Abstract
Xylem hydraulic conductivity (Ks) in stems of tobacco (Nicotiana tabacum) wild-type SR1 was compared to that of PG7 and PG16, two transgenic lines with increased levels of expression of the gene encoding the Aspergillus niger endopolygalacturonase (AnPGII). Activity of AnPGII removes in planta blocks of homogalacturonan (HG) with deesterified carboxyls, thus increasing the degree of neutrality of pectins. The effect of K+ was tested in increasing stem Ks using model plants with more neutral polysaccharides in primary walls and, hence, in intervessel pit membranes. Ks measured with deionized water was compared to that with KCl solutions at increasing concentrations (ΔKs, %). Plants transformed for HG degree of neutrality showed a dwarfed phenotype, but ΔKs did not differ among the three experimental groups. The ion-mediated hydraulic effect saturated at a KCl concentration of 25 mm in SR1 plants. All the three tobacco lines showed ΔKs of around +12.5% and +17.0% when perfused with 10 and 25 mm KCl, respectively. Because modification of HG content did not influence ion-mediated hydraulic enhancement, we suggest that pectin components other than HG, like rhamnogalacturonan-I and/or rhamnogalacturonan-II, might play important roles in the hydrogel behavior of pit membranes.
Long-distance water transport in plants relies on the xylem, a network of conduits (tracheids and vessels) associated with parenchymatous and mechanical tissues and contributing at least by 50% to the hydraulic resistance of the whole plant (Tyree and Zimmermann, 2002). Changes in xylem hydraulic conductivity (Ks) due to cavitation or to mechanical damage have received much attention because they have important effects on the ability of the plant to maintain leaves hydrated during transpiration (Tyree and Sperry, 1989; Salleo et al., 2000; Brodribb et al., 2003; Sack and Holbrook, 2006).
In recent years, our understanding of the physiology of water transport in plants has been enriched by the discovery that the sap ion content affects xylem Ks (Van Ieperen et al., 2000; Zwieniecki et al., 2001). The first report of this phenomenon dates back to Zimmermann (1978), who found that dilute salt solutions injected into excised stem segments prevented the drop in Ks that was observed when pure deionized water was used as a perfusion fluid. Zimmermann hypothesized that this water effect might be a consequence of the swelling of intervessel pit membranes due to their increased hydration. Conversely, increase in the osmotic strength of xylem sap would lead to dehydration and shrinking of pit membranes, thus increasing radial flows through pits due to increased pit membrane porosity. Other scientists became aware of the water effect on Ks, which was prevented using 50 to 100 mm KCl as perfusion fluids during hydraulic measurements. Nonetheless, the mechanisms underlying the water effect were ignored for a long time.
Experimental evidence for pit membranes being responsible for the ion-mediated increase of Ks has been provided by Zwieniecki et al. (2001, 2003) and, more recently, by Gascò et al. (2006). These studies have reported significant increases in xylem Ks when the ion content of the solution injected into the xylem was increased, whereas no effect was observed for nonionic solutes like Suc or ethanol. Ion-mediated increase of Ks ranged from +15% to +150% compared to values obtained with pure deionized water. The effect was sample length dependent (Gascò et al., 2006), suggesting that the ion-mediated increase of Ks (ΔKs) might be related either to interference of ions with some wall component along the entire conduit length or, alternatively, to the number of intervessel pits crossed by the solution. Consistent with the latter hypothesis, an exponential positive relationship was found to exist between the ion-mediated increase in Ks and native loss of Ks (percentage loss of conductivity) of stems of Laurus nobilis (Gascò et al., 2006). Moreover, Zwieniecki et al. (2001) did not observe any ionic effect in the case of single vessels of Fraxinus americana open at both ends, whereas flow between adjacent vessels was strongly enhanced by 100 mm KCl. In a later work, Zwieniecki et al. (2003) came to similar conclusions for single vascular bundles of tomato (Solanum lycopersicum). The potential impact of the ionic effect on plant water relations has been further discussed by Zwieniecki et al. (2004) and Gascò et al. (2006). More recently, the occurrence of ion-mediated flow changes in planta has been questioned by Van Ieperen and Van Gelder (2006) on the basis of the observed suppression of Ks increase when 1 mm CaCl2 solution was used as reference fluid instead of deionized water.
