Abstract
Proton magnetic resonance spectroscopy (1H-MRS) studies showing increased lactate during neural activation support a broader role for lactate in brain energy metabolism than was traditionally recognized. 1H-MRS measures of brain lactate responses have been used to study regional brain metabolism in clinical populations. This study examined whether variations in blood glucose influence the lactate response to visual stimulation in the visual cortex. Six subjects were scanned twice, receiving either saline or 21% glucose intravenously. Using 1H-MRS at 1.5 Tesla with a long echo time (TE=288 msec), the lactate doublet was visible at 1.32 ppm in the visual cortex of all subjects. Lactate increased significantly from resting to visual stimulation. Hyperglycemia had no effect on this increase. The order of the slice-selective gradients for defining the spectroscopy voxel had a pronounced effect on the extent of contamination by signal originating outside the voxel. The results of this preliminary study demonstrate a method for observing a consistent activity-stimulated increase in brain lactate at 1.5 Tesla and show that variations in blood glucose across the normal range have little effect on this response.
Keywords: lactic acid, glycolysis, glycolytic, aerobic, PRESS
1. Introduction
Converging evidence from animal and human studies of both muscle and brain metabolism has stimulated critical reexamination of the role of lactate in energy metabolism (Brooks, 1986; Gladden, 2004; Schurr, 2005). It was previously believed that lactate is produced only when there is insufficient oxygen for mitochondrial metabolism of pyruvate to CO2 and H20, and that lactate is not produced under aerobic conditions (views which still appear medical textbooks (Baynes and Dominiczak, 2005)). However, studies of muscle energy metabolism have established that lactate is almost always produced during intense muscle activity under fully aerobic conditions, and that lactate serves to shuttle energy substrate from one cell or cellular compartment to another (Brooks, 1986; Gladden, 2004). Recent studies have suggested that lactate might serve a similar role in brain energy metabolism (Pellerin et al., 1998; Schurr, 2005). In vivo measures of brain lactate with microsensors in rats (Hu and Wilson, 1997) and with proton magnetic resonance spectroscopy (1H-MRS) in humans (Prichard et al., 1991; Sappey-Marinier et al., 1992; Frahm et al., 1996) have demonstrated brain lactate increases during neuronal activation. These 1H-MRS studies suggests that noninvasive measures of activity-stimulated lactate responses can be used to study brain function in humans. Clinical neuroscientists have begun to use 1H-MRS measures of brain lactate responses to characterize normal and clinical populations, although the strengths and limitations of this method are not well understood. Elevated brain lactate responses to metabolic challenges have consistently been observed in patients with panic disorder (Dager et al., 1995; Dager et al., 1999; Maddock, 2001), and these findings have recently been extended to include elevated visual cortex lactate responses to visual stimulation (Maddock and Buonocore, 2005). Richards and colleagues reported elevated lactate in left frontal regions in dyslexic boys during an oral language task (Richards et al., 1999) and later showed normalization with treatment (Richards et al., 2000). Dynamic lactate responses have also been used to assess patients with hearing loss (Richards et al., 1997) and mitochondrial disorders (Kuwabara et al., 1994), and to assess the effects of sleep deprivation (Urrila et al., 2004).
The most widely used procedure for assessing brain lactate dynamics in humans with 1H-MRS has involved examining visual cortex lactate responses to visual stimulation. However, not all investigators report detection of lactate in normal subjects at rest or consistent lactate increases during visual stimulation, especially with 1.5 Tesla systems (Merboldt et al., 1992; Boucard et al., 2005; Sandor et al., 2005). Thus, a primary goal of the current study was to examine the feasibility of measuring basal and activity-stimulated lactate levels in the visual cortex with a 1.5 Tesla system.
The availability of glucose as a substrate has been shown to influence the systemic lactate response to alkalosis in animals and humans. An alkaline shift in intracellular pH is known to have a powerful disinhibiting effect on phosphofructokinase, a key, rate-limiting enzyme of glycolysis (Trivedi and Danforth, 1966). Alkalosis leads to increased lactate production both systemically and in the brain (Dager et al., 1995; Hood and Tannen, 1998; Maddock, 2001). Both human and animal studies have shown that the increase in serum lactate during respiratory alkalosis is significantly greater during mild hyperglycemia than during a normoglycemic, fasting state (Brautbar et al., 1983; Maddock and Mateo-Bermudez, 1990). Although mild hyperglycemia does not appear to increase brain lactate at rest (Lundbom et al., 1999; Abi-Saab et al., 2002), no prior studies have examined the effect of mild hyperglycemia on activity-stimulated brain lactate responses. Recent studies have demonstrated intracellular alkalinization of glia during neural activation and suggested that this may be one of the mechanisms through which neural activity leads to an increase in glial glycolysis (Itoh et al., 2000; Chesler, 2003). If this is an important mechanism of the activity-stimulated brain lactate response, then mild hyperglycemia may increase the lactate response during brain activation just as it increases systemic lactate responses during intracellular alkaline shifts. Thus, one goal of this study was to test the hypothesis that mild hyperglycemia leads to an increase in the brain lactate response to visual stimulation. If this hypothesis is confirmed, then control or monitoring of blood glucose levels might be required in 1H-MRS studies of the activity-stimulated brain lactate response in clinical populations.
