Abstract
Overexpression of genes that respond to drought stress is a seemingly attractive approach for improving drought resistance in crops. However, the consequences for both water-use efficiency and productivity must be considered if agronomic utility is sought. Here, we characterize two tomato (Solanum lycopersicum) lines (sp12 and sp5) that overexpress a gene encoding 9-cis-epoxycarotenoid dioxygenase, the enzyme that catalyzes a key rate-limiting step in abscisic acid (ABA) biosynthesis. Both lines contained more ABA than the wild type, with sp5 accumulating more than sp12. Both had higher transpiration efficiency because of their lower stomatal conductance, as demonstrated by increases in δ13C and δ18O, and also by gravimetric and gas-exchange methods. They also had greater root hydraulic conductivity. Under well-watered glasshouse conditions, mature sp5 plants were found to have a shoot biomass equal to the wild type despite their lower assimilation rate per unit leaf area. These plants also had longer petioles, larger leaf area, increased specific leaf area, and reduced leaf epinasty. When exposed to root-zone water deficits, line sp12 showed an increase in xylem ABA concentration and a reduction in stomatal conductance to the same final levels as the wild type, but from a different basal level. Indeed, the main difference between the high ABA plants and the wild type was their performance under well-watered conditions: the former conserved soil water by limiting maximum stomatal conductance per unit leaf area, but also, at least in the case of sp5, developed a canopy more suited to light interception, maximizing assimilation per plant, possibly due to improved turgor or suppression of epinasty.
Crop yield is often limited by water availability, and global climate change, together with increasing competition for water resources, makes the genetic improvement of water-use efficiency an increasingly important goal (Parry et al., 2005). Transpiration efficiency (TE) can be defined as crop yield per unit of water transpired or, at the whole-plant level, as the biomass gained per unit of water transpired (TEp). At the individual leaf level, the ratio between CO2 assimilation rate (A) and stomatal conductance (gs) (A/gs) gives the intrinsic TE (TEi; Condon et al., 2002). Progress has been made in defining the genetic loci that control TE (Teulat et al., 2002; Hall et al., 2005; Juenger et al., 2005), and a quantitative trait locus that influences TE has been identified as the erecta gene (Masle et al., 2005). Overexpression of candidate genes has produced improvements in TE, for example, by overexpression of the ABA-inducible group 3 LEA gene HVA1 in wheat (Triticum aestivum; Sivamani et al., 2000; Bahieldin et al., 2005) or the maize (Zea mays) NADP-malic enzyme in tobacco (Nicotiana tabacum; Laporte et al., 2002). There are many reports of mutants and transgenic lines where overexpression of candidate genes reduces gs or improves survival on withholding water (Zhang et al., 2004), but it has been pointed out that little progress has been made in demonstrating that transgenic manipulations provide useful improvements in crop species (Passioura, 2006). It is clearly important to understand the effects of gene manipulation on whole-plant and crop physiology so that predictions of agronomic utility can be made and tested.
Abscisic acid (ABA) is a phytohormone that mediates plant responses to abiotic stresses, including drought, salinity, and low temperature, and acts with other phytohormones to regulate plant growth (Sharp, 2002) and dormancy (Kermode, 2005). The first dedicated step in ABA biosynthesis in plants is the oxidative cleavage of 9-cis-epoxycarotenoids, as catalyzed by 9-cis-epoxycarotenoid dioxygenase (NCED; Schwartz et al., 2003; Taylor et al., 2005). The maize gene VP14 was the first NCED gene to be cloned (Schwartz et al., 1997; Tan et al., 1997), and when the orthologous genes from tomato (Solanum lycopersicum), Arabidopsis (Arabidopsis thaliana), bean (Phaseolus vulgaris), and cowpea (Vigna unguiculata) were overexpressed in plants, the ABA content of leaves or whole plants was enhanced (Thompson et al., 2000; Iuchi et al., 2001; Qin and Zeevaart, 2002; Aswath et al., 2005) and transpiration was reduced (Thompson et al., 2000; Iuchi et al., 2001; Qin and Zeevaart, 2002). ABA was also increased in seeds (Thompson et al., 2000) and germination was delayed (Thompson et al., 2000; Qin and Zeevaart, 2002). Drought tolerance was noted, based on visual observation of transgenic tobacco and Arabidopsis plants when water was withheld (Iuchi et al., 2001; Qin and Zeevaart, 2002), while dry weight accumulation in creeping bent grass (Agrostis palustris) grown under drought or high salinity experiments was enhanced by overexpression of a cowpea NCED (Aswath et al., 2005). These studies all used constitutive promoters to drive expression of NCED genes.
It is thus clear that overproduction of ABA limits gs, but the impact on TE and other physiological traits that influence productivity has not been reported. Because crop yield is often positively related to the total amount of water transpired, it is possible that increasing ABA production and reducing transpiration will simply suppress biomass production (Condon et al., 2004; Blum, 2005). However, the extent to which biomass production is limited by transpiration may vary with genetic background, depending on how profligately water is used by the plant and on the importance of factors other than transpiration that may also limit growth when water is readily available. Although ABA may reduce assimilation by limiting gs, it may also improve growth by increasing water status and cell turgor, or may influence growth and development through direct effects on signaling pathways and cross talk with other hormones. High transpiration rates are also important for leaf cooling in hot environments, and breeding for high yields in such environments has led to selection of cotton (Gossypium hirsutum) and wheat varieties with progressively higher transpiration rates (Radin et al., 1994; Lu et al., 1998). Thus, genetic manipulation to reduce transpiration rate may lead to high temperature stress in some instances.
Exogenous application of ABA and the study of ABA-deficient mutants have established that ABA can have both positive and negative effects on growth, depending on tissue, applied concentration, and interactions with the environment (Arteca and Tsai, 1987; Saab et al., 1990; Mulholland et al., 1996, 1999; Barbour and Farquhar, 2000). It has also been recently reported that, in order to maintain growth during water deficit, ABA acts to antagonize the growth inhibitory effects of ethylene in tomato shoots (Sharp et al., 2000), Arabidopsis shoots (LeNoble et al., 2004), maize roots (Spollen et al., 2000), and also during grain-filling in wheat (Yang et al., 2006). However, this topic remains controversial because the regulation of maize leaf expansion by water deficit during the night did not appear to be regulated by either ABA or ethylene (Voisin et al., 2006).
Exogenously applied ABA is known to increase root hydraulic conductivity (Lpr) both at the organ and the cellular level (Glinka, 1980; Hose et al., 2000), and this is at least partly explained by an up-regulation of aquaporin genes (Zhu et al., 2005). In agreement with this, ABA-deficient mutants are known to have decreased Lpr (Tal and Nevo, 1973). Enhancement of Lpr is a means to optimize the delivery of water from the soil to the shoot. ABA also influences a range of other cellular adaptive processes, such as osmotic adjustment and ion transport, and induces production of metabolites or proteins that function to protect macromolecules from denaturation at lower water activities (Bray, 2002; Chaves and Oliveira, 2004). These ABA-regulated processes have evolved to allow plants to continue to grow and reproduce during periods of low water availability or to survive and recover from dehydration, but may also impact on crop yield in the context of agriculture.
The production and analysis of transgenic genotypes with elevated ABA content provides an approach for the investigation of the function of ABA in whole plants that has advantages over either the use of periodic or short-term exogenous application of ABA, or the study of ABA-deficient mutants where ethylene effects (Sharp, 2002) and wilting have major negative impacts on growth. In this article, we characterize the primary physiological consequences of overproduction of ABA in nonstressed and drought-stressed tomato. Alterations in TE, Lpr, and leaf growth are described, and the implications for agronomic utility are discussed.
RESULTS
We previously described transgenic lines that overexpress LeNCED1 driven by the Gelvin Superpromoter and have a high ABA content (Thompson et al., 2000). In this study, we further investigate two of these high ABA lines, sp5 and sp12, in a homozygous state. Note that sp12 was previously named d9 (see “Materials and Methods”). As sp5 has higher transgene expression than sp12 (Thompson et al., 2007), its responses have been found to be more extreme than sp12 in almost all physiological assays.
