Abstract
Ammonium transport across plant plasma membranes is facilitated by AMT/Rh-type ammonium transporters (AMTs), which also have homologs in most organisms. In the roots of the plant Arabidopsis (Arabidopsis thaliana), AMTs have been identified that function directly in the high-affinity NH4+ acquisition from soil. Here, we show that AtAMT1;2 has a distinct role, as it is located in the plasma membrane of the root endodermis. AtAMT1;2 functions as a comparatively low-affinity NH4+ transporter. Mutations at the highly conserved carboxyl terminus (C terminus) of AMTs, including one that mimics phosphorylation at a putative phosphorylation site, impair NH4+ transport activity. Coexpressing these mutants along with wild-type AtAMT1;2 substantially reduced the activity of the wild-type transporter. A molecular model of AtAMT1;2 provides a plausible explanation for the dominant inhibition, as the C terminus of one monomer directly contacts the neighboring subunit. It is suggested that part of the cytoplasmic C terminus of a single monomer can gate the AMT trimer. This regulatory mechanism for rapid and efficient inactivation of NH4+ transporters may apply to several AMT members to prevent excess influx of cytotoxic ammonium.
Ammonium (NH4+/NH3) transporters of the AMT/Rh family are identified throughout all domains of life, including archae, bacteria, fungi, plants, and mammals (Ludewig et al., 2001; von Wiren and Merrick, 2004). The ammonium transporters (AMTs) from different species appear to have contrasting transport mechanisms, depending on their physiological role (Ludewig, 2006). While the function of AMTs from plants is the net import and accumulation of NH4+ (Mayer et al., 2006), the Rh glycoproteins, in contrast, appear to conduct NH3 or facilitate NH4+/H+ exchange for export and disposal (Westhoff et al., 2002; Zidi-Yahiaoui et al., 2005; Mayer et al., 2006). Similarly, prokaryotic AMT homologs appear to be NH3 channels (Khademi et al., 2004; Zheng et al., 2004; Andrade et al., 2005; Javelle et al., 2005). The genome of many species contains several homologous AMT/Rh genes and six AMTs are found in the model plant Arabidopsis (Arabidopsis thaliana; Loque and von Wiren, 2004).
The transcripts of at least three AMTs from Arabidopsis are regulated by nitrogen availability in roots (Gazzarrini et al., 1999). In several plant species studied, AMT transcripts are up-regulated by nitrogen limitation. In roots AtAMT1;1 is localized in the plasma membrane of the rhizodermis, cortex, and pericycle (Mayer and Ludewig, 2006), and is responsible for about 30% of the ammonium acquisition in Arabidopsis roots (Kaiser et al., 2002). AtAMT1;1 is partially colocalized with AtAMT1;3, which is also expressed in the rhizodermis and cortex and participates in another 30% of NH4+ uptake of roots (Loque et al., 2006). The residual 40% of ammonium influx in roots appears thus to be carried by AtAMT1;2, which is studied here, and AtAMT2;1, which has been mainly localized to the vasculature, but promoter activity was also identified in the cortex and root tip (Sohlenkamp et al., 2002).
Whether plant AMT transporter activity is fine-tuned by posttranscriptional mechanisms is unknown, but the homologous bacterial AmtB is negatively regulated by reversible binding of the signal transduction PII protein GlnK to AmtB (Coutts et al., 2002). The deletion of part of the cytoplasmic tail impairs that interaction and reduces the transport activity by approximately 70% (Coutts et al., 2002). A similar regulation of plant AtAMTs is unlikely since the single PII protein from Arabidopsis is located in chloroplasts (Smith et al., 2002). However, the cytosolic carboxyl terminus (C terminus) has been shown to be important for transport function in AMTs from tomato (Lycopersicon esculentum): The transport by AMTs was inhibited by a specific mutation in that region (Ludewig et al., 2003). An exchange of a conserved Gly by Asp (G458D in LeAMT1;1 and G465D in LeAMT1;2) abolished transport, although the mutation did not affect AMT localization at the plasma membrane (Ludewig et al., 2003).
The importance of this Gly had been initially identified in the homologous Mep transporters from the yeast Saccharomyces cerevisiae. In the mep1-1 mutation, the corresponding Gly was exchanged by Asp (G413D), causing inactivation of Mep1p (Marini et al., 1997, 2000). The mutant had reduced, albeit residual, transport activity. Most notably, the mutant transporter was able to impair the transport of coexpressed Mep2p or Mep3p, indicating cross talk between different Mep transporters (Marini et al., 2000). Similar observations were reported for AMTs in another fungal species, Aspergillus nidulans (Monahan et al., 2002). Likewise, the equivalent Gly mutations in NH4+ transporters from tomato inactivated coexpressed transporters in a dominant way. Cross-inhibition by mutant subunits was taken as evidence for homooligomerization and possibly heterooligomerization by plant AMTs (Ludewig et al., 2003). Although these data had implicated the importance of the cytosolic C terminus in the transport activity of AMTs, the physiological relevance of these observations remained nebulous. Identification of the high-resolution structures of the prokaryotic AmtB from Escherichia coli did not clarify how the C terminus is involved in the transport of NH4+ (Khademi et al., 2004; Zheng et al., 2004). Although the cytosolic C terminus has not structurally been resolved, it appeared that this tail was not a central constituent of the pore, leaving to question its importance for transport activity. The analysis of homologous transporters in the fungal species Candida albicans revealed that the complete deletion of the cytoplasmic C terminus impaired AMT function, but shorter truncations covering the region around the conserved Gly were functional in ammonium transport. Interestingly, the cytoplasmic C terminus was essential for ammonium-related signaling (Biswas and Morschhauser, 2005).
