Abstract
Grape (Vitis vinifera) yield is largely dependent on the fecundity of the cultivar. The average number of inflorescences per shoot (i.e. shoot fruitfulness) is a trait related to fecundity of each grapevine. Berry number and weight per bunch are other features affecting grape yield. An ovule-specific auxin-synthesizing (DefH9-iaaM) transgene that increases the indole-3-acetic acid content of grape transgenic berries was transformed into cultivars Silcora and Thompson Seedless, which differ in the average number of inflorescences per shoots. Thompson Seedless naturally has very low shoot fruitfulness, whereas Silcora has medium shoot fruitfulness. The average number of inflorescences per shoot in DefH9-iaaM Thompson Seedless was doubled compared to its wild-type control. Berry number per bunch was increased in both transgenic cultivars. The quality and nutritional value of transgenic berries were substantially equivalent to their control fruits. The data presented indicate that auxin enhances fecundity in grapes, thus enabling to increase yield with lower production costs.
In the world, 79,550 square kilometers are dedicated to cultivated perennial grape (Vitis vinifera) vines, representing approximately 13% of world fruit and nut production (International Organisation of Vine and Wine, 2006; Food and Agriculture Organization, 2004). Most grape production is used for wine (71%), 27% as fresh fruit, and 2% as dried fruit. More than 10,000 grape cultivars exist worldwide (Vouillamoz and Grando, 2006), and they differ in yielding capacity. Some grape cultivars with high-quality fruit feature low yields mostly due to their low fecundity. Fecundity is determined by the average number of inflorescences per shoot (i.e. shoot fruitfulness) detected on each plant. Thus, shoot fruitfulness defines the number of inflorescences per plant and consequently the whole number of harvestable bunches. Other factors affecting grape fecundity are the number of berries per bunch and their weight. Grapevine fecundity has a genetic basis, but it is also influenced by cultivation practices and by several environmental and biological factors (Buttrose, 1974). Several training and pruning systems have been developed and specifically used for cultivars with different shoot fruitfulness (Mullins et al., 1992).
Grape fruit set and growth is triggered by pollination and correlates with elevated endogenous auxin indole-3-acetic acid (IAA) levels (Cawthon and Morris, 1982). Genes either increasing auxin synthesis or sensitivity or altering auxin signal transduction allow fruit set in the absence of pollination (parthenocarpy; Rotino et al., 1997; Ficcadenti et al., 1999; Acciarri et al., 2002; Pandolfini et al., 2002; Carmi et al., 2003; Mezzetti et al., 2004; Wang et al., 2005; Yin et al., 2006). Thus, auxin might improve fruit set and consequently fruit number per plant. In perennial species, auxin also affects development of the inflorescence. This is indicated by the increased number of inflorescences on raspberry (Rubus idaeus) and strawberry (Fragaria spp.) plants genetically engineered with an ovule-specific auxin-synthesizing gene, DefH9-iaaM (Mezzetti et al., 2004). This gene construct contains an ovule-specific regulatory region from DefH9 isolated from Antirrhinum majus and the iaaM-coding region from Pseudomonas savastonoi. The iaaM gene codes for a Trp-2-monoxygenase enzyme that converts Trp to indole-3-acetamide, which is then hydrolyzed to the auxin IAA (Rotino et al., 1997).
DefH9-iaaM was introduced into the genome of two grape cultivars with different levels of fecundity mainly due to different average number of inflorescences per shoot (Mezzetti et al., 2002). Thompson Seedless, a well-known table grape cultivar, has a very low average number of inflorescences per shoot (Mullins et al., 1992), while Silcora, another table grape cultivar, has shoot fruitfulness higher than Thompson Seedless. Transgenic DefH9-iaaM lines of both cultivars have been cultivated (2001–2006) under open-field conditions to compare their fecundity to that of control nontransgenic plants. Average number of inflorescences per shoot and other relevant parameters were recorded over a 3-year-long production cycle from 2004 to 2006. In Thompson Seedless cultivar, the DefH9-iaaM gene doubled the average number of inflorescences per shoot, while shoot fruitfulness was unaffected in transgenic Silcora. Both transgenic cultivars had an increased number of berries per bunch but more in Thompson Seedless (30%) in comparison with Silcora (15%). Berries of the transgenic cultivars had a substantial equivalent nutritional quality. To our knowledge, this is the first report of a long-term, open-field trial on transgenic grape.
