Abstract
Based on the ability of phytosiderophores to chelate other heavy metals besides iron (Fe), phytosiderophores were suggested to prevent graminaceous plants from cadmium (Cd) toxicity. To assess interactions between Cd and phytosiderophore-mediated Fe acquisition, maize (Zea mays) plants were grown hydroponically under limiting Fe supply. Exposure to Cd decreased uptake rates of 59Fe(III)-phytosiderophores and enhanced the expression of the Fe-phytosiderophore transporter gene ZmYS1 in roots as well as the release of the phytosiderophore 2′-deoxymugineic acid (DMA) from roots under Fe deficiency. However, DMA hardly mobilized Cd from soil or from a Cd-loaded resin in comparison to the synthetic chelators diaminetriaminepentaacetic acid and HEDTA. While nano-electrospray-high resolution mass spectrometry revealed the formation of an intact Cd(II)-DMA complex in aqueous solutions, competition studies with Fe(III) and zinc(II) showed that the formed Cd(II)-DMA complex was weak. Unlike HEDTA, DMA did not protect yeast (Saccharomyces cerevisiae) cells from Cd toxicity but improved yeast growth in the presence of Cd when yeast cells expressed ZmYS1. When supplied with Fe-DMA as a Fe source, transgenic Arabidopsis (Arabidopsis thaliana) plants expressing a cauliflower mosaic virus 35S-ZmYS1 gene construct showed less growth depression than wild-type plants in response to Cd. These results indicate that inhibition of ZmYS1-mediated Fe-DMA transport by Cd is not related to Cd-DMA complex formation and that Cd-induced phytosiderophore release cannot protect maize plants from Cd toxicity. Instead, phytosiderophore-mediated Fe acquisition can improve Fe uptake in the presence of Cd and thereby provides an advantage under Cd stress relative to Fe acquisition via ferrous Fe.
Under certain challenging environmental conditions, plant roots may enhance the release of root exudates that can either increase the mobilization of essential mineral elements in the rhizosphere or inhibit uptake of toxic mineral elements from the soil solution (Marschner, 1995). These contrasting functions might apply even for one molecule or class of molecules depending on the physiological and environmental context. For example, citrate and other dicarboxylates are released by phosphorus-deficient or iron (Fe)-deficient plant roots to mobilize phosphate from sparingly soluble calcium (Ca), Fe, or aluminum (Al) precipitates. While Fe chelation by citrate promotes Fe solubility and subsequent Fe uptake, Al chelation by citrate decreases Al uptake (Neumann and Römheld, 2000). Thus, several plant species release citrate or other carboxylates in response to elevated Al concentrations in the soil solution to protect their roots from Al toxicity (Kochian et al., 2004).
Under Fe deficiency, graminaceous plant species release phytosiderophores, which are hexadentate metal chelators with high affinity for complex formation with Fe(III). The whole Fe(III)-phytosiderophore complex is subsequently taken up by Fe deficiency-inducible transporters of the YS1/YSL protein family (Römheld and Marschner, 1986; Curie et al., 2001). Metal chelation in the rhizosphere soil, however, is not specific for Fe(III), and consequently phytosiderophores have been found to mobilize a wide range of metals, including zinc (Zn), copper (Cu), manganese (Mn), nickel, and cadmium (Cd; Treeby et al., 1989; Awad and Römheld, 2000; Shenker et al., 2001). As assayed by two-electrode voltage clamp, most of these metal-phytosiderophore chelates were also transported via the maize (Zea mays) metal-phytosiderophore transporter ZmYS1 when expressed in Xenopus oocytes (Schaaf et al., 2004b). Whether phytosiderophore release is also directly or indirectly up-regulated under metal stresses other than Fe is currently under debate. In the case of Zn, earlier studies have shown that in certain graminaceous species, the release of phytosiderophores and uptake of Zn phytosiderophores might also be enhanced under Zn deficiency (Zhang et al., 1989; Tolay et al., 2001). By comparative gene expression analysis, it has now been shown that this is not an indirect response to Zn deficiency-induced Fe deficiency (Walter et al., 1994) but rather a direct response to Zn deficiency that is independent of Fe (Suzuki et al., 2006). With regard to toxic concentrations of metals, an enhanced release of phytosiderophores has also been reported for maize plants that were grown in the presence of Cd, and inhibition of Cd uptake via phytosiderophore chelation has been suggested as a mechanism to protect maize roots from Cd toxicity (Hill et al., 2002). Because the extent of phytosiderophore-mediated Cd mobilization strongly depended on the experimental conditions (Awad and Römheld, 2000; Shenker et al., 2001; Collins et al., 2003) and because the existence of a Cd-phytosiderophore complex has never been proven, an evaluation of the physiological importance of Cd-induced phytosiderophore release remains to be evaluated.
Plants represent the major route for the entry of Cd into the food chain. In humans and mammals, Cd might then cause genotoxic and cytotoxic effects, leading finally to the inhibition of cell proliferation and apoptosis. In plants, Cd can cause the inhibition of photosynthesis, respiration, and nitrogen metabolism as well as a decrease in water and mineral nutrient uptake (Deckert, 2005). Inside plant cells, Cd might displace Zn from Zn-binding molecules, such as from Zn-finger transcription factors, or attach to Ca-binding sites in calmodulin and thereby disturb intracellular signaling processes (Clemens, 2006). Already after a few hours of Cd incubation, it can cause oxidative stress, although this transition metal is unable to produce reactive oxygen species via redox reactions and does not participate in Fenton and Haber-Weiss reactions (Clemens, 2006; Garnier et al., 2006).
Inhibitory effects of Cd on the uptake of metal micronutrients have been described for different transport systems and growth conditions. With regard to transport processes across plant membranes, Cd efficiently competed with Fe, Mn, or Zn transport by AtIRT1 (Eide et al., 1996; Korshunova et al., 1999), with Ca transport by TaLCT1 or Ca channels (Clemens et al., 1998; Perfus-Barbeoch et al., 2002) or with Fe transport via AtNRAMPs (Thomine et al., 2003). Competition might also occur in other transporters that have been shown to transport Cd besides other metals (Pence et al., 2000; Mills et al., 2005). Interestingly, a Cd-induced decrease in the long-term accumulation of metal micronutrients was not consistently observed in grasses (Jalil et al., 1994; Shenker et al., 2001), even though Cd efficiently inhibited metal uptake in the short run (Hart et al., 2002) and provoked Fe deficiency (Larbi et al., 2002; Yoshihara et al., 2006). In most studies, however, short- and long-term effects of Cd on metal uptake have not been directly compared, and the role of phytosiderophore-mediated Fe acquisition in Cd uptake and Cd sensitivity of plants remained unaddressed.
It was therefore the aim of this study to evaluate a role of the phytosiderophore 2′-deoxymugineic acid (DMA) in preventing Cd toxicity in maize plants and to analyze possible interactions of Cd with Fe acquisition. To characterize the Cd-chelating potential of DMA, we conducted Cd mobilization tests from different substrates by DMA, carried out competition studies with Fe and Zn, and verified complex formation by high-resolution mass spectrometry (MS). We then investigated whether the presence of DMA protects yeast (Saccharomyces cerevisiae) or plant cells from Cd toxicity and how this relates to Fe uptake systems. Finally, we tested the hypothesis whether phytosiderophore-mediated Fe uptake in transgenic Arabidopsis (Arabidopsis thaliana) plants may decrease Cd sensitivity.
RESULTS
Uptake and Translocation of Fe Are Decreased in the Presence of Cd
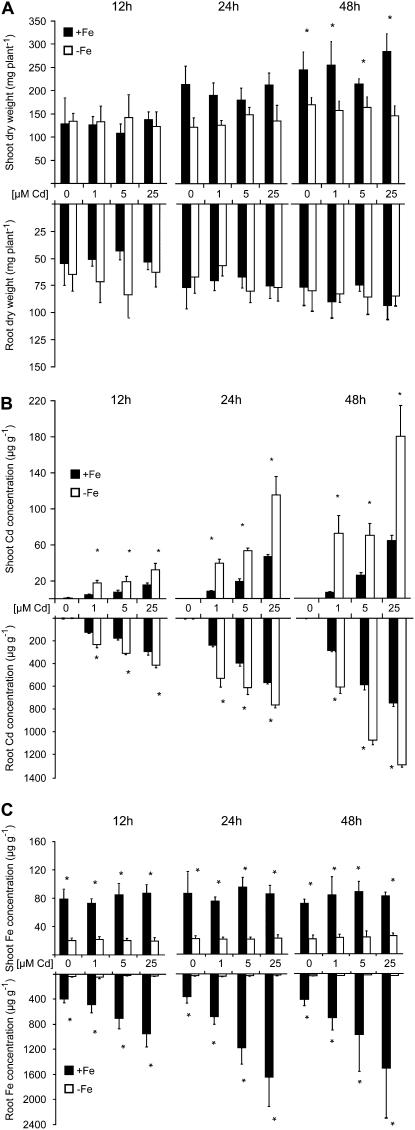
As a prerequisite to verify a role of phytosiderophores in the alleviation of Cd toxicity in strategy II plants, we first investigated the effect of Cd on growth and metal accumulation in maize plants precultured under adequate or limiting Fe supply. Despite 12 or 24 h of growth in the absence or presence of increasing Cd concentrations, biomass formation of roots and shoots was still unaffected by Cd irrespective of the Fe nutritional status (Fig. 1A). As expected, Cd accumulation in roots and shoots increased with the duration of Cd exposure and amount of Cd supply (Fig. 1B). Roots and shoots of plants that were grown under Fe deficiency, however, accumulated on average 2-fold more Cd than Fe-sufficient plants. At the same time, Fe concentrations in the shoot tissue of Cd-treated plants were unaffected by Cd exposure (Fig. 1C) and remained above a critical level of 66 μg g−1 (Marschner, 1995). In contrast, total root Fe accumulation in Fe-adequate plants increased with duration and amount of Cd supply but possibly merely reflected apoplasmic Fe, because extracytosolic Fe was not remobilized prior to analysis (Fig. 1C; Bienfait et al., 1985). Indeed, a separate analysis of apoplastic and symplastic Fe in tobacco (Nicotiana tabacum) roots recently indicated that in particular, apoplastic Fe pools increase during Cd treatment (Yoshihara et al., 2006).