Drought Stress Response
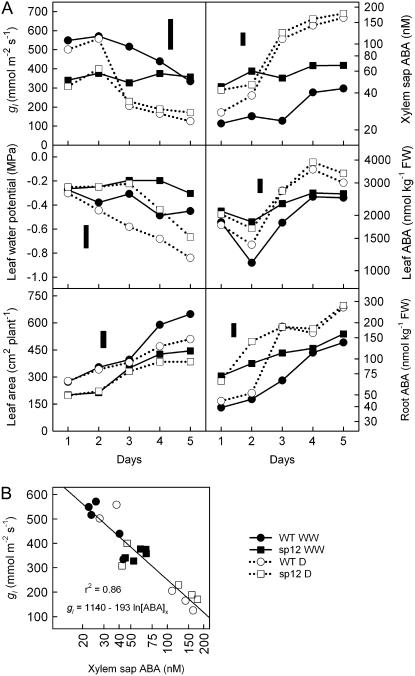
Line sp12 was compared to the wild type in a drought experiment in which plants were either irrigated with a fixed volume of water delivered twice daily throughout the experimental period (the well-watered treatment) or not watered to provide a drought treatment. Leaf conductance (gl), leaf water potential (ψw), leaf area, and ABA content of xylem sap, leaves, and roots were determined 1 to 5 d after withholding irrigation (Fig. 1A). Although we attempted to standardize plant size for both genotypes, the sp12 plants had a 28% lower mean leaf area than the wild type on day 1. The drought treatment reduced leaf expansion in both genotypes, significantly for the wild type (P < 0.05).
Figure 1.
Drought response of ABA-overaccumulating plants. Wild-type and sp12 plants were either well-watered (WW) or not watered (D) for 5 d. A, Time course of gl, ψw, leaf area, and ABA content of leaf, root, and xylem sap. B, Relationship between gl and xylem sap ABA concentration ([ABA]x). ABA data are plotted on a log scale. Floating bars represent lsd at the 5% level for comparing any two means. There were four plant replicates for all measurements except for ABA, for which duplicate assays were performed for each of two plant replicates.
In the well-watered treatment, gl remained high in the wild type on days 1 to 3 and, as expected, was significantly higher than in sp12 (Thompson et al., 2000). However, by days 4 and 5, there was evidence of mild water stress in wild-type plants, even in the well-watered treatment, because both gl and ψw declined (P < 0.05 on day 5 for gl and days 4 and 5 for ψw). Wild-type plants had both a larger leaf area and higher gl on day 1 in the well-watered treatment and would have withdrawn water more rapidly from the vermiculite substrate than the sp12 plants; if we assume that gl did not vary with leaf age, it can be calculated that gl on a per plant basis on day 1 would have been 14.9 and 6.8 mmol plant−1 s−1 for the wild type and sp12, respectively. In addition, mean leaf area for well-watered wild-type plants increased from 272 to 589 cm2 between days 1 and 4. One explanation for the decrease in gl and ψw on days 4 and 5 in well-watered wild-type plants is that, as leaf area increased, transpiration began to exceed the unvarying daily supply of irrigation water and a water deficit consequently began to develop during each day between irrigations. In the case of sp12, with its 28% lower leaf area and 38% lower gl than the wild type on day 1, irrigation is more likely to have been sufficient to avoid a decline in ψw as leaf area increased.
In the drought treatment, ψw declined in wild-type plants throughout the 5-d observation period, becoming significantly lower than the well-watered values on day 3 (P < 0.05), but the decrease was delayed until day 4 for sp12 plants, again presumably because of the slower depletion of irrigation water. By day 5 in the drought treatment, ψw was not significantly different between genotypes, although it was 0.17 MPa higher in sp12. For gl, there was a marked decline from day 3 of the drought treatment, with both genotypes reaching similar values on days 3 to 5.
In the well-watered treatment, sp12 exhibited higher leaf, root, and xylem sap ABA at all time points, significantly for root and xylem sap ABA on days 1 to 3 (P < 0.05), before the wild-type plants became mildly stressed; there was also a general trend of increasing ABA content over the 5-d observation period in the well-watered plants. However, when water was withheld, despite the delayed decline of ψw in sp12 compared to the wild type, ABA values increased in both genotypes and reached similar values on days 3 to 5 (Fig. 1A).
In summary, in nonstressed, well-watered conditions, sp12 had values for ABA content and gl that were comparable to those of mildly stressed wild-type plants, i.e. similar to wild-type plants on days 4 and 5 in the well-watered treatment in which ψw decreased from approximately −0.3 to −0.5 MPa. However, when a larger water deficit was imposed, wild-type and sp12 plants both showed a large response, such that the two genotypes no longer differed significantly in ABA content, gl, and ψw by day 5.
The relationship between xylem sap ABA concentration and gl was similar in the wild type and sp12, indicating that the sensitivity to ABA was not influenced by the higher ABA concentration (Fig. 1B).
TE of Well-Watered Plants
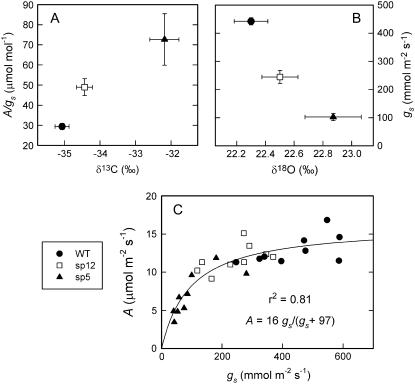
Two measures of TEi were made for well-watered plants of the wild type, sp12, and sp5: first, instantaneous leaf gas exchange using an infrared gas analyzer (IRGA) to record both A and gl, and, second, carbon isotope composition (δ13C), a surrogate measurement that integrates TEi over the development of the sampled leaf (Farquhar et al., 1982; Farquhar and Richards, 1984). gs was obtained from the gl data provided by the IRGA by fitting a value for cuticular conductance (gc; see “Materials and Methods”). A plot of both IRGA and stable isotope methods (Fig. 2A) shows close agreement between the methods; δ13C was significantly larger (less negative) in the transgenic lines, indicating increased TEi compared to the wild type (P < 0.001), and A/gs was also significantly higher (P < 0.05 comparing the wild type and sp5).
Figure 2.
TEi in well-watered wild-type and ABA-overaccumulating plants. A, TEi for the three genotypes as measured by both δ13C and IRGA (A/gs). B, gs as measured by δ18O and IRGA (gs). C, Relationship between A and gs with a rectangular hyperbolic function fitted. IRGA data were collected on three consecutive days for three well-watered plants of each genotype. δ13C and δ18O were determined on six individual plants of each genotype in the same experiment. Error bars represent se of the mean. Plants were grown under standard controlled environment growth conditions.
Similarly, in Figure 2B, instantaneous gs was measured by IRGA and also by measurement of oxygen stable isotope composition (δ18O) of leaf dry matter, an integrative, surrogate measure that is inversely related to gs, assuming constant relative humidity (RH; Barbour and Farquhar, 2000). Again, these two approaches are in agreement, showing that the two transgenic lines had significantly lower gs (P < 0.001) and higher δ18O (significant for the comparison of the wild type to sp5, P < 0.05).
A plot of A against gs for a single genotype where variation in gs is driven by environmental factors typically has a hyperbolic relationship where A is limited by declining gs (Wong et al., 1979). When we plotted A versus gs for the wild type, sp12, and sp5 genotypes grown in the same environment where variation in gs was driven by the genetically increased ABA content, a similar close hyperbolic relationship also was observed (r2 = 0.82), with the three genotypes giving overlapping gs ranges of 247 to 588, 119 to 370, and 40 to 281 mmol m−2 s−1, respectively (Fig. 2C).
In addition to measurements of TEi, whole-plant TE (TEp) was measured gravimetrically in a separate experiment where plants were grown in pots in a glasshouse (Table I). Compared to the wild type, TEp was 27% and 79% higher in sp12 and sp5, respectively (significant at P < 0.05 in both cases).
Table I.