RESULTS
Rationale of the Experiment
The DefH9-iaaM auxin-synthesizing gene has been introduced in tobacco (Nicotiana tabacum), eggplant (Solanum melongena), tomato (Solanum lycopersicum), raspberry, strawberry, and cucumber (Cucumis sativus; Rotino et al., 1997; Ficcadenti et al., 1999; Acciarri et al., 2002; Pandolfini et al., 2002; Mezzetti et al., 2004; Yin et al., 2006). The DefH9-iaaM gene induced parthenocarpic development of the fruit. In strawberry and raspberry, the DefH9-iaaM gene also increased the number of inflorescences per plant (Mezzetti et al., 2004).
We have transformed two grape cultivars to evaluate whether the DefH9-iaaM gene can be used to improve fecundity in grape: Thompson Seedless, a grape cultivar with a very low average number of inflorescences per shoot, and Silcora, a cultivar with a medium average number of inflorescences per shoot (Mezzetti et al., 2002). Both cultivars are curtailed in seed development, i.e. they are marketed as seedless. Thompson Seedless is completely stenospermocarpic (i.e. each berry contains one or more aborted seeds), while Silcora seeds are not fully developed, without lignified teguments, and not germinable. The introduction of the DefH9-iaaM gene in these two cultivars allows an evaluation of whether fecundity can be improved in grape, reducing possible secondary effects caused by alterations of seed development. Cultivation practices were chosen to be appropriate for assessing average number of inflorescences per shoot of the tested genotypes.
DefH9-iaaM Gene Expression and IAA Analysis
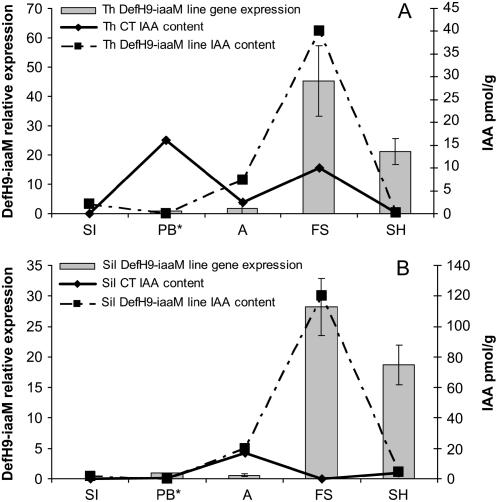
The DefH9-iaaM gene is expressed in both flowers and fruits at different developmental stages (Fig. 1). The steady-state level of DefH9-iaaM mRNA during flower development peaks at fruit set (Fig. 1, A and B). In parallel, total IAA content was measured in flowers and fruits at the same developmental stages (Fig. 1, A and B). A higher IAA content is evident at fruit set in both transgenic lines in comparison to controls. The Thompson Seedless DefH9-iaaM line had 4 times more IAA (Fig. 1A), while Silcora had 120 times more IAA than its control (Fig. 1B). Thus, both DefH9-iaaM gene expression and IAA content are highest at fruit set. It is worthwhile to note that the DefH9-iaaM gene is also expressed in other tissues (e.g. young leaves and tendrils) but at a much lower level (data not shown).
Figure 1.
Total IAA content and relative expression profiles of DefH9-iaaM gene in flowers and berries at different stages of development of Thompson Seedless (A) and Silcora (B). IAA analyses were performed on tissues at the following phenological phases: SI, separated inflorescences; PB, preblooming; A, anthesis; FS, fruit set; SH, small hard fruit. DefH9-iaaM gene relative expression was studied at PB, A, FS, and SH. Expression values were referred to those detected from grape flower tissues at the PB* developmental stage and after normalization with the two housekeeping genes. Data were expressed as means ± se.
Shoot Fruitfulness
The number of fruitful shoots (i.e. shoots bearing inflorescences) was almost doubled to 27% in the transgenic Thompson Seedless line in comparison to control (14%; Table I). As a consequence, shoot fruitfulness of the transgenic Thompson Seedless line also almost doubled, reaching the average of 0.27 inflorescences per shoot in comparison with control.
Table I.