At present, most of the experimental evidence supports the view of intervessel pit membranes as responsible for the ion-mediated increase of Ks. According to the current paradigm, ion-mediated flow enhancement is attributed to the hydrogel nature of pit membrane pectins (Zwieniecki et al., 2001; Boyce et al., 2004; Lòpez-Portillo et al., 2005). Pectins are polysaccharidic polyelectrolytes whose degree of hydration depends on the equilibrium between neutral carboxylic residues (e.g. due to methyl esterification) and exposed negative charges of dissociated carboxyls (Dähnert and Huster, 1999; Ryden et al., 2000). Cations interfere with this equilibrium causing pectins to shrink (Willats et al., 2001a) with consequent increase in the dimensions of pores in the pit membranes and, hence, in Ks. Pectins are major components of primary wall matrix, accounting for about one-third of all wall macromolecules (Ridley et al., 2001; Willats et al., 2001a; Kaczkowski, 2003), and consist of complex polysaccharides rich in GalUA (GalA). GalA can be assembled into two structural types forming the backbone of three main polysaccharide domains that have been isolated and structurally characterized. These are homogalacturonan (HG), rhamnogalacturonan (RG)-I, and RG-II. The most abundant components of primary wall matrix are HG and RG-I, whereas RG-II is generally present in rather low quantities (0.2%–3.6% of wall dry weight; Matsunaga et al., 2004).
Over the last 15 years, increasing availability of cell wall mutants has provided new opportunities to study pectin structure and function (Rhee and Somerville, 1998; Fagard et al., 2000; Ridley et al., 2001; Lao et al., 2003). Specific manipulation of pectin polymers by plant transformation with pectic enzymes (Sorensen et al., 2000; Atkinson et al., 2002; Oomen et al., 2002) can be expected to provide new insights into the role of pectins in the regulation of water flow through the xylem. In this study, plants of tobacco (Nicotiana tabacum) were used with modified HG through expression of the endopolygalacturonase II of Aspergillus niger (AnPGII). These transformed tobacco plants were first described and characterized by Capodicasa et al. (2004). Long regions of HG without methyl esterification are the optimal substrate for AnPGII (Limberg et al., 2000). Transformed lines of tobacco showed the absence of deesterified blocks of HG. Hence, the degree of neutrality of HG was likely to be strongly enhanced. In this study, the hypothesis was tested that such a modification of HG translates into reduced ionic effect as a consequence of the likely modified hydration properties of pit membrane pectins (Zsivánovits et al., 2005).
RESULTS
Tobacco plants transformed with the gene encoding AnPGII that removes blocks of deesterified carboxyls showed strongly reduced vegetative growth leading to a dwarfed phenotype (Table I), in accordance with Capodicasa et al. (2004). In particular, plant height (Hplant), internodal length (L), and whole-plant leaf surface area (AL) of PG16 plants (the line with highest expression of AnPGII) were reduced by about 50%, 40%, and 30%, respectively, compared to SR1 (wild-type) plants. On the contrary, the number of leaves per plant (Nleaves) and stem basal diameter (∅s) were not different among experimental groups. Nleaves ranged between 13 and 16 leaves per plant, whereas ∅s was about 12 mm throughout the tested groups. The above data confirmed that the activity of AnPGII specifically affected stem extension and leaf expansion.
Table I.
Plant height (Hplant), stem basal diameter (∅s), number of leaves (Nleaves), internodal length (Linternode), and whole-plant leaf surface area (AL) as measured in plants of three lines of tobacco
SR1, Wild type; PG7 and PG16 correspond to two increasing levels of expression of the gene AnPGII. Means are reported ±sd (n = 12). Different letters indicate significant differences (P < 0.05).
| Hplant, cm (P < 0.001) | ∅s, mm (n.s.) | Nleaves (n.s.) | Linternode, cm (P = 0.006) | AL, m2 (P = 0.048) | |
|---|---|---|---|---|---|
| SR1 | 91 ± 10 a | 12.47 ± 2.99 | 14 ± 1 | 6.4 ± 1.1 a | 0.2624 ± 0.0565 a |
| PG7 | 69 ± 18 b | 12.45 ± 2.27 | 14 ± 1 | 5.0 ± 1.5 ab | 0.2813 ± 0.0410 a |
| PG16 | 52 ± 3 b | 11.98 ± 1.17 | 14 ± 2 | 3.6 ± 0.5 b | 0.2069 ± 0.0280 b |
Cell wall polysaccharides were analyzed for monosaccharide composition. Total wall material, prepared from stems of transgenic tobacco lines 7 and 16, revealed a content of uronic acids lower than that of stems from untransformed plants with the difference being largest for line 16, where GalA was 30% less than in SR1 plants (Table II). This result is in agreement with previous results obtained when immunodot analysis of cell wall material of untransformed and transgenic lines 7 and 16 was performed by using monoclonal antibodies specific for different pectic polysaccharides (Capodicasa et al., 2004). In parallel with the reduced level of uronic acids and probably as a consequence of a compensatory response, a slightly, but significantly, higher amount of Rha, Ara, and Gal was observed in transgenic plants (Table II). The level of Glc, Xyl, and Man was, instead, not significantly different in untransformed and transgenic plants.
Table II.
