Abstract
AMP deaminase (AMPD) is essential for plant life, but the underlying mechanisms responsible for lethality caused by genetic and herbicide-based limitations in catalytic activity are unknown. Deaminoformycin (DF) is a synthetic modified nucleoside that is taken up by plant cells and 5′-phosphorylated into a potent transition state-type inhibitor of AMPD. Systemic exposure of Arabidopsis (Arabidopsis thaliana) seedlings to DF results in dose-dependent (150–450 nm) and time-dependent decreases in plant growth that are accompanied by 2- to 5-fold increases in the intracellular concentrations of all adenine ribonucleotides. No measurable rescue is observed with either hypoxanthine or xanthine (250 μm), indicating that downstream effects of AMPD inhibition, such as limitations in adenine-to-guanine nucleotide conversion or ureide synthesis, do not play important roles in DF toxicity. However, adenine (250 μm) acts synergistically with a nontoxic dose of DF (150 nm) to produce growth inhibition and adenine nucleotide pool expansion comparable to that observed with a toxic concentration of the herbicide alone (300 nm). Conversely, adenine alone (60–250 μm) has no measurable effects on these parameters. These combined results support the hypothesis that AMPD is the primary intracellular target for this class of herbicides and strongly suggest that adenine nucleotide accumulation is a metabolic trigger for DF toxicity. AMP binds to 14-3-3 proteins and can interrupt client interactions that appear to drive their distributions. Trichome subcellular localization of the phi isoform is disrupted within 8 to 24 h after seedlings are semisubmersed in a solution of DF (100 nm), further suggesting that disrupted 14-3-3 protein function plays a role in the associated herbicidal activity.
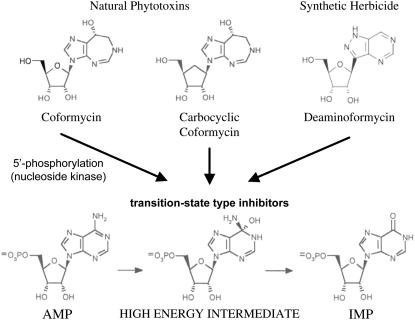
EMBRYONIC FACTOR1 (FAC1) is one of the earliest expressed plant genes and is essential for the zygote-to-embryo transition in Arabidopsis (Arabidopsis thaliana; Xu et al., 2005). The zygote-lethal phenotype is characterized by developmental arrest at the eight to 16 cell stage and mutant embryo shriveling 2 to 3 d after fertilization. The Arabidopsis FAC1 locus encodes an AMP deaminase (AMPD; EC 3.5.4.6), which is a eukaryotic enzyme that catalyzes the hydrolytic deamination of AMP to IMP. This reaction is the initial step in adenine-to-guanine ribonucleotide conversion and in the catabolism of AMP to hypoxanthine, which is then oxidized and linearized to form the recyclable ureides allantoin and allantoic acid. AMPD is also the intracellular target for a class of natural phytotoxins and related synthetic herbicides (Fig. 1). Carbocyclic coformycin was initially discovered in Saccharothrix (Bush et al., 1993), and plant cells can take up this diffusible nucleoside compound and 5′-phosphorylate it into a potent transition state-type inhibitor of AMPD (Dancer et al., 1997). Exposure to carbocyclic coformycin results in cessation of seedling growth, followed by paling and necrosis at the apical meristem (Dancer et al., 1997). Coformycin, a structurally related compound produced by a number of microbes (Nakamura et al., 1974; Isaac et al., 1991), also has herbicidal properties (Isaac et al., 1991). Although the intracellular metabolism of this compound in plants has not been examined, its mode of action is presumably similar because coformycin 5′-phosphate is a potent inhibitor of rabbit muscle AMPD (Frieden et al., 1980). The recently solved x-ray crystal structure of FAC1 complexed with coformycin 5′-phosphate confirmed this mode of inhibition and also provided the first glimpse of a complete AMPD active site in plants (Han et al., 2006). Coformycin and carbocyclic coformycin are also inhibitors of mammalian adenosine deaminase (Frieden et al., 1980; Dancer et al., 1997), but the lack of this enzyme in plants (Le Floc'h et al., 1982; Yabuki and Ashihara, 1991; Dancer et al., 1997) supports the argument that AMPD is the primary intracellular target once these compounds are converted to their respective nucleotide derivatives. The search for more stable and accessible structures led to the synthesis of deaminoformycin (DF), which also has good herbicidal properties and its corresponding 5′-monophosphate is also a strong inhibitor of plant AMPD (Lindell et al., 1999). Taken together, these observations strongly suggest that AMPD is essential for plant life.
Figure 1.
Natural phytotoxins and synthetic herbicide precursors of AMPD inhibitors. These modified nucleoside compounds are taken up by plant cells and 5′-phosphorylated, presumably by a nucleoside kinase. The resulting monophosphate products are transition state-type inhibitors of AMPD. The nucleoside compounds themselves can directly inhibit adenosine deaminase, but the lack of this enzyme in plants supports the hypothesis that their primary intracellular target is AMPD.