To demonstrate a method for measuring brain lactate during baseline and stimulation conditions with a 1.5 Tesla MRI system, and to determine if variations in blood glucose modify the activity-stimulated brain lactate response, the visual cortex lactate response to visual stimulation was measured during fasting normoglycemia and mild hyperglycemia using a 1.5 Tesla system.
2. Methods
2.1 Subjects and Procedures
Six subjects (4 male) aged 21 to 34 years old (mean = 24) gave informed consent and were studied twice, 1 to 7 days apart. A brief medical history (by RJM) established that all subjects were taking no medications and free of current medical illnesses. On each day, the subject arrived between 8:00 and 9:00 AM after a 12 hour fast (attested by self-report) and had an intravenous line placed in a forearm vein. 1H-MRS data were acquired in four scans from each subject. The “baseline” state was defined as the subject resting with eyes closed. First, an initial baseline 1H-MRS scan was acquired. Then, a 155 ml infusion of either 21% glucose or normal saline was started and completed over the subsequent 29 minutes. After 10 minutes of infusion, a second baseline 1H-MRS scan was acquired, followed immediately by two successive scans acquired with eyes open and viewing a visual stimulus (Figure 1). The visual stimulus was a black and white radial checkerboard pattern, with spatial frequencies scaled as a function of eccentricity to match the cortical magnification factor of primary visual cortex. The checkerboard underwent pattern reversal flicker at 8 Hz. The stimulus was projected to a vertical viewing screen at the subject’s feet and was observed via a mirror positioned above the subject’s eyes. Blood glucose level was measured with an Accu-Check monitor (Roche Diagnostics, Basel, Switzerland) using capillary blood obtained by finger stick before and after the infusion. The order of infusions was balanced across subjects.
Figure 1.
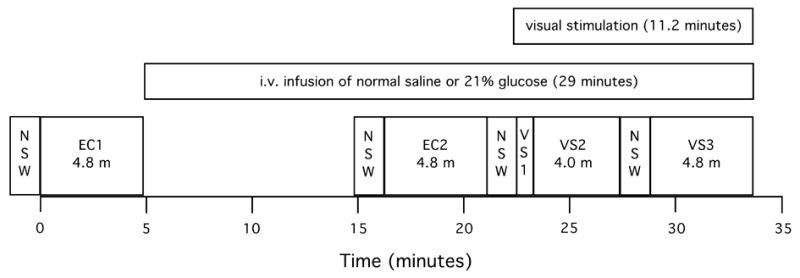
Timing of experimental events. EC1 & EC2 = eyes closed baseline acquisitions; VS1 = the first minute of visual stimulation (duration = 0.8 minutes, no metabolite data were collected during the first 12 seconds of stimulation); VS2 = minutes 2 through 5 of visual stimulation (duration = 4.0 minutes); VS3 = minutes 7 through 11 of visual stimulation (duration = 4.8 minutes); NSW = non-suppressed water scans (1.4 minutes each); m = minutes.
2.2 Scanning Parameters
All MR data were acquired with a 1.5 Tesla magnetic resonance imaging system (Signa Horizon NV/i, OS Version 84M4; GE Medical Systems, Milwaukee, WI) with a 3 inch, receive-only, single-channel, surface coil positioned under the occiput. A sagittal T1-weighted locator scan followed by a coronal T2-weighted fast spin echo scan were acquired for anatomical localization. Four single voxel 1H-MRS measurements were performed using a point-resolved spectroscopy sequence with the following parameters: psd: PROBE-P, TE: 288 msec, TR: 1500 msec, phase cycling: 8, acquisitions: 192, bandwidth: 2500 Hz, acquired points: 2048, outer volume spatial saturation pulses: on. Pilot testing showed that the order of the slice-selective gradients, which defined the spectroscopy voxel via a slice-selective 90°-180°-180° RF pulse sequence, had a pronounced effect on the extent of contamination in the proton spectra from signal originating outside the voxel, as previously reported (Ernst and Chang, 1996). Of the six possible “gradient orders”, only two (R/L, A/P, S/I and S/I, A/P, R/L) produced proton spectra that were free from this artifact, which appeared to include contaminating lipid signal from tissue adjacent to the surface coil (Figure 2). A slice gradient order of R/L, A/P, S/I was used for this study. Automated shimming and RF pulse flip angle optimization procedures preceded the initial MRS scan. Each of the four scans began with an 84 second acquisition of non-suppressed water data (NSW; for subsequent phase optimization), followed by acquisition of metabolite data over 288 seconds, for a total scan duration of 372 seconds. A 30 cc voxel was acquired in the coronal plane and localized in bilateral primary visual cortices centered on the calcarine fissures. Voxel dimensions were 40 mm (L/R) x 25 mm (A/P) x 30 mm (S/I).
