Abstract
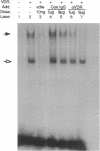
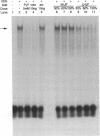
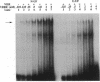
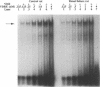
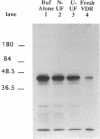
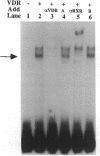
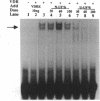
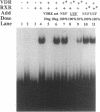
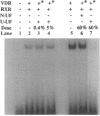
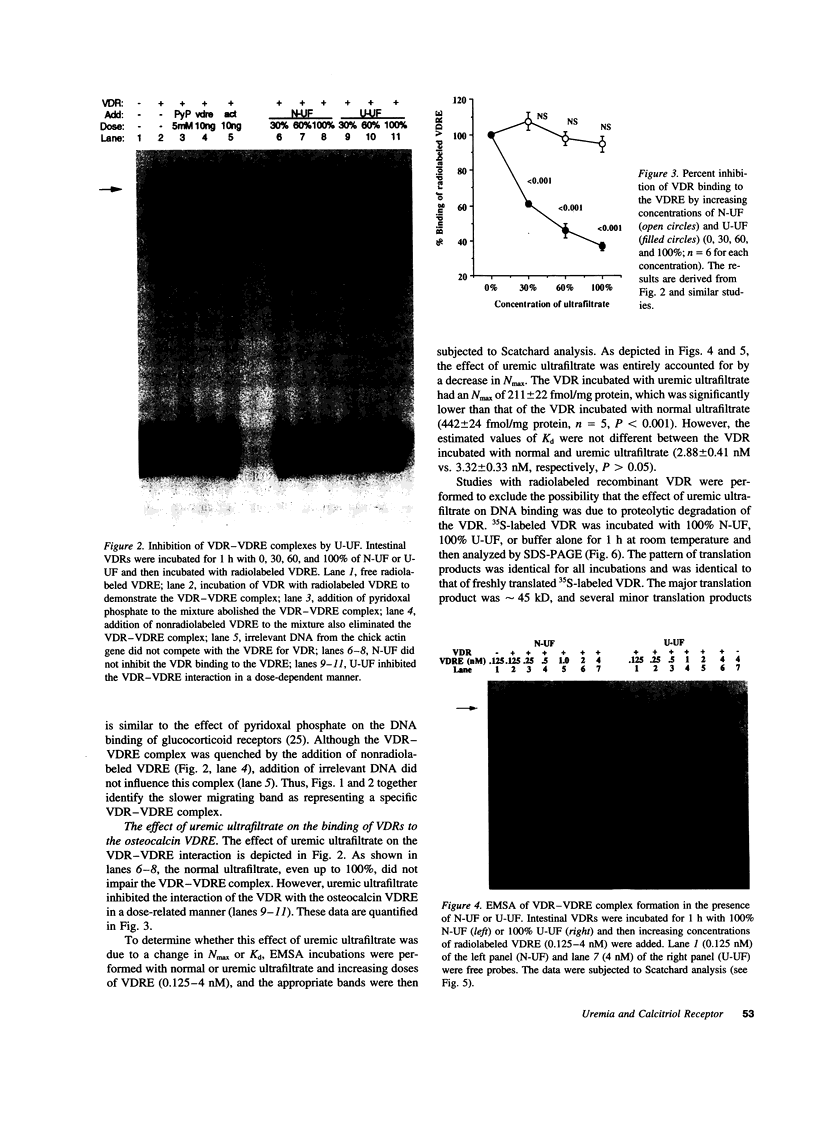
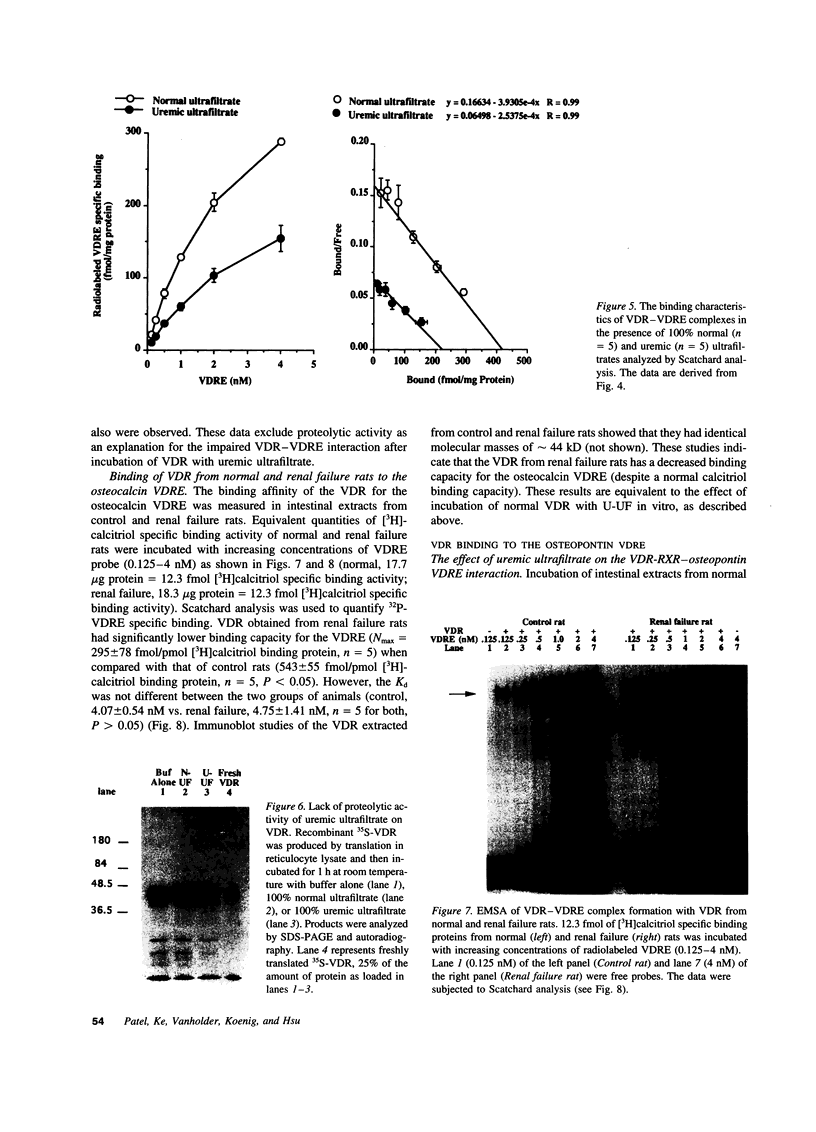
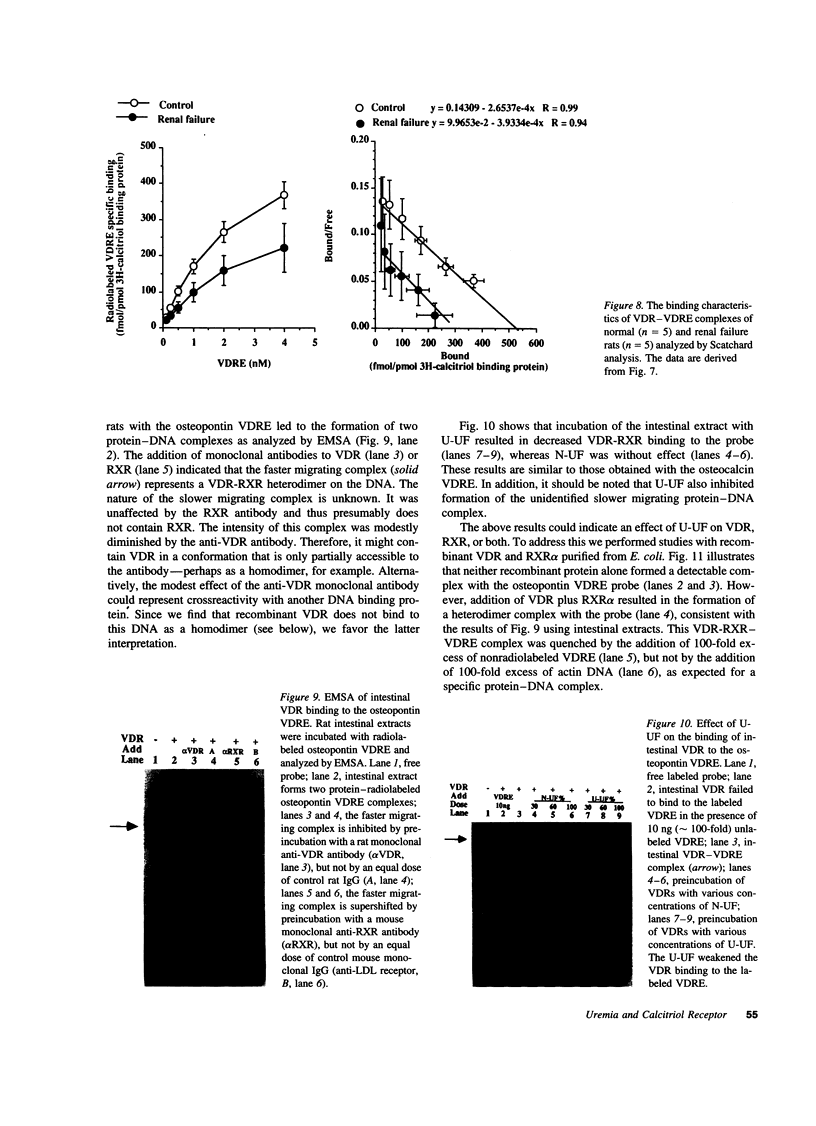
The genomic action of calcitriol (1,25-dihydroxy-vitamin D3) is mediated through the interaction of the calcitriol receptor (VDR) with vitamin D response elements (VDREs). Although renal failure is associated with resistance to the action of calcitriol, the mechanism of this resistance is not well understood. Therefore, we used the electrophoretic mobility shift assay to compare the ability of VDRs from normal and renal failure rats to bind to the osteocalcin gene VDRE. The results indicate that VDRs from renal failure rats have only half the DNA binding capacity as VDRs from control rats, despite identical calcitriol binding. Furthermore, incubation of normal VDRs with a uremic plasma ultrafiltrate resulted in a loss of > 50% of the binding sites for the osteocalcin VDRE. When VDRs bound to DNA as heterodimers with retinoid X receptors, the inhibitory effect of the uremic ultrafiltrate was due to a specific interaction with the VDR, not retinoid X receptors. In addition, uremic ultrafiltrate blocked calcitriol-induced reporter gene activity in transfected JEG-3 cells. Taken together, the results indicate that an inhibitory effect of a uremic toxin(s) on VDR-VDRE binding could underlie the calcitriol resistance of renal failure.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allegretto E. A., Pike J. W., Haussler M. R. Immunochemical detection of unique proteolytic fragments of the chick 1,25-dihydroxyvitamin D3 receptor. Distinct 20-kDa DNA-binding and 45-kDa hormone-binding species. J Biol Chem. 1987 Jan 25;262(3):1312–1319. [PubMed] [Google Scholar]