Chemical analysis of cell walls of transgenic tobacco plants expressing AnPGII
Monosaccharide composition of cell wall from stems of 30-d-old SR1 and transgenic lines PG7 and PG16. Results are expressed in percentage of total moles ±sd (n = 4). Different letters indicate significant differences (P < 0.05).
| GalA | Rha | Ara | Gal | |
|---|---|---|---|---|
| SR1 | 15.5 ± 0.8 a | 4.6 ± 0.8 a | 7.8 ± 0.3 a | 13.2 ± 6.6 a |
| PG7 | 12.3 ± 1.2 ab | 4.8 ± 2.0 a | 8.2 ± 1.5 a | 14.3 ± 6.9 a |
| PG16 | 10.5 ± 2.2 b | 6.4 ± 0.3 b | 13.7 ± 6.6 b | 18.6 ± 4.1 b |
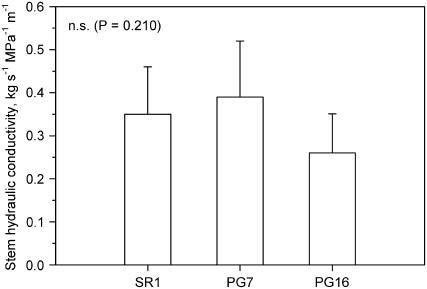
Ks as measured with deionized water and normalized by basal stem cross-sectional area was around 0.40 kg s−1 MPa−1 m−1 in SR1 plants and in plants with intermediate expression of AnPGII (PG7) versus about 0.25 kg s−1 MPa−1 m−1 in PG16 plants (Fig. 1). Although differences were not statistically significant, the lower stem hydraulic efficiency of plants with highest expression of AnPGII compared to that of the wild type (SR1) would be in accordance with similar reduction in the conduit dimensions reported for PG16 plants compared to SR1 plants by Capodicasa et al. (2004).
Figure 1.
Ks of stems of three lines of tobacco. SR1, Wild type; PG7 and PG16 correspond to two increasing levels of expression of the gene AnPGII. Means are reported ±sd (n = 7).
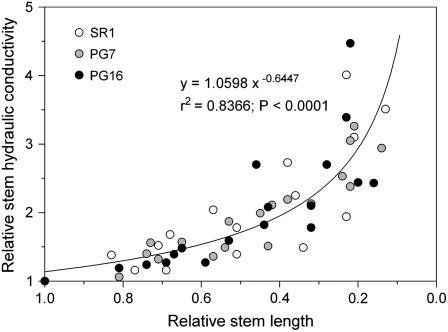
Relative Ks changes versus relative stem length showed the same pattern among the three experimental groups tested, suggesting that the percentage of open vessels versus stem length was very similar in all three groups (Fig. 2). In particular, a marked increase in Ks was observed for stem samples with lengths about 70% of the whole-stem length. This strongly suggests that most conduits were intact in our experimental samples, which were in all cases about 35% as long as the entire stem length. Moreover, preliminary experiments (data not shown) had revealed that the KCl-induced increase in SR1 plants was not different for sample 35 or about 50% as long as the entire stem length.
Figure 2.
Ks versus length of the stem samples, both expressed in relative units. Three lines of tobacco plants were used. SR1, Wild type; PG7 and PG16 correspond to two increasing levels of expression of the gene AnPGII. Each point represents a single experiment. The equation of the curve is reported together with r2 and P values.
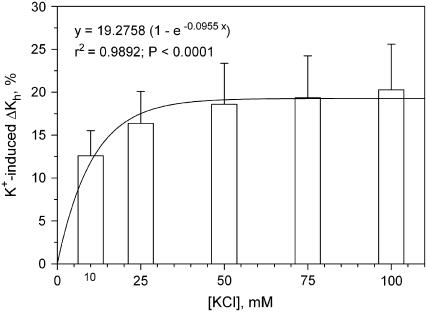
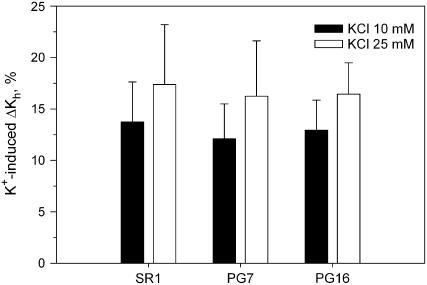
All the KCl solutions tested induced marked increase in the ΔKs of SR1 stems with respect to values obtained with deionized water. The effect was concentration dependent up to 50 mm KCl, which caused ΔKs to increase by +20% compared to water (Fig. 3). The KCl-induced increase in Ks was still detectable even at the lowest salt concentrations tested of 10 and 25 mm (ΔKs was +12.5 and +17%, respectively), whereas [KCl] higher than 50 mm (75 and 100 mm) did not induce any measurable increase in ΔKs. No difference was found to exist in terms of KCl-induced increase of ΔKs among the three experimental groups, despite modification of the electrical charge of HG (Fig. 4) as induced in transformed plants, thus suggesting that expression of AnPGII per se did not affect the ion-mediated increase of Ks.
Figure 3.