In this study, we propose a physiological molecular mechanism of how the C terminus regulates the activity of AMT transporters. We concentrated on AtAMT1;2, which is the only root-expressed AMT in Arabidopsis that has not been cellularly localized; conflicting data on the methylammonium (MeA) transport properties by AtAMT1;2 had been published (Gazzarrini et al., 1999; Shelden et al., 2001). Here, it is shown that AtAMT1;2 is a comparatively low-affinity NH4+ transporter that is preferentially localized in the root endodermis. Specific mutations in the conserved C terminus of AMTs, including an exchange in a putative phosphorylation site, impair transport. Most interestingly, a molecular model shows that part of the carboxyl tail of one monomer attaches to its neighbor; this provides a reasonable explanation for the observed cross-inhibition of functional, coexpressed AMT monomers. It is concluded that this provides a physiological mechanism to effectively prevent excess ammonium influx and toxicity.
RESULTS
Preferential Localization of AtAMT1;2 in the Plasma Membrane of Endodermal Root Cells
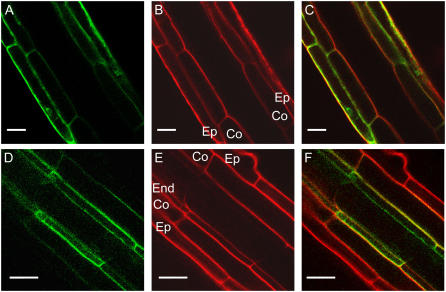
The transcripts of several NH4+ transporters have been identified in roots, including those of AtAMT1;1 and AtAMT1;2 (Gazzarrini et al., 1999). In apical zones of the roots, the green fluorescent protein (GFP) fusion protein with AtAMT1;1 under the control of the endogenous promoter was preferentially identified in epidermal and cortical cell layers (Fig. 1, A–C), as had been shown previously (Loque et al., 2006; Mayer and Ludewig, 2006). In contrast, the GFP-tagged AtAMT1;2 was preferentially found in the plasma membrane of the root endodermis when expressed from the endogenous promoter (Fig. 1, D–F). A minor fraction of AtAMT1;2-GFP fluorescence was also observed in cortical cells. Trans-cellular apoplastic transport across the endodermal cell layer is blocked by the Casparian strip; thus, AtAMT1;2 transfers NH4+ from the apoplast of outer cell layers into the endodermal cytoplasm for further release into the stele.
Figure 1.
Differential expression of AtAMT1;1 and AtAMT1;2 in roots. A, Fluorescence from plant roots expressing AtAMT1;1-GFP from the endogenous promoter. B and C, Same root with propidium iodide cell wall stain (Ep, epidermis; Co, cortex; B) and overlay (C). D, Fluorescence from homozygous plant roots expressing pAtAMT1;2∷AtAMT1;2-GFP. E and F, Propidium iodide stain (End, endodermis; E) and overlay (F). Scale bars = 20 μm.
Transport Characteristics of AtAMT1;2 in Oocytes
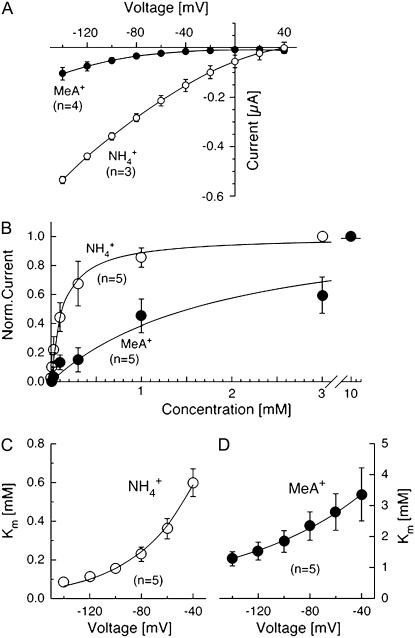
NH4+ induced large inward currents in AtAMT1;2-expressing oocytes (Fig. 2A). Similar to the results with other AMT transporters, ionic currents elicited by addition of ammonium were exclusively inward, even at positive voltages. The inward current elicited by 1 mm ammonium was larger than the current induced by the equivalent amount of MeA (Fig. 2A). At −100 mV, the concentration needed to achieve half-maximal currents was approximately 140 μm for NH4+ and approximately 1.9 mm for MeA+ (Fig. 2B). The NH4+ concentration needed to saturate AtAMT1;2 was much higher than for AtAMT1;1, which has a more than 10-fold higher affinity (Mayer and Ludewig, 2006; Wood et al., 2006). It is possible that the different Km values somewhat reflect the apoplastic ammonium concentrations at the rhizodermis and endodermis.
Figure 2.
Functional characteristics of AtAMT1;2 in Xenopus oocytes. A, Current-voltage plot of currents by AtAMT1;2 induced by ammonium (1 mm, white circles) and MeA (1 mm, black circles). B, Saturation of AtAMT1;2 by NH4+ (white circles) and MeA+ (black circles). C and D, Voltage dependence of the saturation constants for NH4+ (C) and MeA+ transport (D).
The concentration needed to achieve half-maximal currents (“the Km”) was analyzed at each voltage separately and was found to differ. Less ammonium was required to elicit half-maximal currents at more negative voltages. This indicates that both higher ammonium and more negative voltage lead to saturation (Fig. 2C). A similar finding was made with LeAMT1;1 (Ludewig et al., 2002). Assuming a single binding site for NH4+, the transport and saturation are characterized by the entry of NH4+ into the pore and its exit, either back into the external medium or to the cytoplasmic side (this corresponds to transport). The entry (and the exit) rate of NH4+ and, thus, the Km depend on the membrane voltage, as long as the binding site is located within the membrane electric field. The slope of the voltage dependence of the Km was fitted and the fractional electrical distances δNH4+ = 0.56 and δMeA+ = 0.26 were obtained. These values can be interpreted in the way that the binding sites for NH4+ and MeA+ are located 56% and 26%, respectively, inside the membrane electric field, measured from the outside. Interestingly, the steep voltage dependence indicates that NH4+ enters deeply into the pore and that it has to cross more than half of the membrane electric field to reach this site.
AtAMT Transport Activity Is Inactivated by Mutations in the C Terminus
The sequences of AtAMT1;1 and AtAMT1;2 share high similarity that even extends into the cytoplasmic C terminus (Fig. 3A). Specific mutations of a highly conserved Gly in this region have been shown to inactivate AMT/MEP transporters from fungi and tomato (Marini et al., 2000; Ludewig et al., 2003; Smith et al., 2003). However, the role of the cytoplasmic tail is unclear, and how the mutation inhibits the transport function remains obscure.