However, the underlying mechanisms responsible for lethality associated with dramatic reductions (genetic and herbicide induced) in plant AMPD catalytic activity remain to be elucidated. Toward this end, it is reasonable to consider the immediate consequences of an inability to deaminate AMP in a plant cell. Disruption of this reaction could impact on (1) the balance between adenine and guanine nucleotides by interfering with the interconversion pathway, (2) nitrogen metabolism by limiting the production of ureides, (3) hormonal imbalance by promoting substrate accumulation for purine-based cytokinin synthesis (Haberer and Kieber, 2002), and (4) perturbed 14-3-3 protein regulation of key primary metabolic enzymes through the accumulation of AMP (Athwal et al., 1998; Camoni et al., 2001). In considering these possibilities, it is notable that leaf tissue ATP is reportedly elevated within hours after topical application of carbocyclic coformycin to runoff (Dancer et al., 1997) or transpiration feeding of seedlings with DF (Lindell et al., 1999). Consequently, a robust AMPD activity may be necessary to maintain homeostasis of the many processes, located both upstream and downstream in the ATP catabolic pathway, that are impacted by purine metabolism. This study begins to explore this issue by monitoring the effect of systemic DF exposure on Arabidopsis seedling growth and adenine nucleotide pools. In addition, normal purine base compounds are used in an attempt to rescue plants from the toxic effects of DF. Finally, the subcellular distribution of an Arabidopsis 14-3-3/green fluorescent protein (GFP) fusion protein is monitored following semisubmersion of transformed seedlings in a solution of DF. The results of these studies have provided insight regarding the relative importance of upstream and downstream consequences of a limitation in AMPD catalytic activity in a plant cell and the associated processes that interface with purine metabolism.
RESULTS
Dose-Response Effects of Systemic DF
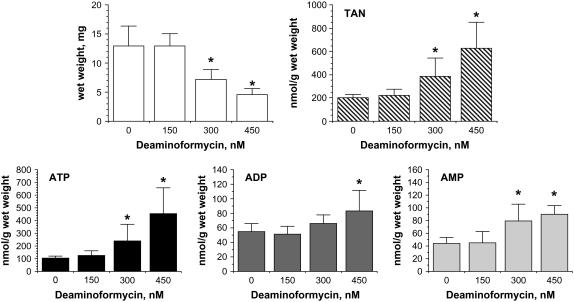
Arabidopsis seedling growth and adenine nucleotide pools were monitored after 9 d of systemic exposure to increasing concentrations of DF (150–450 nm). Figure 2 shows a dose-dependent inverse relationship between these two parameters. Notably, all adenine nucleotides are elevated following systemic exposure to DF. The adenylate energy charge (AEC; [ATP] + 0.5[ADP]/[ATP + ADP + AMP]) could also be calculated from these data. Whereas a high AEC is typically associated with growing cells, this is clearly not the case with AMPD-directed inhibitors in plant cells as this value gradually increased with increasing concentrations of DF, i.e. untreated, 0.65 ± 0.03; 150 nm DF, 0.68 ± 0.03; 300 nm DF, 0.69 ± 0.06; and 450 nm DF, 0.77 ± 0.08* (*, P < 0.05 compared to untreated in an unpaired two-tailed t test). The increase in AEC reflects the more profound elevation in ATP (about 4-fold) relative to that for AMP (about 2-fold) with increasing concentrations of DF.
Figure 2.
Dose-response effects of DF on Arabidopsis seedling growth and adenine nucleotide pools. Five-day-old seedlings were transferred onto Murashige and Skoog salt (0.5×) + 1% Suc agar supplemented with increasing concentrations of DF (0–450 nm) and grown for 9 d under long-day conditions (16 h light/8 h dark). Dose-dependent decrease in seedling growth (top left) is associated with an increase in total adenine nucleotides (TAN; top right), which affects all phosphorylated species: ATP, bottom left; ADP, bottom middle; and AMP, bottom right. Data expressed as the mean ± sd (n = 6). *, P < 0.05 when compared to control in an unpaired two-tailed t test. All metabolites are expressed as nmol/g wet weight.
It was also possible to quantify GTP in extracts prepared from these 9-d-old seedlings. Whereas the levels of all other nucleotides were below the limits of detection, GTP concentrations were similar under all conditions, except at the highest dose of DF, i.e. untreated, 17 ± 2; 150 nm DF, 15 ± 6; 300 nm DF, 16 ± 6; and 450 nm DF, 11 ± 3* nmol/g wet weight (*, P < 0.05 compared to untreated in an unpaired two-tailed t test). However, this modest 35% reduction should be considered as tenuous because of the relative small size of seedlings exposed to 450 nm DF, which resulted in the level of GTP being at the limit of detection in these extracts.