Hydraulic enhancement of ΔKs as induced by increasing concentrations of KCl compared to deionized water in untransformed (SR1) tobacco plants. Means are reported ±sd (n = 4). A curve is also reported through mean values together with equation, r2, and P values.
Figure 4.
Hydraulic enhancement of stem ΔKs as induced by 10 and 25 mm KCl compared to deionized water in three lines of tobacco. SR1, Wild type; PG7 and PG16 correspond to two increasing levels of expression of the gene AnPGII. Means are reported ±sd (n = 7).
DISCUSSION
The hydraulic effect of [K+] on Ks in stems of tobacco confirms analogous data by Zwieniecki et al. (2001). Contrary to expectations, however, constitutive modification of HG electrical charge as caused by expression of the fungal endopolygalacturonase AnPGII did not induce any change in the magnitude of the ionic effect. This opens several questions pertaining to the actual role of pectins (and, specifically, of HG) in the ion-mediated regulation of water flow through plants (Zwieniecki et al., 2004).
In this study, 10 to 50 mm KCl induced 12% to 20% increase in Ks with respect to pure water. This means that xylem hydraulics was affected by ion concentrations well within the physiological range reported for xylem sap of different species (e.g. Tyree et al., 1999; Goodger et al., 2005). Zwieniecki et al. (2001) have reported much larger hydraulic effects at even lower salt concentrations. In fact, Zwieniecki and coworkers found that flow rates through isolated xylem flaps of tobacco stems almost doubled when pure deionized water was substituted for an artificial sap prepared with several salts and with total concentration of 7 mm. The discrepancy might well arise from different experimental setups adopted in the study by Zwieniecki et al. (2001) with respect to our protocol and/or from differences in growth conditions. An alternative explanation for different magnitudes of the recorded ion-mediated hydraulic effect may reside in the multiplicity of cations in the artificial sap used by Zwieniecki et al. (2001).
Introduction of the AnPGII gene into tobacco plants induced important modifications in HG content (Table II). In particular, pectins isolated from PG7 and PG16 plants showed the absence of deesterified blocks of HG (i.e. those carrying negative charges in pectins), which results in a higher degree of neutrality of HG (Capodicasa et al., 2004). According to the polyelectrolite theory, this would influence the hydration properties of pectins and, hence, their swelling-shrinking dynamics (Willats et al., 2001b; Mei and Ballauff, 2005). There is no evidence that the observed altered cell wall composition in PG7 and PG16 plants was specific to a particular tissue or cell type (Capodicasa et al., 2004). Hence, it is likely that the structure of pectins at the pit membrane level was altered as well, and this was expected to modify their swelling-shrinking behavior. In fact, Zsivánovits et al. (2005) have shown that even small changes of the degree of esterification of pectins translate into large changes of their swelling behavior. This did not happen in our case and the ion-mediated ΔKs recorded in PG7 and PG16 plants was not different from that recorded in the wild type (SR1). Therefore, we conclude that HG might not play any key role in the hydrogel behavior of pit membrane pectins. This, however, does not mean that pectins are not responsible for the response of Ks to the ionic strength of the xylem sap. Other pectic components are present in primary walls, namely, RG-I and RG-II, which are possible candidates for the swelling-shrinking dynamics of pit membranes.
RG-I is a family of pectic polysaccharides containing a backbone of a repeating disaccharides consisting of GalA and Rha. Twenty percent to 80% of the rhamnosyl residues are substituted at C-4 for neutral and acidic oligosaccharide side chains (Ridley et al., 2001). Hence, RG-I has negative charges exposed that contribute to confer the polyelectrolite nature to pectins. Moreover, the specific side chains of RG-I are known to have an impact in the water-binding capacity of pectic polymers (Belton, 1997). As an example, a recent study by Ulvskov et al. (2005) has shown that remodeling the side chains of RG-I through transgenic expression of fungal enzymes influences the water-binding capacity of cell walls of potato (Solanum tuberosum) tubers. It is of interest to note that, concomitant with the decrease in uronic acids and of AnPGII-sensitive HG epitopes in tobacco-transformed plants, an increase was recorded of sugars characteristic of RG-I (Table II), which was suggested to reflect a compensatory response. In other words, increased RG-I content in cell walls of lines of tobacco transformed for HG content might act as a mechanism to maintain the hydrogel nature of the primary cell wall.
Another fundamental constituent of plant pectic fraction is RG-II (Ridley et al., 2001). Porosity of the cell wall is known to be strongly affected by the structure of RG-II through its dimerization status (Ishii et al., 2001). It is also worth noting that RG-II is considered a key component in the evolution of vascular plants and its content in plant cell walls has been reported to be highly conserved (for details, see Matsunaga et al., 2004). Nonetheless, RG-II is generally present in low amounts so that it is unlikely that this pectin component may play a significant role in the swelling-shrinking behavior of pit membranes.