Figure 1.
Fe deficiency increases Cd accumulation in maize plants. Fifteen-day-old Fe-sufficient and Fe-deficient plants were harvested after 12, 24, or 48 h following addition of 0, 1, 5, or 25 μm CdCl2 to the nutrient solution. A, Dry matter of shoot and roots. B, Cd concentrations. C, Fe concentrations. Significant differences among Fe-deficient (−Fe) and Fe-sufficient (+Fe) plants as determined by ANOVA followed by the Tukey test are indicated by an asterisk (P < 0.05; n = 4). Bars indicate means ± sd.
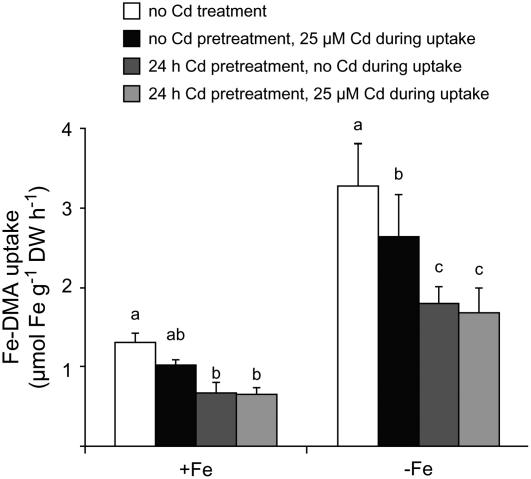
We then investigated a direct effect of Cd on Fe-DMA uptake. Uptake rates of 59Fe-labeled Fe-DMA were determined in the presence of Cd or after pretreating roots for 24 h with Cd. In the presence of 25 μm Cd only during the uptake assay, 59Fe-DMA uptake rates decreased by approximately 20% in Fe-sufficient roots relative to nontreated roots (Fig. 2). However, when roots were preincubated with Cd for 12 h, Fe-DMA uptake rates decreased by approximately 50% irrespective of whether Cd was still present or not during the uptake period. In Fe-deficient roots, uptake rates of Fe(III)-DMA were at an approximately 2.5 times higher level than in Fe-sufficient roots, but Cd showed similar quantitative effects, with Cd preincubation of roots leading to the strongest suppression of Fe(III)-DMA uptake rates. This experiment suggested that inhibition of Fe-DMA uptake is an early event in Cd-mediated toxicity.
Figure 2.
Cd inhibits Fe-phytosiderophore uptake in maize roots. Uptake rates of 59Fe-labeled Fe-DMA in roots of maize plants precultured in the presence or absence of Cd. Fe-sufficient and Fe-deficient plants were either pretreated with 25 μm Cd for 24 h prior to the Fe-DMA uptake experiment or Cd was added during the uptake period, or both treatments were combined. 59Fe-labeled Fe-DMA was supplied at 10 μm for 60 min. Bars indicate means ± sd, and significant differences among different Cd treatments within the Fe-deficient (−Fe) and Fe-sufficient (+Fe) plants as determined by ANOVA followed by the Tukey test are indicated by different letters (P < 0.05, n = 4).
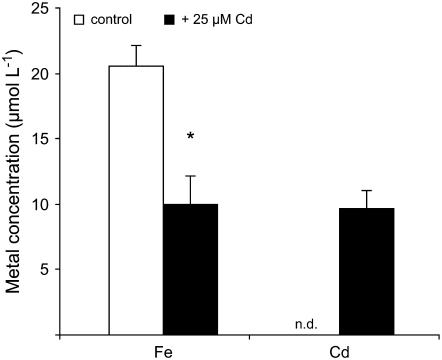
Translocation rates of 59Fe showed exactly the same relative differences among Cd treatments as root uptake rates but at an approximately 20-fold lower level (data not shown). To better quantify this observation, we compared the Fe concentration in the xylem sap of Fe-deficient maize plants after Fe resupply in the presence or absence of Cd. While the bleeding rate of the xylem sap remained unaffected by Cd (data not shown), Fe concentrations in the xylem sap of decapitated maize plants decreased by approximately 50% in response to Cd supply (Fig. 3). Cd-treated plants translocated an almost equimolar amount of Cd and Fe to the shoots. These lower Fe translocation rates in the presence of Cd most likely reflected the inhibition of Fe-DMA uptake by Cd (Fig. 2) and suggested that 48 h of Cd exposure before determining total Fe concentrations in shoots (Fig. 1C) was obviously too short to significantly decrease Fe concentrations in the shoot tissue.
Figure 3.
Cd stress decreases the Fe concentration in the xylem sap of Fe-deficient plants. Fe and Cd concentrations in the xylem bleeding sap of Fe-resupplied plants grown in the absence (control) or presence of 25 μm Cd. Plants were precultured for 23 d under Fe deficiency before Fe and Cd were added to the nutrient solution for a period of 48 h. Significant differences between control and Cd treatments as determined by ANOVA and Tukey test are indicated by an asterisk (P < 0.05, n = 4). n.d., Not detected.
Cd Up-Regulates the Fe-Deficiency Stress Response in Maize Roots
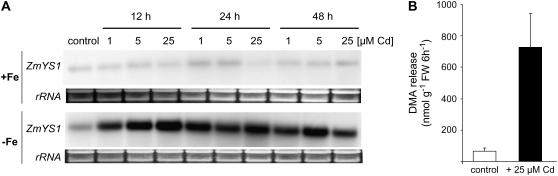
To characterize the Fe nutritional status of Cd-treated maize plants, expression of the major Fe-phytosiderophore transporter in Fe-deficient maize roots, ZmYS1 (Curie et al., 2001), was monitored in response to Cd supply. ZmYS1 mRNA levels were low or not detectable in Fe-sufficient roots and Cd treatment did not substantially alter gene expression levels (Fig. 4A). Transcript levels of ZmYS1 were slightly higher in Fe-deficient roots but dramatically increased in a concentration-dependent manner within the first 12 h of Cd treatment, whereas no further significant effect was observed after prolonged incubation with Cd except a slight decrease of mRNA levels after 48 h at 25 μm Cd. Maize plants were then grown under axenic conditions in the absence of Fe, resulting in a detectable amount of phytosiderophores being released from the roots (Fig. 4B). In the presence of Cd, however, a 7-fold increase in the rate of DMA release was determined. Thus, Cd appeared to up-regulate both components of the strategy II response, phytosiderophore release, and Fe-phytosiderophore transporter gene expression.
Figure 4.
Cd induces Fe-deficiency stress responses in maize. A, RNA gel-blot analysis of ZmYS1 expression in roots from hydroponically grown plants that were precultured for 20 d in absence of Fe or in the presence of 100 μm Fe(III)-EDTA before addition of 0, 1, 5, or 25 μm CdCl2 for 12, 24, or 48 h to the nutrient solution. Plants were harvested at the same time, and total RNA from roots was used for hybridization to the complete ORF of ZmYS1. Ethidium bromide-stained gel blots are shown as a control. B, Rate of DMA release within 6 h from roots of 10-d-old maize plants grown under axenic conditions. The plants were incubated in the absence or presence of 25 μm Cd for 24 h before being transferred to ultrapure water for collection of the exudates. Bars indicate means ± sd (n = 5).
Phytosiderophores Poorly Mobilize Cd
To investigate a role of phytosiderophores in Cd chelation, an aliquot of a soil being naturally rich in heavy metals was extracted by phytosiderophores or by the synthetic chelator diaminetriaminepentaacetic acid (DTPA). In agreement with ammonium nitrate-extractable amounts of metals (see “Materials and Methods”), DTPA efficiently mobilized Zn, Cu, and to a lesser extent also Cd (Table I), confirming that Cd mobilization is increased in the presence of this ligand. In contrast, DMA efficiently mobilized Cu and Zn but almost no Cd, suggesting a particularly low affinity of phytosiderophores to Cd in comparison to synthetic chelators.