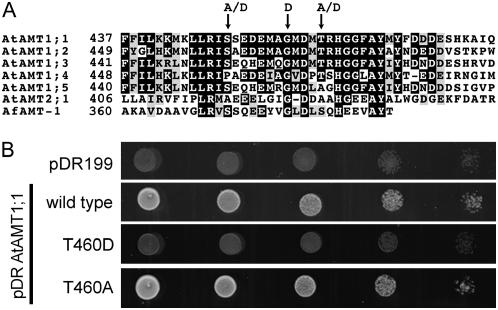
Figure 3.
The cytosolic C terminus of AMT transporters affects NH4+ transport. A, Alignment of amino acid sequences of several plant NH4+ transporters with AfAMT-1. The numbers indicate the amino acid numbers from the start Met. B, Growth of AMT-deficient yeast (31019b) transformed with control plasmid pDR199, AtAMT1;1, and the AtAMT1;1 (wild type) mutants T460D and T460A on 3 mm ammonium as sole nitrogen source.
The sequences of AtAMT1;1 and AtAMT1;2 are identical over a stretch of 17 amino acids in the C terminus (Fig. 3A). Within that sequence, the conserved and functionally indispensable Gly is identified (Fig. 3A). In addition, a partially conserved Thr is recognized, which was phosphorylated in a large-scale screen for plasma membrane phosphoproteins (Nuhse et al., 2004). The phosphorylation of this Thr may suggest that posttranslational modifications are involved in the regulation of AMT function. It is worth mentioning that the identified phosphopeptide (ISSEDEMAGMDMpTR) was isolated from Arabidopsis suspension cells that were grown in ammonium-rich medium (2 mm; Nuhse et al., 2004). When grown in nitrogen-rich medium, the high-affinity uptake systems are efficiently shut off in roots (Loque et al., 2006).
The Thr is conserved in a number of AMTs from different plant species and even in some bacterial and archaeal AMTs (Supplemental Fig. S1). In several other AMTs, including the prokaryotic AMT-1 from Archaeoglobus fulgidus, this Thr is replaced by Ser. Other amino acids are found at the equivalent position in most fungal homologs, while the carboxyl tail of mammalian Rh glycoproteins fails to show significant conservation with AMTs.
Whether modifications in the relevant Thr (Thr-460 in AtAMT1;1) affect NH4+ transport was tested by introducing mutations at this position. The Thr was replaced by Asp, which is negatively charged at physiological pH and may mimic phosphorylation. The exchange in AtAMT1;1 inactivated NH4+ transport, as determined by growth assays of triple-mepΔ yeast expressing the T460D mutant construct (Fig. 3B). By contrast, the mutation of Thr-460 to the uncharged Ala, which is the corresponding residue in AtAMT1;5, yielded a functional transporter (Fig. 3B).
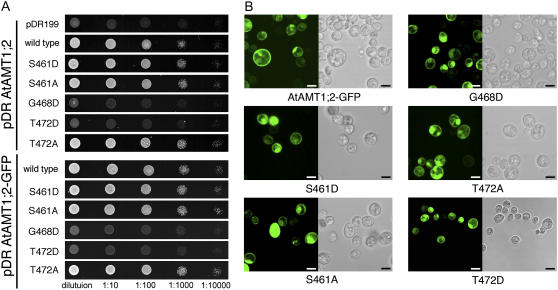
Equivalent mutations in AtAMT1;2 or a GFP-tagged version of AtAMT1;2 had similar effects (Fig. 4A). The T472D mutant was inactive, while the T472A mutant was functional (Fig. 4A). In addition, the mutant in which the adjacent Gly (Gly-468) was exchanged by Asp was also nonfunctional (Fig. 4A). The equivalent mutation inactivates fungal homologs (Marini et al., 2000; Monahan et al., 2002) and two AMTs from tomato (Ludewig et al., 2003). By contrast, mutants having a partially conserved Ser at position 461 (Supplemental Fig. S1) exchanged by Ala or Asp were functional (Fig. 4A).
Figure 4.
Effects of mutations in the C terminus of AtAMT1;2 on NH4+ transport. A, Growth of AMT-deficient yeast transformed with AtAMT1;2 wild type and mutants on 3 mm ammonium as sole nitrogen source (top). The same growth assay for the mutants in an AtAMT1;2-GFP fusion protein (bottom) is also shown. B, Fluorescence pattern of GFP-tagged AtAMT1;2 wild type and mutants in yeast (left) and bright-field images (right). Scale bars = 4 μm. [See online article for color version of this figure.]
The mutations at positions 461, 468, and 472 did not affect the localization pattern of the GFP-tagged AtAMT1;2 proteins expressed in yeast, suggesting that targeting of the membrane proteins was unaffected (Fig. 4B). This resembles the properties of the dominant Gly-to-Asp mutant in yeast (Marini et al., 2000) and tomato (Ludewig et al., 2003).
The transport rates of the AtAMT1;2 wild type and the mutants T472D, T472A, and G468D were also quantified using 14C-MeA transport assays in yeast. The T472D and G468D mutants had no residual transport activity when expressed in yeast (Supplemental Fig. S3A). In contrast, robust 14C-MeA uptake was observed for the AtAMT1;2 wild type and only slightly reduced uptake for the T472A mutant (Supplemental Fig. S3B). Taken together, a specific mutation that mimics phosphorylation at the putative phosphorylation site in the C-terminal tail of AMTs abolishes NH4+ transport.