At present, it is very difficult to envision a clear conceptual model for the role of the different pectic polymers in the hydrogel behavior of the cell wall matrix. This difficulty arises from our poor knowledge of the precise architecture of the different domains as well as of their assembly/disassembly in planta. As an example, it is largely unclear how different pectic constituents are combined into a supramolecular structure (Vincken et al., 2003), although it is generally agreed that they are covalently linked to each other (Ridley et al., 2001). In addition, it has been convincingly shown (Bouton et al., 2002) that plants can compensate for the decrease in one cell wall component by increasing the fractional amount of another one. This poses serious limits to the possibility of using mutants and transformed plants for one wall component to unravel the role of pectic hydrogels in the ion-mediated control of xylem water flow. Nonetheless, the increasing number of plant species transformed for cell walls still appears to be the most powerful tool for elucidating the mechanisms by which plants sense changes in the ionic concentration of xylem fluids and possibly transduce this signal into hydraulic effects.
An alternative explanation for the observed similarity of the ion-mediated changes in Ks among our experimental groups may come from the process of maturation of xylem vessels. At the time of autolysis of xylem vessels, pit membranes are subject to partial hydrolysis that does not occur in other primary cell walls. Classical studies have shown that much of the noncellulosic components of the cell walls are removed during hydrolysis (e.g. O'Brien, 1969). It is possible, therefore, that induced modifications of pectins in transgenic plants were nullified during ontogeny of vessels. This point deserves, in our opinion, more detailed study.
Future studies are needed to quantify the ion-mediated hydraulic effect in planta. This can only be done through hydraulic measurements preceded by accurate measurements of changes in the natural xylem sap concentration that should be applied in the laboratory to realistically reproduce what can occur in nature.
CONCLUSION
Our data further reinforce the hypothesis that ion-mediated enhancement of xylem hydraulic efficiency is a common feature of vascular plants, as first proposed by Zwieniecki et al. (2001, 2003). Nonetheless, the view that dehydration of pit membranes through an increase in pectin neutrality would cause an increase in membrane porosity with a consequent increase in Ks is not supported by our data. Because plants transformed for HG content showed ion-mediated hydraulic enhancement similar to that of the wild type, we suggest that (1) plants may regulate long-distance water transport through enriching ionic concentration of xylem sap; (2) pectin components other than HG (like RG-I and/or RG-II) might play important roles in the hydrogel behavior of pit membranes; and (3) plants transformed for one pectic component may only be useful for studying regulation of xylem water transport if they are also monitored for compensatory changes in other pectic components and supramolecular architecture.
MATERIALS AND METHODS
Plant Material
Plants of tobacco (Nicotiana tabacum) transformed with the gene encoding AnPGII were used. Details about the transformation procedure can be found in Capodicasa et al. (2004). In particular, three experimental groups (12 plants per group) were tested: untransformed plants (wild type [SR1]) and two R2 homozygous lines (PG7 and PG16) expressing increasing levels of AnPGII. Seeds were planted in greenhouse trays. After 14 d, seedlings were transplanted into 3-L pots filled with potting mix and grown in a controlled environmental chamber where air temperatures were adjusted to vary between 25°C and 17°C (day/night), relative humidity was set at 70%, and light was provided by lamps with a photosynthetically active radiation of 400 ± 50 μmol m−2 s−1. The photoperiod was set at 12 h. Plants of the three lines under study were randomly put under lamps to prevent border effects. Plants were irrigated daily with 200 mL tap water. All measurements were performed 6 weeks after transplantation when plants were fully developed and inflorescence was present, but not yet mature.
Preparation of Cell Wall and Monosaccharide Composition Analysis
Cell walls were prepared as described by Stolle-Smits et al. (1997) by adding 80% ethanol to 2 g of plant material homogenized in liquid nitrogen. After heating at 80°C for 30 min, the mixture was blended and centrifuged at 7,000g for 15 min. The pellet was washed four times in 80% ethanol and twice in 95% ethanol. Cell wall material was subsequently washed with acetone and air dried in a fume cupboard. It was then washed again three times with 50 mm sodium acetate buffer, pH 5.2, at 70°C for 1 h, resuspended in 50 mm sodium acetate buffer, pH 5.2, containing 10 mg of α-amylase (1,000 units mL−1), and incubated overnight at 30°C. After inactivation of the enzyme in boiling water for 10 min, the suspension was cooled, centrifuged at 7,000g for 20 min, and the pellet washed twice with SDS (1.5%) in 50 mm sodium metabisulfate. The pellet was treated for 1 h at 30°C with 72% sulfuric acid and then with 1 m sulfuric acid for 3 h at 100°C. Total sugar content was estimated using orcinol assay and uronic acids were determined by using the m-hydroxybiphenyl assay (Schols et al., 1995). To determine the neutral monosaccharide composition, the pellet was hydrolyzed by using 2 m aqueous trifluoroacetic acid for 2 h at 121°C and the monosaccharides analyzed by high-performance anion-exchange chromatography on a Carbo-Pac PA1 column (Dionex) using 16 mm NaOH as an eluant. Sugars were monitored by pulsed-amperometric detection.