Time-Course Effects of Systemic DF
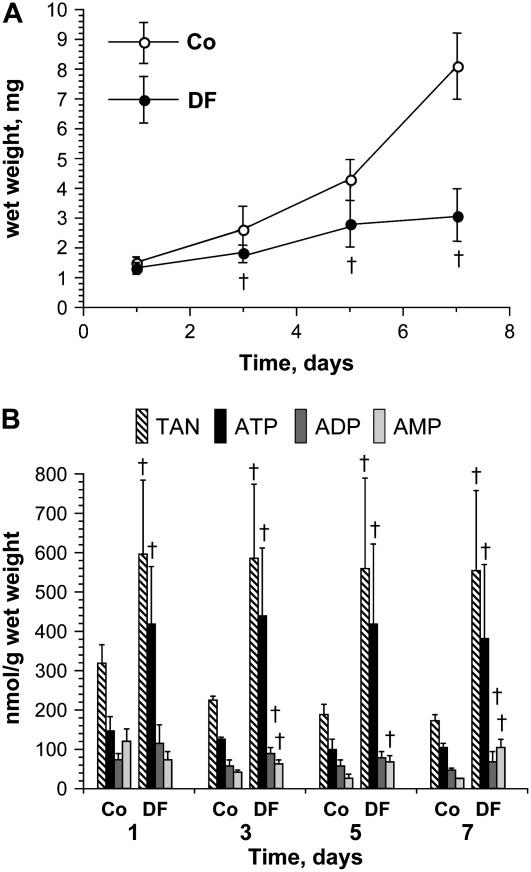
Figure 3 shows the time course of effects produced by systemic exposure to 300 nm DF. The total adenine nucleotide pool was expanded after only 1 d, due predominantly to a significant increase in ATP, whereas growth inhibition was not significant until after 3 d, at which time the concentrations of ADP and AMP were also significantly elevated.
Figure 3.
Time-course effects of DF on Arabidopsis seedling growth and adenine nucleotide pools. Five-day-old seedlings were transferred onto Murashige and Skoog salt (0.5×) + 1% Suc agar with and without 300 nm DF and grown for 1, 3, 5, and 7 d under long-day conditions. A, Seedling growth expressed as mg wet weight following root excision (mean ± sd, n = 6). †, P < 0.05 when compared to the corresponding control (Co) time point in an unpaired two-tailed t test. B, Adenine nucleotide pools in control (Co) and DF-treated seedling tissue. †, P < 0.05 when compared to the corresponding value in control seedlings at that time point in an unpaired two-tailed t test. All metabolites are expressed as nmol/g wet weight (mean ± sd, n = 6).
Effect of Hypoxanthine and Xanthine on DF Toxicity
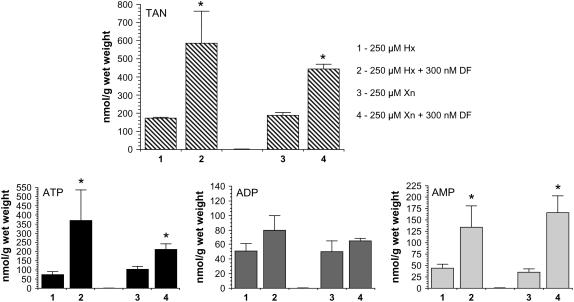
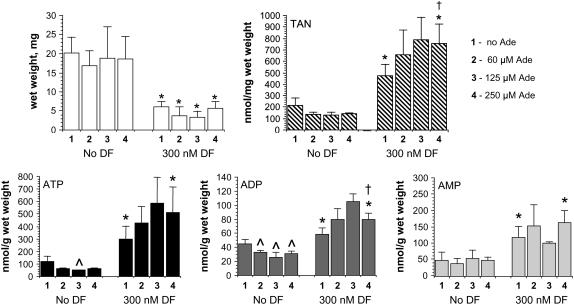
Hypoxanthine and xanthine (250 μm each) were added to the solid growth medium in an attempt to rescue seedlings from DF toxicity. Figure 4 shows that neither of these purine base compounds is able to reverse DF growth inhibition, and Figure 5 confirms that this is again accompanied by increased levels of adenine nucleotides.
Figure 4.
Downstream catabolites are unable to reverse the growth inhibition of Arabidopsis seedlings by DF. Protocol as described in the legend to Figure 2, except that the agar medium was supplemented with 250 μm hypoxanthine alone (top), hypoxanthine and 300 nm DF (upper middle), 250 μm xanthine alone (lower middle), or xanthine and 300 nm DF (bottom). Seedling wet weights following root excision (mean ± sd, n = 4, except hypoxanthine alone [n = 3]) are listed at the right. *, P < 0.05 when compared to the corresponding purine base alone condition in an unpaired two-tailed t test. Seedlings shown represent those closest to the average for the group.
Figure 5.
Increased adenine nucleotide pools in DF-treated seedlings supplemented with hypoxanthine and xanthine. Data were generated from the seedlings shown in Figure 4. Total adenine nucleotides (TAN), top; ATP, bottom left; ADP, bottom middle; AMP, bottom right. Bar 1, 250 μm hypoxanthine; bar 2, 250 μm hypoxanthine + 300 nm DF; bar 3, 250 μm xanthine; bar 4, 250 μm xanthine + 300 nm DF. Data expressed as the mean ± sd, n = 4, except hypoxanthine alone (n = 3). *, P < 0.05 when compared to the corresponding no DF condition in an unpaired two-tailed t test.
Dose-Response Effects of Adenine
Adenine is directly incorporated into the adenine nucleotide pool (Doree, 1973; Le Floc'h et al., 1982). This purine base was also added to the medium (60–250 μm), alone or in combination with 300 nm DF. Figure 6 shows that adenine alone has no effect on the growth of Arabidopsis seedlings, nor does it cause an expansion of adenine nucleotide pools. Conversely, when administered together, adenine and DF have a synergistic effect on the adenine nucleotide pool, which is further elevated relative to that observed with herbicide alone. However, it is difficult to determine from these data whether there is also a synergistic effect on growth due to the degree of inhibition exerted by 300 nm DF alone.