Table I.
Phytosiderophores poorly mobilize Cd from soil
Concentrations of Cd, Cu, and Zn (nanomoles per liter) in the filtrates of a soil naturally rich in heavy metals, after incubation with water, 50 μm DMA, or 50 μm DTPA solution. Values represent means ± sd. Significant differences are indicated for each element by different letters as determined by ANOVA followed by Tukey test (P < 0.05; n = 4).
| Element | Water | DMA | DTPA |
|---|---|---|---|
| Cd | 0.3 ± 0.0 b | 1.2 ± 0.0 b | 109.6 ± 3.4 a |
| Cu | 22.1 ± 1.2 c | 452.7 ± 5.8 a | 264.8 ± 10.3 b |
| Zn | 2.1 ± 2.1 c | 389.2 ± 2.6 b | 462.6 ± 17.2 a |
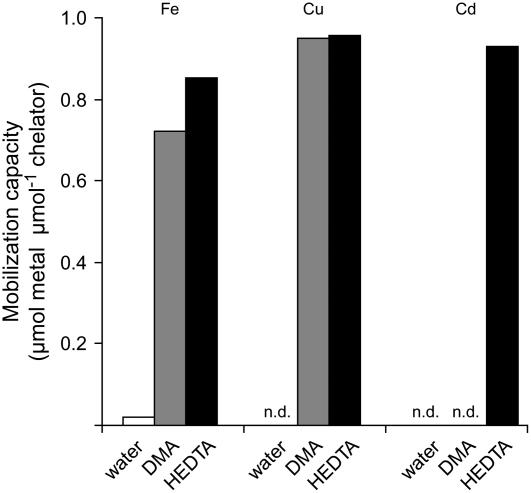
Because metal-binding forms and equilibrium reactions in soils are complex and difficult to predict, we reevaluated the Cd mobilization capacity by phytosiderophores using a synthetic resin loaded with a single metal in incubation assays with DMA for comparison to the synthetic chelator HEDTA that is a weaker ligand for Cd, Zn, and Cu than DTPA (Norvell, 1991). As expected, both chelators mobilized Fe(III) from the resin and even more efficiently Cu(II) (Fig. 5), which is in agreement with a slightly higher complex formation constant of DMA with Cu(II) than with Fe(III) (Murakami et al., 1989). Notably, Cd mobilization was also high for HEDTA but not detectable in the case of DMA (Fig. 5). This was unexpected with regard to the metal adsorption properties reported for the resin, which has been reported to bind Cu even more firmly than Cd (manufacturer's protocol, Bio-Rad). Taken together, two independent mobilization assays showed that the phytosiderophore DMA is apparently a weak or inefficient ligand for the chelation of Cd relative to Zn, Cu, or Fe(III).
Figure 5.
The phytosiderophore DMA does not mobilize Cd from a Cd-loaded resin. Mobilization of Cd, Fe, and Cu was determined from a Cd-, Fe-, or Cu-loaded Chelex resin using water, a HEDTA, or DMA solution at pH 5.5 as eluent for metal extraction. sds in all treatments were too low to become visualized (n = 4).
Low Competitivity of Cd in Metal-Phytosiderophore Complex Formation
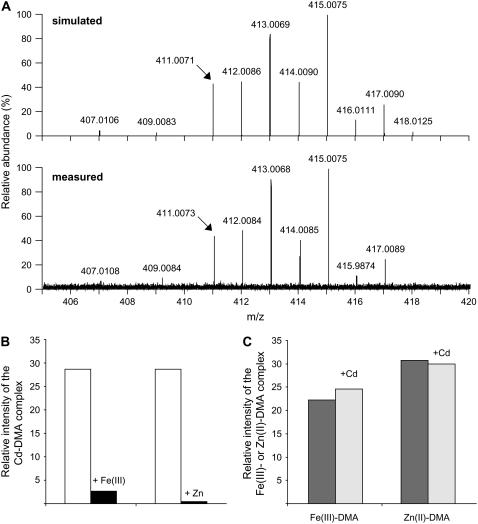
Nano-electrospray-Fourier transform ion cyclotron resonance-MS (nano-ESI-FTICR-MS) was then employed to verify the formation of Cd-DMA complexes in aqueous solutions. This type of high-resolution MS has recently been shown to accurately discriminate among metal chelate species that differ in even less than 0.02 mass-to-charge ratio (m/z; Weber et al., 2006). When the measured nano-ESI-mass spectrum in the negative ionization mode of a Cd(II)-containing DMA solution was compared with the calculated mass spectrum of the Cd-DMA complex ([M − 3H + Cd(II)]−, C12H17O7N2Cd1, m/z 407.0106), the simulated mass spectrum of the Cd(II)-DMA complex was accurately reflected in the measured sample (Fig. 6A). The relative abundance of the naturally occurring stable Cd isotopes is approximately 106Cd (1.3%), 108Cd (0.9%), 110Cd (12.5%), 111Cd (12.8%), 112Cd (24.1%), 113Cd (12.2%), 114Cd (28.7%), and 116Cd (7.5%), which explains the obtained mass distribution. On the basis of the Cd isotope pattern, the nano-ESI-FTICR mass spectrum (m/z 150–800) of a solution made from 20 μm DMA and 10 μm Cd(II) chloride indicated only the presence a single negatively charged 1:1 complex (Fig. 6A).
Figure 6.
Phytosiderophores form a weak complex with Cd as revealed by nano-ESI-FTICR-MS. A, ESI ionization mass spectra of Cd-DMA complexes in the negative ionization mode. Top graph, Calculated mass spectrum of the Cd(II)-DMA complex ([DMA − 3H + Cd(II)]−). Bottom graph, Section of the mass spectrum (m/z 150–800) of a 20-μm DMA solution supplemented with 10 μm Cd(II); resolution (full width at half maximum) 200,000. B and C, Competition studies between Cd and Fe or Zn for phytosiderophore complex formation. Addition of 10 μm Fe(III) or Zn(II) to a 10-μm Cd(II)-DMA solution (left) or of 10 μm Cd(II) to 10 μm Fe(III)-DMA or Zn(II)-DMA solution (right). Mass spectra were recorded by nano-ESI-FTICR after 30 min of incubation. Experiments were repeated several times with similar results, and representative values of one experiment are shown.
As an alternative approach to characterize the complex stability of Cd-DMA relative to other metals, Fe(III) and Zn were added to a 10-μm Cd-DMA solution before the Cd-DMA complex was redetermined by nano-ESI-FTICR. Addition of Fe(III) in an equimolar ratio to Cd led to an almost complete disappearance of the Cd-DMA complex (Fig. 6B). Employing Zn as a competing metal, which has an approximately 5 orders of magnitude lower stability constant with DMA relative to Fe(III) (Murakami et al., 1989), also led to an almost complete dissociation of Cd from the ligand. Even though these competition experiments were conducted in simplified solutions that did not consider the presence of other chelating and competing substances or redox reactions that might occur simultaneously in complex soil solutions, they indicated that Cd was rapidly outcompeted by Fe(III) and Zn from the DMA complex. This was in strong agreement with the observation that Cd was not chelated by DMA in the presence of other heavy metals (Table I). When equimolar Cd concentrations were added to compete with Fe(III) or Zn(II) in a preformed DMA complex, Cd did not significantly displace any Fe(III) or Zn from the DMA complex (Fig. 6C), confirming that DMA plays a minor role in Cd complexation.
The Effect of Phytosiderophore Chelation on Cd Uptake by Maize Roots
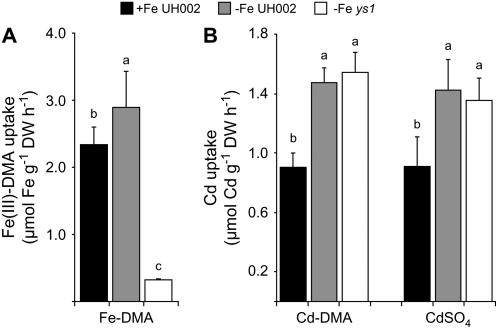
Because the presence of other metals leads to a destabilization of the Cd-DMA complex (Fig. 6C), a short-term uptake experiment was conducted in which root uptake of DMA-chelated 109Cd was compared to that of free 109Cd2+ in the absence of competing metals. For that purpose, wild-type maize plants were starved for Fe, which led to an increase in the uptake capacity for 59Fe-labeled Fe(III)-DMA (Fig. 7A). Fe-deficient ys1 mutant plants lacking functional ZmYS1 expression (Schaaf et al., 2004a) took up Fe(III)-DMA at a 10-fold lower rate. Uptake rates of DMA-chelated, radiolabeled Cd, however, did not differ between Fe-deficient wild-type and ys1 plants (Fig. 7B), indicating that the metal-phytosiderophore transporter ZmYS1 was irrelevant for Cd uptake from Cd-DMA. Nevertheless, Cd uptake rates were higher in Fe-deficient than in Fe-sufficient wild-type plants, showing that a Fe deficiency-inducible transport system contributed to Cd uptake. Interestingly, Cd uptake rates from Cd-DMA in both plant lines exactly matched those from CdSO4, strongly suggesting that phytosiderophore chelation of Cd could not significantly avoid the generation of free Cd2+ and subsequent Cd2+ uptake.