Morphological Measurements
Prior to hydraulic measurements (see below), the following morphological variables were measured for each plant group: plant height and internodal length (Hplant and Linternode, respectively, both measured using a ruler), stem basal diameter (∅s, measured using a digital caliper), number of leaves per plant (Nleaves), and total leaf surface area per plant (AL, measured using a leaf area meter; model LI-3000A; LI-COR).
Hydraulic Measurements
Preliminary experiments to confirm the ion-mediated effect on the Ks of tobacco plants (Zwieniecki et al., 2001) were performed on 30-cm-long stem segments of SR1 plants. Stems were cut off under distilled water to prevent embolism and connected to the apparatus for hydraulic measurements (XYL'EM, Xylem Embolism Meter; Bronkhorst; for details about instrumentation, see Cochard et al., 2000). Samples typically bore four to five leaves that were removed; stems were tightly wrapped with parafilm to prevent leaks from foliar traces. Samples were then flushed with deionized water at a pressure P = 0.1 MPa for 30 min to remove eventual native emboli. Ks was measured as (F/P) × L, where F is the measured flow rate, P is the pressure applied, and L is the length of the stem segment tested. Flow rate was measured at P = 9 kPa first with deionized water and then with solutions at increasing KCl concentrations (10, 25, 50, 75, and 100 mm). KCl was used as perfusion fluid because K+ is the most abundant cation in the xylem sap and represents about 50% of the total inorganic ion concentration (Siebrecht et al., 2003). The entire sequence of solutions was perfused into each of the four stem segments tested.
The ion-mediated increase in Ks (ΔKs) was tested in the three plant groups. Stem segments bearing four to five leaves were collected from SR1, PG7, and PG16 plants as described above. Due to reduced internodal length in transformed plants (see “Results”), stem segments of PG7 and PG16 plants were about 22 and 18 cm long, respectively. In all cases, however, samples tested for hydraulic measurements represented about 35% of Hplant. Samples were connected to the XYL'EM and flushed with deionized water as described above. Then, Ks was measured at P = 9 kPa first with deionized water and then with 10 and 25 mm KCl solutions. The entire sequence of KCl concentrations (see above) was not used in this case as preliminary measurements had shown that 25 mm KCl almost saturated the Ks response (see below). Moreover, 10 to 25 mm KCl is a range of concentration typically found in xylem sap (e.g. Herdel et al., 2001; Goodger et al., 2005). In these experiments, seven plants per group were tested. Because the relative Ks increase has been shown to be influenced by the number of vessel ends in the measured sample (Gascò et al., 2006), stem length-dependent changes of Ks were measured at the end of each experiment by progressively shortening samples and remeasuring Ks until the least sample length of 1.5 cm was reached. This procedure caused the number of intact conduits within the sample to be progressively decreased and was intended to give information about the relative amount of vessel ends in each stem segment tested (Sperry et al., 2005). Finally, the basal diameter of the stem sample was measured and Ks was scaled by the stem cross-sectional area.
Acknowledgments
We are grateful for the visit of A. Gascó to the Department of Biology, University of Trieste, which was granted by Fundación Alfonso Martín Escudero, and to E. Gortan for technical assistance.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is Andrea Nardini (nardini@units.it).
