Abstract
The mutualistic interaction in arbuscular mycorrhiza (AM) is characterized by an exchange of mineral nutrients and carbon. The major benefit of AM, which is the supply of phosphate to the plant, and the stimulation of mycorrhization by low phosphate fertilization has been well studied. However, less is known about the regulatory function of carbon availability on AM formation. Here the effect of enhanced levels of hexoses in the root, the main form of carbohydrate used by the fungus, on AM formation was analyzed. Modulation of the root carbohydrate status was performed by expressing genes encoding a yeast (Saccharomyces cerevisiae)-derived invertase, which was directed to different subcellular locations. Using tobacco (Nicotiana tabacum) alc∷cwINV plants, the yeast invertase was induced in the whole root system or in root parts. Despite increased hexose levels in these roots, we did not detect any effect on the colonization with Glomus intraradices analyzed by assessment of fungal structures and the level of fungus-specific palmitvaccenic acid, indicative for the fungal carbon supply, or the plant phosphate content. Roots of Medicago truncatula, transformed to express genes encoding an apoplast-, cytosol-, or vacuolar-located yeast-derived invertase, had increased hexose-to-sucrose ratios compared to β-glucuronidase-transformed roots. However, transformations with the invertase genes did not affect mycorrhization. These data suggest the carbohydrate supply in AM cannot be improved by root-specifically increased hexose levels, implying that under normal conditions sufficient carbon is available in mycorrhizal roots. In contrast, tobacco rolC∷ppa plants with defective phloem loading and tobacco pyk10∷InvInh plants with decreased acid invertase activity in roots exhibited a diminished mycorrhization.
Arbuscular mycorrhiza (AM) represents a widespread mutualistic association between soil-born fungi of the phylum Glomeromycota and most land plants. The AM interaction enables the plant to improve its supply of water and mineral nutrients, mainly phosphate. In return, the obligate biotrophic AM fungi are provided with carbon. In the Arum-type interaction, as analyzed here between Glomus sp. and tobacco (Nicotiana tabacum) or Medicago truncatula, the AM fungus colonizes the cortical cells by formation of intra- and intercellular hyphae and very characteristic haustoria-like structures, the highly branched intracellular arbuscules. When the carbon supply is sufficient, lipid-rich vesicles are formed intercellularly within the cortex, as fungal storage organs. Intracellular fungal structures are separated from the plant cytoplasm by an extension of the plasma membrane, forming the periarbuscular membrane surrounding the arbuscule. The greatly increased surfaces of host and arbuscule plasma membranes offers optimized conditions for effective nutrient exchange via the established symbiotic interface (for review, see Gianinazzi-Pearson et al., 1996; Harrison, 1999).
The exchange of phosphate via the periarbuscular interface and the induction of AM-specific phosphate transporters are already well characterized (Karandashov and Bucher, 2005; Requena, 2005). The same holds true for the regulation of AM by the phosphate availability for the plant. High levels of available phosphate (Mosse, 1973; Jasper et al., 1979) as well as the inhibition of phosphate exchange by the knockout or down-regulation of AM-inducible phosphate transporters (Maeda et al., 2006; Javot et al., 2007) can suppress the mycorrhization. In contrast, there are only limited data about regulation of AM formation by the carbon aspect of this interaction, although previous studies revealed a link between phosphate and carbon availability. Analysis of monoxenic AM cultures of carrot (Daucus carota) roots fed with isotopically labeled carbon indicated an increased carbon supply of the fungus in roots that were undersupplied with phosphate (Olsson et al., 2002). Moreover, higher carbon availability was shown to stimulate the phosphate allocation (Bücking and Shachar-Hill, 2005). Conversely, limiting light intensity reduced mycorrhization and lead to decreased growth and phosphate uptake (Hayman, 1974; Tester et al., 1985; Son and Smith, 1988).
The fungal carbon uptake and metabolism has intensively been studied using isotopic-labeled substrates (for review, see Bago et al., 2000). Hexoses, in particular Glc, were found to be the major form in which carbon is taken up and metabolized by AM fungi (Solaiman and Saito, 1997; Pfeffer et al., 1999; Douds et al., 2000). Glc can then be directly incorporated into trehalose and glycogen, the first substantial fungal carbon pool (Shachar-Hill et al., 1995). Later on, storage lipids are synthesized. The most abundant form of lipid in AM fungi are triacylglycerols, which also serve as a carbon transport form in the fungus (Bago et al., 2002). In the symbiotic stage, carbohydrates can only be taken up within intraradical structures, no uptake of hexoses could be detected by the extraradical mycelium (Pfeffer et al., 1999; Douds et al., 2000). The high labeling levels in fungal metabolites suggested a direct uptake of isotopic-labeled hexoses from the root apoplast without dilution with the hexoses of the host sugar pool (Shachar-Hill et al., 1995; Douds et al., 2000). The uptake of carbon via the arbuscules is anticipated inter alia by the optimized formation and localization of these highly branched structures for nutrient exchange (for review, see Blee and Anderson, 1998). Moreover, the incorporation of isotopically labeled Glc into trehalose and glycogen was found to depend on the presence of arbuscules (Pfeffer and Shachar-Hill, 1996) and the latter could be correlated with spore formation (Douds, 1994). However, isolated intraradical hyphae showed efficient uptake of Glc, suggesting the putative involvement of hyphae in carbon uptake as well (Solaiman and Saito, 1997).
In addition to labeling experiments, the exchange of carbon in the form of hexoses is further supported by transcript accumulation of a mycorrhiza-induced hexose transporter from M. truncatula in colonized root areas (Harrison, 1996). This suggests an active transport of hexoses into and/or out of colonized cells driven by H+ gradient generated by plasma membrane-located H+-ATPases, which were found to be induced upon mycorrhization (Marx et al., 1982; Gianinazzi-Pearson et al., 1991; Ferrol et al., 2002; Krajinski et al., 2002). The active hexose transport would supplement the potential passive efflux of carbohydrates through the plant plasma membrane into the symbiotic interface. The subsequent uptake of carbon by the AM fungus in form of hexoses is supported by the isolation of a fungal monosaccharide transporter from a member of the Glomeromycota that undergoes AM-like endosymbiosis with cyanobacteria (Schüssler et al., 2006). The presence of orthologous proteins from other glomeromycotan fungi within the arbuscular plasma membrane is very likely. Another indication of fungal uptake of hexoses is given by increased transcript and activity levels of plant Suc-cleaving enzymes in mycorrhizal roots (Wright et al., 1998; Blee and Anderson, 2002; Hohnjec et al., 2003; Ravnskov et al., 2003; Schubert et al., 2003; Schaarschmidt et al., 2006).
In plants, Suc is the major transport form of photosynthetically fixed carbon to sink organs. Utilization of Suc requires cleavage that can either be performed by cytosolic Suc synthases, producing UDP-Glc and Fru, or invertases, producing Glc and Fru. Plant invertases can be classified by their subcellular location and their pH optima into three groups: (1) acidic cell wall-bound apoplastic invertases, (2) acidic soluble vacuolar invertases, and (3) alkaline soluble cytosolic invertases (Tymowska-Lalanne and Kreis, 1998; Roitsch and González, 2004). No AM-fungal Suc-cleaving enzymes have been identified so far, suggesting that plant invertases and Suc synthases play an important role in delivering hexoses to the fungal partner. Particularly, extracellular invertases have a key function in supporting increasing sink strength, a feature of mycorrhizal roots, by phloem unloading (Godt and Roitsch, 1997; Tymowska-Lalanne and Kreis, 1998; Roitsch et al., 2003) and they may directly deliver utilizable carbohydrates to the apoplastic fungal structures. Increased transcript and activity levels of apoplastic invertases in mycorrhizal roots, which are particularly detectable at stages with high carbohydrate demand, corroborate this assumption (Wright et al., 1998; Schaarschmidt et al., 2006).
To analyze the regulatory function of the carbon supply during AM, this study focuses on the modulation of the carbohydrate status of the plant by root-specific overexpression of invertases, particularly an apoplast-located invertase. Increased extracellular hexose levels, achieved by an a priori enhanced apoplastic invertase activity in the root, might cause several alterations in the formation of the AM interaction. On the one hand, establishing a higher sink strength in the root before colonization by the AM fungus and the increase of available carbon might stimulate the mycorrhization and enhance the benefit to the plant as described for the phosphate supply (Bücking and Shachar-Hill, 2005). On the other hand, the supply of the AM fungus with hexoses for free could result in an excessive colonization, changing a mutualistic association into a parasitic one. If the fungus is able to take up hexoses via intraradical hyphae, as shown for isolated hyphae (Solaiman and Saito, 1997), the number of arbuscules might decrease, indicating that the fungus does not fulfill its part in the symbiosis any longer. Another possibility might include activation of defense-related mechanisms in the plant root via hexose sensing (Herbers et al., 1996; Rolland et al., 2002; Roitsch et al., 2003), which can interfere with the mutualistic interaction, resulting in diminished mycorrhization.
To study the impact of altered hexose availability on mycorrhization of tobacco or M. truncatula two general approaches were followed. To increase root hexose content, yeast (Saccharomyces cerevisiae)-derived invertase(s) were expressed either under control of a chemically inducible promoter in tobacco or in hairy roots of M. truncatula following Agrobacterium rhizogenes transformation. Decreased root hexose content was achieved by either expression of a phloem-specific Escherichia coli inorganic pyrophosphatase or root-specific expression of Arabidopsis (Arabidopsis thaliana) invertase inhibitor.
Here we report that an elevated root hexose content does not alter mycorrhization of tobacco or M. truncatula roots, indicating sufficient carbon supply in normal growth conditions. Reducing assimilate supply of roots as consequence of reduced phloem loading of photoassimilates, however, strongly decreases AM growth. By specifically inhibiting root invertase activity the requirement of sufficient hexose supply for AM growth could be documented.