Vegetative developmenta, fecundityb, and total plant fruit production of Thompson Seedless (TS) and Silcora (S) DefH9-iaaM lines (GM) in comparison with controls (CT)
Data collected during the whole production cycle consisting of three years of cultivation: 2004, 2005, and 2006. Mean ± se; means in the same column that are followed by the same letter are not different (P ≤ 0.05) using the lsd test.
| Clones | No. Shoots/Plant | Average Shoot Dry Weight | Fruitful Shoots | Shoot Fruitfulness | No. Bunches/Plant | No. Berries/Bunch | Berry Weight | Bunch Weight | Plant Fruit Production |
|---|---|---|---|---|---|---|---|---|---|
| g | % | g | g | kg | |||||
| TS CT | 17.5 ± 0.36a | 34 ± 2.7a | 14.1 ± 2.5b | 0.14 ± 0.01b | 2.88 ± 0.33b | 189.0 ± 7.7b | 0.95 ± 0.02a | 180.7 ± 8.8b | 0.55 ± 0.05b |
| TS GM | 16.1 ± 0.32b | 31 ± 2.3a | 27.2 ± 7.8a | 0.27 ± 0.02a | 5.97 ± 0.47a | 247.6 ± 10.3a | 0.87 ± 0.01b | 217.2 ± 10.1a | 1.17 ± 0.09a |
| S CT | 16.4 ± 0.58a | 13 ± 1.8a | 70.0 ± 4.6a | 0.86 ± 0.04a | 17.38 ± 1.00a | 98.4 ± 3.7b | 2.88 ± 0.13b | 289.9 ± 20.2b | 4.88 ± 0.43b |
| S GM | 17.0 ± 0.56a | 15 ± 2.2a | 66.6 ± 3.8a | 0.80 ± 0.05a | 15.38 ± 1.25a | 113.5 ± 5.3a | 3.24 ± 0.19a | 376.1 ± 29.3a | 5.31 ± 0.44a |
Expressed as number of shoots per plant and average shoot dry weight (grams).
Expressed as percentage of fruitful shoots, shoot fruitfulness (average number of inflorescences per shoot), number of bunches per plant, number of berries per bunch, berry weight (grams), and bunch weight (grams).
The DefH9-iaaM Thompson Seedless line had somewhat fewer shoots per vine in comparison with Thompson Seedless control (Table I) without altering shoot growth, as expressed by its average dry weight at the end of the season. Thus, vegetative development was only slightly affected in transgenic Thompson Seedless. Shoot fruitfulness and vegetative growth did not differ between Silcora DefH9-iaaM line and its control (Table I). In conclusion, the DefH9-iaaM gene caused a stable and pronounced increase in average number of inflorescences per shoot only in Thompson Seedless, a cultivar with natural low shoot fruitfulness.
Bunches and Berry Number
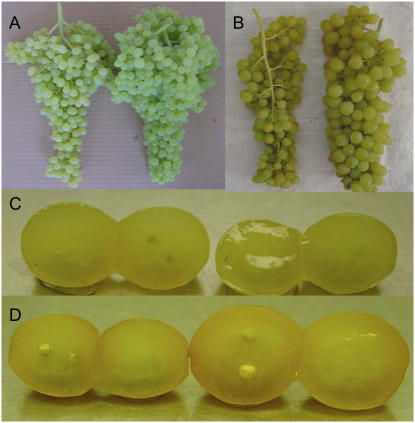
Thompson Seedless DefH9-iaaM line had twice as many bunches per plant than its control (Table I). The increase in bunch number depends on the increased number of inflorescences per shoot detected in the transgenic line (Table I). In the transgenic Thompson Seedless line, the number of berries per bunch was 30% more than its control (Table I). Although the average berry weight was slightly lower in the DefH9-iaaM Thompson Seedless line in comparison with the control (Table I), the average weight of the DefH9-iaaM Thompson Seedless bunch was 25% more than the control (Table I; Fig. 2A).
Figure 2.
Grape bunches harvested from Thompson Seedless control (A, left) and genetically modified (GM; A, right), and Silcora control (B, left) and GM (B, right). Berry section of Thompson Seedless control (C, left) and GM (C, right) and Silcora control (D, left) and GM (D, right).
The Silcora DefH9-iaaM line had the same number of bunches per plant compared to its control. However, berry number per bunch increased by 15% (Table I). Transgenic Silcora berries also had an increased berry weight (Table I). As a result of both the higher number of berries per bunch and the higher weight of the berries, transgenic Silcora bunches had a weight 40% higher than the control (Table I; Fig. 2B).
In conclusion, the number of berries per bunch increased by 30% and 15% in the transgenic Thompson Seedless and Silcora lines, respectively. These data indicate that both transgenic cultivars had more berries.