Effect of LeNCED1 overexpression on TEp
Transpiration was determined gravimetrically over a period of 25 d and the production of aboveground biomass was determined over the same period. Initial biomass was the mean plant dry weight at the beginning of the 25-d period. P values, ses of difference (SED), and lsds (5%) from ANOVA are given (n = 3).
| Genotype
|
ANOVA
|
|||||
|---|---|---|---|---|---|---|
| Wild Type | sp12 | sp5 | P | SED | lsd | |
| Initial biomass (g dry wt) | 3.31 | 3.50 | 3.29 | 0.751 | 0.292 | 0.810 |
| Biomass production (g dry wt) | 37.96 | 31.37 | 34.91 | 0.652 | 6.75 | 18.75 |
| Transpiration (kg H2O) | 8.5 | 5.5 | 4.38 | 0.019 | 0.85 | 2.35 |
| TEp (g dry wt kg H20−1) | 4.49 | 5.72 | 8.04 | 0.002 | 0.37 | 1.02 |
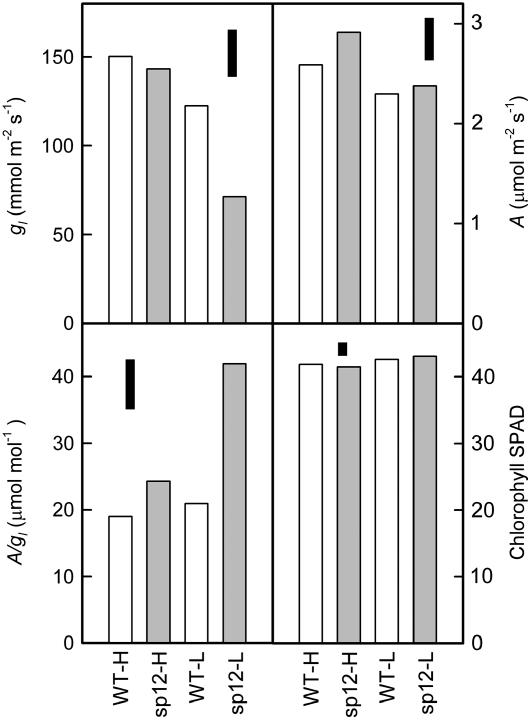
Response to RH
Wild-type and sp12 plants were established under the same low RH conditions (65%) as used in Figures 1 and 2, and then transferred to controlled environment cabinets, either at the same low RH or at higher RH (88%–92%), and kept well-watered. During the light period, the low and high RH treatments were equivalent to 0.92 and 0.48 kPa vapor pressure deficit (VPD). Gas-exchange and leaf chlorophyll measurements were made (Fig. 3). Considering both genotypes together, low humidity reduced gl (P < 0.001), but sp12 showed a greater response than the wild type as low humidity reduced gl by 19% in the wild type compared to 50% in sp12. Thus, as previously observed under low RH (Figs. 1A and 2B), sp12 had lower gl than the wild type (in this case 42% lower). However, there was no genotype effect on gl at high humidity (P > 0.05), and so a significant genotype × humidity interaction was detected (P = 0.023). Assimilation was reduced at lower humidity (P = 0.007), but there was no significant genotype effect (P > 0.05). As a result of lower gl, A/gl was 2-fold higher in sp12 than in the wild type at low humidity (P < 0.05), but there was no significant difference at high humidity, giving a genotype × humidity interaction (P = 0.004). SPAD readings were not significantly different between genotypes (Fig. 3, P > 0.9).
Figure 3.
Interaction between VPD and genotype for gas exchange. Plants were established at 65% RH and on day 0 were either left at low RH (L) or transferred to a high RH cabinet (88%–95% RH; H). IRGA measurements (n = 10 plant replicates) were taken on day 26, when plants had on average 14 leaves. A, gl, and A/gl are plotted. SPAD readings were taken on day 13 (n = 15). Floating black bars represent lsd (5%). White bars, Wild type; gray bars, sp12.
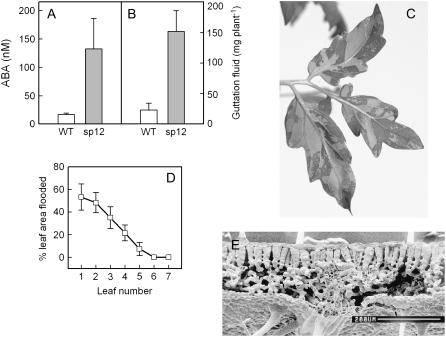
We previously reported increased guttation and interveinal chlorosis when sp12 and sp5 plants were grown at high humidity (Thompson et al., 2000); from closer observation of both lines, it is now apparent that the interveinal chlorosis mapped to the same leaf regions where intercellular air spaces had previously been flooded with sap (leaf flooding). Here, we report observations of guttation and leaf flooding at both RH levels for the wild type and sp12. No plants growing at 65% RH exhibited guttation or leaf flooding, but almost all plants of both sp12 and the wild type guttated during the predawn period under high RH; sp12 plants produced a much larger volume of guttation fluid than the wild type, with a 6.6-fold greater volume recorded on day 5 (Fig. 4B). The guttation fluid had a 7.8-fold higher ABA concentration in sp12 (Fig. 4A). Leaf flooding occurred only in sp12 plants at high RH, and was recognizable as sectors that were dark when observed under reflective light (Fig. 4C) but pale and translucent when observed by transmitted light (not shown). Flooded air spaces were predominantly bordered by veins (Fig. 4C) and were more prevalent in the lower leaves (Fig. 4D). Two weeks after transfer to the high humidity environment, most sp12 plants ceased to show guttation and leaf flooding, presumably due to adaptation, or perhaps the increasing leaf area allowed greater dissipation of root pressure. Figure 4E illustrates that tomato leaves are heterobaric (Lawson and Morison, 2006), i.e. the substomatal air spaces are subdivided by veins or bundle sheath extensions connecting the lower epidermis to the palisade layer, thus providing a barrier to sap flow and explaining the interveinal nature of the flooding.
Figure 4.
ABA concentration of guttation fluid and flooding of intercellular spaces. Wild-type and sp12 plants were transferred from low to high humidity 2 d before collecting data. A, ABA concentration of guttation fluid (n =3); B, mass of collected guttation fluid (n = 5); C, typical symptoms of intercellular flooding for an sp12 leaf photographed under reflective illumination; D, incidence of leaf flooding in sp12 leaves, plotted by leaf position (leaf 1 is the lowest leaf, n = 10); E, freeze-fracture scanning electron micrograph of a wild-type leaf (bar = 200 μm). Error bars show single ses of the mean; n refers to number of independent plants.
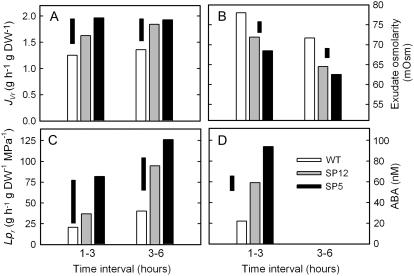
Lpr
Wild type, sp12, and sp5 plants were grown in a hydroponic system, and exudate was collected from root stumps between 1 to 3 and 3 to 6 h after detopping the plants. Osmolarities of exudates and the hydroponic media were determined and Lpr was calculated (Fig. 5). Exudation rate expressed on a root dry weight basis (JVr) and Lpr were both higher in sp12 and sp5 than in the wild type during the 1 to 3 h time interval, although only sp5 differed significantly from the wild type (P < 0.05). However, during the 3 to 6 h period, both transgenic lines had significantly higher Lpr and JVr values than the wild type (P < 0.05; Fig. 5, A and C). Taking both time intervals together, Lpr values were 2.4- and 3.6-fold higher than the wild type in sp12 and sp5, respectively (Fig. 5C). Both lines had lower exudate osmolarities than the wild type at both time intervals (P < 0.05 for all comparisons to the wild type, and P < 0.001 for the overall genotype effect; Fig. 5B). Exudates from sp12 and sp5 contained significantly more ABA than those from wild-type plants (2.7- and 4.3-fold, respectively; P < 0.001 for the overall genotype effect; Fig. 5D).
Figure 5.
Lpr and ABA concentration of root exudates. Plants growing in hydroponics were detopped and exudates collected from the stumps at the indicated time intervals. A, JVr; B, exudate osmolarity; C, Lpr; D, ABA concentration in exudate. ABA was not determined at 3 to 6 h. Floating bars are lsd at 5% level, given separately for each time interval, n = 6.
When transpiration is low, root pressure may contribute significantly to sap flow to the shoot, although this will be influenced by both the mass of the root system and the JVr. Based on the mean values from the two hydroponic experiments, the fraction of dry matter partitioned to the roots, i.e. the root mass fraction (Poorter and Nagel, 2000), for the wild type, sp12, and sp5 was 0.058, 0.056, and 0.068, respectively. The 17% greater root mass fraction in sp5 was significant (P < 0.05, n = 12 plants per genotype) and would have further increased delivery of exudate to the shoot, although the 49% mean increase in JVr would have had a greater impact.