RESULTS
Effect of Root-Specific Enhancement of Apoplastic Invertase Activity on AM Formation
To increase the carbon availability in the root and to analyze its role in the formation of AM, we used transgenic tobacco plants expressing a chimeric gene encoding a yeast-derived invertase that is translocated to the apoplast. In these NT (tobacco) alc∷cwINV plants, the expression is under the control of the alcohol-inducible (alc) promoter system derived from Aspergillus nidulans. The alc promoter can easily be activated in roots and also in root parts by the specific application of low concentrations of acetaldehyde (Schaarschmidt et al., 2004). Drenching with 0.05% (v/v) acetaldehyde at weekly intervals results in long-lasting induction of the invertase and does not apparently affect plant vigor (Schaarschmidt et al., 2004).
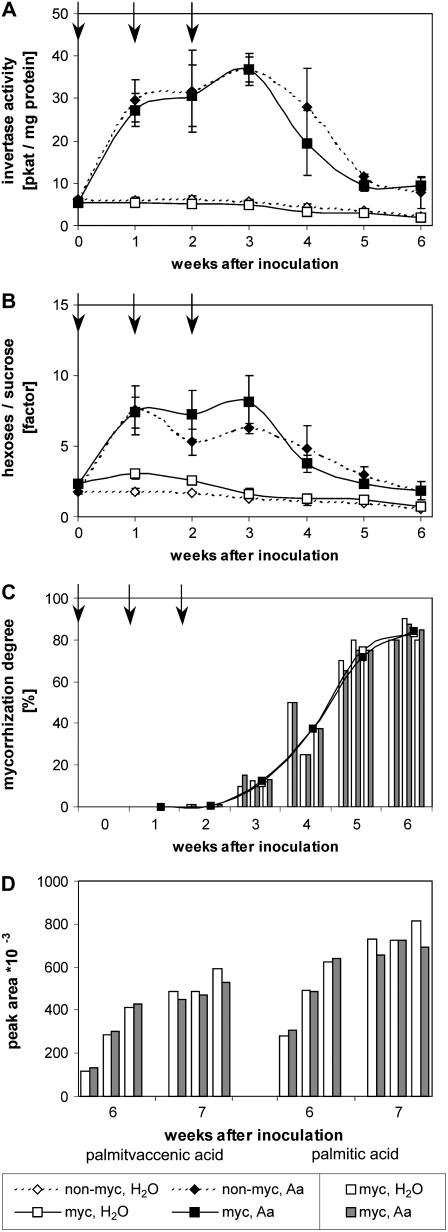
To allow a direct comparison between control roots and roots with elevated invertase activity in one mycorrhizal plant, split-root plants were used, diminishing the high biological variance occurring in the analysis of an interaction between two different organisms. Apoplastic invertase was induced by weekly drenching of one part of the split root with 0.05% (v/v) acetaldehyde starting at the time of inoculation (Fig. 1A) and hexose levels increased. Levels of Glc (2-fold) and Fru (2.5-fold) were increased over a period of at least 6 weeks, whereas Suc content decreased (0.6-fold; Fig. 1B shows the increased hexose-to-Suc ratio). Sugar levels of water-treated roots or leaves were not affected (data not shown). The acetaldehyde application did not detectably interfere with either the vigor of the plant or of the AM fungus (data not shown).
Figure 1.
Induction of an apoplast-located invertase in root parts of transgenic tobacco plants and effects on mycorrhization. A to C, NT alc∷cwINV plants were cultivated using the split-root system where both root parts were either inoculated with G. intraradices 6 weeks after sowing (myc) or left as controls without inoculation (non-myc). To induce expression from the chimeric invertase gene, a defined root from the split-root plants was drenched with 100 mL of 0.05% (v/v) aqueous acetaldehyde solution (Aa). The control root was treated with water (H2O). Soil drenching was performed in weekly intervals 0, 7, and 14 d after inoculation as indicated by arrows. A, Invertase activity of the root parts. B, Ratio of the Glc and Fru contents to the Suc content of the root parts. C, Degree of mycorrhization of the root parts of single plants. D, Content of C16:1Δ11 and C16:0 in single root parts of mycorrhizal plants 6 and 7 weeks after inoculation. Plants harvested 6 weeks after inoculation are described above (A–C), plants harvested at week 7 were cultivated and inoculated in the same way but drenched with acetaldehyde 21, 28, and 35 d after inoculation. Data are expressed in A and B as means ± sd. (n = 3). In C, data of single root parts and the mean values of the three parallel plants are given.
Surprisingly, comparing colonization of high-hexose and control roots with Glomus intraradices revealed no obvious difference (Figs. 1C and 2). The degree of mycorrhization and the formation of fungal structures did not differ from water-treated NT alc∷cwINV roots or from wild-type plants that were drenched either with acetaldehyde or with water (Figs. 1C and 2). The number of arbuscules and fungal vesicles did not change and the colonization was restricted to the root cortex as usual; no fungal structures were found in the root central core (Fig. 2) or in the root tip (data not shown). Furthermore, no significant changes could be detected in the accumulation of the fungus-specific palmitvaccenic acid (C16:1Δ11) or of its precursor palmitic acid (C16:0; Fig. 1D), indicating that the fungal supply of carbon was unchanged. Obviously, the variance between different mycorrhizal plants was in most cases greater than between the differently treated root parts of one plant (Fig. 1D, see also Fig. 1C). Similarly, levels of AM-induced plant secondary metabolites, such as cyclohexenone derivatives (Maier et al., 2000) and mycorradicin derivatives (also called yellow pigment; Klingner et al., 1995), which accumulate in mycorrhizal roots and are suggested to play a functional role in AM (Fester et al., 2002; Strack et al., 2003), were not affected by the increased invertase activity (Table I). In nonmycorrhizal plants these metabolites could not be detected (data not shown; see also Fester et al., 2002, 2005; Schliemann et al., 2006). Induction of invertase activity starting at the beginning of fungal colonization (3 weeks after inoculation) also had no effect on the mycorrhization (Supplemental Fig. S1; for metabolite accumulation, see also Fig. 1D and Table I). Even testing different AM fungi (G. intraradices versus Glomus mosseae) as well as different light conditions (high-light conditions with increased UV-[A + B] intensity or reduced light intensity to limit the carbohydrate supply of mycorrhizal wild-type roots) did not show any effect of increased hexose levels in root parts of NT alc∷cwINV plants on AM formation (Supplemental Figs. S2 and S3). In addition, the analysis of normally grown (nonsplit root) plants also revealed no influence of increased root-specific apoplastic invertase activity on the mycorrhization (see also Supplemental Fig. S3).
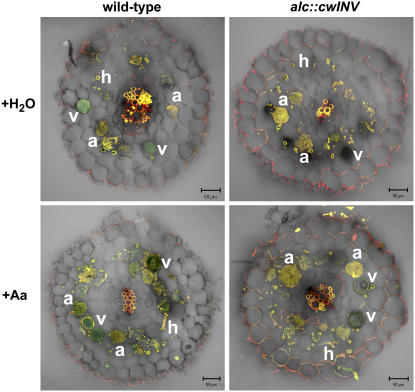
Figure 2.
Formation of fungal structures in water- and acetaldehyde-treated wild-type and alc∷cwINV roots. Cross sections of 140 μm thickness of G. intraradices-colonized roots were stained with two fluorescent-labeled WGAs. The fluorescence of WGA-TRITC showing high affinity to arbuscules and hyphae is shown in red, and the fluorescence of WGA-Alexa Fluor 488, which additionally labeled fungal vesicles (v), is in green. In the overlay, structures labeled by both fluorescent WGAs as arbuscules (a) and hyphae (h) appear in yellow. Plants were harvested 6 weeks after inoculation. Drenching of the whole root system with 100 mL 0.05% (v/v) acetaldehyde for root-specific induction of apoplastic invertase was performed five times at weekly intervals starting with the time point of inoculation (+Aa). Control plants were water treated (+H2O). Bars = 50 μm.
Table I.
AM-specific plant metabolites in tobacco alc∷cwINV roots
Inoculation with G. intraradices and induction of invertase in root parts by acetaldehyde was performed as described in Figure 1. Compounds were detected in mycorrhizal roots via HPLC analysis of methanol extracts. Cyclohexenone and mycorradicin derivatives showed typical UV absorption maxima at 245 and 380 nm, respectively. Aa, Acetaldehyde-treated root parts; H2O, water-treated root parts. Data are presented as mean values ± sd of root parts of three plants harvested 6 and 7 weeks after inoculation and as mean ± sd of the ratios of the acetaldehyde-treated root part to the water-treated root part.
| Metabolite (Peak Area × 10−3) | 6 Weeks
|
7 Weeks
|
||||
|---|---|---|---|---|---|---|
| +H2O | +Aa | +Aa/+H2O | +H2O | +Aa | +Aa/+H2O | |
| Cyclohexenone derivatives | ||||||
| RT 19.5 | 208.8 ± 50.0 | 226.3 ± 53.4 | 1.09 ± 0.10 | 313.4 ± 38.7 | 283.3 ± 47.6 | 0.93 ± 0.22 |
| RT 22.0 | 73.3 ± 21.9 | 68.2 ± 19.5 | 0.94 ± 0.14 | 93.7 ± 14.2 | 79.4 ± 16.1 | 0.88 ± 0.26 |
| RT 27.0 | 61.9 ± 17.1 | 53.7 ± 14.2 | 0.87 ± 0.04 | 66.9 ± 2.3 | 48.1 ± 17.5 | 0.89 ± 0.13 |
| RT 27.7 | 152.5 ± 5.4 | 143.5 ± 1.3 | 0.94 ± 0.03 | 142.1 ± 21.4 | 153.8 ± 11.4 | 1.12 ± 0.27 |
| RT 28.1 | 67.3 ± 22.1 | 63.5 ± 16.2 | 0.98 ± 0.21 | 92.4 ± 16.6 | 79.5 ± 11.3 | 0.90 ± 0.25 |
| RT 30.4 | 43.9 ± 14.1 | 41.3 ± 16.3 | 0.94 ± 0.23 | 67.4 ± 11.8 | 55.8 ± 13.5 | 0.86 ± 0.26 |
| Mycorradicin derivatives | 372.7 ± 104.6 | 359.7 ± 125.1 | 0.96 ± 0.26 | 540.8 ± 120.4 | 455.0 ± 105.2 | 0.91 ± 0.32 |
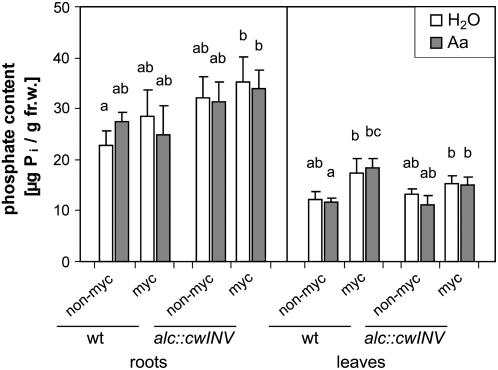
AM not only improves the nutrient availability of the plant, but it can also enhance the plant's tolerance against drought and salt stress (Pfeiffer and Bloss, 1988; Ruiz-Lozano and Azcón, 2000; Cho et al., 2006). Thus, as criteria of the plant's benefit from the symbiotic interaction, the plant phosphate content and the salt stress tolerance of nonsplit root plants in term of transcript accumulation of the salt stress-inducible genes Osmotin and tobacco stress-induced gene 1 (Tsi1; Park et al., 2001) were investigated. G. intraradices-colonized wild-type plants showed a slightly enhanced inorganic phosphate content in the leaves compared to nonmycorrhizal plants (Fig. 3). Phosphate content of the NT alc∷cwINV plants was not altered by the expression of the invertase gene. Additionally, the induction of the yeast invertase had no effect on the gene expression of Osmotin and Tsi1 (Supplemental Fig. S4).
