Abstract
Upon the transition of dark-adapted plants to low light, the energy-dependent quenching (qE) of excitation energy is only transiently induced due to the only transient generation of the transthylakoid pH gradient. We investigated the transient qE (qETR) in different Arabidopsis (Arabidopsis thaliana) mutants. In dark-adapted plants, qETR was absent in the npq4 mutant (deficient in the PsbS protein) and the pgr1 mutant (restricted in lumen acidification). In comparison with wild-type plants, qETR was reduced in the zeaxanthin (Zx)-deficient npq1 mutant and increased in the Zx-accumulating npq2 mutant. After preillumination of plants (to allow the synthesis of large amounts of Zx), the formation and relaxation of qETR was accelerated in all plants (except for npq4) in comparison with the respective dark-adapted plants. The extent of qETR, however, was unchanged in npq1 and npq4, decreased in npq2, but increased in wild-type and pgr1 plants. Even in presence of high levels of Zx, qETR in pgr1 mutants was still lower than that in wild-type plants. In the presence of the uncoupler nigericin, qETR was completely abolished in all genotypes. Thus, the transient qETR shows essentially the same characteristics as the steady-state qE; it is strictly dependent on the PsbS protein and a low lumen pH, but the extent of qETR is largely modulated by Zx. These results indicate that qETR does not represent a different quenching mechanism in comparison with the steady-state qE, but simply reflects the response of qE to the dynamics of the lumen pH during light activation of photosynthesis.
Thermal dissipation of light energy serves important photoprotective functions in plant photosynthesis. Three basic mechanisms contribute to these processes: state transitions (Allen, 1992), the energy or pH-dependent mechanism (Horton et al., 1996; Niyogi et al., 2005), and photoinhibition (Aro et al., 1993; Demmig-Adams and Adams, 2006), giving rise to three components of nonphotochemical quenching (NPQ) of chlorophyll (Chl) fluorescence: qT, qE, and qI, respectively.
The qT mechanism balances the excitation of the two photosystems by reversible phosphorylation of a mobile pool of light-harvesting complex II and is important under nonsaturating light intensities (Haldrup et al., 2001). State transitions are regulated by the redox state of the chloroplast (Hamel et al., 2000; Rintamäki et al., 2000). The thylakoid protein kinase that is responsible for light-harvesting complex II phosphorylation has recently been identified in Chlamydomonas (Chlamydomonas reinhardtii; Bellafiore et al., 2005) and Arabidopsis (Arabidopsis thaliana; Bonardi et al., 2005).
The qI quenching is related to photoinhibition of photosynthesis and develops upon prolonged exposure of leaves to highly excessive light stress conditions (Krause, 1988). However, the nature of qI is not fully clear. In part, qI is caused by an inactivation of the D1 protein in the PSII reaction center (Aro et al., 1993), but also zeaxanthin (Zx) seems to be involved in qI formation (Jahns and Miehe, 1996; Thiele et al., 1996; Verhoeven et al., 1996). The qI component of NPQ is only slowly (30 min to several hours) reversible due to the requirement of turnover of the D1 protein and Zx epoxidation. Zx-dependent forms of sustained energy dissipation are supposed to have important photoprotective functions, particularly in evergreen species (Ebbert et al., 2001, 2005; Demmig-Adams and Adams, 2006; Zarter et al., 2006).
The qE mechanism represents the dominating NPQ component under moderate light stress conditions and is related to energy dissipation processes generated by the energization of the thylakoid membrane (Krause et al., 1982). qE is supposed to be based on energy dissipation in the antenna of PSII and represents an efficient mechanism to reduce the electron pressure on the photosynthetic electron transport chain at saturating light intensities. This process is controlled by the synergistic action of the lumen pH, xanthophyll binding, and conformational changes in the antenna of PSII (Horton et al., 2000). Generation of the maximum qE essentially requires a lumen pH below 6 (Munekage et al., 2001; Jahns et al., 2002), de-epoxidized xanthophylls (Niyogi et al., 1998), the PsbS protein (Li et al., 2000), and other antenna proteins of PSII (Horton et al., 2005; Kalituho et al., 2006). qE is strictly regulated by the pH in the thylakoid lumen and generated within 10 min of illumination at saturating light intensities. The pH regulation of qE allows a flexible and rapid switch of the function of the PSII antenna between light harvesting and energy dissipation upon rapidly changing light conditions. The PsbS protein of PSII has been supposed to act as sensor of the lumen pH (Li et al., 2002b, 2004) and to be the site of energy dissipation (Niyogi et al., 2005). Different models have been proposed for the molecular basis of qE. Horton et al. (2005) favor the idea that pH-induced conformational changes induce the formation of a dissipative state in the antenna of PSII and that Zx acts as an allosteric effector of qE, implying a more indirect role of Zx in energy quenching (Pascal et al., 2005). By contrast, the direct involvement of Zx in the quenching process has been derived from femtosecond transient absorption measurements, assuming that energy transfer to a Chl-Zx heterodimer and subsequent formation of a Zx cation radical in the antenna of PSII is the basis of energy dissipation (Holt et al., 2005).
Recently, a second type of qE quenching has been proposed, which is transiently generated during the transition of dark-adapted plants to nonsaturating light and is supposed to be related to events in the reaction center of PSII (Finazzi et al., 2004). In barley (Hordeum vulgare) leaves, this transient qE (qETR) was found to be dependent on a transthylakoid proton gradient and the presence of the PsbS protein but independent of Zx (Finazzi et al., 2004). It has been proposed that qETR represents an initial energy quenching state of PSII from which transition to either qT, qI, or a stable qE state can be achieved, depending on the light intensity and the physiological conditions (Finazzi et al., 2004). Based on nonlinear regression analysis of NPQ, however, the dynamics of the transient NPQ have been correlated with the dynamics of antheraxanthin (Ax) concentrations (D'Haese et al., 2004), implying a crucial function of the xanthophyll cycle in qETR formation.
We studied the transiently formed qE in more detail in several Arabidopsis mutants that show an altered steady-state qE and investigated the role of the lumen pH and Zx in this process. The following mutants were used: (1) the npq1 mutant (Niyogi et al., 1998), which is affected in the violaxanthin (Vx) de-epoxidase and is unable to form Zx upon illumination; (2) the npq2 mutant (Niyogi et al., 1998), which is affected in the Zx epoxidase and accumulates high levels of Zx under all conditions; (3) the pgr1 mutant (Munekage et al., 2001), which carries a single point mutation in the Rieske subunit of the cytochrome b6/f complex (this mutant is limited in lumen acidification due to an altered pH dependence of linear electron transport [Jahns et al., 2002] and ferredoxin-dependent cyclic electron transport [Okegawa et al., 2005]); and (4) the psbs-1.3 mutant (Grasses et al., 2002), which is affected in the PsbS protein of PSII and thus unable to generate qE. For the latter mutant, which is allelic to the PsbS-deficient npq4 mutant (Li et al., 2000), the more common name npq4 will be used throughout the article.