- Allegretto E. A., Pike J. W. Trypsin cleavage of chick 1,25-dihydroxyvitamin D3 receptors. Generation of discrete polypeptides which retain hormone but are unreactive to DNA and monoclonal antibody. J Biol Chem. 1985 Aug 25;260(18):10139–10145. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cake M. H., DiSorbo D. M., Litwack G. Effect of pyridoxal phosphate on the DNA binding site of activated hepatic glucocorticoid receptor. J Biol Chem. 1978 Jul 25;253(14):4886–4891. [PubMed] [Google Scholar]
- Carlberg C., Bendik I., Wyss A., Meier E., Sturzenbecker L. J., Grippo J. F., Hunziker W. Two nuclear signalling pathways for vitamin D. Nature. 1993 Feb 18;361(6413):657–660. doi: 10.1038/361657a0. [DOI] [PubMed] [Google Scholar]
- Demay M. B., Kiernan M. S., DeLuca H. F., Kronenberg H. M. Characterization of 1,25-dihydroxyvitamin D3 receptor interactions with target sequences in the rat osteocalcin gene. Mol Endocrinol. 1992 Apr;6(4):557–562. doi: 10.1210/mend.6.4.1316548. [DOI] [PubMed] [Google Scholar]
- Fukagawa M., Kaname S., Igarashi T., Ogata E., Kurokawa K. Regulation of parathyroid hormone synthesis in chronic renal failure in rats. Kidney Int. 1991 May;39(5):874–881. doi: 10.1038/ki.1991.110. [DOI] [PubMed] [Google Scholar]
- Fukuda N., Tanaka H., Tominaga Y., Fukagawa M., Kurokawa K., Seino Y. Decreased 1,25-dihydroxyvitamin D3 receptor density is associated with a more severe form of parathyroid hyperplasia in chronic uremic patients. J Clin Invest. 1993 Sep;92(3):1436–1443. doi: 10.1172/JCI116720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst M., Feldman D. Cleavage of the rat intestinal 1,25-dihydroxyvitamin D3 receptor by an endogenous protease to a form with defective DNA binding. Arch Biochem Biophys. 1986 Oct;250(1):153–161. doi: 10.1016/0003-9861(86)90712-5. [DOI] [PubMed] [Google Scholar]