Figure 3.
Inorganic phosphate content of roots and leaves of water- and acetaldehyde-treated wild-type and alc∷cwINV plants. Six-week-old plants were either inoculated with G. intraradices (myc) or left without inoculation (non-myc). Invertase induction in the whole root system was performed as described in Figure 1 for root part-specific induction. Data are presented as mean values + sd (nonmycorrhizal plants: n = 4; mycorrhizal plants: n = 6) and are tested with multiple t tests, including Bonferroni correction. P < 0.05. Means sharing the same letters are not significantly different.
To exclude an induction of defense reactions by elevated extracellular invertase activity in the root, transcript levels of defense-related genes, which are induced by constitutive expression of the apoplast-located yeast invertase (Herbers et al., 1996), were analyzed. In contrast to tobacco leaves with strongly increased invertase activity, the induction of the yeast-derived invertase in the root did not lead to enhanced transcript levels of PAR1, PR-Q, or PR-1b in roots or leaves (Supplemental Fig. S5).
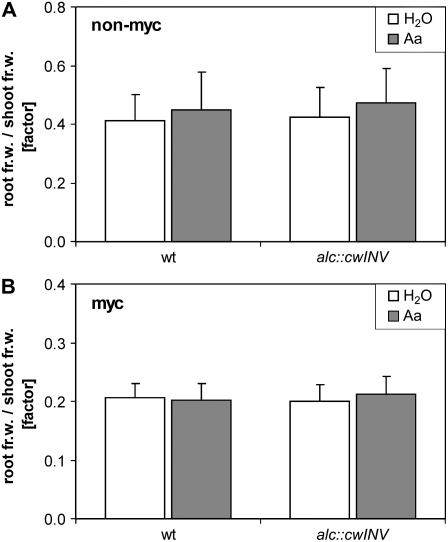
Biomass analysis of nonmycorrhizal or mycorrhizal wild-type and alc∷cwINV plants, treated either with water or acetaldehyde, did not indicate an increased sink function in roots with enhanced apoplastic invertase activity (Fig. 4). The root-to-shoot ratio of the fresh weight and of the dry weight (data not shown) did not change.
Figure 4.
Biomass analysis of nonmycorrhizal and mycorrhizal wild-type and transgenic tobacco plants exhibiting increased apoplastic invertase activity in the root. A, Root-to-shoot ratio of the fresh weight of 8-week-old nonmycorrhizal wild-type and alc∷cwINV plants. These plants were soil drenched twice with 100 mL 0.05% (v/v) acetaldehyde (Aa) or water (H2O) 1 and 2 weeks before harvest. Mean values of two independent experiments + sd are given (wild type: n = 22; alc∷cwINV: mean values of three independent lines with each n = 30). B, Root-to-shoot ratio of the fresh weight of 13-week-old wild-type and alc∷cwINV plants inoculated with G. intraradices 7 weeks before harvest. Acetaldehyde (Aa) or water (H2O) was applied as described for A, but was carried out weekly four times starting 7 d after inoculation. Results are the mean + sd (wild type: n = 24; alc∷cwINV: mean values of three independent lines with each n = 16).
Summarizing, these data suggest that the sink function of the root and the supply of the AM fungus with carbon cannot be improved by root-specifically elevated apoplastic invertase activity leading to increased hexose levels.
Overexpression of Invertases Directed to Three Different Compartments in Root Cells of M. truncatula
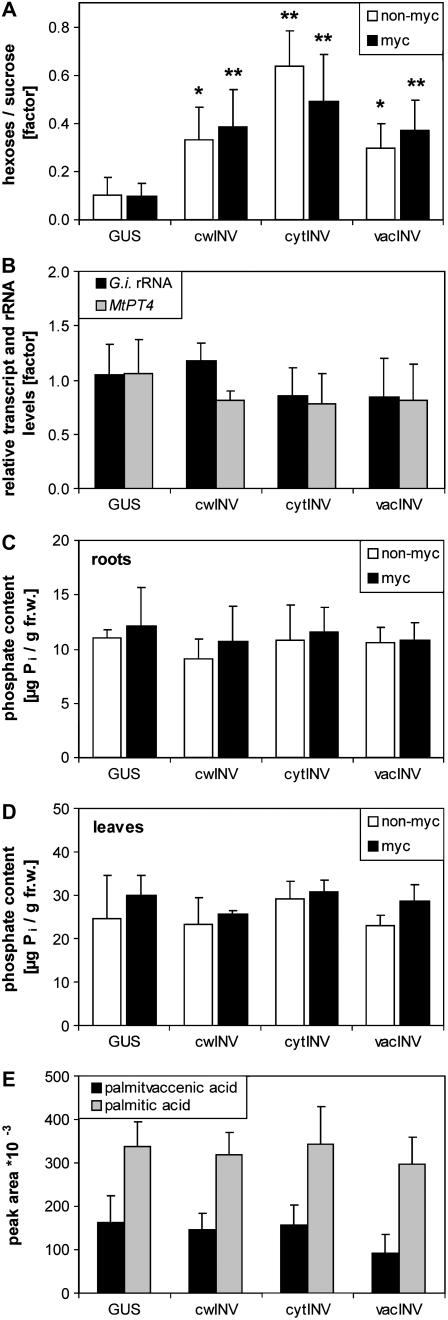
Because increased apoplastic invertase activity of the root had no effect on AM formation, the influence of cytosol- and vacuole-located invertases was tested. In tobacco plants that expressed the yeast gene coding for cytosolic invertase under control of the alc promoter (NT alc∷cytINV; Caddick et al., 1998), cytosolic invertase induction in the root did not alter the extent of colonization with G. intraradices or G. mosseae (Supplemental Figs. S2 and S3). To compare all three types of different subcellular located invertases, A. rhizogenes-mediated root transformation of the model legume M. truncatula was performed using either the 35S∷cwINV, the 35S∷cytINV, or the 35S∷vacINV construct. Control plants were transformed with the 35S∷uidA construct expressing the gene coding for β-glucuronidase (GUS). Yeast invertase expressing roots showed on average an approximately 3- to 6-fold increased hexose level and an enhanced hexose-to-Suc ratio, both compared to GUS-transformed roots (Fig. 5A).
Figure 5.
Analysis of root-transformed M. truncatula plants expressing genes encoding yeast-derived invertase directed to different subcellular locations. A, Ratio of the sum of Glc and Fru to the Suc content of the transformed roots. B, Relative levels of G. intraradices rRNA and of transcripts of the mycorrhiza-induced phosphate transporter MtPT4 in mycorrhizal roots. Transcript and rRNA levels of roots transformed with the GUS construct were set to 1. C and D, Inorganic phosphate content of roots (C) and leaves (D). E, Content of C16:1Δ11 and C16:0 in mycorrhizal roots. A. rhizogenes-mediated root transformation was performed using constructs containing the gene coding for yeast invertase, which is either translocated to the apoplast (cwINV), the cytosol (cytINV), or the vacuole (vacINV), or the gene coding for GUS, all expressed under control of the 35S promoter. Five weeks after transformation, plants were inoculated with G. intraradices (myc) or left without inoculation (non-myc) and were harvested 5 weeks later. All results are presented as means + sd (nonmycorrhizal plants: n = 4; mycorrhizal plants: n = 6). Data of nonmycorrhizal and mycorrhizal plants root transformed with plasmids coding for the different located invertases were compared to nonmycorrhizal and mycorrhizal plants root transformed with the GUS construct, respectively, using the Student's t test. *P < 0.05, **P < 0.01.
However, none of the overexpressing plants showed altered mycorrhization. The content of G. intraradices-specific rRNA did not differ significantly between GUS- and INV-transformed roots. The same was found for transcript levels of the AM-induced phosphate transporter MtPT4 (Fig. 5B). Both parameters can be used for quantification of mycorrhization (Isayenkov et al., 2004). Nonmycorrhizal plants contained no fungal rRNA and negligible MtPT4 transcript levels (data not shown). The inorganic phosphate levels in roots and leaves (Fig. 5, C and D) as well as AM-induced and fungus-specific metabolites, such as C16:1Δ11 and C16:0 (Fig. 5E) or cyclohexenone and mycorradicin derivatives (data not shown), did not change in any of the INV-transformed roots compared to roots of GUS-transformed plants. Thus, it can be concluded that enhanced carbon availability in the root at specified subcellular location did not modify the physiological properties of AM. This finding suggests that the supply of the AM fungus is already optimal in the mutualistic association.