A Molecular Model of AtAMT1;2 Predicts C-Terminal Interactions
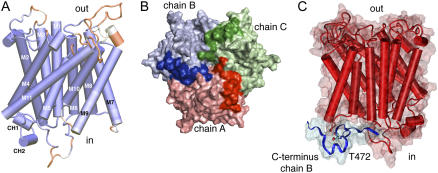
The crystal structures of prokaryotic AMT homologs were used to generate an AtAMT1;2 homology model. The highest resolution structures of E. coli AmtB (1U7G) and A. fulgidus AMT-1 (2B2F) were taken as templates (Khademi et al., 2004; Andrade et al., 2005). The structures aligned well within the core transmembrane region (Fig. 5). Major deviations in the AtAMT1;2 homology model were restricted to the external and internal loops that connect the transmembrane helices. Interestingly, a surprisingly precise alignment and structural model were obtained from the highly conserved cytosolic C terminus, which forms a structure that includes two short helices (CH1 and CH2; Fig. 5A). The C terminus was fully ordered and resolved in the AfAMT-1 structure; it aligns with the cytoplasmic face of the same subunit but also forms hydrogen bonds with the adjacent monomer in the trimer. The same fold is observed in the AtAMT1;2 model; the C terminus of the neighboring chain B (blue) is positioned on top of the cytoplasmic surface of chain A (red; Fig. 5).
Figure 5.
A homology model of AtAMT1;2 predicts interactions by the C terminus. A, Side view of a homology monomer model of AtAMT1;2 based on EcAmtB and AfAMT-1. Deviations in the structure from its templates are colored in red, and closely resembling structures are given in dark blue. The first 53 amino-terminal amino acids and the final 34 cytosolic amino acids have no correlation in the structure of AfAMT-1 and were removed for clarity. The transmembrane helices are numbered (M1–M11) and the two short C-terminal helices, CH1 and CH2, are indicated at the cytoplasmic side. B, View from the cytosolic side on surface and cartoon representations of the trimeric homology model. Chain A (red), chain B (blue), and chain C (green) are shown with the C terminus in darker color. C, Side view from a different angle of monomer chain A in red and the C terminus of the neighboring chain B in blue. Thr-472 from chain B is explicitly shown.
The structure of AfAMT-1 and the model suggest that subunit interactions by the C terminus are of functional importance. A close inspection of the position of the putative phosphorylation site Thr-472 identified tight packing with its neighbors, including two residues from the M7-M8 linker of the adjacent subunit (residues Gly-323 and His-324). The side chain was accessible from the cytoplasm, which opens the possibility for its modification (Supplemental Fig. S2). By contrast, the Ser-461 was positioned further away from the adjacent subunit at the beginning of CH1. Interestingly, Gly-468, which when mutated to Asp inactivates AMTs from many species, is located in the hinge between helices CH1 and CH2 and is adjacent to Thr-472 (Supplemental Fig. S2). It is obvious that a side chain larger than in Gly cannot be accommodated at that position. Any exchange to another amino acid must disrupt the helix-loop-helix structure and the interaction with adjacent residues. Similarly, in AfAMT-1, the oxygen of the corresponding backbone Gly forms two hydrogen bonds with the Ser that corresponds to the Thr in AtAMTs. The first hydrogen bond is formed from the Gly (G379:O) to the backbone amino group of Ser (S383:N) and the second with the hydroxy group of the Ser side chain (S383:OH). Whether modifications in a single subunit also affect proper functioning of coassembled monomers was tested using coexpression of mutant and wild type in Xenopus oocytes.
NH4+ Transport by AtAMT1;2 Mutants in Oocytes
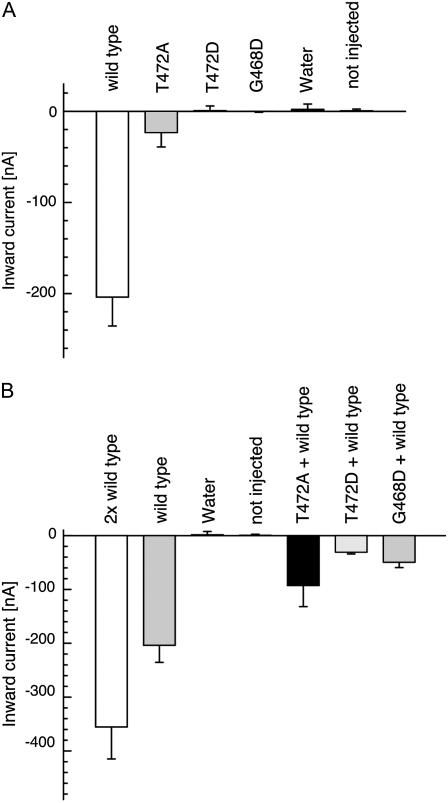
The large magnitude of the currents elicited by ammonium in AtAMT1;2-expressing oocytes allowed a reliable and quantitative comparison with currents by mutant transporters (Fig. 6A). Consistent with the nonfunctionality of the mutants T472D and G468D in yeast, injection of equal amounts of cRNA did not lead to detectable NH4+ currents. The currents from oocytes expressing these mutants were indistinguishable from water-injected and noninjected controls (Fig. 6A). In contrast, NH4+ currents were detected in T472A mutant-expressing oocytes, but these were of almost 10-fold lower magnitude compared to the AtAMT1;2 wild type.
Figure 6.
Coexpression of AMT mutants and AtAMT1;2 wild type in oocytes. Inward currents by 0.2 mm NH4Cl at −100 mV from oocytes injected with equal amounts of cRNA (A) or mixtures of cRNAs (B) are shown. Data are from three to six oocytes. Similar data were obtained in four independent experiments.
Cross-Inhibition by Coexpressed Nonfunctional Monomers
Further analysis was done by coexpression of equal amounts of mutant and wild-type cRNA. Doubling the amount of injected cRNA roughly doubled the NH4+ current by AtAMT1;2 (Fig. 6B). However, coexpression of equal amounts of cRNA from wild type and mutant G468D drastically reduced the NH4+ current to below that of AtAMT1;2 expressed alone. This is consistent with data from tomato AMTs, where the corresponding mutant also inhibited transport by wild-type monomers (Ludewig et al., 2003). NH4+ transport by AtAMT1;2 is reduced to 15% by the coexpressed mutant T472D (Fig. 6B). Assuming equal processing and stability of the wild type and mutant proteins, a binomial distribution of wild type/mutant subunits within the trimeric complexes is expected. If a single mutant monomer is sufficient to inactivate the entire trimer, only the trimers consisting completely of wild-type monomers will be active and only 12.5% of residual current will remain.