- Hsu C. H., Patel S. R., Vanholder R. Mechanism of decreased intestinal calcitriol receptor concentration in renal failure. Am J Physiol. 1993 Apr;264(4 Pt 2):F662–F669. doi: 10.1152/ajprenal.1993.264.4.F662. [DOI] [PubMed] [Google Scholar]
- Hsu C. H., Patel S. R., Young E. W. Mechanism of decreased calcitriol degradation in renal failure. Am J Physiol. 1992 Feb;262(2 Pt 2):F192–F198. doi: 10.1152/ajprenal.1992.262.2.F192. [DOI] [PubMed] [Google Scholar]
- Hsu C. H., Vanholder R., Patel S., De Smet R. R., Sandra P., Ringoir S. M. Subfractions in uremic plasma ultrafiltrate inhibit calcitriol metabolism. Kidney Int. 1991 Nov;40(5):868–873. doi: 10.1038/ki.1991.287. [DOI] [PubMed] [Google Scholar]
- Hughes M. R., Malloy P. J., Kieback D. G., Kesterson R. A., Pike J. W., Feldman D., O'Malley B. W. Point mutations in the human vitamin D receptor gene associated with hypocalcemic rickets. Science. 1988 Dec 23;242(4886):1702–1705. doi: 10.1126/science.2849209. [DOI] [PubMed] [Google Scholar]
- Jurutka P. W., Hsieh J. C., MacDonald P. N., Terpening C. M., Haussler C. A., Haussler M. R., Whitfield G. K. Phosphorylation of serine 208 in the human vitamin D receptor. The predominant amino acid phosphorylated by casein kinase II, in vitro, and identification as a significant phosphorylation site in intact cells. J Biol Chem. 1993 Mar 25;268(9):6791–6799. [PubMed] [Google Scholar]
- Kerner S. A., Scott R. A., Pike J. W. Sequence elements in the human osteocalcin gene confer basal activation and inducible response to hormonal vitamin D3. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4455–4459. doi: 10.1073/pnas.86.12.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Mangelsdorf D. J., Evans R. M. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992 Jan 30;355(6359):446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkor A. B. Reduced binding of [3H]1,25-dihydroxyvitamin D3 in the parathyroid glands of patients with renal failure. N Engl J Med. 1987 Jun 18;316(25):1573–1577. doi: 10.1056/NEJM198706183162504. [DOI] [PubMed] [Google Scholar]