Effect of an Undersupply of the Root on AM
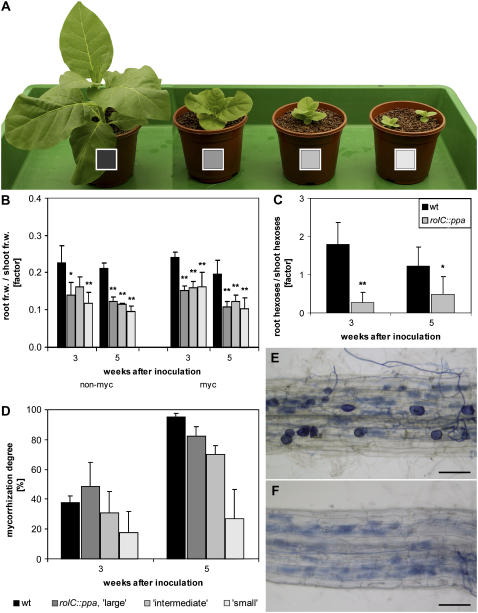
Because increased hexose levels in the root had no effect on mycorrhization, as proof of concept, transgenic tobacco plants with an undersupply of carbon in the root were analyzed. The effect of low root carbohydrate content on AM formation was studied in transgenic tobacco plants expressing the ppa gene from E. coli, encoding inorganic pyrophosphatase, under control of the phloem-specific rolC promoter (NT rolC∷ppa). Phloem-specific expression of ppa inhibits the inorganic pyrophosphate-dependent uptake of Suc into the phloem cells resulting in sugar accumulation in source leaves and an undersupply of the sink organs (Lerchl et al., 1995). Here, heterozygous NT rolC∷ppa plants were investigated. These plants were classified according to the degree of growth reduction (large, intermediate, and small; Fig. 6A). All plants showed stunted growth, and in addition, the root-to-shoot ratio of the fresh weight (Fig. 6B) and of the dry weight (data not shown) was decreased compared to wild-type plants. This clearly indicates an insufficient carbohydrate supply in the root. Furthermore, all rolC∷ppa plants exhibited decreased carbohydrate levels in the root and/or increased levels in the shoot (Fig. 6C shows the decreased root-to-shoot ratio of hexoses).
Figure 6.
Analysis of transgenic tobacco plants with phloem-specific expression of a pyrophosphatase. A, Phenotypes of 9-week-old nonmycorrhizal wild-type (left-most position) and rolC∷ppa plants. Heterozygous NT rolC∷ppa plants were classified by their growth reduction into three groups: large, intermediate, and small (from left to right; see different shadings). B, Biomass analysis. Ratio of root fresh weight to shoot fresh weight of nonmycorrhizal and mycorrhizal wild-type and rolC∷ppa plants 3 and 5 weeks after inoculation. Wild-type and rolC∷ppa plants were inoculated with G. intraradices 6 weeks after sowing (myc) or left without inoculation (non-myc). C, Root-to-shoot ratio of the sum of Glc and Fru in the mycorrhizal plants. Ratios of NT rolC∷ppa plants comprehend data of large, intermediate, and small plants. D, Mycorrhization degree of the inoculated plants. Data in B, C, and D are given as means + sd (n ≥ 6). For each developmental stage, the fresh weight and hexose ratios of NT rolC∷ppa plants were compared to wild-type plants using the Student's t test. *P < 0.05, **P < 0.01. E and F, Ink-stained fungal structures in a wild-type (E) and an intermediate rolC∷ppa plant (F) 4 weeks after inoculation. Bars represent 100 μm.
With increasing growth reduction and therefore lower carbohydrate supply of roots of the heterozygous NT rolC∷ppa plants, decreasing mycorrhization rates were observed upon G. intraradices inoculation (Fig. 6D). Moreover, fewer fungal vesicles and spores were found compared to wild-type plants (Fig. 6, E and F). This indicates an undersupply of the AM fungus leading to decreased formation of fungal storage organs, which rely on the carbon allocation by the plant.
Effect of Root-Specifically Decreased Invertase Activity on AM
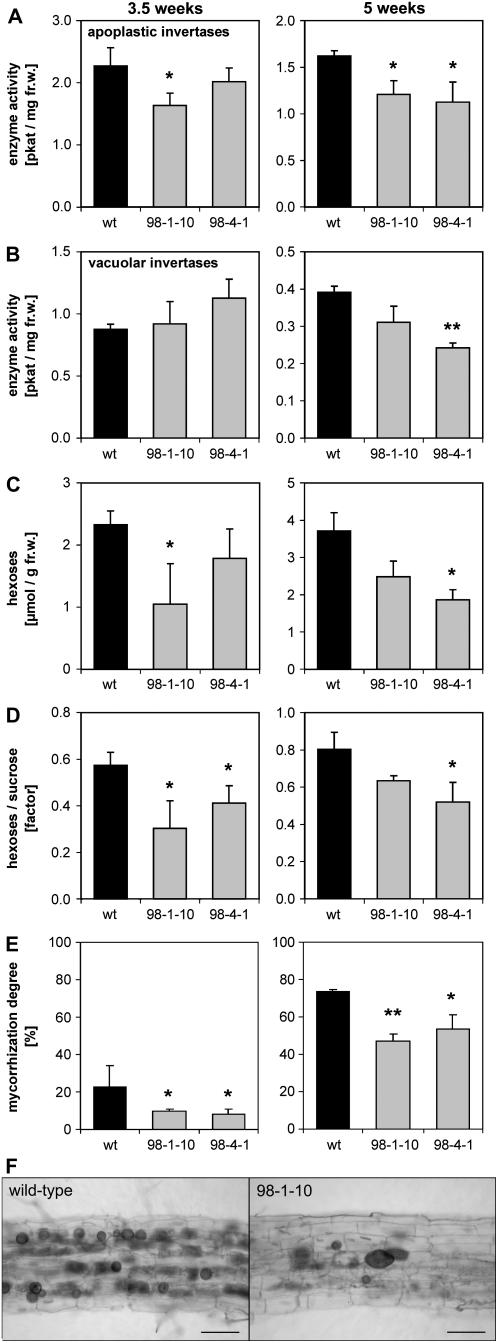
Because a general undersupply of the root with carbon by defective phloem loading resulted in decreased mycorrhization, the analysis of plants with reduced invertase activity and decreased phloem unloading complement this study. This aspect was implemented by expressing the Arabidopsis gene AtC/VIF2 coding for an inhibitor of acid invertases (Link et al., 2004) under control of the root- and seedling-specific pyk10 promoter from Arabidopsis (Nitz et al., 2001) in transgenic tobacco plants (NT pyk10∷InvInh). Recombinant AtC/VIF2 protein was shown to affect apoplastic and vacuolar invertase activities in vitro (Link et al., 2004). Plants of the two independent NT pyk10∷InvInh lines, 98-1-10 and 98-4-1, showed reduced apoplastic invertase activities in the root (Fig. 7A). Vacuolar invertase activity was inhibited in vitro only in one line at later developmental stages (Fig. 7B). Neutral cytosolic invertase activity levels were not affected; the same was true for invertases in leaves (Supplemental Fig. S6). Nonmycorrhizal plants showed a similar affection of invertase activities (Supplemental Fig. S6). According to the reduced apoplastic invertase activity, the roots had lower contents of Glc and Fru (Fig. 7C shows the sum of both hexoses) and a reduced ratio of both hexoses to Suc (Fig. 7D). However, in contrast to rolC∷ppa tobacco, plants with root-specific overexpression of invertase inhibitor were not altered in their vegetative growth. There were no alterations in their total biomass (data not shown) and their root or shoot biomass compared to wild-type plants (Fig. 8 shows the root-to-shoot ratio of the fresh weight). Nevertheless, corresponding to the lower hexose levels in roots of pyk10∷InvInh plants we found a lower mycorrhization level with G. intraradices (Fig. 7E). Moreover, colonized roots of NT pyk10∷InvInh plants showed a lower density of fungal structures compared to the wild type (Fig. 7F), reflected by a significant decrease in fungus-specific rRNA 5 weeks after inoculation (data not shown). This indicates that the carbon supply in the AM interaction depends on the activity of apoplastic invertases that deliver hexoses.
Figure 7.
Analysis of transgenic tobacco plants with root-specific expression of an invertase inhibitor. A and B, Cell wall (A) and vacuolar (B) invertase activity in roots of wild-type SR1 plants and NT pyk10∷InvInh plants of two independent lines (98-1-10 and 98-4-1) 3.5 and 5 weeks after inoculation with G. intraradices. C, Glc and Fru content of the roots. D, Ratio of the Glc and Fru contents to the Suc content of the roots. E, Degree of mycorrhization. To allow statistical analysis, the degree of mycorrhization in percent of the root length was determined for every root system in 50 to 100 root pieces of each 1 cm length. Plants were in two independent experiments inoculated with G. intraradices either 2.5 weeks after sowing and harvested 3.5 weeks later or inoculated 4 weeks after sowing and harvested 5 weeks later. Data are presented as mean values + sd (at 3.5 weeks: n = 5; at 5 weeks: n = 3). The data from the transgenic lines were pairwise compared to the wild type by the Student's t test. *P < 0.05, **P < 0.01. F, Ink-stained fungal structures in a wild-type and a NT pyk10∷InvInh plant of line 98-1-10, each 5 weeks after inoculation. Bars represent 100 μm.
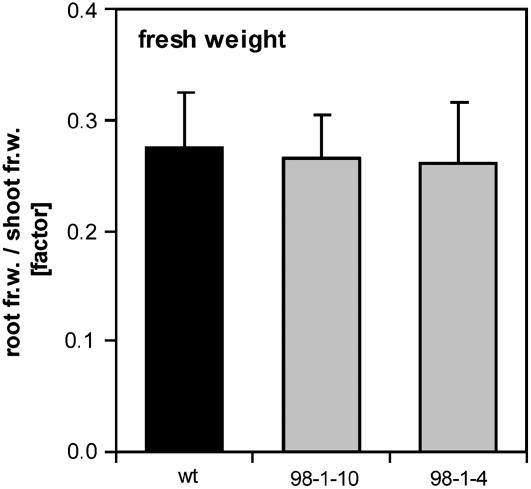
Figure 8.
Biomass analysis of NT pyk10∷InvInh plants. Root-to-shoot ratio of the fresh weight of 6-week-old nonmycorrhizal wild-type SR1 plants and plants of two independent NT pyk10∷InvInh lines (98-1-10 and 98-4-1). Mean values of +sd are given (n ≥ 33).