Nutritional Quality of the Fruit and Production
Thompson Seedless and Silcora are both considered substantially seedless cultivars. Consequently, the DefH9-iaaM gene had negligible effects on seed development and germinability. However, while nontransgenic Thompson Seedless berries still contain some aborted seeds, in transgenic Thompson Seedless they were not easily detectable at ripeness (Fig. 2, C and D).
Several parameters related to berry quality and nutritional value were analyzed in transgenic and control berries. No relevant differences in the tested parameters were found between transgenic and control berries, except for an increased total antioxidant capacity (TAC) and total polyphenol content (TPH) of transgenic Silcora berries (Table II). Minor changes in transgenic Thompson Seedless berries were observed for other parameters, i.e. titratable acidity (TA), pH, malic acid (MA), and citric acid (CA), probably due to a slight delayed ripening of the transgenic Thompson Seedless line. These changes have negligible effects on the overall quality and nutritional value of grape fruit, indicating the substantial equivalence of transgenic and control berries.
Table II.
Berry qualitya and nutritional valueb of Thompson Seedless (TS) and Silcora (S) DefH9-iaaM lines (GM) in comparison with controls (CT)
Data collected at harvest of the three years of cultivation: 2004, 2005, and 2006. Mean ± se; means in the same column that are followed by the same letters are not different (P ≤ 0.05) using the lsd test.
| Clones | SS | TA | pH | TaA | MA | CA | TPH | TAC Trolox |
|---|---|---|---|---|---|---|---|---|
| g/L | g/L | g/L | mg gallic acid/g fruit | μmol/g | ||||
| TS CT | 18.8 ± 0.35a | 9.6 ± 0.35a | 3.1 ± 0.02b | 8.6 ± 0.27a | 3.4 ± 0.23a | 0.30 ± 0.02a | 0.88 ± 0.03b | 3.1 ± 0.08a |
| TS GM | 18.7 ± 0.38a | 8.0 ± 0.24b | 3.2 ± 0.03a | 8.3 ± 0.19a | 2.4 ± 0.32b | 0.22 ± 0.01b | 0.96 ± 0.02a | 2.9 ± 0.06a |
| S CT | 15.2 ± 0.60a | 5.5 ± 0.40a | 3.3 ± 0.05a | 7.0 ± 0.36a | 1.3 ± 0.18a | 0.11 ± 0.01a | 1.41 ± 0.11b | 4.8 ± 0.21b |
| S GM | 15.6 ± 0.39a | 5.4 ± 0.39a | 3.3 ± 0.04a | 6.7 ± 0.32a | 1.5 ± 0.20a | 0.12 ± 0.01a | 1.76 ± 0.06a | 6.6 ± 0.07a |
Expressed as soluble solids contents (SS), TA, pH, tartaric acid (TaA), MA, and CA.
Expressed as TPH and TAC.
The combined effect of enhanced shoot fruitfulness and increased number of berries per bunch in the transgenic Thompson Seedless line caused a doubling in fruit production (Table I). The transgenic Silcora line did have a statistically significant increase (8%) in fruit production (Table I).
DISCUSSION
The study of biological factors and mechanisms affecting flower formation in grape has already highlighted differences from annual model species (i.e. Arabidopsis [Arabidopsis thaliana]). In grapevine, GAs inhibit floral meristem production (Boss and Thomas, 2002), and floral induction in latent buds is stimulated by light and high temperature (Buttrose, 1974). These differences are most likely due to the evolution of a climbing habit of growth in grapevine (Boss and Thomas, 2002).
Grape inflorescences are initiated during late spring within latent bud complexes formed in the axil of every leaf, generally developing either leaf or inflorescence primordia. In the following spring, bud burst occurs and the inflorescence primordia undergo further development. The number of inflorescences and their fruiting capacity have a great influence on grape fecundity.
Grape cultivars differ in their fecundity, which is largely dependent on the degree of shoot fruitfulness. This is related to the cultivar genetic background and influenced by the cultivation practice and the environment (Baldwin, 1964; Sànches and Dokoozlian, 2005). Low average number of inflorescences per shoot has impaired the commercial success of some cultivars. Thus, cultivation practices to improve shoot fruitfulness have been developed (Sànches and Dokoozlian, 2005). Genetic tools have not improved shoot fruitfulness or bunch weight and size in grape. This work used transgenic grape lines with an auxin synthesis-encoding gene (DefH9-iaaM) to investigate whether auxin increases fecundity in grape. With this aim, we have chosen genetic backgrounds with different grades of shoot fruitfulness and bunch features.