The mutant T472A, which elicited less current than the wild type, also partially inhibited the NH4+ current by the coexpressed AtAMT1;2. The current was, however, larger than in coinjections of wild type and nonfunctional mutants. Despite the reduced NH4+ transport by the T472A mutant, the residual activity was sufficient to restore yeast growth to the wild-type level (Fig. 4A). Taken together, the data suggest that the correct C-terminal fold is required for AtAMT activity and that disruption of the carboxyl tail of a single monomer will inactivate the entire AMT trimer.
Weak Temperature Dependence of AtAMT1;2
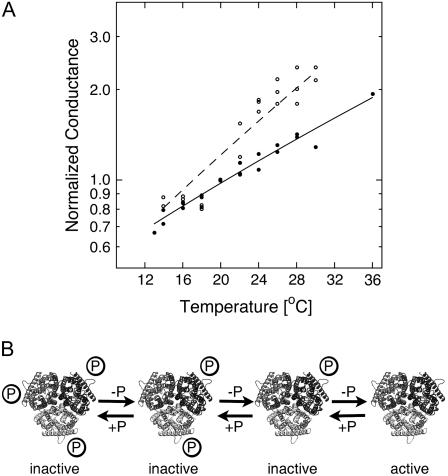
If gating and conformational coupling between coassembled monomers occurs, larger conformational rearrangements might be involved. We tested that hypothesis by measuring the temperature dependence of the NH4+ currents. A weak temperature dependence was observed for AtAMT1;2 currents (Q10 = 1.5), which is consistent with a diffusion-controlled transport process and minimal conformational rearrangements during transport (Fig. 7A).
Figure 7.
Temperature dependence of NH4+ currents and model for the regulation of AMT transporters by the C terminus. A, Normalized ammonium-induced currents (1 mm) at −100 mV by AtAMT1;2 (black circles) and the T472A mutant (white circles). The data were fitted with the Arrhenius equation. B, Each monomer contains an independent pathway for NH4+, but the activation of all subunits within the trimer (e.g. by dephosphorylation) is essential for transport function. Conformational transitions are predicted to occur independent of further modifications in the carboxyl tail of the T472A mutant.
In contrast, a steeper but still weak temperature dependence (Q10 = 1.9) was measured in the partially active T472A mutant, suggesting that some minor conformational rearrangements between active and inactive states may occur. The apparent activation enthalpy was 50 kJ/mol (compared to 33 kJ/mol in AtAMT1;2; Fig. 7A).
DISCUSSION
Nutrients such as ammonium are mostly absorbed at the epidermis (rhizodermis) and move symplastically through the cortex to the stele. However, nutrients may enter the symplasm later in cortical and endodermal cells, but the Casparian strip provides a major barrier for further apoplastic movement. While AtAMT1;1 and AtAMT1;3 are involved in the high-affinity loading of NH4+ at the rhizodermis (Loque et al., 2006; Mayer and Ludewig, 2006; Wood et al., 2006), AtAMT1;2 has a lower affinity to NH4+ and is preferentially localized in the root endodermis. This tissue shares functional aspects with the polarized epithelia in animal tissues. It is worth mentioning that the fluorescence from GFP-tagged AtAMT1;2 was stronger at the cortical side. This observation, however, needs confirmation by independent methods. Further transport in the stele involves AtAMT1;1 (Loque et al., 2006; Mayer and Ludewig, 2006) and AtAMT2;1 (Sohlenkamp et al., 2002).
Although the AtAMT1;2 sequence contains a putative plastid transit peptide (Shelden et al., 2001), the protein is localized to the plasma membrane. The transport properties of AtAMT1;2 for NH4+ were analyzed in oocytes. Half-maximal currents were measured at approximately 140 μm, while the currents by MeA+ were half-maximal in the millimolar range. This contrasts to previous studies where a Km = 40 μm for MeA+ had been determined in yeast (Gazzarrini et al., 1999). In another study, yeast-expressing AtAMT1;2 had biphasic uptake kinetics for 14C-MeA+ with Kms of 36 μm and 3 mm (Shelden et al., 2001). We detected very little current by MeA+ in the micromolar range but observed significant currents at higher MeA concentrations. Monophasic transport characteristics were measured for NH4+.
The previous identification of a phosphorylated Thr in the C terminus of AMTs opened the possibility that the transporter function is regulated by phosphorylation (Nuhse et al., 2004). The mutational exchange of the relevant Thr in AtAMT1;1 and AtAMT1;2 by Asp adds a negative charge at that position; both mutants were nonfunctional. By contrast, the exchange to Ala restored their function. The mutations at Thr-472 in AtAMT1;2 not only impaired the transport within the mutated monomer, but also inhibited coexpressed wild-type monomers. A similar dominant cross-inhibition of NH4+ transport by a Gly-to-Asp exchange in the C terminus had been reported for the tomato orthologs (Ludewig et al., 2003). This inhibition was confirmed here for AtAMT1;2. Importantly, GFP-tagged wild type and mutant proteins did not differ in their subcellular localization properties, in accordance with earlier data (Ludewig et al., 2003).