DISCUSSION
The influence of the carbohydrate status of the plant on AM formation is still poorly understood. In this study, the effect of elevated root hexose levels on the mutualistic interaction in AM was investigated. This was achieved by increased invertase activities in different subcellular locations in the root due to the expression of chimeric genes encoding yeast-derived invertase (Sonnewald et al., 1991). Surprisingly, we were not able to detect any change in the mycorrhization of tobacco or Medicago plants with increased apoplastic, cytosolic, or vacuolar invertase activities in the root, either on the fungal or plant side.
We intensively analyzed NT alc∷cwINV plants with root (part)-specifically increased apoplastic invertase activity, because extracellular invertases have been suggested to play a crucial role in the carbohydrate supply of the obligate biotrophic AM fungus (Schaarschmidt et al., 2006). However, no evidence was found either for forcing the mutualistic association, or for changing it into a parasitic one, or for suppressing AM by activation of defense reactions. All of the measured parameters for AM formation and for the plant's benefit of AM remained unaffected, both in normally grown and in split-root plants. On the first glance this might contradict to previous feeding experiments indicating a stimulation of the phosphate allocation in mycorrhizal roots by increased carbohydrate availability (Bücking and Shachar-Hill, 2005). However, in these experiments with axenic root cultures the authors observed due to feeding of higher Suc amounts an increased carbon transfer from the root to the fungus that was suggested to stimulate P uptake and transfer by the fungus. Here, no indices for a higher carbon transfer from the plant to the AM fungus, deduced from the content of fungus-specific C16:1Δ11 and its precursor C16:0 (Trépanier et al., 2005; van Aarle and Olsson, 2005), were found, although levels of available carbon increased. Moreover, the finding of Bücking and Shachar-Hill (2005) was obtained from experiments with axenic cultures of transformed carrot roots, and it cannot be directly translated to the situation in a normal mycorrhizal root.
A strong induction of extracellular invertases in plants and the severe modulation of source-sink activities could also lead to an activation of defense-related mechanisms via sugar-mediated gene expression (Rolland et al., 2002; Roitsch et al., 2003). As shown previously, constitutive expression of the apoplast-located yeast invertase resulted in a strong accumulation of PR protein transcripts in tobacco leaves, encoded by PAR1, PR-1b, and PR-Q (Herbers et al., 1996). During root-specific expression of apoplast-located yeast invertase, we did not observe transcript accumulation of those PR genes, indicating that there was no activation of plant defense responses interfering with the mutualistic interaction. To check the intensity of sink-source modulation in tobacco plants with elevated extracellular invertase activity in the root, some markers for sink strength were analyzed. However, there was no effect of enhanced apoplastic invertase activity detected on the starch content (data not shown) or on the root biomass. The root-to-shoot ratio of fresh weight and dry weight did not change; the same was found for the total plant biomass (data not shown). This might suggest that the increased apoplastic invertase activity did not change the sink strength. Especially in storage sinks, as rhizomes, tap roots, or tubers, the storage is often limited by the activity of enzymes involved in starch and oil accumulation, like Suc synthase or hexokinases. Nevertheless, increased sink strength by expression of yeast-derived apoplastic invertase leading to higher yields has been shown, e.g. for potato (Solanum tuberosum) tubers (Sonnewald et al., 1997; Hajirezaei et al., 2000) and for Arabidopsis seeds (Heyer et al., 2004). In contrast, transgenic tobacco plants expressing the apoplast-located yeast invertase with a seed-specific promoter did not show increased accumulation of storage products in the oilseeds, even with additional overexpression of hexokinase, indicating no change in the sink strength of these storage sinks (Tomlinson et al., 2004). In Arabidopsis, the root-specific expression of yeast-derived apoplastic invertase had no detectable effect on the plant biomass as well (von Schweinichen and Büttner, 2005). In contrast, Arabidopsis plants expressing an apoplastic invertase of Chenopodium rubrum with the root-specific pyk10 promoter showed only slightly enhanced invertase activity and no changes in the soluble sugar contents, but there was an indirect influence on the whole plant development and an increased total plant biomass (von Schweinichen and Büttner, 2005). The authors observed a similar phenotype when the extracellular-located yeast invertase was driven under a meristem-specific promoter, which was observed in several other studies (e.g. Lerchl et al., 1995; Fukushima et al., 2001; Canam et al., 2006), confirming the principal function of a yeast-derived invertase in plants.
In this study induction of the apoplast-located yeast invertase resulted in root-specifically enhanced hexose levels that were suggested to affect AM. To test whether the subcellular location of increased invertase activity in the root plays an essential role for improving the carbon supply of the AM fungus, A. rhizogenes-mediated root transformation was carried out. In addition, the model legume M. truncatula was used to exclude potential tobacco-specific features in the formation of AM. However, even in Medicago, none of the differently located invertases had an observable effect on AM formation. These results could suggest that hexoses might not be the favored form of AM-fungal carbon uptake and that the fungus is capable of using Suc with the same efficiency. In this case, increased invertase activity in the root would not influence the availability of carbon for the fungus. Nevertheless, this assumption would be in strong contrast to several other studies that examine the induction of Suc-cleaving enzymes in mycorrhizal roots (e.g. Dehne, 1986; Snellgrove et al., 1987; Wright et al., 1998; Hohnjec et al., 2003; Schaarschmidt et al., 2006) and also to the fact that isolated intraradical hyphae showed a high preference for the uptake of Glc compared to Suc and Fru (Solaiman and Saito, 1997). To vitiate this assumption, we have shown the importance of acid invertases in the AM association by repressing their enzymatic activity. Posttranslational inhibitor-mediated inactivation of enzymes represents a powerful tool for analyzing the physiological role of enzyme classes. Proteinaceous inhibitors of extracellular and acid invertases are essential to control acid invertase activities and their regulatory functions in plants (Balibrea et al., 2004; for review, see Rausch and Greiner, 2004). Here, transgenic tobacco plants with root-specific expression of AtC/VIF2 were analyzed. The inhibitory effect of AtC/VIF2 has been demonstrated in vitro using recombinant protein (Link et al., 2004). From this, a higher in vitro affinity of vacuolar invertases compared to apoplastic invertases was shown; however, the inhibitors may behave differently in vivo. Using in vitro assays, we found in the roots of two independent NT pyk10∷InvInh lines a significantly reduced extracellular invertase activity but less inhibition of vacuolar invertases. Along with decreased apoplastic invertase activity, the roots were characterized by reduced hexose contents and a decreased hexose-to-Suc ratio, indicating the in vivo function of AtC/VIF2 in tobacco roots. The plants, however, did not show an altered vegetative growth and root or shoot biomass. Reduced colonization rates with a lower density of fungal structures clearly demonstrate the important role of apoplastic invertases in supplying sufficient amounts of hexoses in the mycorrhizal root.
In conclusion, we suggest that under normal growth conditions the supply of the fungal symbiont with carbon is already optimal in the AM symbiosis and that it cannot readily be improved. This hypothesis is supported by increased levels of soluble sugars in mycorrhizal clover (Trifolium repens) roots (Wright et al., 1998), which argues for a slower uptake of hexoses by the fungus compared to delivering Suc and cleavage into hexoses by the plant. In our study, we were not able to detect significantly increased root hexose or Suc levels in mycorrhizal wild-type or control transgenic plants as water-treated NT alc∷cwINV and GUS-transformed M. truncatula (data not shown). However, constant (instead of decreasing) hexose or Suc levels in mycorrhizal tobacco and Medicago roots indicate the sufficient availability of carbohydrates in wild-type plants under normal growth conditions. In contrast, according to previous experiments that show a reduced mycorrhization upon limiting light intensity (Hayman, 1974; Tester et al., 1985; Son and Smith, 1988), suppression of AM could be observed if the root was severely undersupplied with carbohydrates as shown for NT rolC∷ppa plants. During the disturbed inorganic pyrophosphatase-dependent uptake of Suc into the phloem cells and translocation of Suc to the sink tissue (Lerchl et al., 1995), the formation of AM was characterized by less density of fungal storage organs, indicating a deficiency of carbon.
Summarizing, our findings clearly demonstrate the fungal dependency on the carbohydrate supply of the root controlled by plant invertases and the general regulation of AM formation by carbon availability. Nevertheless, in the functional symbiotic interaction in AM, the carbon supply seems not to be the limiting factor. It is tempting to speculate that other mechanisms such as the phosphate supply of the plant or plant defense responses to limit fungal growth to the root cortex may be of higher importance in regulating that interaction.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Wild-type tobacco (Nicotiana tabacum cv Samsun NN) plants were obtained from Vereinigte Saatzuchten eG and germinated on solid Murashige and Skoog medium (Duchefa). Transgenic tobacco alc∷cwINV (Schaarschmidt et al., 2004) and rolC∷ppa plants (Lerchl et al., 1995) were selected by sowing on solid Murashige and Skoog medium containing 50 mg/L kanamycin A (Duchefa). All tobacco plants were cultivated in a growth chamber with 16 h light (250 μmol photons m−2 s−1; Philips Powerstar HQI 250/D lamps) at 25°C and 50% relative humidity. After 3 weeks, plants were transferred into pots filled with expanded clay of 2 to 5 mm particle size (Original Lamstedt Ton, Fibo ExClay). They were watered with distilled water and fertilized twice per week with 10 mL 10× Long Ashton (20% phosphate corresponding to 2.8 mm; Hewitt, 1966).
Medicago truncatula cv Jemalong seeds (obtained from AustraHort) were scarified by incubation in concentrated H2SO4 for 10 min, washed with water, and surface sterilized in 1:6 diluted sodium hypochlorite solution (12% Cl; Roth) for 5 min. After washing, the seeds were placed on 0.8% (w/v) plant agar (Duchefa) and germinated 2 d at room temperature (RT) in the light and 4 d at 4°C in the dark. The seedling were root transformed as described below and transferred to pots filled with expanded clay as described for tobacco. Plants were cultivated in a growth chamber at 20°C, 50% relative humidity, and 16 h light (250 μmol m−2 s−1)/8 h dark, watered with distilled water, and fertilized twice per week with 5 mL 10× Long Ashton (20% phosphate).