Grape plants were planted in 2001 and grown in open-field cultivation. The data collected over 3 years show that the genetic transformation of Thompson Seedless with the auxin synthesis-encoding DefH9-iaaM gene stably increased average number of inflorescences per shoot. Shoot fruitfulness was unchanged in transgenic Silcora grapes. Thus, the increase in average number of inflorescences per shoot is evident only in Thompson Seedless, the cultivar with a genetic background of low shoot fruitfulness.
An increased number of inflorescences in DefH9-iaaM transgenic plants has been observed in two Rosaceae perennial species (Mezzetti et al., 2004) but not in annual species (Solanaceae; Acciarri et al., 2002). Thus, iaaM must affect inflorescence primordia formation within the latent bud. As the only biochemical function of iaaM is auxin synthesis, we conclude that auxin, either directly or indirectly, improves shoot fruitfulness. To act directly, auxin produced by DefH9-iaaM expression could be synthesized either in the inflorescence primordia within the latent bud or synthesized elsewhere in the plant and transported to the latent buds. The expression results evidenced that DefH9-iaaM expression in grape flowers starts from preblooming and increases up to fruit set. However, a very low level of DefH9-iaaM gene expression is detectable also in leaves and tendrils (E. Costantini, L. Landi, and B. Mezzetti, unpublished data). Thus, it cannot be ruled out that iaaM expression in leaves and tendrils might also be a source of IAA transported to latent buds. An indirect action of auxin could result from several well-known biological effects of auxin; for instance, auxin affects vasculature formation (Mattsson et al., 2003). Whatever the mechanism of auxin action, the DefH9-iaaM auxin synthesis-encoding gene is a genetic tool that could be used to enhance shoot fruitfulness in grape cultivars with low fecundity.
In grape, IAA level in flowers is often elevated at fruit set, and the exogenous application of auxin to flowers promotes early fruit growth (Coombe and Hale, 1973; Davies et al., 1997; Davies and Robinson, 2000). In many plant species, fruit set can be improved by auxin manipulation either chemically or genetically (Spena and Rotino, 2001). In this regard, the increased berry number per bunch observed in both transgenic grape lines is likely a consequence of the increased IAA synthesis during flower development.
It is worthwhile to mention that current cultivation practice with Thompson Seedless and some other table grapes often involves the use of exogenous GA to open grape bunches and to improve fruit yield. This work shows that a very similar effect is achieved by properly synthesized endogenous auxin. In some species and tissues, auxin up-regulates GA biosynthetic genes (Frigerio et al., 2006). Thus, the data can be most parsimoniously explained by an up-regulation of GA biosynthetic genes within the transgenic grape berry. In annual species, auxin interplay with other phytohormones, including GA, is rather complex. Such complexities are bound to be at least conserved also in perennial species.
A prerequisite for any applied use of DefH9-iaaM is the substantial equivalence of transgenic and nontransgenic fruit. Quality and nutritional attributes of transgenic and nontransgenic grape fruit were substantially equivalent. A similar substantial equivalence has been observed also in DefH9-iaaM tomato fruit (Rotino et al., 2005).
The field trial focused on primarily assessing shoot fruitfulness and consequently plant fecundity. Nevertheless, the data indicate that the DefH9-iaaM gene activity does not curtail grape yield. In fact, the data indicate an 8% improved production in transgenic Silcora and a more than doubled production in transgenic Thompson Seedless. Thus, the productivity data hereby presented suggest that it will be worthwhile to test the increased yield with other trials performed under different cultivation conditions.
MATERIALS AND METHODS
Plant Material and Experimental Trial
The open-field trial with transgenic and control grape (Vitis vinifera) clones was established at the Experimental Farm of the Marche Polytechnic University in March 2001 by following the European Commission (EC 2001/18) rules for transgenic plants. The open-field trials were carried out following the protocol approved by Comitato Interministeriale di Valutazione, Biotechnology, Italian Minister of Health B/IT/99/24.