Leaf Growth and Biomass
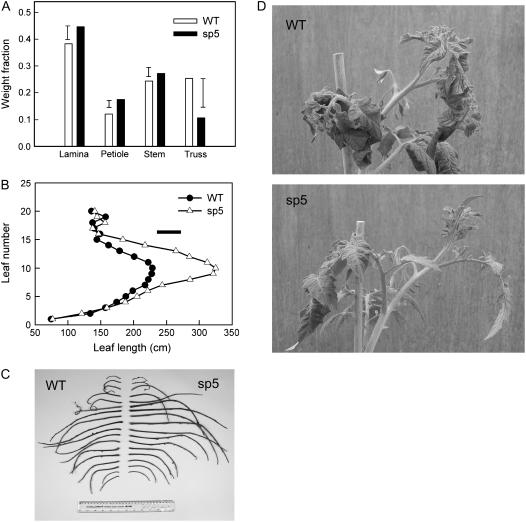
It was clear from numerous observations under glasshouse conditions that sp12 and sp5 take longer to establish than wild-type plants, by about 4 to 10 d between radicle emergence and the four-leaf stage; however, growth rate is subsequently similar in all genotypes under well-watered conditions and transgenic plants often appeared to produce longer leaves. To investigate leaf growth and biomass partitioning within the shoot, germination of wild-type and sp5 seeds was synchronized and plants were harvested after 13 weeks of growth under standard glasshouse conditions.
Final shoot dry weight and plant height were not significantly different between genotypes (21.6 and 21.4 g for wild-type and sp5 shoot dry weight, respectively; P = 0.871). However, the sp5 plants partitioned significantly more biomass to the lamina (P = 0.021) and petiole (P < 0.001) and significantly less to the trusses (P = 0.012), although the developing fruit had only progressed to the immature green fruit stage in the lower trusses at harvest (Fig. 6A). Leaf area was higher in sp5 (2,114 and 2,683 cm2 for the wild type and sp5, respectively; P < 0.001), and there was a suggestion of higher specific leaf area (258.3 and 285.3 cm2 g−1 dry weight for the wild type and sp5, respectively; P = 0.065). There was a noticeable increase in petiole length in sp5 (P < 0.001), which was significant for leaf numbers 8 to 15 (Fig. 6, B and C) in this experiment; similar leaf length data were obtained from plants grown in the hydroponic system (data not shown). There was also a reduction in leaf epinasty in sp5 (Fig. 6D), as was also apparent in many other glasshouse experiments. The angle between the petiole and lower stem was measured for leaves 3, 4, and 5; the mean angle for sp5 (124°) was higher (P = 0.007) than that for the wild type (110°).
Figure 6.
Increased petiole length, reduced epinasty, and altered biomass partitioning in sp5 versus the wild type. A, Biomass partitioning as a fraction of total shoot dry weight; B, leaf length at each leaf position at final harvest; C, petioles from two typical plants with leaflets removed; D, the top portion of typical wild-type and sp5 plants showing the differing extent of epinasty. Error bars in A and B are lsd at 5%, n = 9.
In another experiment where irrigation was set at a suboptimal level for growth of the wild type, mean leaf lengths at final harvest were 33.2, 40.2, and 42.5 cm for the wild type, sp12, and sp5, respectively (P = 0.006 for overall genotype effect, lsd [5%] = 3.7), indicating that the effect on petiole extension is common to the two transgenic lines (R. Smeeton and I. Taylor, unpublished data).
DISCUSSION
Drought Response
Under nonstressed, well-watered conditions, the LeNCED1 overexpression line sp12 exhibited increased xylem sap ABA concentrations and reduced gl values equivalent to mildly stressed wild-type plants (Fig. 1). When a root-zone water deficit was imposed by withholding irrigation, ABA and gl responded, reaching the same final levels in the wild type and sp12, albeit from different basal levels. The response of both the wild type and sp12 to drought was greater than the difference between these two genotypes under well-watered conditions. In agreement with this pattern, the constitutive transgenic expression of LeNCED1 in leaves of well-watered sp12 and sp5 plants was lower than that of the endogenous gene in wilted leaves (S.A. Tung, unpublished data). The important characteristic of these two lines in this context, then, is that they behave differently than the wild type under well-watered conditions, whereas, at least in the study of sp12, the wild-type and transgenic genotypes exhibited similar final values for ABA content and gl, such that differences were no longer apparent once a particular severity of stress was reached and the endogenous mechanisms for ABA accumulation were triggered.
TE and Interaction with VPD
Under well-watered conditions, lines overexpressing LeNCED1 had greatly improved TEi, driven by the reduction in gs, as demonstrated by both instantaneous and integrative methods. There was a positive relationship between δ13C and A/gs, as expected from theory (Farquhar et al., 1982), with the transgenic lines having higher δ13C values, suggesting that the increase in TEi was maintained over the timescale of leaf development. In agreement, ABA-deficient mutants of tomato had lower δ13C values than the wild type that increased when ABA was applied (Bradford et al., 1983). The negative relationship between δ18O and gs is in agreement with both the published model for oxygen isotope discrimination and experimental data for cotton (Barbour and Farquhar, 2000); δ18O therefore provides a promising tool for screening genetic material for differences in gs. These results contradict a puzzling report of a positive relationship between gs and δ18O where gs was manipulated by ABA applications and other treatments (Sheshshayee et al., 2005).
The decline in gs and transpiration is generally proportionally greater than the associated reduction in A (Jones, 1976; Hetherington and Woodward, 2003); an increase in TEi is therefore often observed as gs decreases. Because of this nonlinear relationship, a given reduction in gs has a small effect on A at high gs but has a much larger effect at lower values of gs, as can be seen in Figure 2C. This relationship was apparent when gs in cotton leaves was manipulated by applying ABA (Wong et al., 1979); our data show that the relationship also fits when ABA levels were increased via genetic transformation (Fig. 2C). In the case of the two transgenic lines examined, there was little penalty in A for sp12, whereas sp5 exhibited a substantial reduction in A because of the larger decrease in gs. Compared to the wild type, A/gs was 1.7- and 2.5-fold higher for sp12 and sp5, respectively (Fig. 2A).
A simulation analysis for sorghum (Sorghum bicolor; Sinclair et al., 2005), in which the normal maximum transpiration rate (E) of 1.0 mm h−1 was limited to 0.4 mm h−1, indicated that the imposed lower limit increased average yield over 115 seasons by improving yield in dry, low-yielding years. Water-use efficiency was also improved. Mean E values from the IRGA data presented in Figure 2 were 0.27, 0.22, and 0.12 mm h−1 in the wild type, sp12, and sp5, respectively. Thus, we have created genotypes in which E is limited proportionally to a similar extent as in the model; such transgenic approaches could be used to select lines with a transpiration rate that is capped at a level suitable for specific growing environments.
Setting a maximum limit for E was particularly effective in the simulations described by Sinclair et al. (2005) because it reduced transpiration during periods when atmospheric demand (VPD) was highest, i.e. when water loss would be greatest. This is very similar to the phenotype we have created in sp12 in view of the observed interaction between genotype and VPD when measuring gl (Fig. 3): At high VPD, stomata in sp12 were more closed than the wild type, improving TEi and conserving soil water, but, when VPD was low, gl was similar in both sp12 and the wild type, allowing maximum CO2 assimilation when atmospheric demand for water was low. It will be of interest to determine whether sp5, with its higher transgene expression and ABA content relative to sp12, is able to maintain stomatal closure under low VPD, or, indeed, whether it attains lower gl than sp12 and the wild type during mild drought stress.
It is well known that stomatal closure is induced by increases in VPD (Grantz, 1990). Although some ABA-insensitive mutants appear to show some stomatal response to VPD (Assmann et al., 2000), recent reports suggest that Arabidopsis mutants that lack this response carry defects in OST1, a gene encoding a protein kinase known to be required for ABA-induced stomatal closure, and ABA2, encoding xanthoxin oxidase, the enzyme that catalyzes the penultimate step of ABA biosynthesis (Xie et al., 2006). It therefore appears that ABA is needed to mediate the VPD response; our data extend this observation as increased accumulation of ABA in well-watered transgenic plants enhanced the response to VPD.
Lpr and Leaf Flooding
We previously reported observations of increased guttation in the two NCED-overexpressing lines (Thompson et al., 2000) and have shown here that this is often accompanied by leaf flooding. The guttation and leaf flooding phenotypes only occurred when VPD was low, and we have shown that these plants also had unusually high root exudation rates. This combination provides high water input and low water output for the shoot, clearly resulting in an imbalance in water supply that could not be corrected even by guttation through hydathodes, a release mechanism that may have evolved to prevent leaf flooding under such conditions. That the flooding of intercellular air spaces occurred mainly in the lower leaves (Fig. 4D) and more often in younger plants of the NCED-overexpressing lines suggests that their higher root pressure became dissipated as the distance between the roots and leaves increased. Leaf flooding was not seen at 65% RH even when gl was reduced by 4-fold in line sp5 (Fig. 2), presumably because water loss exceeded delivery from the roots when atmospheric demand for water was high.