After inoculation with Glomus intraradices, plants were cultivated under the same conditions as before, but fertilized with 25 mL 10× Long Ashton (20% phosphate) twice per week in case of tobacco and 10 mL twice per week in case of Medicago. Roots and middle-aged leaves of tobacco and roots and all leaves of Medicago plants were harvested at the end of the light period.
Plasmid Constructions, Stable Plant Transformation, and Determination of Plant Invertase Activities
The pyk10 promoter was amplified by PCR using genomic DNA and subcloned into the vector pTF2-6 (T. Fatima and T. Roitsch, unpublished data) to generate pMB1-18. The cDNA encoding AtC/VIF2 (at5g64620) was amplified by reverse transcription-PCR using total RNA, initially cloned into the vector pBluescript KS+ to generate pMCG2, and subsequently subcloned thereof as Acc65I-KpnI fragment into the binary vector pTF2-6 to generate plasmid pMCG4. To generate a transcriptional fusion between the pyk10 promoter and the cDNA encoding AtC/VIF2, a 1,467 bp pyk10 promoter fragment was subcloned as Acc65I fragment from pMB1-18 into the binary vector pMCG4, linearized by Acc65I, to generated pMCG6. The pyk10∷InvInh construct was transformed in tobacco (cv SR1) using Agrobacterium tumefaciens strain LBA4404 and standard transformation procedures (Horsch et al., 1985). Transgenic lines expressing the pyk10∷InvInh fusion were characterized by PCR (M. Gonzalez and T. Roitsch, unpublished data).
Determination of apoplastic (cell wall bound) and vacuolar plant invertase activities was performed as described by Greiner et al. (1999).
Agrobacterium rhizogenes-Mediated Root Transformation
Construction of plasmids containing the 35S∷cwINV, 35S∷cytINV, and 35S∷vacINV constructs was described previously (von Schaewen et al., 1990; Sonnewald et al., 1991). A plasmid containing the 35S∷uidA construct was kindly provided by Stanislav Isayenkov (IPB Halle; Isayenkov et al., 2005). Agrobacterium rhizogenes Arqua1 (Quandt et al., 1993) was transformed by electroporation. The induction of transgenic hairy roots was performed according to standard procedures (Vieweg et al., 2004). After development of hairy roots, all roots that did not emerge from the infection site and roots that did not show a hairy root phenotype were removed.
Invertase Induction and Determination of Yeast Invertase Activity
Using the alc promoter system, the yeast-derived invertase was induced in NT alc∷cwINV plants root specifically by soil drenching with 100 mL of 0.05% (v/v) aqueous acetaldehyde solution (Schaarschmidt et al., 2004). Unless otherwise mentioned, acetaldehyde was applied three times at weekly intervals starting with the time point of inoculation. Control plants were drenched in the same way with distilled water. Root part-specific invertase induction was carried out using the split-root system, in which the root system was divided onto two pots. Here, only one root part of mycorrhizal or nonmycorrhizal plants was treated with acetaldehyde, whereas the other was drenched with water. Determination of invertase activity was performed as described before (Schaarschmidt et al., 2004).
Inoculation with G. intraradices and Staining of Fungal Structures
The AM fungus G. intraradices Schenk & Smith isolate 49 (Maier et al., 1995) was enriched by previous cocultivation with leek (Allium porrum cv Elefant). For inoculation, plants were transferred after careful removal of the previous substrate to new pots filled with expanded clay containing 5% to 10% (v/v) G. intraradices inoculum freshly harvested from mycorrhizal leek plants. Nonmycorrhizal plants were transferred in the same way to pure expanded clay. All tobacco plants were inoculated 6 weeks after sowing; inoculation of Medicago was performed 5 weeks after root transformation. For the estimation of G. intraradices colonization a representative cross section of each root system was taken. The mycorrhizal structures in the root pieces were stained according to Vierheilig et al. (1998) using 5% (v/v) ink (Sheaffer Skrip jet black, Sheaffer Manufacturing) in 2% (v/v) acetic acid and analyzed using a stereomicroscope. The degree of mycorrhization is given in percent of root length. Micrographs of ink-stained roots were taken using a Zeiss Axioplan microscope equipped with a video camera (Fujix Digital Camera HC-300Z, Fuji Photo Film) and were processed through Photoshop 7.0 (Adobe Systems).
For fluorescent staining of fungal structures with wheat germ agglutinin (WGA), coupled to tetramethyl rhodamine isothiocyanate (TRITC) or Alexa Fluor 488, root pieces of 3 mm were fixed with 4% (w/v) paraformaldehyde and 0.1% (v/v) Triton X-100 in phosphate-buffered saline (PBS) for 30 min at RT and afterward cut into 140-μm thick cross sections using a vibrating blade microtome (VT 1000 S, Leica Microsystems). Cross sections were digested in 1% (w/v) cellulase, 0.1% (w/v) bovine serum albumin, and 0.01% (w/v) pectinase in PBS for 1 h at RT. After washing with PBS, staining was performed using 50 μg/mL WGA-TRITC and 50 μg/mL WGA-Alexa Fluor 488 (both from Molecular Probes) in PBS for 30 min at RT. The formation of mycorrhizal structures was analyzed with a confocal laser-scanning microscope (LSM 510 Meta, Zeiss) using the 488 nm (Alexa Fluor 488) and 543 nm (TRITC) laser lines for excitation. Series of optical sections (z series) were acquired by scanning 19 sections with a distance of 0.2 μm on the z axis; z-series projections were done with the LSM Image Examiner software (Zeiss).
Real-Time Reverse Transcription-PCR Analysis
Total RNA of Medicago root material was isolated using the Qiagen RNeasy plant mini kit including a DNase digestion (RNase-free DNase Set, Qiagen). First-strand cDNA synthesis of 1 μg RNA in a final volume of 20 μL was performed with M-MLV Reverse Transcriptase, RNase H Minus, Point Mutant (Promega) according to the supplier's protocol using an oligo dT (T19) primer for gene expression analysis of MtPT4 and a random hexamer primer for determination of fungal rRNA levels.
For real-time PCR, 4.5 μL of 1:9 diluted cDNA (25 ng reverse-transcribed total RNA) were mixed with 2× TaqMan master mix (Applied Biosystems) and 20× TaqMan probe and primers (Assays-by-Design, Applied Biosystems) in a final volume of 10 μL in three independent replicates. TaqMan probes and primers for the mycorrhiza-induced phosphate transporter MtPT4 of M. truncatula and for G. intraradices-specific rRNA were used as described previously (Isayenkov et al., 2004). As internal control for both cDNA syntheses transcript levels of elongation factor 1-α of M. truncatula were measured (Isayenkov et al., 2004). Real-time PCR was performed using the Mx 3005P QPCR system (Stratagene) according to the Assays-by-Design protocol (Applied Biosystems). Data were evaluated with the MxPro software (Stratagene) and calculated by the comparative Ct method.
Determination of Soluble Sugars and Inorganic Phosphate
The soluble sugar contents were measured photometrically by a coupled enzymatic assay as described previously (Schaarschmidt et al., 2004). The determination of inorganic phosphate was performed according to Taussky and Shorr (1953). Frozen root and leaf material (100–500 mg fresh weight) was homogenized in liquid nitrogen and incubated under shaking with 800 μL 3% (v/v) perchloric acid for 20 min at RT. After centrifugation (14,000g, 5 min, RT), 120 μL of the supernatant was incubated with 80 μL reaction solution containing 10% (v/v) ammonium molybdate stock solution [0.4 m (NH4)6Mo7O24 in 10 n H2SO4] and 0.18 m FeSO4 in a microtitre plate. When the reaction reached a plateau the absorbance was measured at 750 nm using a 96-well microtitre plate reader (Sunrise, Tecan). The standard was KH2PO4. All measurements were carried out in three independent replicates for each sample.
Metabolite Analysis
Homogenized lyophilized root material (30 mg) was first extracted three times with 500 μL hexane. The supernatant (in total 1.5 mL) was collected and 15 μL of a methyl nonadecanoate stock solution (2 mg/mL hexane) (Sigma-Aldrich) were added as internal standard. After washing the pellet with hexane and drying it, the polar components were extracted in the same way using 80% (v/v) aqueous methanol. As internal standard, 75 μL of a ribitol stock solution (2 mg/mL water; Sigma-Aldrich) was added.
Gas chromatography/mass spectroscopy measurements were performed with an Trace 2000 GC equipped with an Autosampler 3000 and a single quadrupole Trace DSQ (ThermoElectron). Hexane extracts (125 μL aliquots) were derivatized after reduction to dryness in glass injection vials with 100 μL N-methyl-N-(trimethylsilyl)-trifluoroacetamide (CS-Chromatographie Service) for 30 min at 70°C and diluted with 400 μL hexane prior to injection. The following conditions were used: EI-voltage 70 eV; source temperature 240°C; column J&W DB-5 MS (30 m × 0.25 mm, i.d., 0.25 μm film thickness; Agilent); carrier gas helium at constant flow of 1 mL/ min; temperature program: 50°C (2 min), 50°C to 260°C (6°C/min), 260°C (3 min), 260°C to 300°C (10°C/min), 300°C (6 min); injection temperature: 240°C, splitless injection 1 μL, mass range of mass-to-charge ratio 40 to 800. Data acquisition and evaluation run with Xcalibur 1.4.1.
Methanol extracts were analyzed by HPLC according to Schliemann et al. (2006). A 10 μL aliquot of each extract was injected onto a 5 μm Nucleosil C18 column (250 × 4 mm i.d.; Macherey-Nagel). Separation was achieved using a 40-min linear gradient at 1 mL/min from 5% to 25% (v/v) acetonitrile in 1.5% (v/v) aqueous H3PO4 followed by a gradient from 25% to 80% (v/v) acetonitrile in 20 min. Cyclohexenone and mycorradicin derivatives were detected photometrically at 245 nm and 380 nm, respectively, by a Waters 2996 photodiode array detector. Data were collected and analyzed using the Millennium software 2010 (Millipore).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number NM_125858.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Mycorrhizal formation in alc∷cwINV tobacco plants after invertase induction in root parts.
Supplemental Figure S2. Influence of UV and total photon irradiance intensity on the mycorrhization of wild-type and transgenic tobacco plants with increased invertase activity in the root.
Supplemental Figure S3. Colonization of transgenic tobacco plants, exhibiting root part-specifically increased apoplastic or cytosolic invertase in roots, with G. mosseae.