Figure 6.
Dose response of adenine on seedling growth and adenine nucleotide pools in the presence and absence of DF. Protocol as described in the legend to Figure 2, except that the agar medium was supplemented with increasing concentrations of adenine (bar 1, none; bar 2, 60 μm; bar 3, 125 μm; bar 4, 250 μm) alone or in combination with 300 nm DF. Seedling wet weights, top left; total adenine nucleotides (TAN), top right; ATP, bottom left; ADP, bottom middle; AMP, bottom right. Data are expressed as the mean ± sd. *, P < 0.05 when compared to the corresponding no adenine condition in an unpaired two-tailed t test (note: no statistical comparisons using the 60 μm adenine, 300 nm DF and 125 μm adenine, 300 nm DF data were possible because there were only two seedlings in each of these groups). ^, P < 0.05 when compared to the corresponding no adenine condition. †, P < 0.05 when compared to the no adenine, 300 nm DF condition in an unpaired two-tailed t test.
Synergistic Effect of Adenine on DF Toxicity
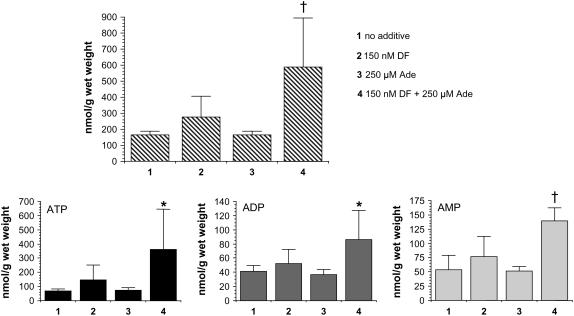
Based on the results shown in Figure 6, the experiment was then repeated with adenine (250 μm), alone or in combination with a lower dose of DF (150 nm) to determine if there was a synergistic effect on seedling growth. Figures 7 and 8 show that neither supplement has any significant effect on growth or adenine nucleotide pools in Arabidopsis seedlings. Conversely, there is significant growth inhibition and adenine nucleotide pool expansion when both compounds are added to the medium at these concentrations.
Figure 7.
Synergistic effect of adenine and DF on growth inhibition of Arabidopsis seedlings. Protocol as described in the legend to Figure 2, except that the agar medium received no supplement (top row), or was supplemented with 150 nm DF (second row), 250 μm adenine (third row), or 150 nm DF and 250 μm adenine (bottom row). Seedling wet weights (mean ± sd, n = 6) following root excision are listed at the right. *, P < 0.05 when compared to all other conditions in unpaired two-tailed t tests. Seedlings shown represent those closest to the average for the group.
Figure 8.
Synergistic effect of adenine and DF on adenine nucleotide pools in Arabidopsis seedling tissue. Data generated from the seedlings in Figure 7. Total adenine nucleotides (TAN), top; ATP, bottom left; ADP, bottom middle; AMP, bottom right. Bar 1, No additive; bar 2, 150 nm DF; bar 3, 250 μm adenine; bar 4, 150 nm DF + 250 μm adenine. Data expressed as the mean ± sd, n = 6. *, P < 0.05 when compared to all other conditions except for the 150 nm DF alone condition in an unpaired two-tailed t test; †, P < 0.05 when compared to all other conditions in unpaired two-tailed t tests.
Effect of DF on the Subcellular Localization of 14-3-3-Phi/GFP
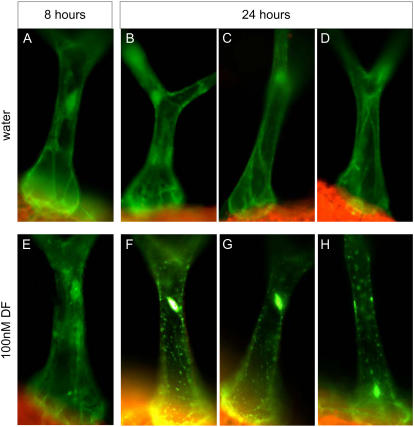
A transformed Arabidopsis line expressing the coding region of the phi isoform coupled to GFP was used to evaluate the effect of DF on the subcellular localization of this 14-3-3 protein in trichomes of 2-week-old seedlings following semisubmersion in a 100 nm solution of herbicide. Compared to the smooth cytoskeletal distribution of 14-3-3-phi/GFP under control conditions, Figure 9 shows a time-dependent stippled distribution of this fusion protein in trichomes of DF-treated seedlings.
Figure 9.
Effects of DF on the subcellular distribution of constitutively expressed 14-3-3-phi/GFP. The top row shows examples of trichomes from 2-week-old plants held semisubmerged in water for 8 h (A) or for 24 h (B–D). The bottom row shows trichomes from plants held similarly in a 100 nm solution of DF for 8 h (E) or for 24 h (F–H).
Ki Determination of DF 5′-Monophosphate Using a Purified Arabidopsis AMPD Recombinant Enzyme
A 3.3 Å x-ray crystal structure of an Arabidopsis AMPD N-truncated (ΔI139M) recombinant enzyme complexed with coformycin 5′-phosphate (Han et al., 2006) has provided direct evidence that 5′-phosphorylated forms of these herbicides bind to the active site. The ΔI139M recombinant enzyme (Km [−ATP] = 12 ± 3 mm [Han et al., 2006]) was assayed at a constant substrate concentration (2.4 mm AMP) in the presence of variable concentrations of DF 5′-phosphate (none, and 100 nm to 4.5 μm). Inhibitor plots ([Vmax/Vo]−1 versus inhibitor concentration) were generated using three independent enzyme preparations to reveal a Ki of 601 ± 340 nm (data not shown).