Conformational coupling between adjacent subunits can be rationalized based on the structure of AfAMT-1 and the homology model of AtAMT1;2, but such static models have clear limitations. Crystal structures of a plant AMT and protein dynamics may be required to prove whether conformational changes occur within the cytoplasmic parts of AMTs. The cytosolic TM5-TM6 and TM9-TM10 linkers were partially disordered and varied within the different crystal structures of EcAmtB, which may indicate some flexibility of these peptides (Zheng et al., 2004). Similarly, the structure of the C terminus of EcAmtB (1U7G) was disordered, which, however, may simply have resulted from the His-tag that was attached to AmtB for purification. EcAmtB interacts with the regulating GlnK protein, which negatively regulates transport. Interestingly, a deletion in the C terminus reduces the activity of AmtB to roughly 30% and impairs the binding of GlnK to AmtB (Coutts et al., 2002). Very recently, the structure of AmtB in complex with GlnK was resolved (Conroy et al., 2007; Gruswitz et al., 2007). This structure revealed that the carboxyl tail of EcAmtB adopts an identical fold as in AfAMT-1 and in the AtAMT1;2 model structure. The effects of mutations in the C-terminal region on EcAmtB function were also recently investigated (Severi et al., 2007). Remarkably similar conclusions regarding potential interactions between AmtB monomers and the possible role of the C-terminal region have been drawn. Two classes of mutants were identified, either with residual (approximately 25%) activity or essentially inactive. These two mutant classes were interpreted to reflect two distinct states of the C terminus and, hence, EcAmtB (Severi et al., 2007).
A weak temperature dependence corresponding to a Q10 of 1.5 was observed for the wild-type currents. A Q10 value of approximately 1.3 to 1.6 is characteristic for diffusion-limited processes, but enzymatic reactions and large conformational changes in proteins are frequently associated with a higher Q10 of 2 to 3 (Liu et al., 1996). The observed Q10 value is compatible with minor conformational changes during transport and a channel-like NH4+ transport mechanism. However, minor conformational changes that would be required in a H+-coupled NH3 cotransporter mechanism (=net NH4+ transport) cannot be excluded. An even lower temperature dependence of transport has been reported for EcAmtB (Javelle et al., 2005) and the structurally related Rh glycoproteins (Zidi-Yahiaoui et al., 2005).
When compared with the wild-type AtAMT1;2, the transport activity of the T472A mutant was more strongly reduced in oocytes than in yeast. It is possible that other cytosolic factors differently regulate wild-type AtAMT1;2 activity in these systems (e.g. one might speculate that AtAMT1;2 is partially phosphorylated in yeast but not in oocytes). Furthermore, the reduced transport activity of T472A correlated with a larger activation enthalpy, which corresponds to a Q10 of 1.9. It is likely that the overall mechanism of NH4+ conduction is not altered. However, it was evident from the structure of the C terminus (Fig. 5) that any exchange in Thr-472 must alter the original fold. We suggest that the active, conducting state is less favored in the mutant T472A and that the equilibrium between the conformations is shifted to the inactive state. This gives a plausible explanation for the reduced current in that mutant, especially at the lower temperatures at which the oocyte experiments were performed. In contrast, protein modifications at the conserved stretch of the C terminus appear to manipulate the ratio between active and inactive states in the wild type (Fig. 7B). If phosphorylation has the same effect as the Asp mutation, a specific kinase will then be required for inactivation to occur. It is likely that the appropriate kinase for a plant protein is not endogenously expressed in oocytes. This explains the weak temperature dependence of the wild type in oocytes, as it is constitutively active.
Regulation by cytosolic peptides is not unprecedented in membrane transport. For example, the molecular mechanism of inactivation in K+ ion channels involves reversible blockage of the conduction pathway by the peptide tail (Hoshi et al., 1990). Similarly, plant aquaporins are gated by phosphorylation at flexible cytosolic loops. Aquaporins are tetramers with each monomer forming a solute channel, and transition between the open and closed conformations leaves the overall pore architecture intact (Tornroth-Horsefield et al., 2006). Interestingly, interactions between aquaporin monomers affect their overall activity (Fetter et al., 2004).
A schematic model of AMT gating is presented in Figure 7B. The posttranslational modification of a single monomer and disruption of the conserved part of its carboxyl-tail fold appear to be sufficient to inactivate its physical neighbors and the entire trimer. This is a much more efficient shut-off than activation mechanism. Its physiological role is likely to minimize excess influx of cytotoxic ammonium; this allows plant roots to rapidly cope with variable levels of nitrogen supply. AtAMT1;1, AtAMT1;2, and AtAMT1;3 are in direct contact with the external root apoplasm. These AMTs are highly conserved in the relevant region, including the Thr. The conservation of the putative phosphorylation site in AMTs from many plant species suggests that the proposed regulation is potentially a feature of many plant AMTs.
MATERIALS AND METHODS
Plasmid Constructs
The promoter and coding regions of AtAMT1;2 (At1g64780; 3,011 bp) were amplified from genomic Col-0 DNA by PCR using Phusion polymerase (New England Biolabs). The sequence was subcloned into the plant transformation binary vector pTkan+GFP. The following primers were used (5′→3′): GGGGATCCTAAACTTCTCTATCCAAAAAAACCCTGTACC and CCTCTAGAAACAGTCAAGGTCGGTGTAGGAGTCGAGCT. The reverse primer was designed to eliminate the STOP codon and to generate a translational fusion with GFP. For oocyte and yeast expression, the open reading frame of AtAMT1;2 was amplified from the genomic fragment and subcloned into the oocyte expression vector pOO2 and the yeast expression vector pDR199. The fragment with eliminated STOP codon was also amplified and inserted in frame in front of the GFP(S65T) sequence in the yeast expression vector. Mutations were introduced by the QuikChange method from Stratagene. All PCR fragments were verified by full-length sequencing.
Plant Growth and Analysis
Arabidopsis (Arabidopsis thaliana) plants (ecotype Col-0) were grown in soil and transformed using the GV3101 Agrobacterium strain by spraying. Transgenic plant selection and segregation for kanamycin-resistance analysis were performed on agar plates with 50 μg/mL kanamycin. Homozygous 10-d-old plants carrying a single T-DNA insertion were analyzed by confocal microscopy (Leica DMRE microscope equipped with a confocal head TCS SP). Before imaging, the cell wall was counterstained for 3 min with a 1:50 dilution from a stock of 1 mg/mL propidium iodide.
Expression in Yeast
The plasmids containing the respective open reading frames were heat shock-transfected in the ura− AMT defective yeast strain (31019b; triple-mepΔ; Marini et al., 1997). The nitrogen-deficient growth medium was yeast nitrogen base without amino acids and ammonium sulfate (Difco), supplemented with 3% Glc and 3 mm NH4Cl as sole nitrogen source. No buffer was added. Growth of yeast was not affected by expression of the different constructs under nonselective conditions. 14C-MeA uptakes were performed at an optical density of 5 using 100 μm MeA as described (Gazzarrini et al., 1999).