RESULTS
Light Dependence of NPQ Formation
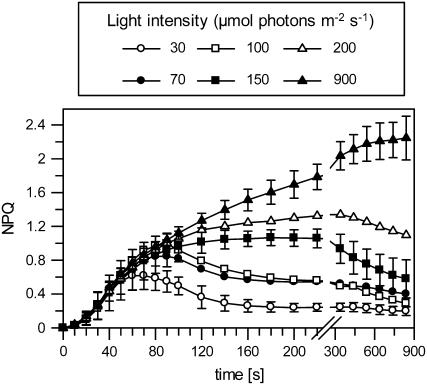
The time course of NPQ formation in dependence on the light intensity is shown in Figure 1 for dark-adapted Arabidopsis plants. The dynamics of the generation and relaxation of NPQ was strongly dependent on the light intensity. At light intensities of 900 μmol photons m−2 s−1, which can be assumed to be sufficient to saturate photosynthetic electron transport in plants grown under 150 μmol photons m−2 s−1, a more or less stable steady-state value of NPQ was reached after about 300 s. This steady-state NPQ has been assigned to energy dissipation processes in the PSII antenna (Horton et al., 1996). At low light intensities, in the range from 30 to 100 μmol photons m−2 s−1, NPQ was only transiently generated, reaching a maximum after about 60 to 100 s and relaxing slowly to a low steady-state level within 200 s. It has been suggested that this transiently formed qETR is related to processes in the reaction center of PSII (Finazzi et al., 2004). The only transient appearance of NPQ under these conditions can simply be explained by the generation and breakdown of the transthylakoid pH gradient at nonsaturating light intensities (Horton, 1983). Up to 100 μmol photons m−2 s−1, the extent of qETR increased gradually (Fig. 1), reflecting the light-dependent increase of the transthylakoid pH gradient upon the dark-light transition. The subsequent relaxation of NPQ in the light can be attributed to the light activation of the NADPH- and ATP-consuming reactions of the Calvin cycle resulting in the breakdown (or reduction) of the ΔpH under nonsaturating light intensities (Slovacek and Hind, 1981; Horton, 1983). At higher saturating light intensities, proton release into the thylakoid lumen exceeds proton consumption by ATP synthesis, leading to the formation of a stable ΔpH and NPQ. For plants grown at 150 μmol photons m−2 s−1, a stable ΔpH is obviously reached at about 200 μmol photons m−2 s−1, as indicated by the generation of a stable NPQ at these light intensities (Fig. 1). As a consequence, qETR can be monitored only at lower, nonsaturating light intensities upon the dark-light transition of dark-adapted plants.
Figure 1.
Time course of NPQ formation in dependence on the actinic light intensity. Leaves from 4-week-old wild-type plants that were dark adapted for at least 10 h were illuminated for 14 min at different light intensities in the range from 30 to 900 μmol photons m−2 s−1 followed by dark exposure for 10 min. The dynamics of NPQ were derived from the maximum fluorescence induced by saturating light flashes at each of the measuring points. Mean values (±sd) of three independent experiments are shown. For clarity, sd is shown for only three light intensities but was in the similar range for the other intensities.
NPQ Formation in Different NPQ Mutants
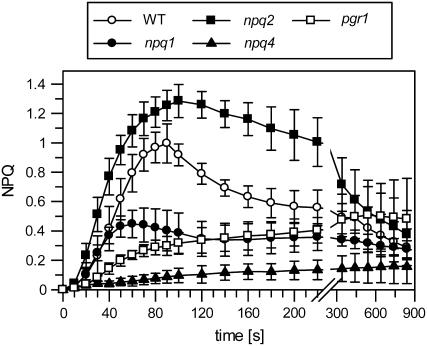
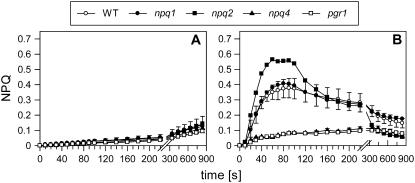
To gain more insight into the characteristics of qETR, we studied the NPQ formation in four Arabidopsis mutants, npq1, npq2, npq4, and pgr1, which have been shown to be affected in the extent and/or kinetics of NPQ formation. The qETR was nearly completely abolished in npq4 (Fig. 2), underlining the requirement of the PsbS protein for qETR. In pgr1 plants, only a very slow rise of NPQ was detectable that was not reversible during the whole illumination period of 840 s. The generation of qETR in this mutant was obviously limited by the reduced transthylakoid pH gradient, supporting the requirement of a low lumen pH for the transient NPQ. In the Zx-deficient npq1 mutant, qETR was reduced to about 50% of the wild-type level, while in the Zx-enriched npq2 mutant, a clearly higher qETR was determined in comparison with the wild type (Fig. 2). These characteristics of qETR imply that, in addition to PsbS and a high ΔpH, de-epoxidized xanthophylls are also required for the induction of the maximal qETR.
Figure 2.
The transient NPQ in different Arabidopsis mutants. The dynamics of the transient NPQ were determined in dark-adapted wild-type and mutant plants. Leaves were illuminated at a light intensity of 100 μmol photons m−2 s−1. At this light intensity, maximum values for the transient NPQ were induced in the wild type (compare with Fig. 1). For initial values of FV/FM, see Table III. Further experimental conditions are given in the legend to Figure 1. Mean values (±sd) of three to four independent experiments are shown.
Vx De-Epoxidation and Electron Transport Rates during NPQ Formation
We investigated the extent and the rate of Zx formation under the same experimental conditions used for the determination of qETR. A reasonable amount of Zx was found to be formed within 1 min of illumination in wild-type and npq4 plants, but a clearly lower amount was determined in pgr1 (Table I). As expected, no Zx was detectable in npq1, while high levels of Zx were present in npq2. These characteristics of the different genotypes were also reflected by the rates of Zx formation during the first minute of illumination; very similar values were found again for npq4 and the wild type, and no changes in the xanthophyll content were detectable for the two xanthophyll cycle mutants npq1 and npq2. In pgr1 plants, a clearly lower rate of Zx formation was found in comparison with the wild type and npq4 (Table I), which can be explained by the reduced capacity of lumen acidification in this mutant in comparison with wild-type plants due to an altered pH dependence of plastoquinol oxidation (Munekage et al., 2001; Jahns et al., 2002). Similar conclusions can be drawn from the determination of the initial rates of electron transport through PSII showing lowest rates for pgr1 and npq2 (Table I). The reduced rate of electron transport in npq2 in comparison with the wild type might be related to sustained energy dissipation due to the accumulation of high levels of Zx in this mutant (Kalituho et al., 2007). It can thus be assumed that, in comparison with wild-type plants, lumen acidification is limited in pgr1 and possibly npq2, but not in npq1 and npq4. The altered characteristics of the qETR observed in npq4, npq1, and npq2 plants are therefore unlikely to be related to changes in the lumen pH in comparison with the wild type.