Figure 7.
Cd uptake by maize roots is not prevented by phytosiderophore chelation. A, Short-term uptake rates of 59Fe-labeled Fe-DMA in roots of 17-d-old plants of wild-type maize (UH002) or of the ys1 mutant precultured under Fe-sufficient (+Fe) or Fe-deficient (−Fe) conditions for the whole growth period. B, Short-term uptake rates of 109Cd-labeled CdSO4 or Cd-DMA in the same plant lines as used in A. An inbred line (UH002) and the ys1 mutant were compared. Bars indicate means ± sd and significant differences are indicated by different letters as determined by ANOVA and Tukey test (P < 0.05; n = 4).
Phytosiderophores Cannot Protect Yeast from Cd Toxicity
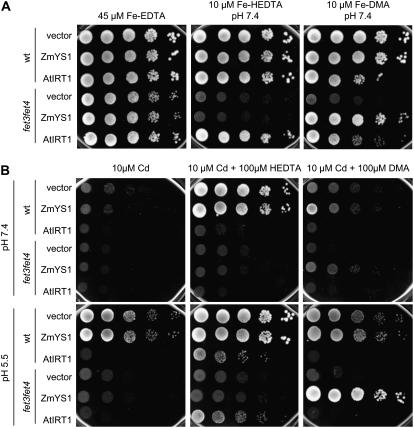
Because Hill et al. (2002) reported that addition of phytosiderophore-containing root exudates from Fe-deficient maize roots alleviated maize plants from Cd stress, we reinvestigated this possibility by addition of phytosiderophores or HEDTA to yeast cells exposed to toxic Cd concentrations. Employing the model system yeast provided the advantage that different yeast strains could be used to distinguish between uptake pathways for Cd or Fe and their corresponding phytosiderophore complexes. Growth complementation of the Fe uptake-defective yeast mutant fet3fet4 on low concentrations of either Fe(III)-HEDTA or Fe(III)-DMA confirmed functionality of the heterologously expressed Arabidopsis Fe2+ transporter AtIRT1 and of the maize Fe(III)-phytosiderophore transporter ZmYS1 and their dependence on the supplied Fe-binding forms (Fig. 8A). Wild-type yeast transformants grew well on any kind of Fe source, except when AtIRT1-expressing cells were supplied with Fe-DMA, which led to a weak growth reduction. Addition of 10 μm Cd in the presence of 10 μm Fe led to complete growth depression of all yeast transformants (Fig. 8B). In the presence of HEDTA, however, wild-type yeast cells overcame Cd stress as long as AtIRT1, which confers high Cd uptake (Connolly et al., 2002), was not ectopically expressed. In contrast, supplementation of DMA could not restore growth of wild-type yeast on toxic Cd concentrations. Thus, Cd chelation by phytosiderophores was apparently not efficient to protect yeast cells from Cd toxicity. Interestingly, ZmYS1-expressing cells tended to overcome Cd toxicity better in the presence of DMA rather than in the presence of HEDTA if the medium pH was buffered at 5.5.
Figure 8.
Phytosiderophores do not protect yeast from Cd toxicity but allow growth of ZmYS1-transformed yeast cells on Cd. A, Growth complementation assay of wild-type yeast and the Fe-uptake defective mutant fet3fet4 transformed with the empty pDR195 vector, pDR196-ZmYS1, or pDR195-AtIRT1. B, Growth complementation assay of the same strains as in A but in the presence of Cd. Except for the 45-μm Fe-EDTA treatment, all media (YNB-ura) were buffered with 50 mm MES-Tris at pH 7.4 or 5.5 and supplemented with 10 μm Fe. For drop tests, yeast precultures in YNB-ura + 45 μm Fe-EDTA were pelleted, washed in Na-EDTA solution, and resuspended in water to achieve an optical density of 1.0 at 600 nm. Cells were diluted by 10-fold, and 10 μL of each dilution were spotted on YNB-ura plates supplemented or not with Cd and/or the chelators HEDTA or DMA. Plates were photographed after 5 d of incubation at 28°C.
Overexpression of the ZmYS1 Gene Alleviates Strategy I Plants from Cd Toxicity in the Presence of Fe-DMA
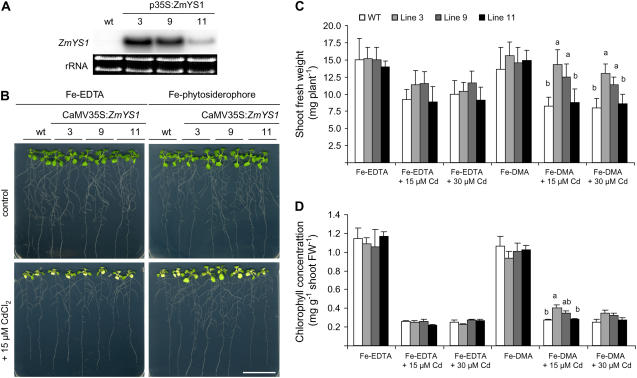
Following the observations that ZmYS1-expressing yeast cells grew slightly better in the presence of Cd (Fig. 8B) and that overall Cd uptake rates were independent of phytosiderophores (Fig. 7), we compared Cd sensitivity of Fe acquisition via Fe2+ or Fe-DMA in planta. For this purpose, the ZmYS1 cDNA was overexpressed under control of a cauliflower mosaic virus (CaMV) 35S promoter in Arabidopsis. Homozygote T2 plants were obtained based on segregation analysis on kanamycin. Three independent lines were selected in the T3 generation and confirmed for expression of the ZmYS1 gene in a northern-blot analysis (Fig. 9A). Transcript levels of ZmYS1 were high in roots of the lines 3 and 9, but low in line 11. Plants of these lines were then pregerminated in 0.5 Murashige and Skoog medium for 8 d before being transferred to agar plates containing either Fe-phytosiderophores or Fe-EDTA as a Fe source and continued to grow for 10 d. In the absence of Cd, wild-type and transgenic lines grew similarly well and free of any visual symptoms under supply of either Fe form (Fig. 9B). In the presence of Cd, however, wild-type plants and transgenic lines grown on Fe-EDTA showed an approximately 30% decrease in shoot biomass accompanied by severe chlorosis (Fig. 9, C and D). These Cd-induced symptoms were similar in all lines. When Fe was supplied as Fe-DMA, however, the two CaMV35S-ZmYS1-transformed lines with highest expression levels exhibited no significant decrease in shoot fresh weight in response to 15 μm Cd and only an approximately 15% decrease in response to 30 μm Cd relative to control plants not exposed to Cd (Fig. 9C). With regard to plant development, shoot biomass of the two transgenic lines 3 and 9 and leaf chlorophyll levels of line 3 were superior to those of wild-type plants grown on Fe-DMA. Taken together, these results indicated that Cd-induced growth repression in wild-type plants is similar under supply of Fe-EDTA or Fe-DMA as Fe source, but that Cd stress is alleviated in transgenic plants expressing ZmYS1 provided that Fe-DMA is made available.
Figure 9.
Functional expression of ZmYS1 improves growth of Arabidopsis plants under Cd stress in the presence of Fe phytosiderophore. A, RNA gel-blot analysis of Arabidopsis root RNA extracted from wild-type or homozygous T3 plants transformed with a CaMV35S:ZmYS1 construct. ZmYS1 expression was detected in hydroponically grown, Fe-sufficient plants. Ethidium bromide-stained gel blots are shown as a loading control. B, Growth phenotype of plants grown on modified 0.5 Murashige and Skoog agar plates supplemented with 20 μm Fe(III)-EDTA or Fe(III)-DMA. Plants in bottom section were grown in the presence of 15 μm CdCl2. Plates of representative plants were photographed 10 d after transfer of seedlings to the treatments. Bar = 3 cm. C, Quantitative analysis of shoot growth after plant cultivation as described in B with different Fe-binding forms and Cd concentrations. D, Chlorophyll concentrations in shoots of plants as grown in B. Significant differences at P < 0.05 as determined by ANOVA followed by Tukey test are indicated by different letters (n = 7). Presented data are representative for three independent experiments.
DISCUSSION
Cd Chelation by Phytosiderophores Is Too Weak to Protect Plant Roots from Cd Uptake
In nongraminaceous plant species, the Fe2+ transporter IRT1 has been identified as a major transport pathway for Cd uptake in Fe-deficient roots (Cohen et al., 1998, 2004; Connolly et al., 2002). With the recent isolation of IRT orthologs from rice (Oryza sativa) that are also up-regulated under Fe deficiency and promising candidates to contribute to Fe2+ uptake (Ishimaru et al., 2006), the question was raised whether this transporter family might also confer Cd sensitivity in graminaceous species. Indeed, both root-expressed proteins, OsIRT1 and OsIRT2, conferred Cd uptake when heterologously expressed in yeast (Nakanishi et al., 2006). To avoid Cd toxicity, it has been suggested that a simultaneous Fe deficiency-induced release of phytosiderophores might protect graminaceous plants against Cd uptake as a consequence of external Cd chelation (Hill et al., 2002). This study was set out to investigate the role of phytosiderophores in Cd tolerance in maize and reports here on the basis of several independent methodological approaches that phytosiderophores form Cd chelates that are too weak to protect plant cells from Cd uptake.