Electrophysiological Measurements, Preparation, and Injection of Oocytes
These methods have been described in more detail elsewhere (Mayer et al., 2006). Briefly, oocytes were taken from adult females, dissected by collagenase treatment (2 μg/mL, 1.5 h), and injected with 50 nL of cRNA (10–50 ng/nL). Oocytes were kept in ND96 for 3 d at 16°C and then placed in a small recording chamber. The recording solution was (in mm): 110 choline chloride, 2 CaCl2, 2 MgCl2, 5 MES, pH adjusted to 5.5 with Tris. Variable ammonium concentrations were added as NH4Cl salt. Currents without added ammonium were subtracted at each voltage. The concentration dependence of currents was fitted using the following equation: 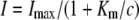 , where Imax is the maximal current at saturating concentration, Km is the substrate concentration permitting half-maximal currents, and c is the experimentally used concentration. The voltage dependence δ of the Km was calculated using the following equation:
, where Imax is the maximal current at saturating concentration, Km is the substrate concentration permitting half-maximal currents, and c is the experimentally used concentration. The voltage dependence δ of the Km was calculated using the following equation: 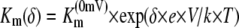 , where δ is fractional electrical distance, e is elementary charge, V is membrane potential, k is Boltzmann's constant, and T is absolute temperature. The temperature was measured with a small thermoelement (EBRO TFN520 coupled to TPN110-30), which was directly positioned in the recording chamber. The NH4+ currents were extracted by subtracting background currents at each temperature. The (apparent) activation enthalpies (ΔH) were extracted from the data using the Arrhenius equation
, where δ is fractional electrical distance, e is elementary charge, V is membrane potential, k is Boltzmann's constant, and T is absolute temperature. The temperature was measured with a small thermoelement (EBRO TFN520 coupled to TPN110-30), which was directly positioned in the recording chamber. The NH4+ currents were extracted by subtracting background currents at each temperature. The (apparent) activation enthalpies (ΔH) were extracted from the data using the Arrhenius equation 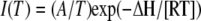 , where I is the current (which corresponds to a transport rate) at the temperature T, R is the gas constant, and A is a constant factor. The Q10 was calculated from ΔH according to
, where I is the current (which corresponds to a transport rate) at the temperature T, R is the gas constant, and A is a constant factor. The Q10 was calculated from ΔH according to 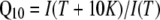 for T = 293°K. In all figures means ± sd are given, except for Figure 2D, which shows means ± SEM.
for T = 293°K. In all figures means ± sd are given, except for Figure 2D, which shows means ± SEM.
Homology Modeling
The primary sequence of AtAMT1;2 was aligned with Archaeoglobus fulgidus AfAMT-1 (22% identity) and EcAmtB (25% identity) using ClustalW. Structural fitting was done using MODELLER 7v7 (available at http://salilab.org/modeller/) and the display of the structures involved VMD, similar to as described (Mayer et al., 2006).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. C-terminal alignment of selected AMTs from different species.
Supplemental Figure S2. Detailed views on the homology-modeled C terminus.
Supplemental Figure S3. 14C-MeA uptake in yeast.
Supplementary Material
Acknowledgments
We thank P. Neumann for excellent technical assistance and F. de Courcy for critically reading the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. Lu673/7–1).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Uwe Ludewig (uwe.ludewig@zmbp.uni-tuebingen.de).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Andrade SL, Dickmanns A, Ficner R, Einsle O (2005) Crystal structure of the archaeal ammonium transporter Amt-1 from Archaeoglobus fulgidus. Proc Natl Acad Sci USA 102 14994–14999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas K, Morschhauser J (2005) The Mep2p ammonium permease controls nitrogen starvation-induced filamentous growth in Candida albicans. Mol Microbiol 56 649–669 [DOI] [PubMed] [Google Scholar]
- Conroy MJ, Durand A, Lupo D, Li XD, Bullough PA, Winkler FK, Merrick M (2007) The crystal structure of the Escherichia coli AmtB-GlnK complex reveals how GlnK regulates the ammonia channel. Proc Natl Acad Sci USA 104 1213–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutts G, Thomas G, Blakey D, Merrick M (2002) Membrane sequestration of the signal transduction protein GlnK by the ammonium transporter AmtB. EMBO J 21 536–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetter K, Van Wilder V, Moshelion M, Chaumont F (2004) Interactions between plasma membrane aquaporins modulate their water channel activity. Plant Cell 16 215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzarrini S, Lejay L, Gojon A, Ninnemann O, Frommer WB, von Wirén N (1999) Three functional transporters for constitutive, diurnally regulated, and starvation-induced uptake of ammonium into Arabidopsis roots. Plant Cell 11 937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruswitz F, O'Connell J III, Stroud RM (2007) Inhibitory complex of the transmembrane ammonia channel, AmtB, and the cytosolic regulatory protein, GlnK, at 1.96 A. Proc Natl Acad Sci USA 104 42–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T, Zagotta WN, Aldrich RW (1990) Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science 250 533–538 [DOI] [PubMed] [Google Scholar]