- Lian J., Stewart C., Puchacz E., Mackowiak S., Shalhoub V., Collart D., Zambetti G., Stein G. Structure of the rat osteocalcin gene and regulation of vitamin D-dependent expression. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1143–1147. doi: 10.1073/pnas.86.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J., Ozono K., Sone T., McDonnell D. P., Pike J. W. Vitamin D receptor interaction with specific DNA requires a nuclear protein and 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9751–9755. doi: 10.1073/pnas.87.24.9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy P. J., Hochberg Z., Pike J. W., Feldman D. Abnormal binding of vitamin D receptors to deoxyribonucleic acid in a kindred with vitamin D-dependent rickets, type II. J Clin Endocrinol Metab. 1989 Feb;68(2):263–269. doi: 10.1210/jcem-68-2-263. [DOI] [PubMed] [Google Scholar]
- McDonnell D. P., Mangelsdorf D. J., Pike J. W., Haussler M. R., O'Malley B. W. Molecular cloning of complementary DNA encoding the avian receptor for vitamin D. Science. 1987 Mar 6;235(4793):1214–1217. doi: 10.1126/science.3029866. [DOI] [PubMed] [Google Scholar]
- Mulder E., Vrij L., Foekens J. A. Inhibition of nucleic acid and chromatin binding of the rat prostate androgen receptor by pyridoxal phosphate, heparin and Cibacron blue. Steroids. 1980 Dec;36(6):633–645. doi: 10.1016/0039-128x(80)90047-1. [DOI] [PubMed] [Google Scholar]
- Müller R. E., Traish A., Wotiz H. H. Effects of pyridoxal 5'-phosphate on uterine estrogen receptor. I. Inhibition of nuclear binding in cell-free system and intact uterus. J Biol Chem. 1980 May 10;255(9):4062–4067. [PubMed] [Google Scholar]
- Nakajima S., Hsieh J. C., MacDonald P. N., Galligan M. A., Haussler C. A., Whitfield G. K., Haussler M. R. The C-terminal region of the vitamin D receptor is essential to form a complex with a receptor auxiliary factor required for high affinity binding to the vitamin D-responsive element. Mol Endocrinol. 1994 Feb;8(2):159–172. doi: 10.1210/mend.8.2.8170472. [DOI] [PubMed] [Google Scholar]