The most likely mechanism for the increase in Lpr is an ABA-induced increased activity of aquaporins (Hose et al., 2000; Javot and Maurel, 2002), either regulated at the transcriptional level (Zhu et al., 2005) or possibly through gating of preexisting channels (Wan et al., 2004). It is also possible that increased conductivity of leaf cells in response to ABA (Morillon and Chrispeels, 2001) influences water balance in the NCED-overexpressing lines. Because exudation rates in sp12 and sp5 were increased proportionally more than the decrease in the osmolarity of the exudates produced, there must also have been an increased solute flux. According to the simple osmotic model of root pressure, whereby solute is loaded into the xylem from root cells, driving subsequent osmotic uptake of water, the additional ABA must have stimulated solute loading to achieve the increased solute flux. In an alternative model of root pressure, uptake of solutes from the soil and xylem into the symplast promotes hydrostatic release of water into the xylem through hydraulic pores that also allow release of hydrated solutes. The solute released to the xylem must then be actively recovered (Pickard, 2003). Under this model, the observed higher exudation rate, combined with the reduced exudate osmolarity, can be explained if the rate of recovery of solute from the xylem is increased.
Leaf Growth and Biomass Partitioning and Their Potential Impact on TEp
A key question is whether the limit on assimilation imposed by low gs has a negative effect on biomass accumulation under optimum conditions. At least under the environmental conditions used here, there was no reduction in aboveground biomass in mature plants when comparing the wild type and sp5; we therefore propose that, although assimilation rate per unit leaf area was reduced, there were counteracting positive effects of ABA on growth. We have also observed in several experiments where roots were washed from soil (A.J. Thompson and J. Andrews, unpublished data) or where plants were grown hydroponically (data given in “Results” section “Lpr”) that the fraction of biomass partitioned to the roots in sp5 or sp12 was either not significantly different to the wild type or was slightly higher, allowing the conclusion that shoot biomass was not maintained in sp5 at the expense of root biomass.
The observed increases in shoot biomass partitioning to the lamina and petiole, reduced epinastic leaf curling, and higher specific leaf area (thinner leaves) in sp5 might be predicted to increase light interception and assimilation per plant (Poorter and Nagel, 2000). One consequence of this, however, is that the large reduction in transpiration per unit leaf area (up to 4-fold in sp5; Fig. 2B) and the associated gain in TEi may have been partially compensated at the plant level by the modest increase in leaf area (27% increase; see “Results” section “Leaf Growth and Biomass”) and more open canopy. In support of this compensatory mechanism, the effect of LeNCED1 overexpression on TEp (1.3- and 1.8-fold higher than the wild type in sp12 and sp5, respectively; Table I) was less extreme than the effect on TEi (corresponding increases of 1.7- and 2.5-fold; Fig. 2A). However, despite this suggestion of compensatory behavior, the effects on TEp were still large, with sp5 using 44% less water than the wild type per unit of biomass produced at the whole-plant level.
The reduction in epinasty in these high ABA lines concurs with observations of exaggerated epinasty in ABA-deficient tomato mutants (Tal and Imber, 1970) and the proposal (based on studies with ABA-deficient and ethylene-insensitive mutants and treatment with inhibitors of either ABA or ethylene biosynthesis and ethylene action) that ABA promotes leaf expansion by suppressing the effects of ethylene (Sharp, 2002; LeNoble et al., 2004). The more upright leaf posture we observed might be a consequence of either increased turgor or reduced epinasty. Turgor is likely to be higher, at least in the leaves of sp12, because they had higher ψw than the wild type, presumably due to their lower gl and the delayed development of root-zone water deficits (Fig. 1A; over all time points and treatments ψw for sp12 was 0.17 MPa higher than the wild type, P < 0.001).
Partitioning of biomass to reproductive tissues was reduced in sp5; our observations suggest that this is likely to be attributable either to a small delay in the initiation of the first truss or to greater flower abortion in the lower trusses. In a collection of Arabidopsis ecotypes, McKay et al. (2003) found that TE (as measured by δ13C) was positively correlated with flowering time, suggesting that in wild populations there may be competing evolutionary strategies for drought avoidance (high TE, late flowering) versus drought escape (low TE, early flowering). More recently, it has been found that FCA is an ABA-binding protein involved in the regulation of flowering time in Arabidopsis (Razem et al., 2006). Thus, by increasing the ABA content of plants, it is possible that we have created a physiological shift toward a drought avoidance strategy. Current investigations are under way to further define the productivity of high ABA plants, including reproductive development, under a range of irrigation regimes.
Implications for Plant Water Status and Growth
Plant water status often declines around midday, and stomata may close due to the inability of the root system to supply sufficient water to the shoot during periods of high evaporative demand. In the lines with elevated ABA (sp5 and sp12), it is likely that water status would have been increased by reductions in transpiration and because their greater Lpr would have improved water supplies to the shoot when atmospheric demand for water was high and Lpr might otherwise have become limiting. In the experiment shown in Figure 6, where plants were grown in 18-cm-diameter pots in a free-draining soil with a single irrigation in the morning, it is likely they experienced diurnal cycles of soil water deficit that increased in severity as the plants grew. The more conservative use of water by sp5 may have had the effect of maintaining a higher soil water status during the afternoon, with positive effects on shoot water status. For these reasons, positive effects of ABA on growth are likely to arise from improvements in water status and enhanced turgor-driven growth.
CONCLUSION
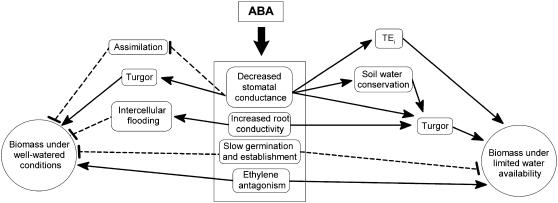
Long-term elevation of ABA in plants produced a range of physiological effects that may impact positively or negatively on crop production depending on water availability (Fig. 7). In well-watered plants, the negative effects of ABA overaccumulation were reduced assimilation rate, leaf flooding and chlorosis (particularly under high humidity environments in young plants), and delayed germination and establishment. However, under standard glasshouse conditions, these effects were insufficient to reduce biomass production, presumably because of counteracting positive effects on leaf expansion through improvements in water status, turgor, and antagonism of epinastic growth. We also hypothesize that under conditions of limited water availability, additional positive factors become relevant; thus, improvements in TEi should allow continued biomass production by conserving soil water and delaying the onset of shoot water deficits, while increased Lpr will improve the supply of water to the shoot, helping to maintain water status when atmospheric demand is high. However, once high ABA plants begin to experience water deficits, they behave as wild-type plants because loss of turgor activates the native ABA biosynthetic pathways and stimulates a greatly increased level of ABA accumulation, swamping the transgene effect displayed by the two transgenic lines under well-watered conditions.
Figure 7.
Primary physiological effects of ABA overproduction and the proposed consequences for productivity when water availability is either nonlimiting or limiting. Dashed lines indicate negative effects and solid lines positive effects.
Optimization of NCED transgene activity for level of expression, developmental timing, and tissue specificity may allow the production of genotypes in which the potential negative physiological effects of high ABA, such as delayed establishment or flowering, can be avoided, while the positive effects on water economy and leaf growth are maintained.
MATERIALS AND METHODS
Plant Materials, Germination, and Establishment
Transgenic primary transformants of tomato (Solanum lycopersicum), sp5 and sp12, and the wild type control (cv Ailsa Craig) were used as described previously (Thompson et al., 2000). Homozygous true-breeding lines were identified for both sp5 and sp12, and these were used for all experiments in this study. Note that line sp12 was originally named d9 and was reported to be derived from a construct where LeNCED1 was driven by the double cauliflower mosaic virus 35S promoter (Thompson et al., 2000); this line has now been shown to have arisen from the construct with LeNCED1 downstream of the Gelvin Superpromoter (Thompson et al., 2007).
For germination, tomato seed were surface sterilized by treatment with 10% (v/v) Domestos household bleach (Lever Faberge) for 30 min to inactivate plant viruses, then washed thoroughly in distilled water. Wild-type and sp12 seed were sown directly onto filter paper soaked in distilled water. The more recalcitrant homozygous sp5 seed, in which the high ABA content severely delayed germination, were first washed for 5 d in tap water using an ebb-and-flow device adapted from a pipette washer, and then transferred to filter paper soaked in distilled water plus 0.5 mg L−1 norflurazon (Syngenta; herbicide grade, 80% purity). For all tomato genotypes, seed were germinated in the dark at 25°C and on the day of radicle emergence seed were sown into 30-mL modules filled with Levington's F2 compost (Levington Horticulture). Typically, radicle emergence took 3 to 4, 10 to 13, or 4 to 8 d for the wild type, sp12, and sp5, respectively (note that emergence often took over 20 d for sp5 in the absence of norflurazon). In the case of sp5, seed were washed for 1 h in tap water to remove residual norflurazon before transfer to compost. After a further 2 to 3 weeks, unless otherwise stated, seedlings of all genotypes were transplanted into vermiculite soaked in nutrient solution (Vitafeed 214; Vitax) in 270-mL or 1,000-mL pots. Genotypes sp5 and sp12 typically required 4 to 10 d longer than the wild type to establish after radicle emergence, but, by careful scheduling and selection, all three genotypes were grown such that they reached the three- to four-leaf stage at approximately the same time.