Figure 2.
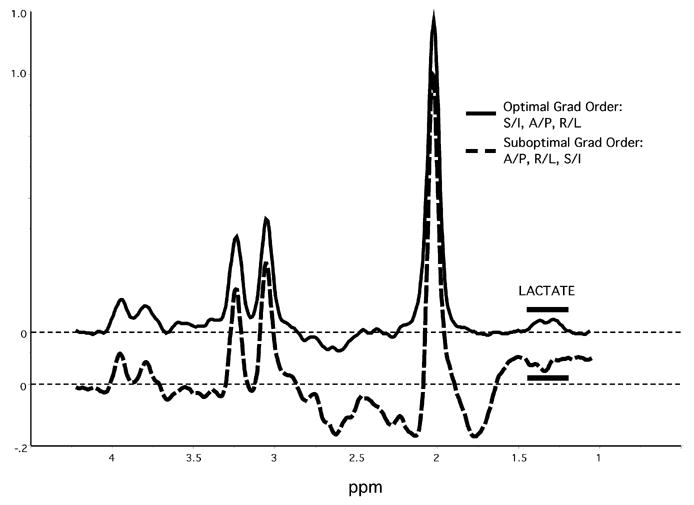
The effect of slice-selective gradient order on contamination by signal originating outside the voxel. The two spectra shown were obtained consecutively from the same voxel in the same subject, with only the gradient order changed. Note that the “default” gradient order (A/P, R/L, S/I) showed extensive contamination making quantification of lactate signal impossible. Spectra amplitudes are normalized to the height of the NAA peak.
2.3 Data Analysis
Five time periods were defined for data analysis (Figure 1): EC1 = eyes closed baseline prior to i.v. infusion (scan #1, duration = 4.8 minutes); EC2 = eyes closed baseline beginning after 11.4 minutes of i.v. infusion (scan #2, duration = 4.8 minutes); VS1 = the first minute of visual stimulation (beginning of scan #3, duration = 48 seconds (no metabolite data were collected during the first 12 seconds of stimulation)); VS2 = minutes 2 through 5 of visual stimulation (remainder of scan #3, duration = 4.0 minutes); and VS3 = minutes 7 through 11 of visual stimulation (scan #4, duration = 4.8 minutes). Data obtained during the first minute of visual stimulation were quantified separately, since pilot studies had shown that the lactate increase did not begin until the second minute.
NAA, creatine, choline and lactate peaks were quantified using MRUI software (MRUI, 2003) to estimate the relative concentrations of these metabolites within the selected voxel. First, individual 12 second “frames” of spectral data from each scan were zero-filled to 4096 and phase-aligned using water as the reference. The 12 second “frames” were then summed across the time period of interest and apodized with a 4 Hz Gaussian function in the time domain. After setting the NAA peak to 2.01 ppm as a reference frequency, lactate and NAA peaks were quantified by custom software implementing an automated, fixed frequency, peak integration procedure, which consisted of setting the NAA peak to 2.01 ppm and summing the spectral values from 1.87 to 2.15 ppm for NAA and from 1.20 to 1.43 ppm for lactate. The Lactate/NAA ratio was then calculated for each time period. Lactate was quantified relative to NAA, rather than creatine, because NAA provides a surrogate marker for neuronal integrity. Lactate responses are expected to result primarily from neuronal activation, and NAA values can serve to normalize lactate to the volume of healthy neuronal tissue in the voxel. In addition, NAA, creatine and choline peaks were quantified using an automated curve fitting algorithm (AMARES) within MRUI. Metabolite data during baseline and visual stimulation periods were analyzed by repeated measures analysis of variance (ANOVAR) with two within subjects variables (glycemic condition and time) followed by directional (one-tailed) paired t-tests.
3. Results
The lactate doublet centered at 1.32 ppm was visible in all subjects (Figure 3 shows example spectra). Mean blood glucose was 94 and 85 mg/dl (5.2 and 4.7 mmol/l) before and after the saline infusion and 96 and 192 mg/dl (5.3 and 10.7 mmol/l) before and after the glucose infusion. The NAA values obtained by curve fitting and by the peak integration method were highly correlated across the 48 scans (r = 0.998).