Table I.
Zx content and initial rates of electron transport and Vx de-epoxidation
Detached leaves were illuminated at a light intensity of 100 μmol photons m−2 s−1. The Zx content was determined after 1 min of illumination. Relative electron transport rates under in vivo conditions were derived from Chl fluorescence measurements during the first minute of illumination. De-epoxidation rates (expressed as percentage of the sum of Vx + Ax + Zx) were calculated from HPLC analysis of the pigment content in the dark and after 0.5 and 1 min. Mean values ± sd of three to four independent experiments are shown. Significant differences in comparison with respective wild-type values are indicated by ** (P < 0.01) or * (P < 0.05).
| Zx Content | Initial Rate
|
|||
|---|---|---|---|---|
| De-Epoxidation
|
Electron Transport | |||
| 0.5 Ax + Zx | Zx | |||
| mmol (mol Chl)−1 | min−1 | |||
| Wild Type | 1.9 ± 0.6 | 8.7 ± 1.2 | 5.7 ± 1.0 | 13.6 ± 1.6 |
| npq1 | 0 | 0 | 0 | 14.8 ± 2.8 |
| npq2 | 113 ± 9.0 | 0 | 0 | 11.7 ± 1.2 |
| npq4 | 1.5 ± 0.2 | 8.0 ± 1.0 | 5.5 ± 0.8 | 12.8 ± 2.1 |
| pgr1 | **1.0 ± 0.2 | **5.7 ± 1.1 | **3.7 ± 0.5 | *10.3 ± 2.1 |
NPQ Formation after Preillumination of the Leaves
To overcome a possible limitation of NPQ formation upon the transition from dark-adapted plants to low light by the rate of Zx synthesis, we investigated the characteristics of qETR after preillumination of the leaves. Leaves were illuminated for 15 min at 20°C to allow the formation of large amounts of Zx prior to the induction of qETR. Subsequently, leaves were redarkened to allow the relaxation of the transthylakoid pH gradient without substantial reconversion of Zx back to Vx. Redarkening for 15 min was found to be sufficient to recover the maximum extent of qETR after 15 min preillumination (data not documented) so that a redarkening of 15 min was used for the following experiments.
We studied the extent of Vx de-epoxidation in wild-type plants at the end of the dark incubation in dependence on the light intensity during preillumination (Table II). The de-epoxidation state (DEPS) of the xanthophyll cycle pigments, defined as (Zx + 0.5 Ax)/(Vx + Ax + Zx), was found to reach values in the range from about 42% (at 300 μmol photons m−2 s−1) to about 54% (at 2,000 μmol photons m−2 s−1). In parallel, we determined the maximum photochemical efficiency of PSII in the dark-adapted state (FV/FM) after this pretreatment to estimate a possible reduction of the PSII quantum efficiency during the illumination period (Table II). Only a slight reduction of the PSII quantum efficiency was detectable up to 1,000 μmol photons m−2 s−1. At 2,000 μmol photons m−2 s−1, however, the reduction was clearly more pronounced (Table II).
Table II.
DEPS and PSII quantum efficiency in preilluminated wild-type plants
Leaves were illuminated for 15 min at indicated light intensities followed by a dark incubation for 15 min. The DEPS is expressed as (Zx + 0.5 Ax)/(Vx + Ax + Zx) × 100. Mean values ± sd of three independent experiments are shown. For FV/FM ratios, significant differences in comparison with the wild type are indicated by ** (P < 0.01) or * (P < 0.05).
| Light Intensity | DEPS | FV/FM |
|---|---|---|
| μmol photons m−2s−1 | ||
| 0 (dark control) | 2.2 ± 0.1 | 0.83 ± 0.01 |
| 300 | 42.6 ± 0.7 | *0.80 ± 0.01 |
| 500 | 41.7 ± 0.8 | *0.80 ± 0.01 |
| 1,000 | 46.3 ± 2.3 | **0.79 ± 0.01 |
| 2,000 | 54.1 ± 0.5 | **0.75 ± 0.01 |
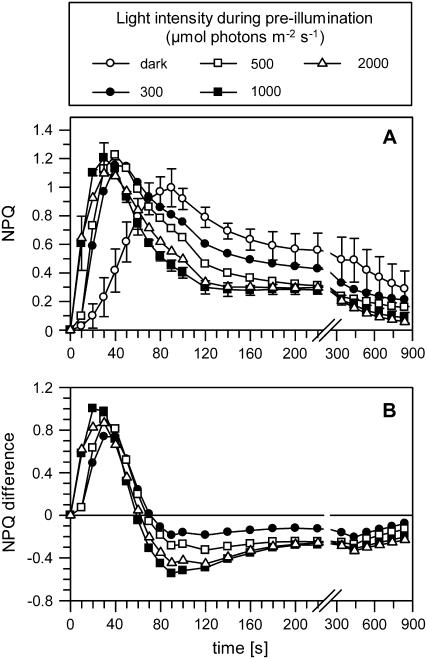
We further determined the dynamics of the NPQ under these conditions (Fig. 3). Preillumination of leaves had two different consequences on the qETR in wild-type plants. First, both the kinetics of NPQ formation and relaxation were accelerated by a factor of about 3 in comparison with dark-adapted samples (Fig. 3). The transient maximum of NPQ was reached after about 30 s in preilluminated leaves, and the major portion of NPQ was relaxed after 120 s. Second, the extent of NPQ was increased in comparison with dark-adapted plants. Notably, the maximal extent of the qETR after preillumination matched the value of the qETR determined for dark-adapted npq2 mutants (compare with Fig. 2). The maximal increase of qETR and the fastest kinetics of qETR formation were observed after preillumination at 1,000 μmol photons m−2 s−1, as visible from the differences of the NPQ induction curves with and without preillumination (Fig. 3B). At the highest light intensity of 2,000 μmol photons m−2 s−1, the extent of qETR was even slightly reduced in comparison with the other light intensities (Fig. 3). This reduction could be based on photoinhibitory processes evolving already during this short illumination period (compare with Table II). It should be noted that the nearly maximal increase of qETR was already observable after preillumination at 300 μmol photons m−2 s−1, although the DEPS was significantly lower than at the two highest light intensities. This implies that a DEPS of roughly 40% is sufficient to induce the maximal qETR. For further experiments, we used a light intensity of 1,000 μmol photons m−2 s−1 during preillumination to allow the most pronounced acceleration of NPQ formation and to avoid any adverse effect on PSII activity at higher light intensities.