DISCUSSION
AMPD catalyzes a reaction that is located at a key position in plant purine nucleotide metabolism. This is underscored by the lethality (genetic or herbicide induced) that accompanies severe reductions in the catalytic activity of this enzyme. However, the mechanistic basis for this toxicity has been obscure. This issue has been addressed in this study by considering the immediate metabolic consequences of AMPD inhibition in Arabidopsis seedlings following systemic exposure to DF, a synthetic herbicide that is taken up by plant cells and 5′-phosphorylated into a potent transition state-type inhibitor of AMPD (Lindell et al., 1999). In addition, normal aglycone bases that interface with metabolic pathways at points either proximal or distal to the AMPD reaction were evaluated for their abilities to affect herbicide toxicity, which has provided insight regarding the relative importance of upstream and downstream consequences of enzyme inhibition.
Although herbicide-induced plant lethality is usually associated with a decrease in the intracellular concentration of ATP that reflects a generalized disruption of cellular energy metabolism (Raymond et al., 1987), this is clearly not the case with AMPD inhibitors. Rather, the dose-dependent and time-dependent decreases in growth rates of Arabidopsis seedlings during systemic exposure to DF are accompanied by increases in the intracellular concentrations of adenine nucleotides. Previous studies with DF (Lindell et al., 1999) or the related phytotoxin, carbocyclic coformycin (Dancer et al., 1997), monitored metabolite pools only during the first 24 h of exposure, and also observed higher levels of ATP but no measurable change in either ADP or AMP. The combined data indicate that AMPD-inhibited plant cells initially attempt to compensate for a limitation in catalytic activity by maintaining the intracellular AEC, which is most effectively accomplished by increasing the level of ATP. However, the continued absence of functional AMPD enzyme over a longer time period ultimately causes ADP and AMP to accumulate. These metabolic effects are not surprising because adenine nucleotide accumulation would be an expected consequence of AMPD inhibition in a plant cell, which has no other avenue for AMP catabolism. Eukaryotes in the animal kingdom and all prokaryotes utilize multiple routes of AMP catabolism, whereas plants lack the additional deaminase enzymes that other organisms use to catalyze adenine-to-hypoxanthine ring conversions, i.e. adenosine deaminase (adenosine to inosine; Le Floc'h et al., 1982; Yabuki and Ashihara, 1991; Dancer et al., 1997) and adenase (adenine to hypoxanthine; Le Floc'h et al., 1982; Yabuki and Ashihara, 1991). Consequently, plants must rely solely on AMPD to accomplish this obligate conversion in the catabolism of adenine nucleotides.
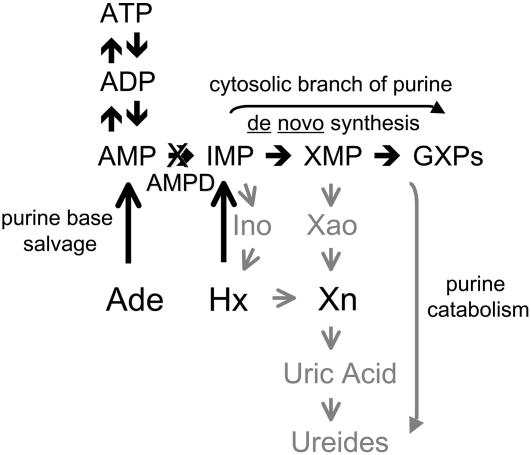
Additional experiments were conducted to determine if purine base supplements had any effect on DF toxicity. Hypoxanthine and xanthine were chosen because they impact on metabolic processes located downstream of the AMPD reaction. As shown in Figure 10, one metabolic fate of hypoxanthine is phosphoribosylation to IMP, which can then be incorporated into the guanine nucleotide pool by the cytosolic branch of the purine de novo pathway. However, hypoxanthine does not rescue seedlings from DF toxicity, nor does the concentration of GTP diminish during growth inhibition in the presence of herbicide alone (300 nm). These combined observations demonstrate that a limitation in adenine-to-guanine nucleotide conversion is not a major factor in the herbicidal activity of DF.
Figure 10.
Metabolic pathways of purine base salvage and catabolism in a plant cell. Ade, Adenine; Hx, hypoxanthine; Xn, xanthine; Ino, inosine; Xao, xanthosine; GXPs, guanine nucleotides. It should be noted that there is also a potential for futile cycling between AMP and adenosine (combined activities of 5′-nucleotidase and adenosine kinase), or between AMP (through adenosine) and adenine (combined activities of 5′-nucleotidase, adenosine nucleosidase, and adenine phosphoribosyltransferase).