Figure 3.
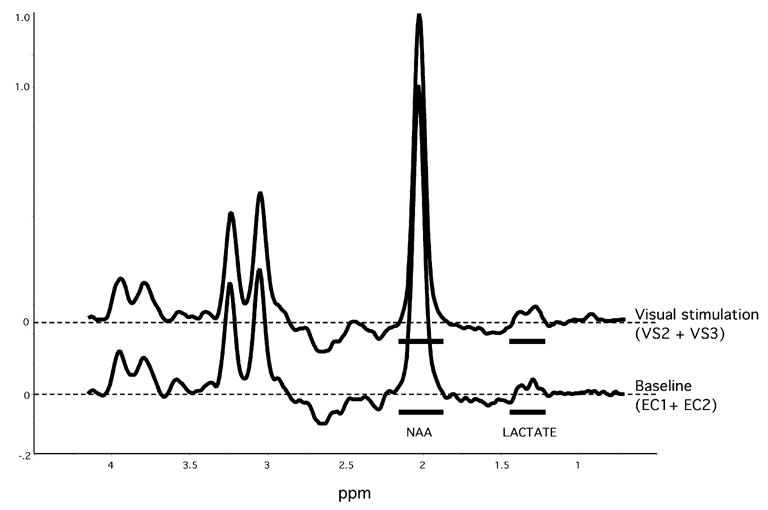
Example spectra during the eyes closed baseline conditions (EC1 + EC2) and the visual stimulation conditions (VS2 + VS3) from one subject. The integration limits for lactate and NAA are shown (solid bars). Spectra amplitudes are normalized to the height of the NAA peak.
Two-way ANOVAR of Lactate/NAA values showed a significant main effect for time (F = 4.3, df = 4, 20, P = 0.011) (Figure 4) but no main effect for glycemic condition (F = 1.4, df = 1, 5, NS) or interaction between glycemic condition and time (F = 1.7, df = 4, 20, NS). Directional (one-tailed) paired t-tests showed that the main effect for time was due to significant increases in lactate from EC1 to VS2 and VS3 (t = 2.13, df = 5, P = 0.043 and t = 3.84, df = 5, P = 0.006 respectively) and from EC2 to VS2 and VS3 (t = 4.19, df = 5, P = 0.004 and t = 3.61, df = 5, P = 0.008 respectively). The overall increase in lactate, quantified as the difference between the weighted average of VS2 and VS3 compared to the average of EC1 and EC2, was highly significant (t = 4.9, df = 5, P = 0.002, OT) and had an effect size of 2.00 (quantified as the mean change from baseline normalized to the standard deviation of the changes from baseline). Figure 3 shows example spectra during these conditions (the sum of EC1 and EC2 compared to the sum of VS2 and VS3) from one subject. Lactate/NAA during the first minute of visual stimulation (VS1) was slightly but not significantly lower than EC1 and EC2. Inspection of the Lactate/NAA values during each minute of visual stimulation showed that lactate increased during the second minute of stimulation and then changed little throughout the subsequent 9 minutes of stimulation. No change in the quality of the spectra was observed as the experiment progressed from the eyes closed to the visual stimulation conditions. Correlation between the mean baseline Lactate/NAA values on the two test days demonstrated a high test-retest reliability (r = 0.881).
Figure 4.
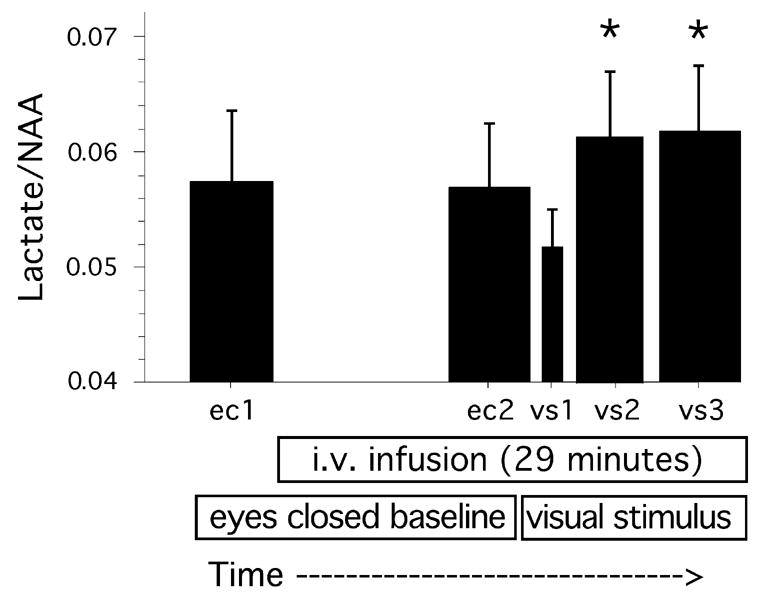
The effect of visual stimulation on Lactate/NAA levels, across glycemic conditions (F = 4.3, df = 4, 20, P = 0.011). *Post hoc tests showed that Lactate/NAA was significantly higher during VS2 and VS3 than during EC1 and EC2 (all P <0.05, see text). Durations: VS1 = 0.8 minutes, VS2 = 4 minutes, all other conditions = 4.8 minutes. EC = eyes closed, VS = visual stimulation. Note that Y axis scale does not begin at zero. Mean and s.e.m. values are shown.