Note that a slightly modified protocol for germination and establishment was employed for the glasshouse experiment presented in Table I (see “Gravimetric Measurement of TEp”).
Standard Controlled Environment Growth Conditions
Unless otherwise stated, growth cabinet conditions were: 65% RH, 22°C/18°C day/night temperature, 14-h photoperiod, 300 μmol m−2 s−1 photosynthetically active radiation, and 360 μmol mol−1 CO2. Plants were irrigated daily by hand with Vitafeed 214 (Vitax). Side shoots and flower trusses were removed as soon as they were apparent, except for the experiment shown in Figure 6.
Drought Experiment
Plants were grown to the four- or five-leaf stage in a walk-in controlled environment cabinet, arranged in a split-plot Trojan Square design (Edmondson, 1998), in 500-mL free-draining pots. There were 40 split-plots, each containing one well-watered plant and one drought-treated plant of the same genotype. This allowed four replicates of the two genotypes for each of five independent harvests. During establishment, all plants were irrigated twice each day with 75 mL of nutrient solution. Irrigations occurred 4 h into the light period and at the beginning of the night period. Drought-treatment plants received no further irrigation after the first irrigation of day 0, whereas the well-watered treatment continued to receive 2 × 75 mL per day. Harvests took place on the afternoon of each of days 1 to 5. On each day, gas exchange was measured (see below), and then, at harvest, ψw (SKPM pressure chamber 1400; Skye Instruments) was determined, samples were taken for leaf, root, and xylem sap ABA determination, and then leaf area and fresh and dry weights were recorded as described previously (Mulholland et al., 2003).
Humidity Experiment: Guttation and Leaf Flooding
Wild-type and sp12 plants were established in a growth cabinet under the standard controlled environment conditions (65% RH). Plants were selected at the four- to five-leaf stage to provide uniform size between and within genotype. At this time (day 0), plants were split into two growth cabinets, one at low RH (65% day and night) and one at high RH (82%–90% day-night), using standard lighting and temperature regimes. The incidence of guttation and interveinal flooding was scored daily at dawn. For interveinal flooding, the percentage of leaf area that was flooded was estimated in 10% increments for each leaf by visual observation. The mass of guttation fluid was measured following collection by absorption on to preweighed filter paper. Guttation fluid for ABA analysis was harvested by pipette. Chlorophyll measurements were made on day 13 with a chlorophyll meter (SPAD-502; Minolta). On day 26, gas-exchange measurements were taken. Five randomized blocks per cabinet were used throughout.
Measurement of Lpr
Plants were raised in 9-cm-diameter pots containing F2 compost and then transferred to a recirculating, aerated, nutrient-film hydroponic system (Winsor et al., 1979) in a glasshouse. Plants were selected to provide a uniform size and then the bottom of each pot was cut away to allow roots to freely grow into the hydroponic system; the pots were placed on angled troughs carrying the recirculating media. Pots and roots were covered with opaque polythene to exclude light. The recirculating nutrient solution was analyzed and adjusted periodically to maintain the original nutrient levels. The glasshouse was partially environmentally controlled (Thompson et al., 2004), and the plants were arranged in three randomized blocks, each consisting of an independent hydroponic system supporting six plants in a single row plus a guard plant at each end. Nineteen days after transfer to the hydroponic system, plants were detopped just below cotyledons, rubber tubing was attached to the root stump, and exudate was collected in foil-covered containers in an ice bath. Exudate collected during the first hour was discarded; exudates collected between 1 to 3 and 3 to 6 h after detopping were frozen immediately at −80°C for analysis. At the end of the collection period, the compost was removed by washing and root and shoot dry weights were recorded. JVr was calculated per gram root dry weight. The osmolarity of exudates and the hydroponic medium was determined with a Type 13 freezing point depression micro-osmometer (Roebling), and then osmotic pressure of media (πm) and exudates (πx) were calculated from the van't Hoff equation (T = 21°C). The experiment was performed twice. Mean osmolarities of the hydroponic media in the two experiments were 54.1 and 52.0 mOsm. Lpr was calculated as (Glinka, 1980):
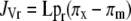 |
ABA Assay
ABA was determined by radioimmunoassay directly upon xylem sap or root exudate, or upon aqueous extracts of root or leaf tissues, using appropriate dilutions, as described by Mulholland et al. (2003). To collect xylem sap (Fig. 1), root systems were detopped and placed in a pressure chamber (SKPM 1400; Skye Instruments) and a pressure applied such that a sap flow rate was achieved that approximated to transpiration by the previously intact plant. Sap was collected within the following 10 min.
Gas-Exchange Measurements and Data Analysis
For Figures 1 and 2, plants were analyzed using a CIRAS-1 IRGA (PP-Systems). The leaf chamber was illuminated under ambient photosynthetically active radiation levels (300 μmol m−2 s−1). CO2 concentration was set to the same concentration as the cabinet (360 ppm). For Figure 3, measurements were made with an LCA-4 IRGA (Analytical Development Company) using ambient light, CO2, and humidity. All measurements were taken on the youngest fully expanded leaf.
IRGAs provide measurements of gl, the sum of gs and gc, with gc often considered to be negligible. However, in Figure 2 we plotted A versus gl and fitted a three-parameter rectangular hyperbolae, providing an estimate of gc = 18.9 mmol m−2 s−1 when A = 0, and then calculated gs = gl − gc. In Figure 2C we fitted Amax and k as A = Amax gs/(gs + k), where k/1.6 is equivalent to a carboxylation conductance; this model assumes that gc was the same for each genotype. We have measured the stomatal and cuticular components of water loss in the wild type and sp5 during biphasic “Hygen” drying curves of detached leaflets (Weyers and Meidner, 1990) and found this assumption to be correct (data not shown).
Gravimetric Measurement of TEp
Wild-type, sp12, and sp5 plants were established so that plant size was synchronized at the beginning of the assessment of TEp (see “Plant Materials, Germination, and Establishment”). In this case, seeds of sp5 were sown 19 d before sp12 and 24 d before the wild type, directly into 7-cm-diameter pots of Levington's M3 compost (Levington Horticulture), with 9-cm petri dish lids placed over the top of the pots to create a humid environment until the hypocotyl emerged. Seeds of sp5 were imbibed in 0.1 mg L−1 norflurazon for 1 d and then thoroughly washed before sowing.
During early growth, plants were irrigated each day to field capacity. On the first day of TEp assessment, three plants per genotype were destructively harvested to determine initial aboveground dry weight. Three further plants were potted into 17-cm-diameter, 2-L pots, also using Levington's M3 compost, and Uvi ground cover discs (Growing Technologies) were placed on top of the compost to limit loss of water by evaporation from the soil. The pots were watered until saturated, allowed to drain to reach field capacity, and weighed. On each of the following 25 d, pots were weighed to record the mass of water lost and then returned to field capacity before reweighing. After this period, all plants were then destructively harvested and aboveground dry weights recorded. Biomass production during the 25 d was calculated as the difference between final and initial aboveground dry weight. TEp was calculated for each plant.
Growth and Leaf Length Analysis
Wild-type and sp5 plants that had germinated on the same day were selected and potted into 7-cm and then 18-cm pots using a free-draining compost (John Innes Potting Compost No. 2 made with grit rather than sand). Nine plants per genotype were arranged in three randomized blocks. After 13 weeks growth in the partially environmentally controlled glasshouse, with daily irrigation by hand, leaf angles were measured using a protractor for leaves 3, 4, and 5; the plants were then photographed and their shoots harvested. Whole leaves were detached and their length was measured with a ruler. Shoots were divided into lamina, petiole, stem, and truss; laminar area was determined using a ΔT area meter MKII (Delta T Devices) and dry weights were recorded. Weight data are presented as a fraction of total shoot biomass (Poorter and Nagel, 2000).