- Nishigori H., Toft D. Chemical modification of the avian progesterone receptor by pyridoxal 5'-phosphate. J Biol Chem. 1979 Sep 25;254(18):9155–9161. [PubMed] [Google Scholar]
- Noda M., Vogel R. L., Craig A. M., Prahl J., DeLuca H. F., Denhardt D. T. Identification of a DNA sequence responsible for binding of the 1,25-dihydroxyvitamin D3 receptor and 1,25-dihydroxyvitamin D3 enhancement of mouse secreted phosphoprotein 1 (SPP-1 or osteopontin) gene expression. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9995–9999. doi: 10.1073/pnas.87.24.9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley B. The steroid receptor superfamily: more excitement predicted for the future. Mol Endocrinol. 1990 Mar;4(3):363–369. doi: 10.1210/mend-4-3-363. [DOI] [PubMed] [Google Scholar]
- Patel S. R., Ke H. Q., Hsu C. H. Effect of vitamin D metabolites on calcitriol degradative enzymes in renal failure. Kidney Int. 1994 Feb;45(2):509–514. doi: 10.1038/ki.1994.66. [DOI] [PubMed] [Google Scholar]
- Patel S. R., Ke H. Q., Vanholder R., Hsu C. H. Inhibition of nuclear uptake of calcitriol receptor by uremic ultrafiltrate. Kidney Int. 1994 Jul;46(1):129–133. doi: 10.1038/ki.1994.252. [DOI] [PubMed] [Google Scholar]
- Patel S., Simpson R. U., Hsu C. H. Effect of vitamin D metabolites on calcitriol metabolism in experimental renal failure. Kidney Int. 1989 Aug;36(2):234–239. doi: 10.1038/ki.1989.185. [DOI] [PubMed] [Google Scholar]
- Pike J. W. Emerging concepts on the biologic role and mechanism of action of 1,25-dihydroxyvitamin D3. Steroids. 1987 Jan-Mar;49(1-3):3–27. doi: 10.1016/0039-128x(87)90077-8. [DOI] [PubMed] [Google Scholar]
- Pike J. W. Evidence for a reactive sulfhydryl in the DNA binding domain of the 1,25-dihydroxyvitamin D3 receptor. Biochem Biophys Res Commun. 1981 Jun;100(4):1713–1719. doi: 10.1016/0006-291x(81)90716-6. [DOI] [PubMed] [Google Scholar]