Neither visual stimulation nor glycemic condition significantly affected NAA/Cr or Ch/Cr ratios (Table 1). Reanalysis of the lactate data calculated as Lactate/Creatine ratio produced an identical pattern of results. As a control for the possibility that changes in lipid contamination across the experiment may have confounded the measurement of lactate changes, signal centered at 0.9 ppm was examined. Mobile lipids can give rise to prominent and broad resonances at 1.3, and 0.9 ppm, especially at short TEs (Auer et al., 2001). We calculated the signal at 0.9 ppm (by integration from 0.78 to 1.02 ppm and normalization to the NAA value) during the eyes closed baseline and visual stimulation periods of the experiment. If an increase in lipid contamination at 1.3 ppm during the visual stimulation period was confounding our measure of lactate changes, the increased lipid signal should also be observed at 0.9 ppm. There were no significant changes or trends in the signal at this frequency (Table 1).
Table 1.
Metabolite values during baseline and visual stimulation, averaged across glycemic conditions
| EC1 | EC2 | VS2 | VS3 | F ratio | P value | |
|---|---|---|---|---|---|---|
| Lactate/NAA | 0.058 | 0.057 | 0.061 | 0.062 | 6.59 | 0.0046 |
| Lactate/Cr | 0.160 | 0.158 | 0.172 | 0.173 | 7.05 | 0.0035 |
| NAA/Cr | 2.772 | 2.769 | 2.809 | 2.789 | 0.77 | NS |
| Ch/Cr | 0.9174 | 0.9203 | 0.9399 | 0.9367 | 1.98 | NS |
| 0.9 ppm/NAA | 0.024 | 0.024 | 0.023 | 0.023 | 0.35 | NS |
EC1 and EC2 are eyes closed baseline conditions, VS2 and VS3 are visual stimulation conditions. F ratio calculated by ANOVAR over the 4 time periods shown (df = 3, 15 for all). Note that this analysis excludes data from the first minute following the onset of visual stimulation (VS1). Signal centered at 0.9 ppm (normalized to NAA) serves as a control for possible changes in lipid signal over time.
To examine the rate of return to baseline lactate levels after the offset of visual stimulation, spectral data were obtained during two consecutive 5 minute post-stimulation recovery periods (EC3 and EC4) in five of the six subjects. Recovery data were obtained in the sixth subject during the first (EC3), but not the second (EC4), recovery period. We defined “percent recovery” as percent return of the lactate/NAA value from the value observed during VS3 to the mean baseline value. Thus, percent recovery during EC3 was quantified as:
and percent recovery during EC4 was quantified as:
These data show mean recovery = 35.1% during the first 5 minutes following the offset of visual stimulation (EC3). Using the EC3 value in place of the missing EC4 value in subject 6, the mean recovery was 65.5% during the second 5 minutes following stimulation (EC4).
4. Discussion
The 1H-MRS methods used in this study demonstrated a consistent increase in lactate in the human visual cortex during visual stimulation. The effect size of 2.00 for the increase in visual cortex lactate in our study agrees with the effect size of 1.88 observed in an earlier study using similar procedures (Prichard et al., 1991). However, other studies have reported a higher percent change in lactate than we observed in our study (Prichard et al., 1991; Sappey-Marinier et al., 1992; Frahm et al., 1996). Differences in field strength, acquisition parameters, and/or the visual stimulus may account for this difference. Since sustained visual stimulation is not associated with hypoxia (Frahm et al., 1996; Kruger et al., 1999), the findings of our study and prior 1H-MRS studies argue against the historical view that lactate is not produced under aerobic conditions (Baynes and Dominiczak, 2005). It has been well established in studies of muscle metabolism that lactate is regularly produced during muscle activity under fully aerobic conditions. Our findings add to a growing literature showing that this is also true of brain metabolism during neuronal activation (Brooks, 1986; Gladden, 2004; Schurr, 2005).