References
- Atkinson RC, Schröder R, Hallett IC, Cohen D, MacRae EA (2002) Overexpression of polygalacturonase in transgenic apple leads to a range of novel phenotypes involving changes in cell adhesion. Plant Physiol 129 122–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belton PS (1997) NMR and the mobility of water in polysaccharide gels. Int J Biol Macromol 21 81–88 [DOI] [PubMed] [Google Scholar]
- Bouton S, Lebouef E, Mouille G, Leydecker MT, Talbotec J, Granier F, Lahaye M, Hofte H, Truong HN (2002) QUASIMODO1 encodes a putative membrane-bound glycosyltransferase required for normal pectin synthesis and cell adhesion in Arabidopsis. Plant Cell 14 2577–2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce CK, Zwieniecki MA, Cody GD, Jacobsen C, Wirik S, Knoll AH, Holbrook NM (2004) Evolution of xylem lignification and hydrogel transport regulation. Proc Natl Acad Sci USA 101 17555–17558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM, Edwards EJ, Gutiérrez MV (2003) Relations between stomatal closure, leaf turgor and xylem vulnerability in eight tropical dry forest trees. Plant Cell Environ 26 443–450 [Google Scholar]
- Capodicasa C, Vairo D, Zabotina O, McCartney L, Caprari C, Mattei B, Manfredini C, Aracri B, Benen J, Knox JP, et al (2004) Targeted modification of homogalacturonan by transgenic expression of a fungal polygalacturonase alters plant growth. Plant Physiol 135 1294–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochard H, Bodet C, Améglio T, Cruiziat P (2000) Cryo-scanning electron microscopy observations of vessel contents during transpiration in walnut petioles. Facts or artifacts? Plant Physiol 124 1191–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dähnert K, Huster D (1999) Comparison of the Poisson-Boltzmann model and the Donnan equilibrium of a polyelectrolite in salt solution. J Colloid Interface Sci 215 131–139 [DOI] [PubMed] [Google Scholar]
- Fagard M, Hofte H, Vernhettes S (2000) Cell wall mutants. Plant Physiol Biochem 38 15–25 [Google Scholar]
- Gascò A, Nardini A, Gortan E, Salleo S (2006) Ion-mediated increase in the hydraulic conductivity of Laurel stems: role of pits and consequences for the impact of cavitation on water transport. Plant Cell Environ 29 1946–1955 [DOI] [PubMed] [Google Scholar]
- Goodger JQD, Sharp RE, Marsh EL, Schachtman DP (2005) Relationships between xylem sap constituents and leaf conductance of well-watered and water-stressed maize across three xylem sap sampling techniques. J Exp Bot 56 2389–2400 [DOI] [PubMed] [Google Scholar]
- Herdel K, Schmidt P, Feil R, Mohr A, Schurr U (2001) Dynamics of concentration and nutrient fluxes in the xylem of Ricinus communis—diurnal course, impact of nutrient availability and nutrient uptake. Plant Cell Environ 24 41–52 [Google Scholar]
- Ishii T, Matsunaga T, Hayashi N (2001) Formation of rhamnogalacturonan II-borate dimer in pectin determines cell wall thickness of pumpkin tissue. Plant Physiol 126 1698–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczkowski J (2003) Structure, function and metabolism of plant cell wall. Acta Physiol Plant 25 287–305 [Google Scholar]
- Lao NT, Long D, Kiang S, Coupland G, Shoue DA, Carpita NC, Kavanagh TA (2003) Mutation of a family 8 glycosyltransferase gene alters cell wall carbohydrate composition and causes a humidity-sensitive semi-sterile dwarf phenotype in Arabidopsis. Plant Mol Biol 53 687–701 [DOI] [PubMed] [Google Scholar]
- Limberg G, Korner R, Buchholt HC, Christensen TM, Roepstorff P, Mikkelsen JD (2000) Analysis of different de-esterification mechanisms for pectin by enzymatic fingerprinting using endopectin lyase and endopolygalacturonase II from A. niger. Carbohydr Res 327 293–307 [DOI] [PubMed] [Google Scholar]
- Lòpez-Portillo J, Ewers F, Angeles G (2005) Sap salinity effects on xylem conductivity in two mangrove species. Plant Cell Environ 28 1285–1292 [Google Scholar]
- Matsunaga T, Ishii T, Matsumoto S, Higuchi M, Darvill A, Albersheim P, O'Neill MA (2004) Occurrence of the primary cell wall polysaccharide rhamnogalacturonan II in Pteridophytes, Lycophytes, and Bryophytes. Implications of the evolution of vascular plants. Plant Physiol 134 339–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Y, Ballauff M (2005) Effect of counterions on the swelling of spherical polyelectrolyte brushes. Eur Phys J E 16 341–349 [DOI] [PubMed] [Google Scholar]
- O'Brien TP (1969) Further observations on hydrolysis of the cell wall in the xylem. Protoplasma 69 1–14 [Google Scholar]
- Oomen RJ, Doeswijk-Voragen CH, Bush MS, Vincken JP, Borkhardt B, van den Broek LA, Corsar J, Ulvskov P, Voragen AG, McCann MC, et al (2002) In muro fragmentation of the rhamnogalacturonan I backbone in potato (Solanum tuberosum L.) results in a reduction and altered location of the galactan and arabinan side-chains and abnormal periderm development. Plant J 30 403–413 [DOI] [PubMed] [Google Scholar]