Supplemental Figure S4. Expression analysis of the salt stress-inducible genes Osmotin and Tsi1 in wild-type and alc∷cwINV tobacco plants.
Supplemental Figure S5. Expression analysis of PR genes in wild-type and alc∷cwINV tobacco plants.
Supplemental Figure S6. Invertase activities in nonmycorrhizal and mycorrhizal NT pyk10∷InvInh plants.
Supplementary Material
Acknowledgments
We gratefully acknowledge Dr. Willibald Schliemann for helpful support with metabolite analysis and critically reading the manuscript. We also thank Dr. Margaret Rice for critically reading the manuscript. Furthermore, Dr. Stanislav Isayenkov is acknowledged for providing plasmid containing the 35S∷uidA construct.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Bettina Hause (bhause@ipb-halle.de).
The online version of this article contains Web-only data.
References
- Bago B, Pfeffer PE, Shachar-Hill Y (2000) Carbon metabolism and transport in arbuscular mycorrhizas. Plant Physiol 124 949–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bago B, Zipfel W, Williams RM, Jun J, Arreola R, Lammers PJ, Pfeffer PE, Shachar-Hill Y (2002) Translocation and utilization of fungal storage lipid in the arbuscular mycorrhizal symbiosis. Plant Physiol 128 108–124 [PMC free article] [PubMed] [Google Scholar]
- Balibrea ME, Gonzalez MC, Fatima T, Lee TK, Proels R, Tanner W, Roitsch T (2004) Extracellular invertase is an essential component of cytokinin-mediated delay of senescence. Plant Cell 16 1276–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blee KA, Anderson AJ (1998) Regulation of arbuscule formation by carbon in the plant. Plant J 16 523–530 [Google Scholar]
- Blee KA, Anderson AJ (2002) Transcripts for genes encoding soluble acid invertase and sucrose synthase accumulate in root tip and cortical cells containing mycorrhizal arbuscules. Plant Mol Biol 50 197–211 [DOI] [PubMed] [Google Scholar]
- Bücking H, Shachar-Hill Y (2005) Phosphate uptake, transport and transfer by the arbuscular mycorrhizal fungus Glomus intraradices is stimulated by increased carbohydrate availability. New Phytol 165 899–912 [DOI] [PubMed] [Google Scholar]
- Caddick MX, Greenland AJ, Jepson l, Krause K-P, Qu N, Riddell KV, Salter MG, Schuch W, Sonnewald U, Tomsett AB (1998) An ethanol inducible gene switch for plants used to manipulate carbon metabolism. Nat Biotechnol 16 177–180 [DOI] [PubMed] [Google Scholar]
- Canam T, Park J-Y, Yu K, Campbell M, Ellis D, Mansfield S (2006) Varied growth, biomass and cellulose content in tobacco expressing yeast-derived invertases. Planta 224 1315–1327 [DOI] [PubMed] [Google Scholar]
- Cho K, Toler H, Lee J, Ownley B, Stutz JC, Moore JL, Augé RM (2006) Mycorrhizal symbiosis and response of sorghum plants to combined drought and salinity stresses. J Plant Physiol 163 517–528 [DOI] [PubMed] [Google Scholar]
- Dehne HW (1986) Influence of VA mycorrhizae on host plant physiology. In V Gianinazzi-Pearson, S Gianinazzi, eds, Physiological and Genetical Aspects of Mycorrhizae. Institut National de la Recherche Agronomique, Paris, pp 431–435
- Douds D, Pfeffer P, Shachar-Hill Y (2000) Application of in vitro methods to study carbon uptake and transport by AM fungi. Plant Soil 226 255–261 [Google Scholar]
- Douds DD Jr (1994) Relationship between hyphal and arbuscular colonization and sporulation in a mycorrhiza of Paspalum notatum Flugge. New Phytol 126 233–237 [Google Scholar]
- Ferrol N, Pozo MJ, Antelo M, Azcon-Aguilar C (2002) Arbuscular mycorrhizal symbiosis regulates plasma membrane H+-ATPase gene expression in tomato plants. J Exp Bot 53 1683–1687 [DOI] [PubMed] [Google Scholar]
- Fester T, Schmidt D, Lohse S, Walter M, Giuliano G, Bramley P, Fraser P, Hause B, Strack D (2002) Stimulation of carotenoid metabolism in arbuscular mycorrhizal roots. Planta 216 148–154 [DOI] [PubMed] [Google Scholar]
- Fester T, Wray V, Nimtz M, Strack D (2005) Is stimulation of carotenoid biosynthesis in arbuscular mycorrhizal roots a general phenomenon? Phytochemistry 66 1781–1786 [DOI] [PubMed] [Google Scholar]
- Fukushima E, Arata Y, Endo T, Sonnewald U, Sato F (2001) Improved salt tolerance of transgenic tobacco expressing apoplastic yeast-derived invertase. Plant Cell Physiol 42 245–249 [DOI] [PubMed] [Google Scholar]
- Gianinazzi-Pearson V, Dumas-Gaudot E, Gollotte A, Tahiri-Alaoui A, Gianinazzi S (1996) Cellular and molecular defence-related root responses to invasion by arbuscular mycorrhizal fungi. New Phytol 133 45–57 [Google Scholar]
- Gianinazzi-Pearson V, Smith SE, Gianinazzi S, Smith FA (1991) Enzymatic studies on the metabolism of vesicular-arbuscular mycorrhizas. V. Is H+-ATPase a component of ATP-hydrolysing enzyme activities in plant-fungus interfaces? New Phytol 117 61–74 [Google Scholar]
- Godt DE, Roitsch T (1997) Regulation and tissue-specific distribution of mRNAs for three extracellular invertase isoenzymes of tomato suggests an important function in establishing and maintaining sink metabolism. Plant Physiol 115 273–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner S, Rausch T, Sonnewald U, Herbers K (1999) Ectopic expression of a tobacco invertase inhibitor homolog prevents cold-induced sweetening of potato tubers. Nat Biotechnol 17 708–711 [DOI] [PubMed] [Google Scholar]
- Hajirezaei M-R, Takahata Y, Trethewey RN, Willmitzer L, Sonnewald U (2000) Impact of elevated cytosolic and apoplastic invertase activity on carbon metabolism during potato tuber development. J Exp Bot 51 439–445 [DOI] [PubMed] [Google Scholar]
- Harrison M (1996) A sugar transporter from Medicago truncatula: altered expression pattern in roots during vesicular-arbuscular (VA) mycorrhizal associations. Plant J 9 491–503 [DOI] [PubMed] [Google Scholar]
- Harrison M (1999) Molecular and cellular aspects of the arbuscular mycorrhizal symbiosis. Annu Rev Plant Physiol Plant Mol Biol 50 361–389 [DOI] [PubMed] [Google Scholar]
- Hayman DS (1974) Plant growth responses to vesicular-arbuscular mycorrhiza. New Phytol 73 71–80 [Google Scholar]
- Herbers K, Meuwly P, Frommer W, Métraux J-P, Sonnewald U (1996) Systemic acquired resistance mediated by the ectopic expression of invertase: possible hexose sensing in the secretory pathway. Plant Cell 8 793–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt EJ (1966) Sand and Water Culture Methods Used in the Study of Plant Nutrition. Commonwealth Agricultural Bureaux, Farnhan Royal, Bucks, UK, pp 187–237
- Heyer AG, Raap M, Schroeer B, Marty B, Willmitzer L (2004) Cell wall invertase expression at the apical meristem alters floral, architectural, and reproductive traits in Arabidopsis thaliana. Plant J 39 161–169 [DOI] [PubMed] [Google Scholar]
- Hohnjec N, Perlick AM, Pühler A, Küster H (2003) The Medicago truncatula sucrose synthase gene MtSucS1 is activated both in the infected region of root nodules and in the cortex of roots colonized by arbuscular mycorrhizal fungi. Mol Plant Microbe Interact 16 903–915 [DOI] [PubMed] [Google Scholar]
- Horsch R, Fry FB, Hoffmann NL, Eichholtz P, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227 1229–1231 [DOI] [PubMed] [Google Scholar]
- Isayenkov S, Fester T, Hause B (2004) Rapid determination of fungal colonization and arbuscule formation in roots of Medicago truncatula using real-time (RT) PCR. J Plant Physiol 161 1379–1383 [DOI] [PubMed] [Google Scholar]
- Isayenkov S, Mrosk C, Stenzel I, Strack D, Hause B (2005) Suppression of allene oxide cyclase in hairy roots of Medicago truncatula reduces jasmonate levels and the degree of mycorrhization with Glomus intraradices. Plant Physiol 139 1401–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper DA, Robson AD, Abbott LK (1979) Phosphorus and the formation of vesicular-arbuscular mycorrhizas. Soil Biol Biochem 11 501–505 [Google Scholar]
- Javot H, Penmetsa RV, Terzaghi N, Cook DR, Harrison MJ (2007) A Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci USA 104 1720–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karandashov V, Bucher M (2005) Symbiotic phosphate transport in arbuscular mycorrhizas. Trends Plant Sci 10 22–29 [DOI] [PubMed] [Google Scholar]
- Klingner A, Bothe H, Wray V, Marner F-J (1995) Identification of a yellow pigment formed in maize roots upon mycorrhizal colonization. Phytochemistry 38 53–55 [Google Scholar]
- Krajinski F, Hause B, Gianinazzi-Pearson V, Franken P (2002) Mtha1, a plasma membrane H+-ATPase gene from Medicago truncatula, shows arbuscule-specific induced expression in mycorrhizal tissue. Plant Biol 4 754–761 [Google Scholar]
- Lerchl J, Geigenberger P, Stitt M, Sonnewald U (1995) Impaired photoassimilate partitioning caused by phloem-specific removal of pyrophosphate can be complemented by a phloem-specific cytosolic yeast-derived invertase in transgenic plants. Plant Cell 7 259–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link M, Rausch T, Greiner S (2004) In Arabidopsis thaliana, the invertase inhibitors AtC/VIF1 and 2 exhibit distinct target enzyme specificities and expression profiles. FEBS Lett 27 105–109 [DOI] [PubMed] [Google Scholar]