The increase in visual cortex lactate during visual stimulation was unaffected by the change in blood glucose concentration from fasting normoglycemia to mild hyperglycemia. When lactate production in systemic tissues is triggered by an intracellular alkaline shift, such as occurs during respiratory alkalosis, mild hyperglycemia leads to significantly greater lactate responses (Brautbar et al., 1983; Maddock and Mateo-Bermudez, 1990). Some studies have suggested that intracellular alkalinization of glia during neural activation may trigger an acceleration of glial lactate production (Itoh et al., 2000; Chesler, 2003). This proposed mechanism led us to predict that hyperglycemia would lead to greater brain lactate responses during neural activation. However, we saw no evidence that the visual cortex lactate response to visual stimulation was increased during mild hyperglycemia. This suggests that normal variation in blood glucose levels will not be a significant confounding variable in clinical MRS studies of brain lactate responses during neural activation.
Our results contrast with earlier studies that did not demonstrate brain lactate increases during visual stimulation (Merboldt et al., 1992; Boucard et al., 2005; Sandor et al., 2005). Boucard et al (Boucard et al., 2005) concluded that low lactate signal precluded reliable measures of visual cortex lactate responses with a 1.5 Tesla system. Our study, like several prior studies successfully demonstrating activity-stimulated visual cortex lactate responses, made use of the higher sensitivity achieved with a surface coil placed directly under the occiput (Prichard et al., 1991; Sappey-Marinier et al., 1992). The lower signal-to-noise ration of occipital cortex metabolite signals using a standard quadrature head coil may account for the inability to detect lactate in some prior studies (Boucard et al., 2005; Sandor et al., 2005). Boucard et al (Boucard et al., 2005) suggested that prior claims about visual cortex lactate responses may have resulted from lipid contamination erroneously attributed to lactate. Examination of our spectra and quantification of changes in lipid signal at 0.9 ppm show that lipid contamination does not explain the observed changes in lactate. Our preliminary studies showed that the order of the slice-selective gradients had a dramatic effect on the extent of contamination from signal originating outside the voxel (Figure 1), as previously described (Ernst and Chang, 1996). Use of a surface coil positioned under the occiput, combined with an optimal gradient order, allowed us to detect consistent and uncontaminated increases in lactate during visual stimulation with a 1.5 Tesla system.
A previous study using a fast-response lactate microsensor in rats reported a biphasic lactate response, with a decrease in lactate during the first seconds following neural activation followed by a later and more sustained increase (Hu and Wilson, 1997). Mangia et al (Mangia et al., 2003) also reported a decrease in lactate during the first seconds following visual stimulation in humans with 1H-MRS using a signal averaging technique. Our methods and procedures were unable to demonstrate a consistent early decrease in lactate during visual stimulation. However, a variable lactate response with a non-significant overall decrease was observed during the first minute of stimulation. To reliably investigate the lactate response during the first seconds following visual stimulation in human subjects, methods with higher SNR and temporal resolution would be required.
In summary, we replicated the finding of a significant increase in lactate in the visual cortex during visual stimulation using 1H-MRS. Our findings add to growing evidence that hypoxia is not a precondition for lactate production in brain, and that aerobic production of lactate occurs during neural activation. Acute hyperglycemia had no effect on the lactate response, suggesting that 1H-MRS studies of brain lactate responses in subjects without metabolic disease are unlikely to be confounded by variations in blood glucose level within the normal range. The choice of slice-selective gradient order was critical for minimizing contamination by artifact in the proton spectra from signal originating outside the voxel. When 1H-MRS spectra are acquired with a surface coil and an optimal slice-selective gradient order, both baseline lactate and increased lactate during visual stimulation can be consistently observed in the visual cortex using a 1.5 Tesla MRI system.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (MH-69699 to RJM) The authors thank Drs. Thomas Nordahl and Greg Harrington for advice and assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abi-Saab WM, Maggs DG, Jones T, Jacob R, Srihari V, Thompson J, Kerr D, Leone P, Krystal JH, Spencer DD, During MJ, Sherwin RS. Striking differences in glucose and lactate levels between brain extracellular fluid and plasma in conscious human subjects: Effects of hyperglycemia and hypoglycemia. Journal of Cerebral Blood Flow and Metabolism. 2002;22:271–279. doi: 10.1097/00004647-200203000-00004. [DOI] [PubMed] [Google Scholar]