First, the phytosiderophore DMA hardly mobilized any Cd from a soil substrate that was naturally rich in Cd (Table I). While phytosiderophores may solubilize Cd from precipitated Cd3(PO4)2 even in the presence of Fe(OH)3 (Shenker et al., 2001), a poor mobilization capacity for Cd from Cd-enriched soil substrates has been reported for root exudates from Fe-deficient maize or wheat (Triticum aestivum) plants (Mench and Martin, 1991; Awad and Römheld, 2000). Obviously, a different composition of the test solutions might be responsible for these variations. Second, DMA efficiently mobilized Fe(III) and Cu(II) but not Cd(II) from a metal-loaded resin, although binding affinities to the resin decreased in the order Fe(III) > Cd(II) > Cu(II) (manufacturer's protocol, Bio-Rad). In contrast, HEDTA mobilized all three metals at a similar rate (Fig. 5). Phytosiderophores thus appeared as inefficient Cd-mobilizing compounds relative to synthetic chelators.
A weak complex formation was then identified as a major reason for the inefficiency of phytosiderophores to mobilize Cd. Chemical analysis by nano-ESI-FTICR MS of an aqueous solution to which Cd(II) and DMA were added separately proved formation of an intact complex (Fig. 6A). This Cd(II)-DMA complex was formed in a 1:1 stoichiometry, comparable to that of Fe(III)-DMA (Weber et al., 2006). However, after supply of equimolar amounts of Fe(III) or Zn, Cd-DMA complexes almost completely disappeared, while Cd itself was not sufficiently competitive to replace Fe(III) or Zn in a DMA complex (Fig. 6, B and C). Although the affinity constant for Cd-DMA still remains to be determined, these observations indicate that Cd-DMA complex formation will become physiologically irrelevant in a complex soil solution when other divalent cations, heavy metals, or protons are present. Other than proposed by Hill et al. (2002), our observations lead to the conclusion that phytosiderophores do not have a significant potential for the protection of plant roots against Cd toxicity due to weak and noncompetitive complex formation. The inefficiency of phytosiderophores to protect plant roots from Cd uptake was finally confirmed in planta by the observation that short-term uptake rates of radiolabeled Cd were independent of the form (Cd-DMA or CdSO4) in which Cd was supplied to the nutrient solution (Fig. 7). Furthermore, these results indicate that phytosiderophores are probably not a promising target for phytoremediation of Cd-contaminated soils irrespective of whether Cd immobilization or phytoextraction is envisaged.
Most likely as a consequence of weak complex formation, DMA also failed to protect yeast cells from Cd toxicity. In contrast to the addition of the synthetic chelator HEDTA, DMA supplementation did not alleviate yeast wild-type cells from Cd stress (Fig. 8). The inability of DMA to alleviate yeast cells from Cd toxicity was recently reported by Nakanishi et al. (2006) and found to be independent of whether the metal-DMA transporter OsYSL15 was expressed in these cells. These findings strongly agreed with the fact that DMA complexation did not influence root uptake rates of radiolabeled Cd, which were identical to the uptake rates of free Cd (Fig. 7). Nevertheless, small currents provoked by Cd-DMA in ZmYS1-expressing oocytes, as determined by two-electrode voltage clamp, indicated that ZmYS1 has the potential to take up Cd-DMA (Schaaf et al., 2004b). We therefore conclude that it is not the selectivity of the metal-phytosiderophore transporter ZmYS1 that restricted Cd-DMA uptake by ZmYS1 (Schaaf et al., 2004b; Fig. 6) but rather the weak affinity of DMA to this metal. In other recent experiments, different metal-complex stabilities might also have influenced reported substrate specificities, as deduced from comparative transport assays with metal-phytosiderophores or metal-nicotianamine and YS1/YSL proteins expressed in heterologous systems (DiDonato et al., 2004; Koike et al., 2004; Roberts et al., 2004). It is therefore recommended that these substrate specificities should be verified after binding constants have been determined and complex formation has been assured under the given experimental conditions.
Fe Acquisition via Phytosiderophores Decreases Cd Sensitivity in Plants
With regard to transport processes across plant membranes, Cd has been reported to efficiently compete with Fe, Mn, or Zn transport by AtIRT1 (Eide et al., 1996; Korshunova et al., 1999) or with Fe transport via AtNRAMPs (Thomine et al., 2003). In the case of graminaceous species, short-term effects of Cd on Fe-phytosiderophore uptake have so far not been reported, to our knowledge, but when Cd was added to hydroponically grown plants, Fe accumulation was neither significantly nor consistently affected irrespective of the Fe nutritional status of the plants (Shenker et al., 2001; Hill et al., 2002). When we cultured maize plants at increasing Cd supply, we also observed that Fe concentrations in shoots were not significantly affected after 2 d of Cd supply (Fig. 1C). Short-term uptake experiments with radiolabeled Fe-DMA, however, showed that Cd exerts an instantaneous inhibition on Fe-DMA uptake in maize roots. At first glance, a decrease of Fe(III)-DMA uptake rates by Cd (Fig. 2) was unexpected with regard to an up-regulation of ZmYS1 gene expression in the presence of Cd (Fig. 4A). Based on the inability of Cd to outcompete Fe(III) from a DMA complex (Fig. 6C), reduced Fe-DMA uptake rates were not the consequence of a lower Fe(III)-DMA concentration in solution but rather the consequence of an inhibitory action of Cd on the Fe-phytosiderophore transport system. Indeed, the presence of Cd during the 59Fe(III)-DMA transport assay had a weaker inhibitory effect than preincubation of roots with Cd and absence of Cd during the uptake assay (Fig. 2). As a consequence, a lower Fe concentration in the xylem sap was found in plants incubated with Cd, indicating a lower overall Fe translocation to shoots (Fig. 3). This might have impaired the Fe nutritional status of the shoot (Schmidt, 2003; Yoshihara et al., 2006) and, in turn, led to enhanced DMA release and enhanced expression of the Fe-phytosiderophore transporter gene ZmYS1 (Fig. 4). However, a systemic signal from the shoot might not be sufficiently rapid to explain the ZmYS1 induction that occurred within a few hours under Cd stress. Alternatively, it might be possible that a Cd-induced change in the labile Fe pool of root cells triggered the induction of Fe-deficiency responses. In Arabidopsis, transcript and protein levels of the Fe2+ transporter IRT1 were down-regulated already after 12 h of Cd supply even under Fe-limiting growth conditions (Connolly et al., 2002). This rapid response represents a protective mechanism at the transcriptional level against further Cd uptake by IRT1. In contrast, ZmYS1 expression in maize roots was not down-regulated after Cd supply but remained most likely under transcriptional control of Fe deficiency, which, itself, was enhanced by the presence of Cd. Taken together, these experiments indicated that inhibition of Fe(III)-phytosiderophore transport across the root plasma membrane is an early target of Cd-mediated toxicity, to which maize plants respond with an increased expression of the Fe-phytosiderophore transporter gene.
Based on the findings that Cd induces Fe-deficiency stress responses and IRT1-mediated Cd accumulation in strategy I plants (Cohen et al., 1998; Larbi et al., 2002; Vert et al., 2002; Yoshihara et al., 2006) and that phytosiderophores did not significantly contribute to overall Cd uptake (Fig. 7), we hypothesized that Fe uptake via phytosiderophores might decrease Cd sensitivity in a strategy I plant. We verified this hypothesis first in yeast and then in transgenic Arabidopsis plants. Yeast cells mainly employ strategy I-like mechanisms for Fe acquisition and express with Fet4p an unspecific metal transporter that confers Cd sensitivity, similar to IRT1 from plants (Li and Kaplan, 1998; Jensen and Culotta, 2002; Fig. 8B). When Fe-efficient wild-type cells were transformed with AtIRT1, Cd sensitivity increased, and yeast growth could not be restored by the presence of HEDTA. In contrast, HEDTA alleviated control transformants from Cd stress. Arrested growth of control transformants in the presence of Cd and DMA revealed that DMA was inefficient to decrease Cd availability. In the presence of DMA, only the expression of ZmYS1 appeared to improve yeast growth on toxic Cd levels (Fig. 8B). The relatively better growth of ZmYS1, relative to control transformants on Cd, suggested that DMA did not interfere with Cd uptake but improved Fe nutrition of yeast cells during Cd stress by bypassing Cd inhibition of Fe uptake. In contrast, Cd-mediated inhibition of Zn uptake in wheat was explained by a competitive interaction at the root plasma membrane transport level (Hart et al., 2002), and field experiments showed that Zn resupply alleviated wheat plants from Cd toxicity (Koleli et al., 2004). Interestingly, this alleviation by Zn was not accompanied by a decrease in Cd concentrations in shoots. Taken together, these observations may indicate that in the long run, Cd-induced inhibition of Fe and Zn uptake and subsequent Fe and Zn deficiency might contribute to the frequently reported production of reactive oxygen species in Cd-treated plants (Deckert, 2005; Garnier et al., 2006). In addition to GSH inactivation due to Cd binding as suggested by Clemens (2006), a lack of superoxide dismutase activities and other Fe- and Zn-dependent antioxidative enzymes might also promote the evolution of reactive oxygen species in response to Cd.