Stable Isotope Measurements
A whole fully expanded leaf, either the fourth of fifth from the top of the plant (the first leaf greater than 2 cm long was counted as leaf 1), was taken and dried at 80°C for 24 h. The entire sample was ground using an Apex rotary mill (Apex Construction); a subsample was further ground with a ball mill (Glen Creston) and sieved to produce a sample of particle size <0.1 mm. Samples were analyzed for 13C/12C and 18O/16O and expressed relative to Pee Dee Belemnite and Vienna Standard Mean Ocean Water to give δ13C and δ18O, respectively (Farquhar et al., 1997; Barbour and Farquhar, 2000). Note that our δ13C values are considerably more negative than typical values for C3 plants because our controlled environment cabinets were supplemented with CO2 of plant origin that was already depleted in 13C. However, all genotypes were grown in the same environment and values between genotypes were comparable.
Statistical Analysis
In the drought experiment (Fig. 1), a split-plot Trojan Square design was employed as described above. For other experiments (Figs. 2–6; Table I), randomized block designs were used. Data analysis was by ANOVA using GenStat Release 7 (Lawes Agricultural Trust). A log transformation of ABA data was used in the drought experiment prior to ANOVA because variability increased with the magnitude in the untransformed data. Means of logs were back-transformed for plots in Figure 1.
P values quoted in the text are for overall genotype, or genotype × treatment effects. Additional comparisons between individual means are made at the 5% level using lsd values. For analysis of data for hydraulic conductivity experiments (Fig. 5), two experiments were analyzed together using experiment as a factor; the P values quoted were obtained after taking mean values over the two time intervals (1–3 and 3–6 h) for each experiment, except for ABA where data were only collected for the first time interval.
Acknowledgments
We thank Steve Quarrie (John Innes Institute) for supplying anti-ABA antibody MAC252; Alison Jackson, Linda Brown, Angela Hambidge, and Colin Clay (Warwick HRI) for technical assistance; Steven Clayton and Sue Woods (Australian National University) for stable isotope analyses; and Rodney Edmondson (Warwick HRI) for statistical advice.
This work was supported by the Biotechnology and Biological Sciences Research Council (Competitive Strategic Grant to A.J.T.) and by the Department for Environment, Food and Rural Affairs (project no. HH1332SPC; B.J.M. and A.J.T.). G.F. acknowledges Discovery support from the Australian Research Council.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Andrew J. Thompson (a.j.thompson@warwick.ac.uk).
Open Access articles can be viewed online without a subscription.
References
- Arteca RN, Tsai D-S (1987) Effects of abscisic acid on the photosynthesis, transpiration and growth of tomato plants. Crop Res 27 91–96 [Google Scholar]
- Assmann SM, Snyder JA, Lee YRJ (2000) ABA-deficient (aba1) and ABA-insensitive (abi1-1, abi2-1) mutants of Arabidopsis have a wild-type stomatal response to humidity. Plant Cell Environ 23 387–395 [Google Scholar]
- Aswath CR, Kim SH, Mo SY, Kim DH (2005) Transgenic plants of creeping bent grass harboring the stress inducible gene, 9-cis-epoxycarotenoid dioxygenase, are highly tolerant to drought and NaCl stress. Plant Growth Regul 47 129–139 [Google Scholar]
- Bahieldin A, Mahfouz HT, Eissa HF, Saleh OM, Ramadan AM, Ahmed IA, Dyer WE, El-Itriby HA, Madkour MA (2005) Field evaluation of transgenic wheat plants stably expressing the HVA1 gene for drought tolerance. Physiol Plant 123 421–427 [Google Scholar]
- Barbour MM, Farquhar GD (2000) Relative humidity- and ABA-induced variation in carbon and oxygen isotope ratios of cotton leaves. Plant Cell Environ 23 473–485 [Google Scholar]
- Blum A (2005) Drought resistance, water-use efficiency, and yield potential: Are they compatible, dissonant, or mutually exclusive? Aust J Agric Res 56 1159–1168 [Google Scholar]
- Bradford KJ, Sharkey TD, Farquhar GD (1983) Gas exchange, stomatal behavior, and δ13C values of the flacca tomato mutant in relation to abscisic acid. Plant Physiol 72 245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray EA (2002) Abscisic acid regulation of gene expression during water-deficit stress in the era of the Arabidopsis genome. Plant Cell Environ 25 153–161 [DOI] [PubMed] [Google Scholar]
- Chaves MM, Oliveira MM (2004) Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. J Exp Bot 55 2365–2384 [DOI] [PubMed] [Google Scholar]
- Condon AG, Richards RA, Rebetzke GJ, Farquhar GD (2002) Improving intrinsic water-use efficiency and crop yield. Crop Sci 42 122–131 [DOI] [PubMed] [Google Scholar]
- Condon AG, Richards RA, Rebetzke GJ, Farquhar GD (2004) Breeding for high water-use efficiency. J Exp Bot 55 2447–2460 [DOI] [PubMed] [Google Scholar]
- Edmondson RN (1998) Trojan square and incomplete Trojan square designs for crop research. J Agric Sci 131 135–142 [Google Scholar]
- Farquhar GD, Henry BK, Styles JM (1997) A rapid on-line technique for determination of oxygen isotope composition of nitrogen-containing organic matter and water. Rapid Commun Mass Spectrom 11 1554–1560 [Google Scholar]
- Farquhar GD, O'Leary MH, Berry JA (1982) On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust J Plant Physiol 9 121–137 [Google Scholar]
- Farquhar GD, Richards RA (1984) Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust J Plant Physiol 11 539–552 [Google Scholar]
- Glinka Z (1980) Abscisic acid promotes both volume flow and ion release to the xylem in sunflower roots. Plant Physiol 65 537–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantz DA (1990) Plant response to atmospheric humidity. Plant Cell Environ 13 667–679 [Google Scholar]
- Hall NM, Griffiths H, Corlett JA, Jones HG, Lynn J, King GJ (2005) Relationships between water-use traits and photosynthesis in Brassica oleracea L. resolved by quantitative genetic analysis. Plant Breed 124 557–564 [Google Scholar]
- Hetherington AM, Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424 901–908 [DOI] [PubMed] [Google Scholar]
- Hose E, Steudle E, Hartung W (2000) Abscisic acid and hydraulic conductivity of maize roots: a study using cell- and root-pressure probes. Planta 211 874–882 [DOI] [PubMed] [Google Scholar]
- Iuchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, Tabata S, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K (2001) Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J 27 325–333 [DOI] [PubMed] [Google Scholar]
- Javot H, Maurel C (2002) The role of aquaporins in root water uptake. Ann Bot (Lond) 90 301–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HG (1976) Crop characteristics and the ratio between assimilation and transpiration. J Appl Ecol 13 605–622 [Google Scholar]
- Juenger TE, McKay JK, Hausmann N, Keurentjes JJB, Sen S, Stowe KA, Dawson TE, Simms EL, Richards JH (2005) Identification and characterization of QTL underlying whole-plant physiology in Arabidopsis thaliana: δ13C, stomatal conductance and transpiration efficiency. Plant Cell Environ 28 697–708 [Google Scholar]
- Kermode AR (2005) Role of abscisic acid in seed dormancy. J Plant Growth Regul 24 319–344 [Google Scholar]
- Laporte MM, Shen B, Tarczynski MC (2002) Engineering for drought avoidance: expression of maize NADP-malic enzyme in tobacco results in altered stomatal function. J Exp Bot 53 699–705 [DOI] [PubMed] [Google Scholar]
- Lawson T, Morison J (2006) Visualising patterns of CO2 diffusion in leaves. New Phytol 169 641–643 [DOI] [PubMed] [Google Scholar]
- LeNoble ME, Spollen WG, Sharp RE (2004) Maintenance of shoot growth by endogenous ABA: genetic assessment of the involvement of ethylene suppression. J Exp Bot 55 237–245 [DOI] [PubMed] [Google Scholar]
- Lu ZM, Percy RG, Qualset CO, Zeiger E (1998) Stomatal conductance predicts yields in irrigated Pima cotton and bread wheat grown at high temperatures. J Exp Bot 49 453–460 [Google Scholar]
- Masle J, Gilmore SR, Farquhar GD (2005) The ERECTA gene regulates plant transpiration efficiency in Arabidopsis. Nature 436 866–870 [DOI] [PubMed] [Google Scholar]