- Auer DP, Gossl C, Schirmer T, Czisch M. Improved analysis of 1h-mr spectra in the presence of mobile lipids. Magnetic Resonance in Medicine. 2001;46:615–618. doi: 10.1002/mrm.1235. [DOI] [PubMed] [Google Scholar]
- Baynes J, Dominiczak M. Medical biochemistry. Elsevier Mosby; Philadelphia: 2005. [Google Scholar]
- Boucard CC, Mostert JP, Cornelissen FW, De Keyser J, Oudkerk M, Sijens PE. Visual stimulation, (1)h mr spectroscopy and fmri of the human visual pathways. European Radiology. 2005;15:47–52. doi: 10.1007/s00330-004-2494-y. [DOI] [PubMed] [Google Scholar]
- Brautbar N, Leibovici H, Massry SG. On the mechanism of hypophosphatemia during acute hyperventilation: Evidence for increased muscle glycolysis. Miner Electrolyte Metab. 1983;9:45–50. [PubMed] [Google Scholar]
- Brooks GA. Lactate production under fully aerobic conditions: The lactate shuttle during rest and exercise. Federation Proceedings. 1986;45:2924–2929. [PubMed] [Google Scholar]
- Chesler M. Regulation and modulation of ph in the brain. Physiological Reviews. 2003;83:1183–1221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- Dager SR, Friedman SD, Heide A, Layton ME, Richards T, Artru A, Strauss W, Hayes C, Posse S. Two-dimensional proton echo-planar spectroscopic imaging of brain metabolic changes during lactate-induced panic. Archives of General Psychiatry. 1999;56:70–77. doi: 10.1001/archpsyc.56.1.70. [DOI] [PubMed] [Google Scholar]
- Dager SR, Strauss WL, Marro KI, Richards TL, Metzger GD, Artru AA. Proton magnetic resonance spectroscopy investigation of hyperventilation in subjects with panic disorder and comparison subjects. American Journal of Psychiatry. 1995;152:666–672. doi: 10.1176/ajp.152.5.666. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L. Elimination of artifacts in short echo time 1h mr spectroscopy of the frontal lobe. Magnetic Resonance in Medicine. 1996;36:462–468. doi: 10.1002/mrm.1910360320. [DOI] [PubMed] [Google Scholar]
- Frahm J, Kruger G, Merboldt KD, Kleinschmidt A. Dynamic uncoupling and recoupling of perfusion and oxidative metabolism during focal brain activation in man. Magnetic Resonance in Medicine. 1996;35:143–148. doi: 10.1002/mrm.1910350202. [DOI] [PubMed] [Google Scholar]
- Gladden LB. Lactate metabolism: A new paradigm for the third millennium. Journal of Physiology. 2004;558:5–30. doi: 10.1113/jphysiol.2003.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood VL, Tannen RL. Protection of acid-base balance by ph regulation of acid production. New England Journal of Medicine. 1998;339:819–826. doi: 10.1056/NEJM199809173391207. [DOI] [PubMed] [Google Scholar]
- Hu Y, Wilson GS. A temporary local energy pool coupled to neuronal activity: Fluctuations of extracellular lactate levels in rat brain monitored with rapid-response enzyme-based sensor. Journal of Neurochemistry. 1997;69:1484–1490. doi: 10.1046/j.1471-4159.1997.69041484.x. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Law MJ, Sokoloff L. Effects of the na(+)/h(+) exchanger monensin on intracellular ph in astroglia. Brain Research. 2000;882:226–229. doi: 10.1016/s0006-8993(00)02822-5. [DOI] [PubMed] [Google Scholar]
- Kruger G, Kastrup A, Takahashi A, Glover G. Simultaneous monitoring of dynamic changes in cerebral blood flow and oxygenation during sustained activation of the human visual cortex. Neuroreport. 1999;10:2939–2943. doi: 10.1097/00001756-199909290-00012. [DOI] [PubMed] [Google Scholar]
- Kuwabara T, Watanabe H, Tanaka K, Tsuji S, Ohkubo M, Ito T, Sakai K, Yuasa T. Mitochondrial encephalomyopathy: Elevated visual cortex lactate unresponsive to photic stimulation--a localized 1h-mrs study. Neurology. 1994;44:557–559. doi: 10.1212/wnl.44.3_part_1.557. [DOI] [PubMed] [Google Scholar]
- Lundbom NM, Manner T, Komu M, Peltola O, Leino KA, Kirvela OA. Barbiturate anesthesia and brain proton spectroscopy. AJNR Am J Neuroradiol. 1999;20:1543–1546. [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ. The lactic acid response to alkalosis in panic disorder: An integrative review. Journal of Neuropsychiatry and Clinical Neurosciences. 2001;13:22–34. doi: 10.1176/jnp.13.1.22. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Buonocore MH. Elevated lactate response during neural activation in panic disorder. Presented at International Society for Magnetic Resonance in Medicine Workshop on MR Spectroscopy in Neuropsychiatric Disorders (October 2005); ISMRM, Banff, Alberta, Canada. 2005. [Google Scholar]
- Maddock RJ, Mateo-Bermudez J. Elevated serum lactate following hyperventilation during glucose infusion in panic disorder. Biological Psychiatry. 1990;27:411–418. doi: 10.1016/0006-3223(90)90551-c. [DOI] [PubMed] [Google Scholar]
- Mangia S, Garreffa G, Bianciardi M, Giove F, Di Salle F, Maraviglia B. The aerobic brain: Lactate decrease at the onset of neural activity. Neuroscience. 2003;118:7–10. doi: 10.1016/s0306-4522(02)00792-3. [DOI] [PubMed] [Google Scholar]
- Merboldt KD, Bruhn H, Hanicke W, Michaelis T, Frahm J. Decrease of glucose in the human visual cortex during photic stimulation. Magnetic Resonance in Medicine. 1992;25:187–194. doi: 10.1002/mrm.1910250119. [DOI] [PubMed] [Google Scholar]
- MRUI. Magnetic resonance user interface. 2003;2003 Available at http://carbon.uab.es/mrui/mrui_Overview.shtml.