As ZmYS1 did not contribute to Cd uptake (Fig. 7), Fe-phytosiderophore transport in general might improve Fe nutrition under Cd stress in grasses relative to nongraminaceous plants. To confer transgenic Arabidopsis plants the possibility of taking up Fe via phytosiderophores, we expressed ZmYS1 constitutively and grew these plants on Fe(III)-EDTA or Fe(III)-DMA. Under supply of Fe(III)-EDTA and in the presence of Cd, wild-type and transgenic Arabidopsis lines exhibited severe chlorosis, whereas transgenic lines expressing ZmYS1 at higher levels remained greener and showed less growth suppression when Fe(III)-DMA was the Fe source (Fig. 9, B and C). In comparison, Connolly et al. (2002) reported that 35S-AtIRT1 Arabidopsis transgenic plants were more sensitive to Cd than wild-type plants, due to a higher accumulation of this metal in the tissue. Thus, phytosiderophore-mediated Fe acquisition is less sensitive to the presence of Cd in the growth medium than IRT1-dependent Fe acquisition. This is additionally supported by the observations that Cd-DMA is not transported by ZmYS1 and that the transcriptional up-regulation of ZmYS1 in response to Cd can compensate for the Cd-mediated inhibition of Fe-DMA transport in maize.
MATERIALS AND METHODS
Plant Culture and Growth Conditions
Maize (Zea mays) seeds (UH002 inbred line or ys1) were rinsed for 3 min in 70% ethanol for 10 min in 15% hydrogen peroxide solution and finally in distilled water. Seeds were germinated in the dark between filter papers soaked with CaSO4-saturated solution for 4 to 5 d and transferred to a half-strength nutrient solution without Fe. After 2 d, seedlings were transferred to a full nutrient solution containing 2.0 mm Ca(NO3)2, 0.7 mm K2SO4, 0.5 mm MgSO4, 0.1 mm KCl, 0.1 mm KH2PO4, 1.0 μm H3BO3, 0.5 μm MnSO4, 0.5 μm ZnSO4, 0.2 μm CuSO4, and 0.01 μm (NH4)6Mo7O24. In Fe-sufficient treatments, 100 μm Fe(III)-EDTA was supplied. The nutrient solution was renewed every 2 or 3 d. Plants were grown hydroponically under aerated and nonsterile conditions in a climate chamber under the following conditions: 16/8 h light/dark; light intensity 280 μmol photons m−2 s−1; temperature 25°C/20°C; and 60% humidity. In all experiments, plants were harvested at the same time of day (usually 7 h after onset of light).
Radiotracer Uptake Studies in Maize Plants
For short-term uptake experiments of radiolabeled substrates, plants were transferred 1 d before the experiment to 0.5-L pots containing nutrient solution without micronutrients. Plants were then transferred to fresh nutrient solution supplemented with 10 μm 109CdSO4, 109Cd-DMA, or 10 μm 59Fe(III)-DMA (the latter two with 10% excess of DMA), which had been prepared 1 d before the experiment and shaken overnight. The specific activity was 6.2 GBq/mol for both radioisotopes. To remove the extraplasmatic Cd, plants were rinsed with 1 mm CaSO4 solution for 10 min, whereas the Fe-DMA-treated plants were washed with 1 mm CaSO4 solution and bipyridyl solution under N2 flushing to create reductive conditions and remove apoplasmic Fe (Bienfait et al., 1985). Thereafter, roots and shoots were separated and dried at 60°C for determination of dry mass followed by ashing at 500°C for 4 h. For determination of radioactivity, samples were dissolved in 5 mL 1% HCl and 5 mL diisopropylnaphtalene (Quick Safe 5, Zinsser Analytic) and analyzed by a liquid scintillation counter (Wallac).
Xylem Sap Collection
Xylem sap was collected from maize plants precultured under Fe deficiency for 23 d. Plants were resupplied with 100 μm Fe(III)-EDTA in the presence or absence of 25 μm CdCl2 48 h before being detopped. Liquid appearing on the excised stem surface during the first few minutes was taken off before a flexible tube was fixed onto the cut end of the stem. Xylem sap exuded for 3 h was collected with a Pasteur pipette and stored at −20°C until further analysis.
Collection of Wheat Root Exudates and Purification of the Phytosiderophore DMA
The phytosiderophore DMA used in all experiments was obtained from root exudates of wheat (Triticum aestivum) cv Ares plants, because wheat roots release large amounts of DMA. Seeds were surface rinsed as above and germinated for 6 d in the dark in quartz sand moistened with saturated CaSO4 solution. Seedlings were transferred to continuously aerated nutrient solution. Plants were grown for 2 weeks and nutrient solution was exchanged every 2 to 3 d. After 1 week of preculture under Fe deficiency, root exudates were collected daily. The root system of approximately 50 plants per pot was first rinsed in deionized water for 30 min and then transferred to 500 mL deionized water for 4 h under continuous aeration, starting 2 h after onset of the light period (Cakmak et al., 1998). Micropur (10 mg/L; Roth) was added to the exudates to prevent microbial degradation during processing of the exudates, which were filtered (Blue ribbon no. 5893, Schleicher and Schüll) and concentrated at 50°C to 10% of the initial volume. DMA was prepurified by cation exchange chromatography (Dowex 50 WX column, Serva) according to von Wirén et al. (1995). Qualitative and quantitative analysis of the DMA solution was done by HPLC as described by Neumann et al. (1999) and further verified by nano-ESI-FTICR-MS according to Weber et al. (2006).
Collection of DMA from Maize Roots under Sterile Conditions
Maize seeds (cv UH002) were surface sterilized with 96% ethanol for 3 min followed by incubation in 3% sodium hypochloride for 30 min. Seeds were then soaked with autoclaved CaSO4-saturated solution at room temperature during 2 h before being placed on solid half-strength modified Murashige and Skoog medium (without Fe) containing 1% Suc and 0.7% agar (Difco, Becton Diekison). Seeds were incubated in the dark at 28°C for 3 d. Individual seedlings were then transferred to sterile glass tubes containing 12 mL Fe-free nutrient solution in which the plantlets were held by inserted 5-mL pipette tips. Plantlets continued to grow during 4 d before transfer to Fe-free nutrient solution in the absence or presence of 25 μm CdCl2 for 24 h. For collection of root exudates, plants were transferred to sterile ultrapure water (Elga) for a period of 6 h. A drop test on solid Luria-Bertani medium from collected exudates of each sample was performed to verify that the solution was free of microbial contamination. DMA concentrations were determined by HPLC according to Neumann et al. (1999).
Heavy Metal Mobilization Tests with Metal-Loaded Resins or Soil
To obtain Cd or Cu-loaded Chelex 100, 4.4 g of Na-loaded resin (Bio-Rad) were washed with 100 mL 1-m HCl in a glass bead column and rinsed with an excess of ultrapure water (Elga) until water drops reached a pH of 5.5 to 6.0. Resins were stirred in 200 mL 50-μm CdCl2 or CuSO4 for 15 min. The suspensions obtained were poured back onto a glass bead column and washed with ultrapure water until washing solutions were free of Cd or Cu, as verified by atomic absorption spectroscopy. In addition, the commercially available Fe-loaded resin Chelex 100 was used (Bio-Rad). The metal-loaded resins were then kept in a 10-mm MES-Tris buffered solution, pH 5.5. For the mobilization test, 2 mL of resin suspension were added to 8 mL water or chelate solution (5 μm HEDTA or DMA at pH 5.5) and shaken at 100 rpm at room temperature for 1 h. Filtrates (Blue ribbon no. 5893, Schleich und Schüll) were analyzed for Cd and Cu concentrations by inductively coupled plasma optical emission spectrometry and Fe by atomic absorption spectroscopy.
A Rendzina soil with a pH (in CaCl2) of 6.4 and 1.4% total organic matter was collected from an agricultural area in Bonndorf, Germany and used for metal mobilization tests. The total concentrations of Cd, Cu, and Zn were 17.0, 53.0, and 443.0 mg kg−1, respectively, while NH4NO3-extractable concentrations of Cd, Cu, and Zn were 96.0, 137.5, and 193.3 μg kg−1, respectively. Five grams of homogenized and dried soil was shaken in 20 mL water (control) or 20 mL 50-μm chelate (DTPA or DMA) at 175 rpm for 1 h and filtered (Blue ribbon no. 5893, Schleich und Schüll). Filtrates were analyzed for Cd, Cu, and Zn concentrations by inductively coupled plasma optical emission spectrometry.
