well as the restorative forces attributed to passive tissue and active muscle intrinsic stiffness. If the spine and torso are disturbed from the equilibrium posture, then intrinsic stiffness, kINT, provides restorative forces that tend to return the posture toward the equilibrium state, i.e. positive contribution to PINT. Conversely, gravitational mass contributes destabilizing forces that tend to drive the posture away from the equilibrium state following a small disturbance, i.e. negative contribution to PINT.To be considered stable, the system must be drawn toward the equilibrium posture (Neyfeh and Balachandran, 2005). The negative contribution of gravitational mass was often greater than the positive contribution of the intrinsic muscle and passive tissue stiffness. Therefore, without the reflex response, the system was often unstable.
PINT increased significantly from 415 N/m at the lowest exertion level to +421 N/m at the highest exertion level (Table 1). This agrees with previously published trends wherein effective trunk stiffness increases with the voluntary effort and muscle activity (Gardner-Morse and Stokes, 2001). At efforts of 20% MVE, the PINT was negative, thereby indicating unstable intrinsic mechanics. The unstable intrinsic system became stable on average with increased voluntary trunk extension effort. However, in a lifting environment, it is possible that PINT may not achieve positive stable behavior with increased exertion. The experiment was designed to recruit muscle-generated trunk extension force so as to resist an external horizontal flexion load from a servomotor and load cell. The destabilizing effects of the gravitational torso mass were therefore independent of the exertion effort. Conversely, if increased extension effort had been achieved by requiring subjects to hold weights in their hands, then the destabilizing gravitational mass effects must increase in proportion to the exertion effort. It is possible in that case that PINT may not become positive and remain unstable with increased effort in a lifting paradigm.
Eight of the 11 subjects demonstrated negative values of PINT. Three subjects demonstrated negative PINT under all experimental conditions, including the 50% MVE trials. The other five exhibited a positive value of PINT only at the 50% MVE condition. The three remaining subjects exhibited a positive value of PINT under all conditions. However, in those subjects who demonstrated positive values of PINT, the values of were small. Moreover, estimates of proportional intrinsic response may be conservative, i.e. more intrinsically stable than normal. While trying to maintain trunk extension force in the novel experimental conditions, subjects may have recruited higher than normal antagonistic co-contraction despite the instructions to relax their trunk flexor muscles. Recruitment of antagonistic co-contraction increases torso stiffness (Lee et al., 2006) and would contribute to more positive values of PINT. Future studies should investigate normalized co-active recruitment during these exertions.
In all subjects, the reflex response contributed to the stabilizing control of the spine and torso. PINT consists of the antagonistic effects of the intrinsic muscle stiffness and gravitational effects. An estimate of the magnitude of the gravitational contribution can be obtained by calculating the trunk mass, M, and the trunk center of mass, h, from subject anthropometry (Winter, 1990). The mean value for the gravitational contribution to PINT was 1695±249 N/m. Therefore, from Eq. (2b), the mean value of intrinsic muscle stiffness was kINT = 1280±240 N/m during the 20% MVE exertions. This increased significantly in proportion to PINT up to a value of kINT = 2116±710 N/m during the 50% MVE exertions. The reflex contribution to stiffness is readily computed from Eq. (3) using the measured parameters of reflex dynamics (Table 1). The mean value of reflex stiffness kREFLEX = 1398±963 N/m failed to demonstrate a significant change with exertion. Recognizing that effective trunk stiffness is the sum of kINT and kREFLEX, one can estimate the effective stiffness from results in Table 1. At the 20% MVE exertion level, the mean extension force was 96±20 N and the estimated effective stiffness of 2486±1052 N/m. This agrees with published measurements. Moorhouse and Granata (2005) applied force perturbations to the trunk rather than position disturbances and obtained an effective trunk stiffness value of 2200±680 N/m during trunk extension exertions of 100 N (Moorhouse and Granata, 2005). Therefore, 42% of the total trunk stiffness can be attributed to the reflex response. There was no statistical difference in the percent of reflex contribution to trunk stiffness between exertion levels. This estimate regarding the role of reflex stiffness is somewhat limited because gravitational contributions were not directly measured but were calculated based on estimates of the subject anthropometry. However, sensitivity analyses indicate that a 10% error in the estimation of the gravitational moment would result in only a 2% error in the percentage contribution of reflexes. Therefore, it is clear that reflexes play a notable role in the stabilizing control of the torso and spine.