Figure 3.
NPQ formation in preilluminated wild-type plants. A, Time course of NPQ dynamics under different preillumination conditions. B, Difference between preilluminated and dark-adapted samples. The dynamics of the transient NPQ were determined in wild-type plants after preillumination for 15 min at different light intensities. Detached leaves were preilluminated for 15 min at light intensities of 300 to 2,000 μmol photons m−2 s−1 (to allow the formation of Zx) and subsequently dark adapted for 15 min (to allow the relaxation of qE without pronounced epoxidation of Zx). All other experimental conditions are as described in the legend to Figure 1. Mean values (±sd) of three to four independent experiments are shown. For clarity, sd is shown for only dark controls and samples preilluminated at a light intensity of 1,000 μmol photons m−2 s−1 but was in the similar range for the other intensities.
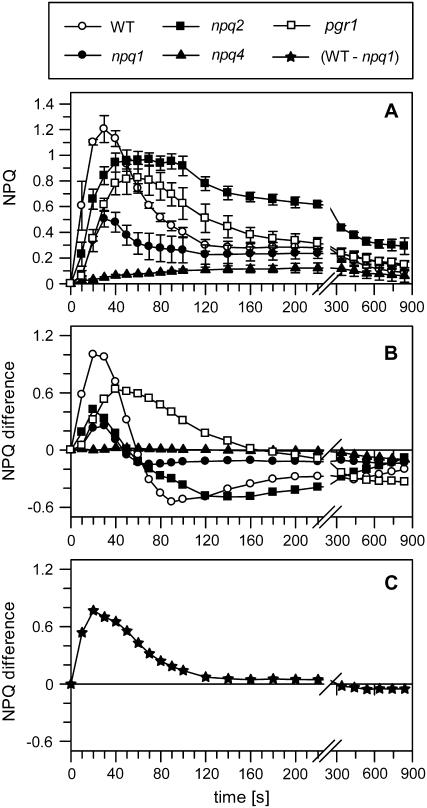
The effect of preillumination on qETR in the different mutant plants is summarized in Table III and Figure 4. In all genotypes, the PSII quantum efficiency was slightly reduced by the preillumination treatment, with the most pronounced reduction occurring in npq2 (Table III). The DEPS after preillumination, on the other hand, was found to be similar in wild type (46.3%) and npq4 (46.7%) but somewhat reduced in pgr1 (35.7%), while no changes in the DEPS were detectable for the two xanthophyll cycle mutants npq1 and npq2 (Table III). In general, an acceleration of NPQ formation was induced by preillumination in all genotypes except npq4, which showed no NPQ formation at all. Very similar to the wild type, the relaxation of qETR was accelerated by the preillumination treatment in the mutants (Fig. 4B). Like in wild-type plants, the maximum qE was induced after about 30 s in preilluminated mutant plants, but each of the mutant plants showed specific characteristics of the NPQ transient. In npq1 plants, light-induced acceleration of NPQ formation was not accompanied by an increase of the extent of qETR. Obviously, the acceleration of qETR formation is not (or not only) simply an effect of Zx accumulation, but rather reflects changes induced by the light activation of the chloroplast. In pgr1 plants, preillumination induced the appearance of a pronounced qETR (Fig. 4A), which was not observable in dark-adapted plants (Figs. 2 and 4B). Most likely, the formation of large amounts of Zx (Table III) prior to NPQ induction was responsible for this behavior. The still lower extent of qETR in pgr1 in comparison with the wild type (Fig. 4A) can be explained by the lower transthylakoid pH gradient in the mutant. An acceleration of qETR due to preillumination was also found in npq2, underlining again the Zx-independent light activation of the leaves as observed for npq1 plants. The reason for the significant reduction of the extent of qETR upon preillumination of npq2 plants from about 1.3 in dark-adapted plants (Fig. 2) to about 1.0 in preilluminated plants (Fig. 4A) is unclear. Possibly, the more pronounced reduction of the PSII quantum efficiency after preillumination in this mutant (Table II) can account for this phenomenon.
Table III.
DEPS and PSII quantum yield in preilluminated wild-type and mutant plants
Leaves were illuminated for 15 min at a light intensity of 1,000 μmol m−2 s−1 followed by a dark incubation for 15 min. The DEPS is expressed as (Zx + 0.5 Ax)/(Vx + Ax + Zx) × 100. Mean values ± sd of three independent experiments are shown. For FV/FM ratios, significant differences in comparison with the wild type are indicated by ** (P < 0.01) or * (P < 0.05).
| DEPS |
FV/FM
|
||
|---|---|---|---|
| Preilluminated | Dark Control | ||
| Wild Type | 46.3 ± 2.3 | 0.79 ± 0.01 | 0.83 ± 0.01 |
| npq1 | 0 ± 0 | 0.78 ± 0.01 | 0.83 ± 0.01 |
| npq2 | 100 ± 0 | **0.72 ± 0.01 | **0.79 ± 0.01 |
| npq4 | 46.7 ± 0.6 | **0.75 ± 0.01 | 0.83 ± 0.01 |
| pgr1 | 35.7 ± 2.0 | *0.76 ± 0.01 | 0.83 ± 0.01 |
Figure 4.
NPQ formation in preilluminated mutant plants. A, Time course of NPQ dynamics in the different genotypes. B, Difference between preilluminated (this fig.) and dark-adapted (compare with Fig. 2) samples. C, Difference between preilluminated wild-type and npq1 plants. Detached leaves were preilluminated for 15 min at a light intensity of 1,000 μmol photons m−2 s−1 and subsequently dark adapted for 15 min. After this treatment, FV/FM ratios were slightly reduced in comparison with dark-adapted samples, but similar levels of Zx were found in wild-type, npq4, and pgr1 plants (compare with Table III). NPQ formation was induced by illumination of the leaves at a light intensity of 100 μmol photons m−2 s−1. All other experimental conditions are as described in the legend to Figure 1. Mean values (± sd) of three to four independent experiments are shown.
Nevertheless, the increase of qETR in preilluminated wild-type plants to the level of dark-adapted npq2 plants (Fig. 2) indicates that the generation of qETR in dark-adapted wild-type plants (Fig. 2) was indeed limited by the rate of Zx synthesis. On the other hand, the complete absence of the transient qETR in preilluminated npq4 plants clearly shows that qETR is essentially dependent on the presence of the PsbS protein. The still-reduced magnitude of qETR in pgr1 and npq1 plants in comparison with the wild type can then be understood by the regulation of qETR by both the transthylakoid pH gradient (partly reduced in pgr1) and Zx (completely absent in npq1). The still-detectable qETR in npq1 plants thus represents a Zx-independent portion of qETR, while the difference between the NPQ transients of wild-type and npq1 plants (Fig. 4C) refers to the portion of qETR that is modulated by Zx.