The DefH9-iaaM (one gene copy) transgenic clones of both Thompson Seedless and Silcora were identified, propagated, and compared in field trial with their corresponding nontransgenic controls (Mezzetti et al., 2002). The experimental trial utilized micropropagated plants of the controls and transgenic lines. Thirty-two well-developed, self-rooted vines of each Thompson Seedless clone (control and DefH9-iaaM line) and 16 vines for each Silcora clone (control and DefH9-iaaM line) were arranged in a completely randomized block design with four plots of either eight or four plants, respectively. Vines were planted at 2.5- × 1.5-m spacing, pruned leaving two canes bearing a maximum of 12 to 13 nodes each, and positioned to sustain vertical shoot development. Horticultural care suitable for commercial production was provided, including fertilization, irrigation, pest control, and dormant-season pruning. The DefH9-iaaM effects on plant vegetative development, fecundity, and fruit nutritional quality were studied from the third to the fifth production cycles (2004, 2005, and 2006).
Gene Expression Studies
The expression level of DefH9-iaaM gene (iaaM coding region) was studied by using the quantitative real-time (qRT)-PCR method. Flowers and berries of Thompson Seedless and Silcora control and transgenic clones were collected at different developmental phases from the experimental vineyard following a random sampling. Flowers and fruits were immediately frozen in dry ice and kept at −80°C until RNA extraction. Samples corresponding to different physiological phases, i.e. separated inflorescences (inflorescences enlarged but with flowers still clumped), preblooming, anthesis, fruit set, small hard fruit, and also young leaves and tendrils, were used for RNA extraction using the protocol developed by Iandolino et al. (2004). A total of 10 to 50 ng of RNA was used for cDNA synthesis with reverse transcription-PCR using the QuantiTect Reverse Transcription kit according to the manufacturer's instructions (Qiagen). A specific primer set was designed from the iaaM gene sequence (Rotino et al., 1997). Expression of both the constitutive 18S rRNA (NCBI-AJ421474) and β-tubulin gene (NCBI-BE846422), two housekeeping genes, were used to normalize the expression results using gene expression analysis with the iCycler iQ Multicolor Real-Time PCR Detection system (Bio-Rad; Vandesompele et al., 2002). Differences in DefH9-iaaM gene expression among flowers and fruits at different developmental stages were calculated according to the delta-delta Ct method (Pfaffl, 2001). Expression values of each developmental stage were referred to those detected from grape flower tissues sampled at the preblooming developmental stage, resulting with the lowest expression level. qRT-PCR was performed using iQ SYBR Green Supermix (Bio-Rad) on a iQ-Cycler (Bio-Rad) under the following conditions: an initial denaturating cycle (8 min at 95°C), followed by 45 cycles constituted by three steps of denaturation, annealing, and polymerization (20 s at 95°C, 30 s at 55°, and 30 s 72°C). PCR amplification was done in a 22-μL total volume, containing 9 μL of diluted cDNA (duplicate), 0.5 μm of each primer, and 11 μL of 2× iQ SYBR Green Supermix. In each experiment, target and housekeeping genes were amplified in the same plate with a series of diluted cDNA (10−5–10−9 ng) to generate calibration-specific curves. Three independent experiments were performed for each sample.
IAA Analysis
The auxin (IAA total) content was analyzed in both control and transgenic plants of the two table grape cultivars. Flowers and fruits were collected at the same developmental stages analyzed by qRT-PCR. Gas chromatography-mass spectrometry of the tissue total IAA content was performed as described by Mezzetti et al. (2004). This value was expressed in picomoles per gram of tissue (fresh weight) and includes both free and conjugated IAA.
Shoot Fruitfulness and Plant Vegetative Development
The number of shoots bearing inflorescences over the total was counted to determine the percentage of fruitful shoots, while shoot fruitfulness was determined by counting the number of inflorescences per plant over the total number of shoots per plant. The vegetative development of control and DefH9-iaaM lines was also compared by detecting the number of shoots per plant and their dry weight at the end of the third and fourth production years (January 2004 and 2005).
Bunches and Berry Features and Plant Production
At harvest time of the three production cycles, average bunch weight (grams) was obtained by weighing all bunches collected from each plant and dividing it by the number of bunches per vine. Berry weight (grams) was determined by weighing 100 berries per each vine collected from the distal, medium, and basal parts of the bunch. The number of berries per bunch was obtained by dividing the bunch weight for the berry weight determined for each plant.