Reflex gain, GR, represents a measure of the magnitude of restorative forces that were contributed by the reflex response, analogous to the intrinsic stiffness. GR increased significantly with exertion. Neuromuscular research suggests that reflex activation is influenced by muscle tone (Matthews, 1986; Bennett et al., 1994). Reflex excitation tends to increase with the pre-activation of muscle until a saturation point of 50% MVE. Likewise, we observed increased reflex gain with the exertion levels up to 50% MVE in a similar manner to the intrinsic stiffness. This contributes to stabilizing control of the spine throughout a broad range of measured extension efforts.
This study revealed some interesting insight into the significant role that reflexes play in trunk dynamics and associated spinal stability. Existing spinal stability models neglect the mechanical effects of reflex responses. Recognizing that reflexes may account for up to 42% of the stabilizing dynamics of the torso, future models should include reflexes. Results may also indicate that individuals with disturbed reflex response may be more susceptible to spinal instability events. Postures and tasks that disturb reflex response may contribute to risk of injury. Solomonow (Solomonow et al., 1999) concluded that prolonged spinal flexion causes tissue creep deformation that subsequently attenuates paraspinal reflex activation. Similar trends have been noted in humans (Rogers and Granata, 2006; Granata et al., 2005). Thus, prolonged flexion may inhibit neuro-muscular control of spinal stability. Future studies should further examine the effect of reflex dynamics in its role of stabilizing neuromuscular control and risk of low-back pain.
Acknowledgments
This research was supported in part by a Grant R01 AR46111 from CDC/National Institute for Occupational Safety and Health. We wish to thank T. Franklin and S. Hanson for their assistance in data collection and M. Diersing for study coordination.
References
- Arjmand N, Shirazi-Adl A. Model and in vivo studies on human trunk load partitioning and stability in isometric forward flexions. Journal of Biomechanics. 2006;39:510–521. doi: 10.1016/j.jbiomech.2004.11.030. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Gorassini M, Prochazka A. Catching a ball: contributions of intrinsic muscle stiffness, reflexes, and higher order responses. Canadian Journal of Physiology and Pharmacology. 1994;72:525–534. doi: 10.1139/y94-076. [DOI] [PubMed] [Google Scholar]
- Bergmark A. Stability of the lumbar spine: a study in mechanical engineering. Acta Orthopaedica Scandinavica Supplementrum. 1989;230:1–54. doi: 10.3109/17453678909154177. [DOI] [PubMed] [Google Scholar]
- Cholewicki J, McGill SM. Mechanical stability on the in vivo lumbar spine: implications for injury and chronic low back pain. Clinical Biomechanics. 1996;11:1–15. doi: 10.1016/0268-0033(95)00035-6. [DOI] [PubMed] [Google Scholar]
- Gardner-Morse MG, Stokes IAF. Trunk stiffness increases with steady-state effort. Journal of Biomechanics. 2001;34:457–463. doi: 10.1016/s0021-9290(00)00226-8. [DOI] [PubMed] [Google Scholar]
- Gardner-Morse MG, Stokes IAF, Laible JP. Role of muscles in lumbar stability in maximum extension efforts. Journal of Orthopaedic Research. 1995;13:802–808. doi: 10.1002/jor.1100130521. [DOI] [PubMed] [Google Scholar]
- Granata KP, Rogers E, Moorhouse K. Effects of static flexion-relaxation on paraspinal reflex behavior. Clinical Biomechaa nics (Bristol, Avon) 2005;20:16–24. doi: 10.1016/j.clinbiomech.2004.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granata KP, Slota GP, Bennett BE, Kang HG. Paraspinal muscle reflex dynamics. Journal of Biomechanics. 2004;37:241–247. doi: 10.1016/s0021-9290(03)00249-5. [DOI] [PubMed] [Google Scholar]
- Granata KP, Wilson SE. Trunk Posture and Spinal Stability. Journal of Biomechanics. 2001;16:650–659. doi: 10.1016/s0268-0033(01)00064-x. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Richardson CA. Inefficient muscular stabilization of the lumbar spine associated with low back pain. A motor control evaluation of transversus abdominis. Spine. 1996;21:2640–2650. doi: 10.1097/00007632-199611150-00014. [DOI] [PubMed] [Google Scholar]