NPQ Formation in the Presence of Nigericin and Dithiothreitol
We further investigated the role of the ΔpH and Zx by eliminating lumen acidification (incubation with nigericin; Fig. 5A) and Zx synthesis (incubation with dithiothreitol [DTT]; Fig. 5B). In control experiments with wild-type and mutant plants that were treated in the same way but infiltrated with water, no differences were detectable between untreated samples and water-infiltrated samples (data not shown), so that any adverse effect due to the infiltration procedure can be excluded. In the absence of any transthylakoid pH gradient, qETR was completely abolished in all genotypes (Fig. 5A), underlining the essential role of the lumen pH for the generation of the transient NPQ. In the presence of DTT, on the other hand, qETR was differently effected in each of the plants (Fig. 5B). HPLC analysis of the pigment content confirmed that Zx synthesis was completely blocked in all genotypes under these experimental conditions (data not shown). While no effects on the NPQ transients were observed for DTT-treated npq1 and npq4 plants in comparison with the respective untreated control (compare with Fig. 2), a strong reduction of NPQ formation was found in the other genotypes. DTT-treated wild-type plants showed exactly the same characteristics of qETR as npq1 plants (in the absence and presence of DTT), supporting the assumption that the observed differences between untreated wild-type and npq1 plants (Fig. 2) were indeed related to the generally inhibited Zx synthesis in npq1. The complete absence of NPQ in DTT-treated pgr1 plants indicates that the slow rise of NPQ in dark-adapted pgr1 leaves (compare with Fig. 2) was related to slow Zx formation. Unexpectedly, however, we also found a strong reduction of qETR in npq2 upon DTT treatment. Because the activity of the Vx de-epoxidase should be negligible due to the absence of any substrate in this mutant, the reduction might be based on an unspecific side effect of DTT in npq2. We can conclude from these experiments that the generation of qETR essentially requires a transthylakoid pH gradient, while the extent of qETR is modulated at a given ΔpH by the Zx content.
Figure 5.
The effect of nigericin and DTT on NPQ formation. The dynamics of the transient NPQ were determined in wild-type and NPQ-mutant plants in the presence of 50 μm nigericin (A) and 5 mm DTT (B). Uptake of inhibitors was performed by vacuum infiltration. NPQ formation was induced by illumination of the leaves at a light intensity of 100 μmol photons m−2 s−1. All other conditions are as described in the legend to Figure 1. Mean values (±sd) of three to four independent experiments are shown. For clarity, sd is shown for only wild-type plants in A but was in the similar range for all other genotypes. In B, sd for npq1 plants is not shown but was in the same range as shown for wild-type plants. It should be noted that the scale of the y axis is spread by a factor of 2 in comparison with all other figures.
DISCUSSION
Our data provide clear evidence that the qETR, which is generated upon the transition of dark-adapted leaves to low light, is modulated by Zx. This conclusion can be derived from the following specific features of qETR in comparison with the qETR in dark-adapted wild-type plants: (1) the reduced qETR in the Zx-deficient npq1 mutant; (2) the increased qETR in the Zx-accumulating npq2 mutant; (3) the increased and accelerated qETR in preilluminated wild-type plants; and (4) the reduced qETR in the presence of DTT. These results are in line with the proposed role of epoxidized xanthophylls in qETR (D'Haese et al., 2004) but in clear contrast to the recently suggested Zx-independent nature of qETR (Finazzi et al., 2004). The most striking difference from the former work by Finazzi et al. (2004) was the detection of reasonable amounts of Zx formed in the wild type during the first 2 min of illumination of the leaves at 100 μmol photons m−2 s−1. It remains to be tested whether these differences are related to different illumination conditions (green light was used by Finazzi et al. [2004], while white light was used here) or different plant material (barley in the earlier work and Arabidopsis in this study). Preliminary studies with Arabidopsis wild-type plants indicated, however, that reasonable amounts of Zx were also formed upon illumination with green light, although the rate of Zx formation was reduced by a factor of about 2 in comparison with white light (data not documented). It is thus more likely that the contradictory results are mainly related to the different plant material.
Independent of these discrepancies, we have to conclude from our data that qETR shows identical characteristics to the steady-state qE formed at saturating light intensities; it is strictly dependent on the presence of the PsbS protein and a transthylakoid pH gradient and is modulated dependent on the amount of de-epoxidized xanthophylls. Therefore, it is very unlikely that qETR represents a specific quenching process in the reaction center of PSII but rather reflects the response of the antenna-related qE to the dynamics of the lumen pH during the induction of photosynthesis upon illumination of dark-adapted plants.
Kinetics of qE Formation
The comparison of the dynamics of NPQ in dark-adapted and preilluminated wild-type plants (Fig. 3B) showed that the kinetics of NPQ formation is significantly accelerated after preillumination of leaves. Our data further indicated that preillumination has at least two distinct effects on NPQ transients at low light intensities. First, it provides the formation of large amounts of Zx, and, second, it leads to the light activation of the chloroplast. The pronounced acceleration of NPQ formation after preillumination in comparison with dark-adapted plants is obviously not only related to the light-induced formation of large amounts of Zx because the Zx-deficient npq1 mutant and the Zx-enriched npq2 mutant also showed the same acceleration as the wild type. Thus, only the magnitude of qETR seems to be modified by the Zx content or the DEPS, while the kinetics of NPQ formation may be determined by the rate of lumen acidification. The more rapid lumen acidification in preilluminated plants can then be understood by the light activation of photosynthetic electron transport. It is known from earlier work that the kinetics of the induction of photosynthetic carbon assimilation (and thus of electron transport) and also of Chl fluorescence is strongly dependent on the preillumination of leaves (e.g. van der Veen, 1951; Walker, 1981; Demmig-Adams et al., 1989; Ruban and Horton, 1999). In dark-adapted plants, the rate of NPQ formation is therefore limited by both the rate of lumen acidification and the rate of Zx formation.
This interpretation is also supported by the results obtained with the pgr1 mutant. In dark-adapted plants, qE formation is strongly retarded in pgr1 (Fig. 2) due to the limited capacity of lumen acidification (Jahns et al., 2002), which leads not only to a reduced pH gradient across the membrane but also slows down the formation of Zx. Even in presence of large amounts of Zx after preillumination (DEPS of about 36%; Table III), the lower rate of lumen acidification in pgr1 is reflected by the slower qE formation in preilluminated pgr1 in comparison with wild-type plants (Fig. 4). Because a DEPS of about 40% was found to be sufficient to induce rapid formation of the maximum qE in wild-type plants (Fig. 3; Table II), we conclude that even after preillumination the lower rate of lumen acidification is limiting NPQ formation in pgr1 plants. This conclusion is further supported by the fact that the pgr1 mutant is not only affected in linear electron transport (Jahns et al., 2002) but also in ferredoxin-dependent cyclic electron transport (Okegawa et al., 2005), which has been shown to contribute significantly to lumen acidification during the induction phase of photosynthesis (Slovacek et al., 1980).