- Pellerin L, Pellegri G, Bittar PG, Charnay Y, Bouras C, Martin JL, Stella NJMP. Evidence supporting the existence of an activity dependent astrocyte-neuron lactate shuttle. Developmental Neuroscience. 1998;20:291–299. doi: 10.1159/000017324. [DOI] [PubMed] [Google Scholar]
- Prichard J, Rothman D, Novotny E, Petroff O, Kuwabara T, Avison M, Howseman A, Hanstock C, Shulman R. Lactate rise detected by 1h nmr in human visual cortex during physiologic stimulation. Proceedings of the National Academy of Sciences, USA. 1991;88:5829–5831. doi: 10.1073/pnas.88.13.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards T, Dager SR, Corina D, Serafini S, Heide AC, Steury K, Strauss W, Hayes CE, Abbott RD, Craft S, Shaw D, Posse S, Berninger VW. Dyslexic children have abnormal brain lactate response to reading-related language tasks. American Journal of Neuroradiology. 1999;20:1393–1398. [PMC free article] [PubMed] [Google Scholar]
- Richards TL, Corina D, Serafini S, Steury K, Echelard DR, Dager SR, Marro K, Abbott RD, Maravilla KR, Berninger VW. Effects of a phonologically driven treatment for dyslexia on lactate levels measured by proton mr spectroscopic imaging. American Journal of Neuroradiology. 2000;21:916–922. [PMC free article] [PubMed] [Google Scholar]
- Richards TL, Gates GA, Gardner JC, Merrill T, Hayes CE, Panagiotides H, Serafini S, Rubel EW. Functional mr spectroscopy of the auditory cortex in healthy subjects and patients with sudden hearing loss. American Journal of Neuroradiology. 1997;18:611–620. [PMC free article] [PubMed] [Google Scholar]
- Sandor PS, Dydak U, Schoenen J, Kollias SS, Hess K, Boesiger P, Agosti RM. Mr-spectroscopic imaging during visual stimulation in subgroups of migraine with aura. Cephalalgia. 2005;25:507–518. doi: 10.1111/j.1468-2982.2005.00900.x. [DOI] [PubMed] [Google Scholar]
- Sappey-Marinier D, Calabrese G, Fein G, Hugg JW, Biggins C, Weiner MW. Effect of photic stimulation on human visual cortex lactate and phosphates using 1h and 31p magnetic resonance spectroscopy. Jouirnal of Cerebral Blood Flow and Metabolism. 1992;12:584–592. doi: 10.1038/jcbfm.1992.82. [DOI] [PubMed] [Google Scholar]
- Schurr A. Lactate: The ultimate cerebral oxidative energy substrate? Journal of Cerebral Blood Flow and Metabolism. 2005 doi: 10.1038/sj.jcbfm.9600174. 10.1038/sj.jcbfm.9600174. [DOI] [PubMed] [Google Scholar]
- Trivedi B, Danforth WH. Effect of ph on the kinetics of frog muscle phosphofructokinase. Journal of Biological Chemistry. 1966;241:4110–4114. [PubMed] [Google Scholar]
- Urrila AS, Hakkarainen A, Heikkinen S, Vuori K, Stenberg D, Hakkinen AM, Lundbom N, Porkka-Heiskanen T. Stimulus-induced brain lactate: Effects of aging and prolonged wakefulness. Journal of Sleep Research. 2004;13:111–119. doi: 10.1111/j.1365-2869.2004.00401.x. [DOI] [PubMed] [Google Scholar]