Determination of Metal-DMA Complexes by Nano-ESI-FTICR-MS
The Cd(II) complex was prepared from an aqueous DMA solution (20 μm in 50 mm ammonium bicarbonate, pH 7.3) by adding an appropriate amount of a 1-mm aqueous solution of Cd(II) chloride (Merck). The pH was readjusted to 7.3 with HCl or aqueous ammonia solution, if necessary. The competition experiments with Fe(III) and Zn(II) were carried out by adding the respective amount of a 1-mm solution of Fe(III) chloride hexahydrate (Roth) or Zn(II) acetate dihydrate (Roth). To improve the comparability of the different measurements, l-Trpe (Roth) was added as an internal standard yielding a final concentration of 20 μm. All signal intensities were analyzed relative to the l-Trpe signal intensity (m/z 203.0826). Before measurement, the samples were diluted with methanol (liquid chromatography-MS grade, Riedel-de Haën) to 70% to avoid problems induced by high surface tension of pure water and to facilitate a stable spray at reduced needle voltage (without electrical discharge). The theoretical masses and isotope distributions for the complexes were calculated using the Xcalibur software LTQ FT version 1.4.2 (Thermo Electron).
All nano-ESI-FTICR-MS experiments were carried out using a LCQ FT FTICR hybrid mass spectrometer (Thermo Electron) equipped with a 7.0 Tesla actively shielded superconducting magnet and nano-ESI source. Gold-plated nano-ESI pipettes (tip i.d. approximately 1 μm) were from MasCom. The instrument was operated in negative ionization mode. Ion transmission into the linear trap and signal intensity was automatically optimized for maximum ion signal of the Fe(III)-DMA complex. The parameters were: source voltage, 0.8 to 1.0 kV; capillary voltage, 20 V; capillary temperature, 100°C; and tube lens voltage, 80 V. The targets for the full scan linear trap and FTICR cell were 3 × 104 and 2 × 105, respectively. The resolving power of the FTICR mass analyzer was set to 200,000 (full width at half maximum at m/z = 400). Full scan FTICR mass spectra in the mass range m/z 150 to 800 were acquired using a single microscan, and displayed mass spectra were averaged from 50 single spectra. The instrument was calibrated externally using 0.01% solution of 85% phosphoric acid in water/methanol (1:1, v/v).
Yeast Complementation
For growth complementation, the yeast (Saccharomyces cerevisiae) strains fet3fet4 (DEY1453, MATa/MATα ade2/+can1/can1 his3/his3 leu2/leu2 trp1/trp1 ura3/ura3 fet3-2:HIS3/fet3-2:HIS3/fet4-1:LEU2/fet4-1:LEU2) and wild type (DY1457, MATα ade6 can1 his3 leu2 trp1 ura3) were used. To support growth of the fet3fet4 mutant, solid yeast nitrogen base (YNB) medium contained 45 μm Fe-EDTA, while the liquid yeast peptone dextrose medium was acidified with concentrated HCl (0.1% v/v). Yeast was transformed either with pDR195, pDR196-ZmYS1 (Schaaf et al., 2004b), or pDR195-AtIRT1 (Schaaf et al., 2006) using the LiAc method (Gietz et al., 1992), and transformants were selected on uracil-deficient YNB (YNB-ura) containing 0.1% Arg as a nitrogen source and the appropriate supplements. The complementation and growth assays on Fe and Cd with chelates were performed on solid YNB-ura medium supplemented with 3% Glc, 0.1% Arg, 0.01% adenine, 0.01% His, 0.01% Leu, and 0.01% Trpe with the Fe, Cd, and chelates added as indicated.
Generation of ZmYS1-Overexpressing Arabidopsis Plants, RNA Gel-Blot Analysis, and Growth Tests on Agar Plates
Using a BamHI restriction site, the open reading frame (ORF) containing a 32-bp 5′-untranslated region of ZmYS1 in pDR196 (Schaaf et al., 2004b) was subcloned into the pPTKan+ vector (Schaaf et al., 2006) containing a p35CaMV-mcs-tCaMV cassette to generate p35S-ZmYS1. Arabidopsis (Arabidopsis thaliana) plants were transformed by the floral dip method (Clough and Bent, 1998). Independent kanamycin-resistant lines (T0) were isolated and amplified. Homozygous T3 plants were cultured in hydroponics according to Loqué et al. (2005).
Total RNA was isolated by Trizol extraction (Invitrogen). RNA (20 μg per lane) was separated by electrophoresis on MOPS-formaldehyde agarose gels, blotted onto Hybond-N+ nylon membranes (Amersham Biosciences), and cross-linked to the membrane by incubation at 80°C for 2 h. The ORF of ZmYS1 was used as a probe for hybridization to total RNA. Membrane hybridization, probe labeling, and membrane washing were conducted essentially as described (Loqué et al., 2005).
For growth test of Arabidopsis plants on agar plates, seeds of wild-type and transgenic plants were germinated and grown for 8 d on modified 0.5 Murashige and Skoog medium (Duchefa), 0.5% Suc, solidified with 1% Difco agar (Becton Diekison), and transferred to the same medium supplemented with metals at indicated concentrations. Plants were grown in a growth chamber with the following conditions: 10/14 h light/dark; light intensity, 120 μmol photons m−2 s−1; and temperature 22°C/19°C at day/night. Chlorophyll concentrations were measured according to Moran and Porath (1980).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers U27590 and AF186234.
Acknowledgments
We thank Susanne Reiner, Jennifer Haeberle, Silvia Kirchner, and Maria Ruckwied, University of Hohenheim, for excellent technical assistance, and David Eide, University of Wisconsin-Madison, for providing the yeast strains.
This work was supported by the Deutsche Forschungsgemeinschaft, Bonn (grant nos. WI1728/6–1 to N.v.W. and WE 2422/5–1 to G.W.), and by the German Academic Exchange Agency, Bonn (fellowship to A.R.M.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Nicolaus von Wirén (vonwiren@uni-hohenheim.de).
Open Access articles can be viewed online without a subscription.
References
- Awad F, Römheld V (2000) Mobilization of heavy metals from contaminated calcareous soils by plant born, microbial and synthetic chelators and their uptake by wheat plants. J Plant Nutr 23 1847–1855 [Google Scholar]
- Bienfait HF, Briel W, Mesland-Mul NT (1985) Free space iron pools in roots: generation and mobilization. Plant Physiol 78 596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakmak I, Erenoglu B, Gülüt KY, Derici R, Römheld V (1998) Light-mediated release of phytosiderophores in wheat and barley under iron or zinc deficiency. Plant Soil 202 309–315 [Google Scholar]
- Clemens S (2006) Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88 1707–1719 [DOI] [PubMed] [Google Scholar]
- Clemens S, Antosiewicz DM, Ward JM, Schachtman DP, Schroeder JI (1998) The plant cDNA LCT1 mediates the uptake of calcium and cadmium in yeast. Proc Natl Acad Sci USA 95 12043–12048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- Cohen CK, Fox TC, Garvin DF, Kochian LV (1998) The role of iron-deficiency stress responses in stimulating heavy-metal transport in plants. Plant Physiol 116 1063–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen CK, Garvin DF, Kochian LV (2004) Kinetic properties of a micronutrient transporter from Pisum sativum indicate a primary function in Fe uptake from the soil. Planta 218 784–792 [DOI] [PubMed] [Google Scholar]
- Collins RN, Merrington G, Mclaughlin MJ, Morel JL (2003) Organic ligand and pH effects on isotopically exchangeable cadmium in polluted soils. Soil Sci Soc Am J 67 112–121 [Google Scholar]
- Connolly EL, Fett JP, Guerinot ML (2002) Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell 14 1347–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curie C, Panaviene Z, Loulergue C, Dellaporta SL, Briat JF, Walker EL (2001) Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature 409 346–349 [DOI] [PubMed] [Google Scholar]
- Deckert J (2005) Cadmium toxicity in plants: is there any analogy to its carcinogenic effect in mammalian cells? Biometals 18 475–481 [DOI] [PubMed] [Google Scholar]
- DiDonato RJ, Roberts LA, Sanderson T, Eisley RB, Walker EL (2004) Arabidopsis yellow stripe-like2 (YSL2): a metal-regulated gene encoding a plasma membrane transporter of nicotianamine-metal complexes. Plant J 39 403–414 [DOI] [PubMed] [Google Scholar]
- Eide D, Broderius M, Fett J, Guerinot ML (1996) A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci USA 93 5624–5628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier L, Simon-Plas F, Thuleau P, Agnel JP, Blein JP, Ranjeva R, Montillet JL (2006) Cadmium affects tobacco cells by a series of three waves of reactive oxygen species that contribute to cytotoxicity. Plant Cell Environ 29 1956–1969 [DOI] [PubMed] [Google Scholar]