- McKay JK, Richards JH, Mitchell-Olds T (2003) Genetics of drought adaptation in Arabidopsis thaliana: I. Pleiotropy contributes to genetic correlations among ecological traits. Mol Ecol 12 1137–1151 [DOI] [PubMed] [Google Scholar]
- Morillon R, Chrispeels MJ (2001) The role of ABA and the transpiration stream in the regulation of the osmotic water permeability of leaf cells. Proc Natl Acad Sci USA 98 14138–14143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland BJ, Black CR, Taylor IB, Roberts JA, Lenton JR (1996) Effect of soil compaction on barley (Hordeum vulgare L) growth. 1. Possible role for ABA as a root-sourced chemical signal. J Exp Bot 47 539–549 [Google Scholar]
- Mulholland BJ, Hussain A, Black CR, Taylor IB, Roberts JA (1999) Does root-sourced ABA have a role in mediating growth and stomatal responses to soil compaction in tomato (Lycopersicon esculentum)? Physiol Plant 107 267–276 [Google Scholar]
- Mulholland BJ, Taylor IB, Jackson AC, Thompson AJ (2003) Can ABA mediate responses of salinity stressed tomato? Environ Exp Bot 50 17–28 [Google Scholar]
- Parry MAJ, Flexas J, Medrano H (2005) Prospects for crop production under drought: research priorities and future directions. Ann Appl Biol 147 211–226 [Google Scholar]
- Passioura J (2006) Increasing crop productivity when water is scarce: from breeding to field management. Agric Water Manage 80 176–196 [Google Scholar]
- Pickard WF (2003) The riddle of root pressure. I. Putting Maxwell's demon to rest. Funct Plant Biol 30 121–134 [DOI] [PubMed] [Google Scholar]
- Poorter H, Nagel O (2000) The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Aust J Plant Physiol 27 595–607 [Google Scholar]
- Qin X, Zeevaart JAD (2002) Overexpression of a 9-cis-epoxycarotenoid dioxygenase gene in Nicotiana plumbaginifolia increases abscisic acid and phaseic acid levels and enhances drought tolerance. Plant Physiol 128 544–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin JW, Lu ZM, Percy RG, Zeiger E (1994) Genetic variability for stomatal conductance in Pima cotton and its relation to improvements of heat adaptation. Proc Natl Acad Sci USA 91 7217–7221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razem FA, El-Kereamy A, Abrams SR, Hill RD (2006) The RNA-binding protein FCA is an abscisic acid receptor. Nature 439 290–294 [DOI] [PubMed] [Google Scholar]
- Saab IN, Sharp RE, Pritchard J, Voetberg GS (1990) Increased endogenous abscisic acid maintains primary root growth and inhibits shoot growth of maize seedlings at low water potentials. Plant Physiol 93 1329–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SH, Qin XQ, Zeevaart JAD (2003) Elucidation of the indirect pathway of abscisic acid biosynthesis by mutants, genes, and enzymes. Plant Physiol 131 1591–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SH, Tan BC, Gage DA, Zeevaart JAD, McCarty DR (1997) Specific oxidative cleavage of carotenoids by VP14 of maize. Science 276 1872–1874 [DOI] [PubMed] [Google Scholar]
- Sharp RE (2002) Interaction with ethylene: changing views on the role of abscisic acid in root and shoot growth responses to water stress. Plant Cell Environ 25 211–222 [DOI] [PubMed] [Google Scholar]
- Sharp RE, LeNoble ME, Else MA, Thorne ET, Gherardi F (2000) Endogenous ABA maintains shoot growth in tomato independently of effects on plant water balance: evidence for an interaction with ethylene. J Exp Bot 51 1575–1584 [DOI] [PubMed] [Google Scholar]
- Sheshshayee MS, Bindumadhava H, Ramesh R, Prasad TG, Lakshminarayana MR, Udayakumar M (2005) Oxygen isotope enrichment (Δ18O) as a measure of time-averaged transpiration rate. J Exp Bot 56 3033–3039 [DOI] [PubMed] [Google Scholar]
- Sinclair TR, Hammer GL, van Oosterom EJ (2005) Potential yield and water-use efficiency benefits in sorghum from limited maximum transpiration rate. Funct Plant Biol 32 945–952 [DOI] [PubMed] [Google Scholar]
- Sivamani E, Bahieldin A, Wraith JM, Al-Niemi T, Dyer WE, Ho THD, Qu RD (2000) Improved biomass productivity and water use efficiency under water deficit conditions in transgenic wheat constitutively expressing the barley HVA1 gene. Plant Sci 155 1–9 [DOI] [PubMed] [Google Scholar]
- Spollen WG, LeNoble ME, Samuels TD, Bernstein N, Sharp RE (2000) Abscisic acid accumulation maintains maize primary root elongation at low water potentials by restricting ethylene production. Plant Physiol 122 967–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal M, Imber D (1970) Abnormal stomatal behavior and hormonal imbalance in flacca, a wilty mutant of tomato. II. Auxin- and abscisic acid-like activity. Plant Physiol 46 373–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal M, Nevo Y (1973) Abnormal stomatal behaviour and root resistance, and hormonal imbalance in three wilty mutants of tomato. Biochem Genet 8 291–300 [DOI] [PubMed] [Google Scholar]
- Tan BC, Schwartz SH, Zeevaart JAD, McCarty DR (1997) Genetic control of abscisic acid biosynthesis in maize. Proc Natl Acad Sci USA 94 12235–12240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor IB, Sonneveld T, Bugg TDH, Thompson AJ (2005) Regulation and manipulation of the biosynthesis of abscisic acid, including the supply of xanthophyll precursors. J Plant Growth Regul 24 253–273 [Google Scholar]
- Teulat B, Merah O, Sirault X, Borries C, Waugh R, This D (2002) QTLs for grain carbon isotope discrimination in field-grown barley. Theor Appl Genet 106 118–126 [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Jackson AC, Symonds RC, Mulholland BJ, Dadswell AR, Blake PS, Burbidge A, Taylor IB (2000) Ectopic expression of a tomato 9-cis-epoxycarotenoid dioxygenase gene causes over-production of abscisic acid. Plant J 23 363–374 [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Mulholland BJ, Jackson AC, McKee JMT, Hilton HW, Symonds RC, Sonneveld T, Burbidge A, Stevenson P, Taylor IB (2007) Regulation and manipulation of ABA biosynthesis in roots. Plant Cell Environ 30 67–78 [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Thorne ET, Burbidge A, Jackson AC, Sharp RE, Taylor IB (2004) Complementation of notabilis, an abscisic acid-deficient mutant of tomato: importance of sequence context and utility of partial complementation. Plant Cell Environ 27 459–471 [Google Scholar]
- Voisin A-S, Reidy B, Parent B, Rolland G, Redondo E, Gerentes D, Tardieu F, Muller B (2006) Are ABA, ethylene or their interaction involved in the response of leaf growth to soil water deficit? An analysis using naturally occurring variation or genetic transformation of ABA production in maize. Plant Cell Environ 29 1829–1840 [DOI] [PubMed] [Google Scholar]
- Wan XC, Steudle E, Hartung W (2004) Gating of water channels (aquaporins) in cortical cells of young corn roots by mechanical stimuli (pressure pulses): effects of ABA and of HgCl2. J Exp Bot 55 411–422 [DOI] [PubMed] [Google Scholar]
- Weyers J, Meidner H (1990) Gravimetric and potometric methods. In Methods in Stomatal Research. Longman Scientific and Technical, Harlow, UK, pp 63–77
- Winsor GW, Hurd RG, Price D (1979) Nutrient film technique. In Growers' Bulletin, Vol 5. Glasshouse Crops Research Institute, Littlehampton, West Sussex, UK
- Wong SC, Cowan IR, Farquhar GD (1979) Stomatal conductance correlates with photosynthetic capacity. Nature 282 424–426 [Google Scholar]
- Xie XD, Wang YB, Williamson L, Holroyd GH, Tagliavia C, Murchie E, Theobald J, Knight MR, Davies WJ, Leyser HMO, et al (2006) The identification of genes involved in the stomatal response to reduced atmospheric relative humidity. Curr Biol 16 882–887 [DOI] [PubMed] [Google Scholar]
- Yang JC, Zhang JH, Liu K, Wang ZQ, Liu LJ (2006) Abscisic acid and ethylene interact in wheat grains in response to soil drying during grain filling. New Phytol 171 293–303 [DOI] [PubMed] [Google Scholar]
- Zhang JZ, Creelman RA, Zhu JK (2004) From laboratory to field. Using information from Arabidopsis to engineer salt, cold, and drought tolerance in crops. Plant Physiol 135 615–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CF, Schraut D, Hartung W, Schaffner AR (2005) Differential responses of maize MIP genes to salt stress and ABA. J Exp Bot 56 2971–2981 [DOI] [PubMed] [Google Scholar]