- Maeda D, Ashida K, Iguchi K, Chechetka SA, Hijikata A, Okusako Y, Deguchi Y, Izui K, Hata S (2006) Knockdown of an arbuscular mycorrhiza-inducible phosphate transporter gene of Lotus japonicus suppresses mutualistic symbiosis. Plant Cell Physiol 47 807–817 [DOI] [PubMed] [Google Scholar]
- Maier W, Peipp H, Schmidt J, Wray V, Strack D (1995) Levels of a terpenoid glycoside (blumenin) and cell wall-bound phenolics in some cereal mycorrhizas. Plant Physiol 109 465–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier W, Schmidt J, Nimtz M, Wray V, Strack D (2000) Secondary products in mycorrhizal roots of tobacco and tomato. Phytochemistry 54 473–479 [DOI] [PubMed] [Google Scholar]
- Marx C, Dexheimer J, Gianinazzi-Pearson V, Gianinazzi S (1982) Enzymatic studies on the metabolism of vesicular-arbuscular mycorrhizas. IV. Ultracytoenzymological evidence (ATPase) for active transfer processes in the host-arbuscule interface. New Phytol 90 37–43 [Google Scholar]
- Mosse B (1973) Plant growth responses to vesicular-arbuscular mycorrhiza. New Phytol 72 127–136 [Google Scholar]
- Nitz I, Berkefeld H, Puzio PS, Grundler FM (2001) Pyk10, a seedling and root specific gene and promoter from Arabidopsis thaliana. Plant Sci 161 337–346 [DOI] [PubMed] [Google Scholar]
- Olsson PA, van Aarle IM, Allaway WG, Ashford AE, Rouhier H (2002) Phosphorus effects on metabolic processes in monoxenic arbuscular mycorrhiza cultures. Plant Physiol 130 1162–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JM, Park C-J, Lee S-B, Ham B-K, Shin R, Paek K-H (2001) Overexpression of the tobacco Tsi1 gene encoding an EREBP/AP2-Type transcription factor enhances resistance against pathogen attack and osmotic stress in tobacco. Plant Cell 13 1035–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer PE, Douds DDJ, Bécard G, Shachar-Hill Y (1999) Carbon uptake and the metabolism and transport of lipids in an arbuscular mycorrhiza. Plant Physiol 120 587–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer PE, Shachar-Hill Y (1996) Plant/microbe symbiosis. In Y Shachar-Hill, PE Pfeffer, eds, Nuclear Magnetic Resonance in Plant Biology, Current Topics in Plant Physiology, ASPP Series, Volume 16. American Society of Plant Physiologists, Rockville, MD, pp 77–107
- Pfeiffer CM, Bloss HE (1988) Growth and nutrition of guayule (Parthenium argentatum) in a saline soil as influenced by vesicular-arbuscular mycorrhiza and phosphorus fertilization. New Phytol 108 315–321 [DOI] [PubMed] [Google Scholar]
- Quandt HJ, Pühler A, Broer I (1993) Transgenic root nodules of Vicia hirsuta: a fast and efficient system for the study of gene expression in indeterminate-type nodules. Mol Plant Microbe Interact 6 699–706 [Google Scholar]
- Rausch T, Greiner S (2004) Plant protein inhibitors of invertases. Biochim Biophys Acta 1696 253–261 [DOI] [PubMed] [Google Scholar]
- Ravnskov S, Wu Y, Graham JH (2003) Arbuscular mycorrhizal fungi differentially affect expression of genes coding for sucrose synthases in maize roots. New Phytol 157 539–545 [DOI] [PubMed] [Google Scholar]
- Requena N (2005) Measuring quality of service: phosphate “à la carte” by arbuscular mycorrhizal fungi. New Phytol 168 268–271 [DOI] [PubMed] [Google Scholar]
- Roitsch T, Balibrea ME, Hofmann M, Proels R, Sinha AK (2003) Extracellular invertase: key metabolic enzyme and PR protein. J Exp Bot 54 513–524 [DOI] [PubMed] [Google Scholar]
- Roitsch T, González M-C (2004) Function and regulation of plant invertases: sweet sensations. Trends Plant Sci 9 606–613 [DOI] [PubMed] [Google Scholar]
- Rolland F, Moore B, Sheen J (2002) Sugar sensing and signaling in plants. Plant Cell 14 S185–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Lozano JM, Azcón R (2000) Symbiotic efficiency and infectivity of an autochthonous arbuscular mycorrhizal Glomus sp. from saline soils and Glomus deserticola under salinity. Mycorrhiza 10 137–143 [Google Scholar]
- Schaarschmidt S, Qu N, Strack D, Sonnewald U, Hause B (2004) Local induction of the alc gene switch in transgenic tobacco plants by acetaldehyde. Plant Cell Physiol 45 1566–1577 [DOI] [PubMed] [Google Scholar]
- Schaarschmidt S, Roitsch T, Hause B (2006) Arbuscular mycorrhiza induces gene expression of the apoplastic invertase LIN6 in tomato (Lycopersicon esculentum) roots. J Exp Bot 57 4015–4023 [DOI] [PubMed] [Google Scholar]
- Schliemann W, Schmidt J, Nimtz M, Wray V, Fester T, Strack D (2006) Accumulation of apocarotenoids in mycorrhizal roots of Ornithogalum umbellatum. Phytochemistry 67 1196–1205 [DOI] [PubMed] [Google Scholar]
- Schubert A, Allara P, Morte A (2003) Cleavage of sucrose in roots of soybean (Glycine max) colonized by an arbuscular mycorrhizal fungus. New Phytol 161 495–501 [DOI] [PubMed] [Google Scholar]
- Schüssler A, Martin H, Cohen D, Fitz M, Wipf D (2006) Characterization of a carbohydrate transporter from symbiotic glomeromycotan fungi. Nature 444 933–936 [DOI] [PubMed] [Google Scholar]
- Shachar-Hill Y, Pfeffer PE, Douds D, Osman SF, Doner LW, Ratcliffe RG (1995) Partitioning of intermediary carbon metabolism in vesicular-arbuscular mycorrhizal leek. Plant Physiol 108 7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snellgrove RC, Stribley DP, Hepper CM (1987) Host-endophyte relationships: invertase in roots. Rothamsted Exp Stn Rep 1986 142 [Google Scholar]
- Solaiman MZ, Saito M (1997) Use of sugars by intraradical hyphae of arbuscular mycorrhizal fungi revealed by radiorespirometry. New Phytol 136 533–538 [DOI] [PubMed] [Google Scholar]
- Son CL, Smith SE (1988) Mycorrhizal growth responses: interactions between photon irradiance and phosphorus nutrition. New Phytol 108 305–314 [DOI] [PubMed] [Google Scholar]
- Sonnewald U, Brauer M, von Schaewen A, Stitt M, Willmitzer L (1991) Transgenic tobacco plants expressing yeast-derived invertase in either the cytosol, vacuole or apoplast: a powerful tool for studying sucrose metabolism and sink/source interactions. Plant J 1 95–106 [DOI] [PubMed] [Google Scholar]
- Sonnewald U, Hajirezaei M-R, Kossmann J, Heyer A, Trethewey RN, Willmitzer L (1997) Increased potato tuber size resulting from apoplastic expression of a yeast invertase. Nat Biotechnol 15 794–797 [DOI] [PubMed] [Google Scholar]
- Strack D, Fester T, Hause B, Schliemann W, Walter MH (2003) Arbuscular mycorrhiza: biological, chemical, and molecular aspects. J Chem Ecol 29 1955–1979 [DOI] [PubMed] [Google Scholar]
- Taussky HH, Shorr E (1953) A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem 202 675–685 [PubMed] [Google Scholar]
- Tester M, Smith FA, Smith SE (1985) Phosphate inflow into Trifolium subterraneum L.: effects of photon irradiance and mycorrhizal infection. Soil Biol Biochem 17 807–810 [Google Scholar]
- Tomlinson KL, McHugh S, Labbe H, Grainger JL, James LE, Pomeroy KM, Mullin JW, Miller SS, Dennis DT, Miki BLA (2004) Evidence that the hexose-to-sucrose ratio does not control the switch to storage product accumulation in oilseeds: analysis of tobacco seed development and effects of overexpressing apoplastic invertase. J Exp Bot 55 2291–2303 [DOI] [PubMed] [Google Scholar]
- Trépanier M, Bécard G, Moutoglis P, Willemot C, Gagné S, Avis TJ, Rioux J-A (2005) Dependence of arbuscular-mycorrhizal fungi on their plant host for palmitic acid synthesis. Appl Environ Microbiol 71 5341–5347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymowska-Lalanne Z, Kreis M (1998) The plant invertases: physiology, biochemistry and molecular biology. Adv Bot Res 28 71–117 [Google Scholar]
- van Aarle IM, Olsson PA (2005) Fungal lipid accumulation and development of mycelial structures by two arbuscular mycorrhizal fungi. Appl Environ Microbiol 69 6762–6767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierheilig H, Coughlan AP, Wyss U, Piché Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol 64 5004–5007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieweg M, Frühling M, Quandt H-J, Heim U, Bäumlein H, Pühler A, Küster H, Perlick A (2004) The promoter of the Vicia faba L. leghemoglobin gene VfLb29 is specifically activated in the infected cells of root nodules and in the arbuscule-containing cells of mycorrhizal roots from different legume and nonlegume plants. Mol Plant Microbe Interact 17 62–69 [DOI] [PubMed] [Google Scholar]
- von Schaewen A, Stitt M, Schmidt R, Sonnewald U, Willmitzer L (1990) Expression of a yeast-derived invertase in the cell wall of tobacco and Arabidopsis plants leads to accumulation of carbohydrate and inhibition of photosynthesis and strongly influences growth and phenotype of transgenic tobacco plants. EMBO J 9 3033–3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schweinichen C, Büttner M (2005) Expression of a plant cell wall invertase in roots of Arabidopsis leads to early flowering and an increase in whole plant biomass. Plant Biol 7 469–475 [DOI] [PubMed] [Google Scholar]
- Wright DP, Read DJ, Scholes JD (1998) Mycorrhizal sink strength influences whole plant carbon balance of Trifolium repens L. Plant Cell Environ 21 881–891 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.