- Rhee SY, Somerville CR (1998) Tetrad pollen formation in quartet mutants of Arabidopsis thaliana is associated with persistence of pectic polysaccharides of the pollen mother cell wall. Plant J 15 79–88 [DOI] [PubMed] [Google Scholar]
- Ridley BL, O'Neill MA, Mohnen D (2001) Pectins: structure, biogenesis, and oligogalacturonide-related signaling. Phytochemistry 57 929–967 [DOI] [PubMed] [Google Scholar]
- Ryden P, MacDougall AJ, Tibbits CW, Ring SG (2000) Hydration of pectic polysaccharides. Biopolymers 54 398–405 [DOI] [PubMed] [Google Scholar]
- Sack L, Holbrook NM (2006) Leaf hydraulics. Annu Rev Plant Biol 57 361–381 [DOI] [PubMed] [Google Scholar]
- Salleo S, Nardini A, Pitt F, Lo Gullo MA (2000) Xylem cavitation and hydraulic control of stomatal conductance in laurel (Laurus nobilis L.). Plant Cell Environ 23 71–79 [Google Scholar]
- Schols HA, Vierhuis E, Bakx EJ, Voragen AGJ (1995) Different populations of pectic hairy regions occur in apple cell walls. Carbohydr Res 275 343–360 [DOI] [PubMed] [Google Scholar]
- Siebrecht S, Herdel K, Schurr U, Tischner R (2003) Nutrient translocation in the xylem of poplar—diurnal variations and spatial distribution along the shoot axis. Planta 217 783–793 [DOI] [PubMed] [Google Scholar]
- Sorensen SO, Pauly M, Bush M, Skjot M, McCann MC, Borkhardt B, Ulvskov P (2000) Pectin engineering: modification of potato pectin by in vivo expression of an endo-1,4-β-D-galactanase. Proc Natl Acad Sci USA 97 7639–7644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry JS, Hacke UG, Wheeler JK (2005) Comparative analysis of end wall resistivity in xylem conduits. Plant Cell Environ 28 456–465 [Google Scholar]
- Stolle-Smits T, Beekhuizen JG, Recourt K, Voragen AGJ, Van Dijk C (1997) Changes in pectic and hemicellulosic polymers of green beans (Phaseolus vulgaris L.) during industrial processing. J Agric Food Chem 45 4790–4799 [Google Scholar]
- Tyree MT, Salleo S, Nardini A, Lo Gullo MA, Mosca R (1999) Refilling of embolized vessels in young stems of Laurel. Do we need a new paradigm? Plant Physiol 120 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Sperry JS (1989) The vulnerability of xylem to cavitation and embolism. Annu Rev Plant Physiol Plant Mol Biol 40 19–38 [Google Scholar]
- Tyree MT, Zimmermann MH (2002) Xylem Structure and the Ascent of Sap. Springer-Verlag, New York
- Ulvskov P, Wium H, Bruce D, Jorgensen B, Qvist KB, Skjot M, Hepworth D, Borkhardt M, Sorensen SO (2005) Biophysical consequences of remodeling the neutral side chains of rhamnogalacturonan I in tubers of transgenic potatoes. Planta 220 609–620 [DOI] [PubMed] [Google Scholar]
- Van Ieperen W, Van Gelder A (2006) Ion-mediated flow changes suppressed by minimal calcium presence in xylem sap in Chrysanthemum and Prunus laurocerasus. J Exp Bot 57 2743–2750 [DOI] [PubMed] [Google Scholar]
- Van Ieperen W, Van Meeteren U, Van Gelder H (2000) Fluid ionic composition influences hydraulic conductance of xylem conduits. J Exp Bot 51 769–776 [DOI] [PubMed] [Google Scholar]
- Vincken JP, Schols HA, Oomen RJFJ, McCann MC, Ulvskov P, Voragen AGJ, Visser RGF (2003) If homogalacturonan were a side chain of rhamnogalacturonan I. Implications for cell wall architecture. Plant Physiol 132 1781–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willats WGT, McCartney L, Mackie W, Knox JP (2001. a) Pectin: cell biology and prospects for functional analysis. Plant Mol Biol 47 9–27 [PubMed] [Google Scholar]
- Willats WGT, Orfila C, Limberg G, Buchholt HC, van Alebeek GJWM, Voragen AGJ, Marcus SE, Christensen TMIE, Mikkelsen JD, Murray BS, et al (2001. b) Modulation of the degree and pattern of methyl-esterification of pectic homogalacturonan in plant cell walls. J Biol Chem 276 19404–19413 [DOI] [PubMed] [Google Scholar]
- Zimmermann MH (1978) Hydraulic architecture of some diffuse-porous trees. Can J Bot 56 2286–2295 [Google Scholar]
- Zsivánovits G, Marudova M, Ring S (2005) Influence of the mechanical properties of pectin films on charge density and charge density distribution in pectin macromolecule. Colloid Polym Sci 284 301–308 [Google Scholar]
- Zwieniecki MA, Melcher PJ, Field TS, Holbrook NM (2004) A potential role for xylem-phloem interactions in the hydraulic architecture of trees: effects of phloem girdling on xylem hydraulic conductance. Tree Physiol 24 911–917 [DOI] [PubMed] [Google Scholar]
- Zwieniecki MA, Melcher PJ, Holbrook NM (2001) Hydrogel control of xylem hydraulic resistance in plants. Science 291 1059–1062 [DOI] [PubMed] [Google Scholar]
- Zwieniecki MA, Orians CM, Melcher PJ, Holbrook NM (2003) Ionic control of the lateral exchange of water between vascular bundles in tomato. J Exp Bot 54 1399–1405 [DOI] [PubMed] [Google Scholar]