An alternate metabolic fate for hypoxanthine is oxidation to xanthine, which is then further oxidized to uric acid, the immediate precursor for peroxisomal synthesis of the linear ureides allantoin and allantoic acid (see Fig. 10). Ureides are major transported forms of fixed nitrogen in tropical legumes, such as soybean (Glycine max) and cowpea (Vigna unguiculata; Schubert, 1986). Moreover, a ureide nitrogen cycle probably operates in all plants because allantoin transporters have been identified in Arabidopsis (Desimone et al., 2002) and allantoin and allantoic acid represent 15% of total nitrogen in the sieve tube sap of several nonlegume species (Ziegler, 1975). However, neither hypoxanthine nor xanthine rescues seedlings from DF toxicity, which demonstrates that a limitation in ureide synthesis is not a major factor in the herbicidal effects exerted by this class of modified nucleosides.
Adenine was chosen as a supplement because it can be phosphoribosylated and incorporated directly into the adenine nucleotide pool (Doree, 1973; Le Floc'h et al., 1982) upstream of the AMPD reaction (see Fig. 10). Studies conducted with this purine base strongly suggest that DF toxicity is triggered by adenine nucleotide accumulation, rather than some other heretofore unrecognized effect of this class of herbicides. In the presence of a DF concentration that does not produce any significant effect on Arabidopsis seedling growth or adenine nucleotide pools (150 nm), adenine (250 μm) exerts a synergistic effect on these parameters that is comparable to what is observed with a higher dose (300 nm) of herbicide alone. Conversely, adenine alone (60–250 μm) has no significant effects on Arabidopsis seedling growth or adenine nucleotide levels. These combined observations demonstrate that a functional AMPD enzyme effectively prevents the detrimental expansion of the adenine nucleotide pool in response to increased salvage synthesis of adenine. This functional significance can be attributed to a dramatic Km and Vmax ATP activation, as reported for the endogenous Catharanthus roseus (Yabuki and Ashihara, 1992) and recombinant Arabidopsis (Han et al., 2006) enzymes. This combined regulatory effect is not seen with orthologs in the animal kingdom, which suggests that plant AMPD may have evolved this property to compensate for the lack of an alternate AMP catabolic pathway. However, this putative adaptation is negated in an AMPD-inhibited plant cell, in which incorporation into the nucleotide pool becomes the primary metabolic end point of salvaged adenine.
A potential mechanistic possibility for DF toxicity that may be attributed to adenine nucleotide accumulation is altered function of 14-3-3 proteins, which in turn regulate many essential enzymes (Darling et al., 2005). 14-3-3 proteins contain a binding site for AMP (Athwal et al., 1998), and in vitro studies have shown that this purine nucleotide can disrupt their functional associations with key enzymes of primary metabolism (Athwal et al., 1998, 2000; Camoni et al., 2001; Camparo et al., 2003). In vivo studies with 5-aminoimidazole-4-carboxamide riboside (AICAR) support this latter hypothesis. AICAR is taken up and 5′-phosphorylated in cells to form AICAR-MP, and the intracellular accumulation of this AMP mimetic is associated with alterations in 14-3-3 protein distributions (Paul et al., 2005) and changes in target enzyme activities that are consistent with disrupted interactions (Huber and Kaiser, 1996; Man and Kaiser, 2001). Therefore, the accumulation of AMP in response to DF may have a similar effect on 14-3-3 proteins. The premise for DF treatment in the fluorescence microscopy experiments presented in this study, and for AICAR treatment in a previous study (Paul et al., 2005), is that if the subcellular distribution of a 14-3-3/GFP fusion is reliant on partner binding, then disruption of that partnering will also disrupt the subcellular distribution of the fusion protein. AICAR treatment of leaves disrupts the smooth cytoskeletal 14-3-3-phi/GFP localization within trichomes, resulting in distinctively globular regions of fluorescence within the cell. DF treatment also disrupts 14-3-3-phi/GFP localization within trichomes, but the altered fluorescence appears stippled rather than globular. While the reason for different patterns of disruption is unclear, the observation that DF treatment alters 14-3-3-phi/GFP localization indicates a disturbed function of this isoform in trichomes. It is tempting to speculate that the accumulation of AMP in DF-treated plant tissue is involved in this altered distribution.
Finally, if adenine nucleotide accumulation is the metabolic trigger that results in lethality due to limitations in AMPD catalytic activity, then selective inhibitors would also possess the desirable herbicidal feature of being toxic only toward plants. All other organisms have the capacity to use alternative routes of AMP catabolism, which would minimize adenine nucleotide accumulation and the associated negative consequences of AMPD inhibition.
CONCLUSION
AMPD is essential for plant life and functions to maintain steady-state levels of adenine nucleotides. The accumulation of these metabolites in an AMPD-inhibited plant cell acts as a trigger that results in growth inhibition and subsequent lethality. Downstream effects of this metabolic dysregulation may include disrupted 14-3-3 protein function.
MATERIALS AND METHODS
Plants
Wild-type Arabidopsis (Arabidopsis thaliana; ecotype Columbia) plants were used in all metabolic studies. For the fluorescent localization study, Arabidopsis (Wassilewskija) plants were transformed with a fusion protein composed of the coding region for the 14-3-3 phi isoform coupled to GFP (S65T) and driven by the cauliflower mosaic virus 35s promoter (Sehnke et al., 2002).