- Hunter IW, Korenberg MJ. The identification of nonlinear biological systems: Wiener and Hammerstein cascade models. Biological Cybernetics. 1986;55:135–144. doi: 10.1007/BF00341929. [DOI] [PubMed] [Google Scholar]
- Lee PJ, Rogers EL, Granata KP. Active trunk stiffness increases with co-contraction. Journal of Electromyography and Kinesiology. 2006;16:51–57. doi: 10.1016/j.jelekin.2005.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoto S, Taimela S, Hurri A, Aalto H, Pyykko I, Alaranta H . Psychomotor speed and postural control in chronic low back pain patients. A controlled follow-up study. Spine. 1996;21:2621–2627. doi: 10.1097/00007632-199611150-00012. [DOI] [PubMed] [Google Scholar]
- Matthews PBC. Observations on the automatic compensation of reflex gain on varying the pre-existing level of motor discharge in man. Journal of Physiology. 1986;374:73–90. doi: 10.1113/jphysiol.1986.sp016066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill SM, Seguin JP, Bennett G. Passive stiffness of the lumbar torso in flexion, extension, lateral bending, and axial rotation. Effect of belt wearing and breath holding. Spine. 1994;19:696–704. doi: 10.1097/00007632-199403001-00009. [DOI] [PubMed] [Google Scholar]
- Mirbagheri MM, Barbeau H, Kearney RE. Intrinsic and reflex contributions to human ankle stiffness: variation with activation level and position. Experimantal Brain Research. 2000;135:423–436. doi: 10.1007/s002210000534. [DOI] [PubMed] [Google Scholar]
- Moorhouse KM, Granata KP. Trunk stiffness and dynamics during active extension exertions. Journal of Biomechanical. 2005;38:2000–2007. doi: 10.1016/j.jbiomech.2004.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyfeh AH, Balachandran B. Applied Nonlinear Dynamics: Analytical, Computational and Experimental Methods. Wiley; New York: 2005. [Google Scholar]
- Nichols TR, Houk JC. Improvement of linearity and regulation of stiffness that results from the actions of the stretch reflex. Journal of Neurophysiology. 1976;39:119–142. doi: 10.1152/jn.1976.39.1.119. [DOI] [PubMed] [Google Scholar]
- Ogata K. Modern Control Engineering. Prentice Hall, Upper Saddle River; NJ: 2002. [Google Scholar]
- Panjabi MM. The stabilizing system of the spine. Part I Function, dysfunction, adaptation and enhancement. Journal of Spinal Disorders. 1992a;5:383–389. doi: 10.1097/00002517-199212000-00001. [DOI] [PubMed] [Google Scholar]
- Panjabi MM. The stabilizing system of the spine. Part II Neutral zone and instability hypothesis. Journal of Spinal Disorders. 1992b;5:390–397. doi: 10.1097/00002517-199212000-00002. [DOI] [PubMed] [Google Scholar]
- Radebold A, Cholewicki J, Polzhofer GA, Green TP. Impaired postural control of the lumbar spine is associated with delayed muscle response times in patients with chronic idiopathic low back pain. Spine. 2001;26:724–730. doi: 10.1097/00007632-200104010-00004. [DOI] [PubMed] [Google Scholar]
- Rogers E, Granata KP. Disturbed paraspinal reflex following flexion-relaxation and recovery. Spine. 2006;31:839–845. doi: 10.1097/01.brs.0000206361.53451.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomonow M, Zhou B, Baratta RV, Lu Y, Harras M. Biomechanics of increased exposure to lumbar injury caused by cyclic loading: part 1. loss of reflexive muscular stabilization. Spine. 1999;24:2426–2434. doi: 10.1097/00007632-199912010-00003. [DOI] [PubMed] [Google Scholar]
- Solomonow M, Zhou B, Harras M, Lu Y, Baratta RV. The ligamento-muscular stabilizing system of the spine. Spine. 1998;23:2552–2562. doi: 10.1097/00007632-199812010-00010. [DOI] [PubMed] [Google Scholar]
- Stokes IAF, Gardner-Morse MG. Lumbar spine maximum efforts and muscle recruitment patterns predicted by a model with multijoint muscles and joints with stiffness. Spine. 1995;28:173–186. doi: 10.1016/0021-9290(94)e0040-a. [DOI] [PubMed] [Google Scholar]
- Westwick DT, Kearney RE. Identification of Nonlinear Physiologic Systems. IEEE Press, Piscataway; NJ: 2003. [Google Scholar]
- Winter DA. Biomechanics and Motor Control of Human xsMovement. Wiley-Interscience Publication; New York: 1990. [Google Scholar]