- Gietz D, St Jean A, Woods RA, Schiestl RH (1992) Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res 20 1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart JJ, Welch RM, Norvell WA, Kochian LV (2002) Transport interactions between cadmium and zinc in roots of bread and durum wheat seedlings. Physiol Plant 116 73–78 [DOI] [PubMed] [Google Scholar]
- Hill KA, Lion LW, Ahner BA (2002) Reduced Cd accumulation in Zea mays: a protective role for phytosiderophores? Environ Sci Technol 36 5363–5368 [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Suzuki M, Tsukamoto T, Suzuki K, Nakazono M, Kobayashi T, Wada Y, Watanabe S, Matsuhashi S, Takahashi M, et al (2006) Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+. Plant J 45 335–346 [DOI] [PubMed] [Google Scholar]
- Jalil A, Selles F, Clarke JM (1994) Effect of cadmium on growth and the uptake of cadmium and other elements by durum wheat. J Plant Nutr 17 1839–1858 [Google Scholar]
- Jensen LT, Culotta VC (2002) Regulation of Saccharomyces cerevisiae FET4 by oxygen and iron. J Mol Biol 318 251–260 [DOI] [PubMed] [Google Scholar]
- Kochian LV, Hoekenga OA, Pineros MA (2004) How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu Rev Plant Biol 55 459–493 [DOI] [PubMed] [Google Scholar]
- Koike S, Inoue H, Mizuno D, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2004) OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the phloem. Plant J 39 415–424 [DOI] [PubMed] [Google Scholar]
- Koleli N, Eker S, Cakmak I (2004) Effect of zinc fertilization on cadmium toxicity in durum and bread wheat grown in zinc-deficient soil. Environ Pollut 131 453–459 [DOI] [PubMed] [Google Scholar]
- Korshunova YO, Eide D, Clark WG, Guerinot ML, Pakrasi HB (1999) The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Mol Biol 40 37–44 [DOI] [PubMed] [Google Scholar]
- Larbi A, Morales F, Abadia A, Gogorcena Y, Lucena JJ, Abadia J (2002) Effects of Cd and Pb in sugar beet plants grown in nutrient solution: induced Fe deficiency and growth inhibition. Funct Plant Biol 29 1453–1464 [DOI] [PubMed] [Google Scholar]
- Li L, Kaplan J (1998) Defects in the yeast high affinity iron transport system result in increased metal sensitivity because of the increased expression of transporters with a broad transition metal specificity. J Biol Chem 273 22181–22187 [DOI] [PubMed] [Google Scholar]
- Loqué D, Ludewig U, Yuan L, von Wirén N (2005) Tonoplast intrinsic proteins AtTIP2;1 and AtTIP2;3 facilitate NH3 transport into the vacuole. Plant Physiol 137 671–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H (1995) Mineral Nutrition of Higher Plants. Academic Press, New York
- Mench M, Martin E (1991) Mobilization of cadmium and other metals from two soils by root exudates of Zea mays L., Nicotiana tabacum L. and Nicotiana rustica L. Plant Soil 132 187–196 [Google Scholar]
- Mills RF, Francini A, Ferreira da Rocha PSC, Baccarini PJ, Aylett M, Krijger GC, Williams LE (2005) The plant P1B-type ATPase AtHMA4 transports Zn and Cd and plays a role in detoxification of transition metals supplied at elevated levels. FEBS Lett 579 783–791 [DOI] [PubMed] [Google Scholar]
- Moran R, Porath D (1980) Chlorophyll determination in intact tissues using N,N-dimethylformamide. Plant Physiol 65 478–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Ise K, Hayakawa M, Kamei S, Takagi S (1989) Stabilities of metal complexes of mugineic acid and their specific affinities for iron(III). Chem Lett (Jpn) 18 2137–2140 [Google Scholar]
- Nakanishi H, Ogawa I, Ishimaru Y, Mori S, Nishizawa NK (2006) Iron deficiency enhances cadmium uptake and translocation mediated by the Fe2+ transporters OsIRT1 and OsIRT2 in rice. Soil Sci Plant Nutr 52 464–469 [Google Scholar]
- Neumann G, Haake C, Römheld V (1999) Improved HPLC-method for determination of phytosiderophores in root washings and tissue extracts. J Plant Nutr 22 1389–1402 [Google Scholar]
- Neumann G, Römheld V (2000) The release of root exudates as affected by the plant's physiological status. In R Pinton, Z Varanini, Z Nannipieri, Z Varanni, eds, The Rhizosphere: Biochemistry and Organic Substances at the Soil-Plant Interface. Marcel Dekker, New York, pp 41–94
- Norvell W (1991) Reactions of metal chelates in soils and nutrient solutions. In JJ Mortvedt, ed, Micronutrients in Agriculture, Ed 2. Soil Science Society of America, Madison, WI, pp 187–228
- Pence NS, Larsen PB, Ebbs SD, Letham DLD, Lasat MM, Garvin DF, Eide D, Kochian LV (2000) The molecular physiology of heavy metal transport in the Zn/Cd hyperaccumulator Thlaspi caerulescens. Proc Natl Acad Sci USA 97 4956–4960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfus-Barbeoch L, Leonhardt N, Vavasseur A, Forestier C (2002) Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the plant water status. Plant J 32 539–548 [DOI] [PubMed] [Google Scholar]
- Roberts LA, Pierson AJ, Panaviene Z, Walker EL (2004) Yellow stripe1: expanded roles for the maize iron-phytosiderophore transporter. Plant Physiol 135 112–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römheld V, Marschner H (1986) Evidence for a specific uptake system for iron phytosiderophores in roots of grasses. Plant Physiol 80 175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf G, Erenoglu BE, von Wirén N (2004. a) Physiological and biochemical characterization of metal-phytosiderophore transport in graminaceous species. Soil Sci Plant Nutr 50 989–995 [Google Scholar]
- Schaaf G, Honsbein A, Meda AR, Kirchner S, Wipf D, von Wirén N (2006) AtIREG2 encodes a tonoplast transport protein involved in iron-dependent nickel detoxification in Arabidopsis thaliana roots. J Biol Chem 281 25532–25540 [DOI] [PubMed] [Google Scholar]
- Schaaf G, Ludewig U, Erenoglu BE, Mori S, Kitahara T, von Wirén N (2004. b) ZmYS1 functions as a proton-coupled symporter for phytosiderophore- and nicotianamine-chelated metals. J Biol Chem 279 9091–9096 [DOI] [PubMed] [Google Scholar]
- Schmidt W (2003) Iron solutions: acquisition strategies and signaling pathways in plants. Trends Plant Sci 8 188–193 [DOI] [PubMed] [Google Scholar]
- Shenker M, Fan TWM, Crowley DE (2001) Phytosiderophores influence on cadmium mobilization and uptake by wheat and barley plants. J Environ Qual 30 2091–2098 [DOI] [PubMed] [Google Scholar]
- Suzuki M, Takahashi M, Tsukamoto T, Watanabe S, Matsuhashi S, Yazaki J, Kishimoto N, Kikuchi S, Nakanishi H, Mori S, et al (2006) Biosynthesis and secretion of mugineic acid family phytosiderophores in zinc-deficient barley. Plant J 48 85–97 [DOI] [PubMed] [Google Scholar]
- Thomine S, Lelievre F, Debarbieux E, Schroeder JI, Barbier-Brygoo H (2003) AtNRAMP3, a multispecific vacuolar metal transporter involved in plant responses to iron deficiency. Plant J 34 685–695 [DOI] [PubMed] [Google Scholar]
- Tolay I, Erenoglu B, Römheld V, Braun HJ, Cakmak I (2001) Phytosiderophore release in Aegilops tauschii and Triticum species under zinc and iron deficiencies. J Exp Bot 52 1093–1099 [DOI] [PubMed] [Google Scholar]
- Treeby M, Marschner H, Römheld V (1989) Mobilization of iron and other micronutrient cations from a calcareous soil by plant-borne, microbial, and synthetic metal chelators. Plant Soil 114 217–226 [Google Scholar]
- Vert G, Grotz N, Dedaldechamp F, Gaymard F, Guerinot ML, Briat JF, Curie C (2002) IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14 1223–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wirén N, Römheld V, Shioiri T, Marschner H (1995) Competition between micro-organisms and roots of barley and sorghum for iron accumulated in the root apoplasm. New Phytol 130 511–521 [DOI] [PubMed] [Google Scholar]
- Walter A, Romheld V, Marschner H, Mori S (1994) Is the release of phytosiderophores in zinc-deficient wheat plants a response to impaired iron utilization? Physiol Plant 92 493–500 [Google Scholar]
- Weber G, von Wirén N, Hayen H (2006) Analysis of iron(II)/iron(III) phytosiderophore complexes by nano-electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Rapid Commun Mass Spectrom 20 973–980 [DOI] [PubMed] [Google Scholar]
- Yoshihara T, Hodoshima H, Miyano Y, Shoji K, Shimada H, Goto F (2006) Cadmium inducible Fe deficiency responses observed from macro and molecular views in tobacco plants. Plant Cell Rep 25 365–373 [DOI] [PubMed] [Google Scholar]
- Zhang FS, Römheld V, Marschner H (1989) Effect of zinc deficiency in wheat on the release of zinc and iron mobilizing root exudates. J Plant Nutr Soil Sci 152 205–210 [Google Scholar]