Growth Conditions
For all metabolic studies, seeds collected from dried siliques of wild-type plants were surface sterilized, resuspended in sterile water, and imbibed at 4°C in the dark for 2 d. Imbibed seeds were placed on Murashige and Skoog salt (0.5×) + 1% Suc agar plates, which were then positioned vertically 12 to 15 inches under three 25W fluorescent bulbs for 5 d at constant light. Five-day-old seedlings were transferred into 24-well Murashige and Skoog salt + 1% Suc agar plates with and without 150 to 450 nm DF and 60 to 250 μm purine bases (adenine, hypoxanthine, or xanthine), and grown under long-day conditions (16 h light/8 h dark) for up to nine more days.
For the fluorescent localization study, plants were grown horizontally in plates on nutrient agar (Paul et al., 2001), and then 2-week-old seedlings were held semisubmerged for up to 24 h in a 100 nm solution of DF or water as a control.
Quantification of Seedling Growth and Adenine Nucleotide Pools
Roots were excised and the remaining tissue was weighed and ground to a powder under liquid nitrogen. Ice-cold 10% (w/v) TCA was added and the frozen tissue was homogenized on ice for 2 min with a motorized pestle. The homogenate was centrifuged at 14,000g for 2 min at 4°C, and the supernatant was neutralized with an equal volume of 0.5 m tri-n-octylamine in freon. All samples were immediately frozen in dry ice and stored at −80°C. Adenine nucleotides were separated by anion-exchange HPLC, as described previously (Mahnke and Sabina, 2005). Briefly, 75 μL of sample was injected onto a Partisil-10 SAX anion-exchange column (Whatman) and developed with a 40-min linear gradient of 5 mm NH4H2PO4, pH 3.4, to 750 mm NH4H2PO4, pH 4.0. The column was run at a flow rate of 2 mL/min and eluate was monitored at 254 nm. The various peaks in the extract were identified by comparison of retention times to known external standards and the results were expressed in nmol/g wet tissue weight.
Microscopy and Photography
Following semisubmersion in a 100 nm solution of DF or water control, trichomes of transformed plants were examined with an Olympus BX51 fluorescent microscope coupled to an Evolution MP cooled CCD camera with Q-capture 2.60 software (Quantitative Imaging). Photos were taken through the 20× objective and captured with no binning. The specific region of interest was subsequently cropped from the resulting large (14.2-MB) digital file. Slide samples were prepared in water.
Kinetic Analysis of DF 5′-Phosphate Inhibition of Arabidopsis AMPD
The 5′-phosphorylated derivative of DF was synthesized from formycin B as described previously (Lindell et al., 1999). An N-truncated Arabidopsis recombinant enzyme (ΔI139M) was expressed from a baculoviral vector transfected into insect cells and the resulting activity purified by phosphocellulose chromatography using salt gradient elution (0.15–2.4 m potassium chloride) as described previously (Han et al., 2006). Approximately 0.15 units of purified enzyme was assayed in the absence and presence of increasing concentrations of DF 5′-phosphate (100 nm–4.5 μm) under conditions that consumed less than 20% of available substrate. Assays were performed in 100-μL volumes that also contained 25 mm imidazole, pH 6.5, 150 mm potassium chloride, 2.4 mm AMP, and 0.2 mg/mL bovine serum albumin. Reactions were terminated by immersing the assay tubes in a dry ice/ethanol bath. Frozen assay mixtures were stored at −80°C until analysis, which involved substrate and product (IMP) separation by anion-exchange HPLC using the conditions described above.
Statistical Analysis
All quantitative data were compiled as the mean ± sd, and statistical significance between groups and conditions was evaluated in two-tailed unpaired t tests.
This work was supported by a cooperative arrangement between Bayer CropScience GmbH and the Medical College of Wisconsin, a Public Health Service supplemental grant to R.L.S. through the Center for Eukaryotic Structural Genomics (University of Wisconsin, Madison; U54–GM074901–01, J.L. Markely, P.I.), a Research Affairs Committee at the Medical College of Wisconsin grant, and the National Aeronautics and Space Administration (grant no. NAG 10–291).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Richard L. Sabina (sabinar@mcw.edu).
Open Access articles can be viewed online without a subscription.