The crucial role of the rate of lumen acidification for the kinetics of NPQ formation is also obvious from the data obtained with npq2 plants. Strikingly, NPQ formation in dark-adapted and preilluminated plants seemed to be slightly slower (but definitely not faster) in npq2 when compared with the wild type (Figs. 2 and 4), although maximal amounts of Zx are present in npq2 under all conditions. The even slower rate of NPQ formation in npq2 might reflect the less efficient utilization of absorbed light energy due to sustained heat dissipation of excitation energy (Kalituho et al., 2007), resulting in a reduced electron transport rate and by that of lumen acidification in npq2 (Table I).
Kinetics of qE Relaxation
In general, the relaxation of qETR in dark-adapted leaves was rather slow in all genotypes (Fig. 2). Only about 50% of the amplitude of NPQ relaxed within about 5 min of illumination in wild-type plants, while the relaxing portion was even lower in npq1 and npq2. Due to the strict dependence of NPQ on the lumen pH, this slow relaxation must be understood as a slow rate of proton consumption upon the dark-to-light transition of dark-adapted plants at a light intensity of 100 μmol photons m−2 s−1. For Arabidopsis wild-type plants, similar kinetics of NPQ relaxation have been determined by Finazzi et al. (2004), while a much faster relaxation of qE was observed for barley leaves in the same study. Thus, the characteristics of qETR relaxation seem to be strongly dependent on the plant material. We cannot decide whether these differences represent specific properties of the two species or rather may be related to different developmental stages of the plants. It should be noted, however, that both the kinetics of qETR formation and relaxation were found to be strongly dependent on the age of the plants in Arabidopsis. In general, the kinetics became slower with increasing age of the plants (data not documented).
Notably, NPQ relaxation was found to be strongly accelerated by preillumination. In preilluminated wild-type and npq1 plants, the major portion of qE was reversible within 120 s after onset of illumination. Obviously, preillumination led to the activation of proton-consuming reactions in these two genotypes, most likely by light activation of the ATP synthase. The only very slowly relaxing portion of qETR in these two genotypes, on the other hand, may be related to a residual small transthylakoid pH gradient that is permanently present under these experimental conditions. The generally slower relaxation of qETR in preilluminated pgr1 and npq2 plants has then to be explained by the generally reduced proton consumption in these two mutants. It is reasonable to assume that the lower electron transport rates in these mutants account for these differences. Either light activation of ATP synthesis is limited in pgr1 and npq2, or the light-induced ΔpH is not sufficient to drive rapid proton consumption in these two mutants.
Energy Dissipation and the Role of Zx
Although the specific role of Zx in energy dissipation is still under discussion (Holt et al., 2005; Horton et al., 2005; Niyogi et al., 2005; Demmig-Adams and Adams, 2006), it is undoubtedly accepted that Zx is required for the generation of the maximum steady-state qE. In various studies, a clear correlation of the amount of Zx (and Ax) and the extent of qE has been shown (e.g. Demmig-Adams et al., 1990; Gilmore and Yamamoto, 1993; D'Haese et al., 2004), but recent work brought evidence that only a few molecules of Zx per PSII are essentially required for qE (Bukhov et al., 2001; Ruban et al., 2002). Our data show that the major portion of the qETR in Arabidopsis wild-type plants can also be generated in the presence of rather low amounts of Zx (DEPS of less than 10%), which are formed during the first 2 min of illumination at low light intensities (Fig. 1; Table I). A further increase of the DEPS to about 45% (as in preilluminated wild-type plants; Fig. 3; Table II) was accompanied by only a small increase of the extent of qETR. This indicates that no linear correlation between the extent of qETR and the DEPS or Zx content exists, at least at higher Zx concentrations, under our experimental conditions. It can therefore be suggested that only a small pool of Zx is involved in qETR. However, more detailed quantitative analyses would be required to clarify this point.
The residual Zx-independent qE that is detectable in npq1 plants from Arabidopsis has been shown to vary from about 10% to 20% (Niyogi et al., 1998; Pogson and Rissler, 2000; Kalituho et al., 2007) up to nearly 50% (Dall'Osto et al., 2005) of the respective wild-type levels. Interestingly, absolute values of qE in npq1 were in all cases in the range from 0.2 to 0.4 in those former studies, which is similar to the magnitude of the transient qETR determined in this work for npq1 (Figs. 2 and 4). Moreover, qE formation in npq1 occurred very rapidly within about 1 min in those former studies. It can thus be speculated that the residual Zx-independent qE in npq1 is identical with the Zx-independent portion of the transient qETR. The extent of this rapidly formed Zx-independent portion of qE has been shown to depend not only on the lumen pH but also on the amount of the PsbS protein (Crouchman et al., 2006), like the overall NPQ (Li et al., 2002a; Crouchman et al., 2006). Horton et al. (2005) put forward an allosteric model of NPQ in which both PsbS and Zx act as indirect regulators of a quenching site in the antenna of PSII. Indeed, all findings in this work are consistent with this allosteric model of NPQ. It is thus very likely that both components of qE, the steady state and the qETR, are based on the same quenching mechanism in the antenna of PSII.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Wild-type and mutant Arabidopsis (Arabidopsis thaliana) ecotype Columbia-0 plants were grown in soil at a light intensity of 150 μmol photons m−2 s−1 and a constant temperature of 20°C under long-day conditions (14 h light/10 h dark). The following mutants were used: npq1 and npq2 (Niyogi et al., 1998), pgr1 (Munekage et al., 2001), and psbs-1.3 (Grasses et al., 2002). For the latter mutant, which is allelic to the PsbS-deficient npq4 mutant (Li et al., 2000), the more common name npq4 is used throughout the article.
Fluorescence Measurements
Room temperature Chl a fluorescence was measured under in vivo conditions at 20°C using a pulse-amplitude-modulated fluorometer (PAM 101, Walz). For quenching analyses, leaves were illuminated for 14 min at indicated intensities of white actinic light, followed by 10 min of dark incubation. For the determination of the induction of qE, saturating white light pulses (2,000 μmol photons m−2 s−1, duration 1 s) were applied every 10 s during the first 100 s of illumination, followed by seven flashes given every 20 s and six flashes every 100 s. The relaxation of qE was determined by saturating light pulses spaced 100 s during 10 min of dark incubation. Stern-Volmer type of NPQ was calculated as described by Krause and Jahns (2004).