- Rosen E. D., Beninghof E. G., Koenig R. J. Dimerization interfaces of thyroid hormone, retinoic acid, vitamin D, and retinoid X receptors. J Biol Chem. 1993 Jun 5;268(16):11534–11541. [PubMed] [Google Scholar]
- Ross T. K., Moss V. E., Prahl J. M., DeLuca H. F. A nuclear protein essential for binding of rat 1,25-dihydroxyvitamin D3 receptor to its response elements. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):256–260. doi: 10.1073/pnas.89.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone T., Kerner S., Pike J. W. Vitamin D receptor interaction with specific DNA. Association as a 1,25-dihydroxyvitamin D3-modulated heterodimer. J Biol Chem. 1991 Dec 5;266(34):23296–23305. [PubMed] [Google Scholar]
- Sone T., Scott R. A., Hughes M. R., Malloy P. J., Feldman D., O'Malley B. W., Pike J. W. Mutant vitamin D receptors which confer hereditary resistance to 1,25-dihydroxyvitamin D3 in humans are transcriptionally inactive in vitro. J Biol Chem. 1989 Dec 5;264(34):20230–20234. [PubMed] [Google Scholar]
- Subauste J. S., Katz R. W., Koenig R. J. DNA binding specificity and function of retinoid X receptor alpha. J Biol Chem. 1994 Dec 2;269(48):30232–30237. [PubMed] [Google Scholar]
- Szabo A., Merke J., Thomasset M., Ritz E. No decrease of 1,25(OH)2D3 receptors and duodenal calbindin-D9k in uraemic rats. Eur J Clin Invest. 1991 Oct;21(5):521–526. doi: 10.1111/j.1365-2362.1991.tb01404.x. [DOI] [PubMed] [Google Scholar]
- Walling M. W., Kimberg D. V., Wasserman R. H., Feinberg R. R. Duodenal active transport of calcium and phosphate in vitamin D-deficient rats: effects of nephrectomy, Cestrum diurnum, and 1alpha,25-dihydroxyvitamin D3. Endocrinology. 1976 May;98(5):1130–1134. doi: 10.1210/endo-98-5-1130. [DOI] [PubMed] [Google Scholar]
- Wiese R. J., Uhland-Smith A., Ross T. K., Prahl J. M., DeLuca H. F. Up-regulation of the vitamin D receptor in response to 1,25-dihydroxyvitamin D3 results from ligand-induced stabilization. J Biol Chem. 1992 Oct 5;267(28):20082–20086. [PubMed] [Google Scholar]