References
- Athwal GS, Huber JL, Huber SC (1998) Phosphorylated nitrate reductase and 14-3-3 proteins. Site of interaction, effect of ions, and evidence of an AMP-binding site on 14-3-3 proteins. Plant Physiol 118 1041–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athwal GS, Lombardo CR, Huber JL, Masters SC, Fu H, Huber SC (2000) Modulation of 14-3-3 protein interactions with target polypeptides by physical and metabolic effectors. Plant Cell Physiol 41 523–533 [DOI] [PubMed] [Google Scholar]
- Bush BD, Fitchett GV, Gates DA, Langley D (1993) Carbocyclic nucleosides from a species of Saccharothrix. Phytochemistry 32 737–739 [Google Scholar]
- Camoni L, Visconti S, Marra M, Aducci P (2001) Adenosine 5′-monophosphate inhibits the association of 14-3-3 proteins with the plant plasma membrane H(+)-ATPase. J Biol Chem 276 31709–31712 [DOI] [PubMed] [Google Scholar]
- Camparo S, Lingiah G, Martin T (2003) Function and specificity of 14-3-3 proteins in the regulation of carbohydrate and nitrogen metabolism. J Exp Bot 54 595–604 [DOI] [PubMed] [Google Scholar]
- Dancer JE, Hughes RG, Lindell SD (1997) Adenosine-5′-phosphate deaminase: a novel herbicide target. Plant Physiol 114 119–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling DL, Yingling J, Wynshaw-Boris A (2005) Role of 14-3-3 proteins in eukaryotic signaling and development. Curr Top Dev Biol 68 281–315 [DOI] [PubMed] [Google Scholar]
- Desimone M, Catoni E, Ludewig U, Hilpert M, Schneider A, Kunze R, Tegeder M, Frommer WB, Schumacher K (2002) A novel superfamily of transporters for allantoin and other oxo derivatives of nitrogen heterocyclic compounds in Arabidopsis. Plant Cell 14 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doree M (1973) Metabolism of exogenous adenine by Acer pseudoplatanus cells. Phytochemistry 12 2101–2108 [Google Scholar]
- Frieden C, Kurz LC, Gilbert HR (1980) Adenosine deaminase and adenylate deaminase: comparative kinetic studies with transition state and ground state analogue inhibitors. Biochemistry 19 5303–5309 [DOI] [PubMed] [Google Scholar]
- Haberer G, Kieber JJ (2002) Cytokinins. New insights into a classic phytohormone. Plant Physiol 128 354–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B-W, Bingman CA, Mahnke DK, Bannen RM, Bednarek SY, Sabina RL, Phillips GN Jr (2006) Membrane association, mechanism of action, and structure of Arabidopsis Embryonic Factor 1 (FAC1). J Biol Chem 281 14939–14947 [DOI] [PubMed] [Google Scholar]
- Huber SC, Kaiser WM (1996) 5-Amino-4-carboxamide riboside activates nitrate reductase in darkened spinach and pea leaves. Physiol Plant 98 833–837 [Google Scholar]
- Isaac BG, Ayer SW, Letendre LJ, Stonard RJ (1991) Herbicidal nucleosides from microbial sources. J Antibiot (Tokyo) 44 729–732 [DOI] [PubMed] [Google Scholar]
- Le Floc'h F, Lafleuriel J, Guillot A (1982) Interconversion of purine nucleotides in Jerusalem artichoke shoots. Plant Sci Lett 27 309–316 [Google Scholar]
- Lindell SD, Moloney BA, Hewitt BD, Earnshaw CG, Dudfield PJ, Dancer JE (1999) The design and synthesis of inhibitors of adenosine 5′-monophosphate deaminase. Bioorg Med Chem Lett 9 1985–1999 [DOI] [PubMed] [Google Scholar]
- Mahnke DK, Sabina RL (2005) Calcium activates erythrocyte AMP deaminase [isoform E] through a protein-protein interaction between calmodulin and the N-terminal domain of the AMPD3 polypeptide. Biochemistry 44 5551–5559 [DOI] [PubMed] [Google Scholar]
- Man H-M, Kaiser WM (2001) Increased glutamine synthetase activity and changes in amino acid pools in leaves treated with 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR). Physiol Plant 111 291–296 [DOI] [PubMed] [Google Scholar]
- Nakamura H, Koyama G, Iitaka Y, Ono M, Yagiawa N (1974) Structure of coformycin, an unusual nucleoside of microbial origin. J Am Chem Soc 96 4327–4328 [DOI] [PubMed] [Google Scholar]
- Paul AL, Daugherty CJ, Bihn EA, Chapman DK, Norwood KL, Ferl RJ (2001) Transgene expression patterns indicate that spaceflight affects stress signal perception and transduction in Arabidopsis. Plant Physiol 126 613–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A-L, Sehnke PC, Ferl RJ (2005) Isoform-specific localization among 14-3-3 proteins in Arabidopsis seems driven by client interactions. Mol Biol Cell 16 1735–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond P, Gidrol X, Salon C, Pradet A (1987) Control involving adenine and pyridine nucleotides. In PK Stumpf, EE Conn, eds, The Biochemistry of Plants, Vol 11. Academic Press, New York, pp 129–175
- Schubert K (1986) Products of biological nitrogen fixation in higher plants: synthesis, transport and metabolism. Annu Rev Plant Physiol 37 539–574 [Google Scholar]
- Sehnke PC, Rosenquist M, Alsterfjord M, DeLille J, Sommarin M, Larsson C, Ferl RJ (2002) Evolution and isoform specificity of plant 14-3-3 proteins. Plant Mol Biol 50 1011–1018 [DOI] [PubMed] [Google Scholar]
- Xu J, Zhang HY, Xie CH, Xue HW, Dijkhuis P, Liu CM (2005) EMBRYONIC FACTOR 1 encodes an AMP deaminase and is essential for the zygote to embryo transition in Arabidopsis. Plant J 42 743–758 [DOI] [PubMed] [Google Scholar]
- Yabuki N, Ashihara H (1991) Catabolism of adenine nucleotides in suspension-cultured plant cells. Biochim Biophys Acta 1073 474–480 [DOI] [PubMed] [Google Scholar]
- Yabuki N, Ashihara H (1992) AMP deaminase and the control of adenylate catabolism in suspension-cultured Catharanthus roseus cells. Phytochemistry 31 1905–1909 [Google Scholar]
- Ziegler H (1975) Nature of transported substances. In MH Zimmerman, JA Milburn, eds, Encyclopedia of Plant Physiology. Transport in Plants. I. Phloem Transport. Springer-Verlag, Berlin, pp 59–100