Electron transport rates through PSII, JF, at each of the saturating light pulses during the illumination period were estimated from the quantum efficiency of PSII-driven electron transport, ΦPSII, (Genty et al., 1989), according to the equation
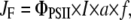 |
where I is the incident photosynthetically active radiation (mol photons m−2 s−1), a is the fraction of light absorbed by the leaf, and f the fraction of absorbed light energy distributed to PSII (Krall and Edwards, 1992). Calculations were based on the assumption of an even distribution of excitation energy between PSII and PSI, i.e. f = 0.5, as given for mature leaves of C3 plants, and that a = 0.84 (see Krause and Jahns [2003] for further details).
Pigment Analysis
For pigment analysis, leaves were frozen in liquid N2 and stored at −80°C. Pigments were extracted with acetone and quantified by reverse-phase HPLC (Färber et al., 1997).
Infiltration with Nigericin and DTT
To induce the uptake of nigericin or DTT, leaf discs were vacuum infiltrated in a syringe containing 50 μm nigericin or 5 mm DTT, respectively. After infiltration, leaf discs were incubated in the same solutions for 10 min in the dark.
This work was supported by the Deutsche Forschungsgemeinschaft (grant nos. Ja 665/2–4 and SFB 663, TPB2).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Peter Jahns (pjahns@uni-duesseldorf.de).
References
- Allen JF (1992) Protein phosphorylation in regulation of photosynthesis. Biochim Biophys Acta 1098 275–335 [DOI] [PubMed] [Google Scholar]
- Aro E-M, Virgin I, Andersson B (1993) Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta 1143 113–134 [DOI] [PubMed] [Google Scholar]
- Bellafiore S, Bameche F, Peltier G, Rochaix JD (2005) State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433 892–895 [DOI] [PubMed] [Google Scholar]
- Bonardi V, Pesaresi P, Becker T, Schleiff E, Wagner R, Pfannschmidt T, Jahns P, Leister D (2005) Photosystem II core phosphorylation and photosynthetic acclimation require two different protein kinases. Nature 437 1179–1182 [DOI] [PubMed] [Google Scholar]
- Bukhov NG, Kopecky J, Pfündel EE, Klughammer C, Heber U (2001) A few molecules of zeaxanthin per reaction centre of photosystem II permit effective thermal dissipation of light energy in photosystem II of a poikilohydric moss. Planta 212 739–748 [DOI] [PubMed] [Google Scholar]
- Crouchman S, Ruban A, Horton P (2006) PsbS enhances nonphotochemical fluorescence quenching in the absence of zeaxanthin. FEBS Lett 580 2053–2058 [DOI] [PubMed] [Google Scholar]
- D'Haese D, Vandermeiren K, Caubergs RJ, Guisez Y, De Temmerman L, Horemans N (2004) Non-photochemical quenching kinetics during the dark to light transition in relation to the formation of antheraxanthin and zeaxanthin. J Theor Biol 227 175–186 [DOI] [PubMed] [Google Scholar]
- Dall'Osto L, Caffarri S, Bassi R (2005) A mechanism of nonphotochemical energy dissipation, independent from PsbS, revealed by a conformational change in the antenna protein CP26. Plant Cell 17 1217–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW (2006) Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation. New Phytol 172 11–21 [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW, Heber U, Neimanis S, Winter K, Krüger A, Czygan F-C, Bilger W, Björkman O (1990) Inhibition of zeaxanthin formation and of rapid changes in radiationless energy dissipation by dithiothreitol in spinach leaves and chloroplasts. Plant Physiol 92 293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig-Adams B, Winter K, Krüger A, Czygan FC (1989) Zeaxanthin and the induction and relaxation kinetics of the dissipation of excess excitation-energy in leaves in 2% O2, 0% CO2. Plant Physiol 90 887–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbert V, Adams WW, Mattoo AK, Sokolenko A, Demmig-Adams B (2005) Up-regulation of a photosystem II core protein phosphatase inhibitor and sustained D1 phosphorylation in zeaxanthin-retaining, photoinhibited needles of overwintering Douglas fir. Plant Cell Environ 28 232–240 [Google Scholar]
- Ebbert V, Demmig-Adams B, Adams WWI, Mueh KE, Staehelin LA (2001) Correlation between persistent forms of zexanthin-dependent energy dissipation and thylakoid protein phosphorylation. Photosynth Res 67 63–78 [DOI] [PubMed] [Google Scholar]
- Färber A, Young AJ, Ruban AV, Horton P, Jahns P (1997) Dynamics of xanthophyll-cycle activity in different antenna subcomplexes in the photosynthetic membranes of higher plants: the relationship between zeaxanthin conversion and nonphotochemical fluorescence quenching. Plant Physiol 115 1609–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finazzi G, Johnson GN, Dallosto L, Joliot P, Wollman FA, Bassi R (2004) A zeaxanthin-independent nonphotochemical quenching mechanism localized in the photosystem II core complex. Proc Natl Acad Sci USA 101 12375–12380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990 87–92 [Google Scholar]
- Gilmore AM, Yamamoto HY (1993) Linear models relating xanthophylls and lumen acidity to non- photochemical fluorescence quenching. Evidence that antheraxanthin explains zeaxanthin-independent quenching. Photosynth Res 35 67–78 [DOI] [PubMed] [Google Scholar]
- Grasses T, Pesaresi P, Schiavon F, Varotto C, Salamini F, Jahns P, Leister D (2002) The role of Delta pH-dependent dissipation of excitation energy in protecting photosystem II against light-induced damage in Arabidopsis thaliana. Plant Physiol Biochem 40 41–49 [Google Scholar]
- Haldrup A, Jensen PE, Lunde C, Scheller HV (2001) Balance of power: a view of the mechanism of photosynthetic state transitions. Trends Plant Sci 6 301–305 [DOI] [PubMed] [Google Scholar]
- Hamel P, Olive J, Pierre Y, Wollman FA, de Vitry C (2000) A new subunit of cytochrome b(6)f complex undergoes reversible phosphorylation upon state transition. J Biol Chem 275 17072–17079 [DOI] [PubMed] [Google Scholar]
- Holt NE, Zigmantas D, Valkunas L, Li XP, Niyogi KK, Fleming GR (2005) Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science 307 433–436 [DOI] [PubMed] [Google Scholar]
- Horton P (1983) Effects of changes in the capacity for photosynthetic electron transfer and photophosphorylation on the kinetics of fluorescence induction in isolated chloroplasts. Biochim Biophys Acta 724 404–410 [Google Scholar]
- Horton P, Ruban AV, Walters RG (1996) Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol 47 655–684 [DOI] [PubMed] [Google Scholar]