Berry Quality and Nutritional Values
At harvest of the three production cycles, berries were sampled and immediately frozen at −20° for quality and nutritional analysis. Differences in fruit nutritional quality among transgenic and control lines were studied by analyzing: soluble solids contents, TA, pH, tartaric acid, MA, CA, TPH, and TAC. Berry juice was extracted from samples from each of the four plots (three analyses for each sample). A small portion of homogenized berries was used to measure the soluble solids with a BS model RFM 81 refractometer. Different portions of 10 g of juice extracts were used for the determinations of berry juice pH and TA according to AOAC methods (AOAC, 1980). CA and MA contents were measured using enzyme kits (Diffchamb), and tartaric acid was measured by a colorimetric vanadic acid reaction and read at 500 nm. TPH was determined in fruit extracts (Scalzo et al., 2005) by the Folin-Ciocalteu-based method (Slinkard and Singleton, 1997), using gallic acid as a standard for the calibration curve. Results were calculated as gallic acid equivalent in fresh fruit (milligrams gallic acid/gram). TAC was evaluated according to the Trolox equivalent antioxidant capacity modified assay (Re et al., 1999) and expressed as micromole Trolox (an analog of tocopherol) equivalents per gram of fresh weight (Scalzo et al., 2005). The total fruit antioxidant capacity was assessed by analyzing hydrophilic and lipophilic extracts. The hydrophilic phase extraction was performed by homogenizing (with a T25 Ultra-turrax blender) frozen fruit samples with extraction solution ethanol:water (80:20) to a final ratio of 1:10 (w/v). Homogenate was centrifuged (2,000g for 10 min) and the supernatant (hydrophilic extract) transferred to vials and stored (−20°C) until assayed. Three extracts were collected. The lipophilic phase extraction was made on pellet adding acetone (1:4 w/v) and then centrifuged (2,000g for 15 min), and the supernatant was transferred to vials and stored (−20°C) until assayed. Three fruit extracts for each phase were recovered, and the antioxidant capacity was measured separately by recording the A734 in a spectrophotometer. Data are expressed as TAC obtained by the total of the two phases.
Statistical Analysis
Data were subjected to one-way ANOVA for means comparison, and sds were calculated according to lsd test (STATISTICA Statsoft). Data are reported as means ± se. Differences at P ≤ 0.05 were considered significant.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers NCBI-AJ421474 and NCBI-BE846422.
Acknowledgments
We thank G. Murri and L. Borghesi of the Marche Polytechnic University's Experimental Farm for management of the open-field trials. Furthermore, we thank P. Pucci and A. Flagello (CEINGE, University of Naples, Federico II) for IAA analysis.
This work was supported by the Fondo per gli Investimenti della Ricerca di Base project RBAUOIJTHS of the Italian Ministry of University and Research.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Bruno Mezzetti (b.mezzetti@univpm.it).
Open Access articles can be viewed online without a subscription.
References
- Acciarri N, Restaino F, Vitelli G, Perrone D, Zottini M, Pandolfini T, Spena A, Rotino GL (2002) Genetically modified parthenocarpic eggplants: improved fruit productivity under both greenhouse and open field cultivation. BMC Biotechnol 2 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC (1980) Official Methods of Analysis, Ed 13. Association of Analytical Chemistry, Washington, DC
- Baldwin JG (1964) The relation between weather and fruitfulness of the Sultana vine. Aust J Agric Res 15 920–929 [Google Scholar]
- Boss PK, Thomas MR (2002) Association of dwarfism and floral induction with a grape “green revolution” mutation. Nature 416 847–850 [DOI] [PubMed] [Google Scholar]
- Buttrose MS (1974) Climatic factors and fruitfulness in grapevines. Hortic Abstr 44 319–325 [Google Scholar]
- Carmi N, Salts Y, Dedicova B, Shabtai S, Barg R (2003) Induction of parthenocarpy in tomato via specific expression of the rolB gene in the ovary. Planta 217 726–735 [DOI] [PubMed] [Google Scholar]
- Cawthon DL, Morris JR (1982) Relationship of seed number and maturity to berry development, fruit maturation, hormonal changes and uneven ripening of ‘Concord’ (Vitis vinifera L.) grapes. J Am Soc Hortic Sci 107 1097–1104 [Google Scholar]