- Horton P, Ruban AV, Wentworth M (2000) Allosteric regulation of the light-harvesting system of photosystem II. Philos Trans R Soc Lond B Biol Sci 355 1361–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P, Wentworth M, Ruban A (2005) Control of the light harvesting function of chloroplast membranes: the LHCII-aggregation model for non-photochemical quenching. FEBS Lett 579 4201–4206 [DOI] [PubMed] [Google Scholar]
- Jahns P, Graf M, Munekage Y, Shikanai T (2002) Single point mutation in the Rieske iron-sulfur subunit of cytochrome b(6)/f leads to an altered pH dependence of plastoquinol oxidation in Arabidopsis. FEBS Lett 519 99–102 [DOI] [PubMed] [Google Scholar]
- Jahns P, Miehe B (1996) Kinetic correlation of recovery from photoinhibition and zeaxanthin epoxidation. Planta 198 202–210 [Google Scholar]
- Kalituho L, Grasses T, Graf M, Rech J, Jahns P (2006) Characterization of a nonphotochemical quenching-deficient Arabidopsis mutant possessing an intact PsbS protein, xanthophyll cycle and lumen acidification. Planta 223 532–541 [DOI] [PubMed] [Google Scholar]
- Kalituho L, Rech J, Jahns P (2007) The role of specific xanthophylls in light utilization. Planta 225 423–439 [DOI] [PubMed] [Google Scholar]
- Krall JP, Edwards GE (1992) Relationship between photosystem-II activity and CO2 fixation in leaves. Physiol Plant 86 180–187 [Google Scholar]
- Krause GH (1988) Photoinhibition of photosynthesis. An evaluation of damaging and protective mechanisms. Physiol Plant 74 566–574 [Google Scholar]
- Krause GH, Jahns P (2003) Pulse amplitude modulated fluorometry and its application in plant science. In BR Green, W Parson, eds, Light Harvesting Antennas in Photosynthesis. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 373–399
- Krause GH, Jahns P (2004) Non-photochemical energy dissipation determined by chlorophyll fluorescence quenching: characterization and function. In GC Papageorgiou, Govindjee, eds, Chlorophyll a Fluorescence: A Signature of Photosynthesis. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 463–495
- Krause GH, Vernotte C, Briantais J-M (1982) Photoinduced quenching of chlorophyll fluorescence in intact chloroplasts and algae. Resolution into two components. Biochim Biophys Acta 679 116–124 [Google Scholar]
- Li X-P, Björkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK (2000) A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403 391–395 [DOI] [PubMed] [Google Scholar]
- Li XP, Gilmore AM, Caffarri S, Bassi R, Golan T, Kramer D, Niyogi KK (2004) Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J Biol Chem 279 22866–22874 [DOI] [PubMed] [Google Scholar]
- Li XP, Müller-Moule P, Gilmore AM, Niyogi KK (2002. a) PsbS-dependent enhancement of feedback de-excitation protects photosystem II from photoinhibition. Proc Natl Acad Sci USA 99 15222–15227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XP, Phippard A, Pasari J, Niyogi KK (2002. b) Structure-function analysis of photosystem II subunit S (PsbS) in vivo. Funct Plant Biol 29 1131–1139 [DOI] [PubMed] [Google Scholar]
- Munekage Y, Takeda S, Endo T, Jahns P, Hashimoto T, Shikanai T (2001) Cytochrome b(6)f mutation specifically affects thermal dissipation of absorbed light energy in Arabidopsis. Plant J 28 351–359 [DOI] [PubMed] [Google Scholar]
- Niyogi KK, Grossman AR, Björkman O (1998) Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10 1121–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK, Li XP, Rosenberg V, Jung HS (2005) Is PsbS the site of non-photochemical quenching in photosynthesis? J Exp Bot 56 375–382 [DOI] [PubMed] [Google Scholar]
- Okegawa Y, Tsuyama M, Kobayashi Y, Shikanai T (2005) The pgr1 mutation in the Rieske subunit of the cytochrome b(6)f complex does not affect PGR5-dependent cyclic electron transport around photosystem I. J Biol Chem 280 28332–28336 [DOI] [PubMed] [Google Scholar]
- Pascal AA, Liu ZF, Broess K, van Oort B, van Amerongen H, Wang C, Horton P, Robert B, Chang WR, Ruban A (2005) Molecular basis of photoprotection and control of photosynthetic light-harvesting. Nature 436 134–137 [DOI] [PubMed] [Google Scholar]
- Pogson BJ, Rissler HM (2000) Genetic manipulation of carotenoid biosynthesis and photoprotection. Philos Trans Rl Soc Lond B Biol Sci 355 1395–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintamäki E, Martinsuo P, Pursiheimo S, Aro EM (2000) Cooperative regulation of light-harvesting complex II phosphorylation via the plastoquinol and ferredoxin-thioredoxin system in chloroplasts. Proc Natl Acad Sci USA 97 11644–11649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruban AV, Horton P (1999) The xanthophyll cycle modulates the kinetics of nonphotochemical energy dissipation in isolated light-harvesting complexes, intact chloroplasts, and leaves of spinach. Plant Physiol 119 531–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruban AV, Pascal AA, Robert B, Horton P (2002) Activation of zeaxanthin is an obligatory event in the regulation of photosynthetic light harvesting. J Biol Chem 277 7785–7789 [DOI] [PubMed] [Google Scholar]
- Slovacek RE, Crowther D, Hind G (1980) Relative activities of linear and cyclic electron flows during chloroplast Co2-fixation. Biochim Biophys Acta 592 495–505 [DOI] [PubMed] [Google Scholar]
- Slovacek RE, Hind G (1981) Correlation between photosynthesis and the transthylakoid proton gradient. Biochim Biophys Acta 635 393–404 [DOI] [PubMed] [Google Scholar]
- Thiele A, Schirwitz K, Winter K, Krause GH (1996) Increased xanthophyll cycle activity and reduced D1 protein inactivation related to photoinhibition in two plant systems acclimated to excess light. Plant Sci 115 237–250 [Google Scholar]
- van der Veen R (1951) Fluorescence and induction phenomena in photosynthesis. Physiol Plant 4 486–494 [Google Scholar]
- Verhoeven AS, Adams WWI, Demmig-Adams B (1996) Close relationship between the state of the xanthophyll cycle pigments and photosystem II efficiency during recovery from winter stress. Physiol Plant 96 567–576 [Google Scholar]
- Walker DA (1981) Secondary fluorescence kinetics of spinach leaves in relation to the onset of photosynthetic carbon assimilation. Planta 153 273–278 [DOI] [PubMed] [Google Scholar]
- Zarter CR, Adams WW, Ebbert V, Adamska I, Jansson S, Demmig-Adams B (2006) Winter acclimation of PsbS and related proteins in the evergreen Arctostaphylos uva-ursi as influenced by altitude and light environment. Plant Cell Environ 29 869–878 [DOI] [PubMed] [Google Scholar]