- Coombe BG, Hale CR (1973) The hormone content of ripening grape berries and the effects of growth substance treatments. Plant Physiol 51 629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C, Boss PK, Robinson SP (1997) Treatment of grape berries, a nonclimacteric fruit with a synthetic auxin, retards ripening and alters the expression of developmentally regulated genes. Plant Physiol 115 1155–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C, Robinson SP (2000) Differential screening indicates a dramatic change in mRNA profiles during grape berry ripening. Cloning and characterization of cDNAs encoding putative cell wall and stress response proteins. Plant Physiol 122 803–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficcadenti N, Sestili S, Pandolfini T, Cirillo C, Rotino GL, Spena A (1999) Genetic engineering of parthenocarpic fruit development in tomato. Mol Breed 5 463–470 [Google Scholar]
- Food and Agriculture Organization (2004) FAO yearbook: production. International statistics. Crop production by country, includes fruit, vegetables, grape & vine. http://www.fao.org/documents/
- Frigerio M, Alabadì D, Pérez-Gomez J, Garcìa-Càrcel L, Philips AL, Hedden P, Blazquez MA (2006) Transcriptional regulation of gibberellin metabolism genes by auxin signaling in Arabidopsis. Plant Physiol 142 553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iandolino AB, Goes da Silva F, Lim H, Choi H, Williams LE, Cook DR (2004) High-quality RNA, cDNA, and derived EST libraries from grapevine (Vitis vinifera L.). Plant Mol Biol 22 269–278 [Google Scholar]
- International Organisation of Vine and Wine (2006) Organization of vine and wine: situation and statistics of the world vitiviniculture sector. http://www.oiv.int/uk/accueil/index.php
- Mattsson J, Ckurshumova W, Berleth T (2003) Auxin signaling in Arabidopsis leaf vascular development. Plant Physiol 131 1327–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzetti B, Landi L, Pandolfini T, Spena A (2004) The DefH9-iaaM auxin-synthesizing gene increases plant fecundity and fruit production in strawberry and raspberry. BMC Biotechnol 4 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzetti B, Pandolfini T, Navacchi O, Landi L (2002) Genetic transformation of Vitis vinifera via organogenesis. BMC Biotechnol 2 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins MG, Bouquet A, Williams LE (1992) Biology of the Grapevine. Cambridge University Press, Cambridge, UK
- Pandolfini T, Rotino GL, Camerini S, Defez R, Spena A (2002) Optimization of transgene action at the post-transcriptional level: high quality parthenocarpic fruits in industrial tomatoes. BMC Biotechnol 2 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW (2001) A new mathematical model for relative quantification in real time RT-PCR. Nucleic Acids Res 29 2002–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26 1231–1237 [DOI] [PubMed] [Google Scholar]
- Rotino GL, Acciarri N, Sabatini E, Mennella G, Lo Scalzo R, Maestrelli A, Molesini B, Pandolfini T, Scalzo J, Mezzetti B, et al (2005) Open field trial of genetically modified parthenocarpic tomato: seedlessness and fruit quality. BMC Biotechnol 5 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotino GL, Perri E, Zottini M, Sommer H, Spena A (1997) Genetic engineering of parthenocarpic plants. Nat Biotechnol 15 1398–1401 [DOI] [PubMed] [Google Scholar]
- Sànches LA, Dokoozlian NK (2005) Bud microclimate and fruitfulness in Vitis vinifera L. Am J Enol Vitic 56 319–329 [Google Scholar]
- Scalzo J, Politi A, Mezzetti B, Battino M (2005) Plant genotype and cultural condition interactions affecting fruits total antioxidant potential and poliphenolic contents. Nutrition 21 207–213 [DOI] [PubMed] [Google Scholar]
- Slinkard K, Singleton VL (1997) Total phenol analysis: automation and comparison with manual methods. Am J Enol Vitic 28 49–55 [Google Scholar]
- Spena A, Rotino GL (2001) Parthenocarpy: state of the art. In SS Bhojwani, WY Soh, eds, Current Trends in the Embryology of Angiosperms. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 435–450
- Vandesompele J, De Preter K, Pattyn Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3 research 0034 [DOI] [PMC free article] [PubMed]
- Vouillamoz JF, Grando MS (2006) Genealogy of wine grape cultivars: “Pinot” is related to “Syrah”. Heredity 97 102–110 [DOI] [PubMed] [Google Scholar]
- Wang H, Jones B, Li Z, Frasse P, Delalande C, Regad F, Chaabouni S, Latche A, Pech JC, Bouzayen M (2005) The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell 17 2676–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Malinowski R, Ziolkowska A, Sommer H, Plader W, Malepszi S (2006) The DefH9-iaaM-containing construct efficiently induces parthenocarpy in cucumber. Cell Mol Biol 11 279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]