- Javelle A, Thomas G, Marini AM, Kramer R, Merrick M (2005) In vivo functional characterisation of the E. coli ammonium channel AmtB: evidence for metabolic coupling of AmtB to glutamine synthetase. Biochem J 360 215–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser BN, Rawat SR, Siddiqi MY, Masle J, Glass AD (2002) Functional analysis of an Arabidopsis T-DNA “knockout” of the high-affinity NH4+ transporter AtAMT1;1. Plant Physiol 130 1263–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khademi S, O'Connell J III, Remis J, Robles-Colmenares Y, Miercke LJ, Stroud RM (2004) Mechanism of ammonia transport by Amt/MEP/Rh: structure of AmtB at 1.35 Å. Science 305 1587–1594 [DOI] [PubMed] [Google Scholar]
- Liu G, Hinch B, Davatol-Hag H, Lu Y, Powers M, Beavis AD (1996) Temperature dependence of the mitochondrial inner membrane anion channel. The relationship between temperature and inhibition by protons. J Biol Chem 271 19717–19723 [DOI] [PubMed] [Google Scholar]
- Loque D, von Wiren N (2004) Regulatory levels for the transport of ammonium in plant roots. J Exp Bot 55 1293–1305 [DOI] [PubMed] [Google Scholar]
- Loque D, Yuan L, Kojima S, Gojon A, Wirth J, Gazzarrini S, Ishiyama K, Takahashi H, von Wiren N (2006) Additive contribution of AMT1;1 and AMT1;3 to high-affinity ammonium uptake across the plasma membrane of nitrogen-deficient Arabidopsis roots. Plant J 48 522–534 [DOI] [PubMed] [Google Scholar]
- Ludewig U (2006) Ion transport versus gas conduction: function of AMT/Rh-type proteins. Transfus Clin Biol 13 111–116 [DOI] [PubMed] [Google Scholar]
- Ludewig U, von Wirén N, Frommer WB (2002) Uniport of NH4+ by the root hair plasma membrane ammonium transporter LeAMT1;1. J Biol Chem 277 13548–13555 [DOI] [PubMed] [Google Scholar]
- Ludewig U, von Wirén N, Rentsch D, Frommer WB (2001) Rhesus factors and ammonium: a function in efflux? Genome Biol 2 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig U, Wilken S, Wu B, Jost W, Obrdlik P, El Bakkoury M, Marini AM, Andre B, Hamacher T, Boles E, et al (2003) Homo- and hetero-oligomerization of ammonium transporter-1 NH4+ uniporters. J Biol Chem 278 45603–45610 [DOI] [PubMed] [Google Scholar]
- Marini AM, Soussi-Boudekou S, Vissers S, André B (1997) A family of ammonium transporters in Saccharomyces cerevisiae. Mol Cell Biol 17 4282–4293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini AM, Springael JY, Frommer WB, André B (2000) Cross-talk between ammonium transporters in yeast and interference by the soybean SAT1 protein. Mol Microbiol 35 378–385 [DOI] [PubMed] [Google Scholar]
- Mayer M, Dynowski M, Ludewig U (2006) Ammonium ion transport by the AMT/Rh homolog LeAMT1;1. Biochem J 396 431–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M, Ludewig U (2006) Role of AMT1;1 in NH4+-acquisition in Arabidopsis thaliana. Plant Biol (Stuttg) 8 522–528 [DOI] [PubMed] [Google Scholar]
- Mayer M, Schaaf G, Mouro I, Lopez C, Colin Y, Neumann P, Cartron JP, Ludewig U (2006) Different transport mechanism in plant and human AMT/Rh-type ammonium transporters. J Gen Physiol 127 133–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan BJ, Unkles SE, Tsing IT, Kinghorn JR, Hynes MJ, Davis MA (2002) Mutation and functional analysis of the Aspergillus nidulans ammonium permease MeaA and evidence for interaction with itself and MepA. Fungal Genet Biol 36 35–46 [DOI] [PubMed] [Google Scholar]
- Nuhse TS, Stensballe A, Jensen ON, Peck SC (2004) Phosphoproteomics of the Arabidopsis plasma membrane and a new phosphorylation site database. Plant Cell 16 2394–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severi E, Javelle A, Merrick M (2007) The conserved carboxy-terminal region of the ammonia channel AmtB plays a critical role in channel function. Mol Membr Biol (in press) [DOI] [PubMed]
- Shelden MC, Dong B, de Bruxelles GL, Trevaskis B, Whelan J, Ryan PR, Howitt SM, Udvardi MK (2001) Arabidopsis ammonium transporters, AtAMT1;1 and AtAMT1;2, have different biochemical properties and functional roles. Plant Soil 321 151–160 [Google Scholar]
- Smith CS, Zaplachinski ST, Muench DG, Moorhead GB (2002) Expression and purification of the chloroplast putative nitrogen sensor, PII, of Arabidopsis thaliana. Protein Expr Purif 25 342–347 [DOI] [PubMed] [Google Scholar]
- Smith DG, Garcia-Pedrajas MD, Gold SE, Perlin MH (2003) Isolation and characterization from pathogenic fungi of genes encoding ammonium permeases and their roles in dimorphism. Mol Microbiol 50 259–275 [DOI] [PubMed] [Google Scholar]
- Sohlenkamp C, Wood CC, Roeb GW, Udvardi MK (2002) Characterization of Arabidopsis AtAMT2, a high-affinity ammonium transporter of the plasma membrane. Plant Physiol 130 1788–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornroth-Horsefield S, Wang Y, Hedfalk K, Johanson U, Karlsson M, Tajkhorshid E, Neutze R, Kjellbom P (2006) Structural mechanism of plant aquaporin gating. Nature 439 688–694 [DOI] [PubMed] [Google Scholar]
- von Wiren N, Merrick M (2004) Regulation and function of ammonium carriers in bacteria, fungi and plants. Top Curr Genet 9 95–120 [Google Scholar]
- Westhoff CM, Ferreri-Jacobia M, Mak DO, Foskett JK (2002) Identification of the erythrocyte Rh blood group glycoprotein as a mammalian ammonium transporter. J Biol Chem 277 12499–12502 [DOI] [PubMed] [Google Scholar]
- Wood CC, Poree F, Dreyer I, Koehler GJ, Udvardi MK (2006) Mechanisms of ammonium transport, accumulation, and retention in oocytes and yeast cells expressing Arabidopsis AtAMT1;1. FEBS Lett 580 3931–3936 [DOI] [PubMed] [Google Scholar]
- Zheng L, Kostrewa D, Berneche S, Winkler FK, Li XD (2004) The mechanism of ammonia transport based on the crystal structure of AmtB of Escherichia coli. Proc Natl Acad Sci USA 101 17090–17095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidi-Yahiaoui N, Mouro-Chanteloup I, D'Ambrosio AM, Lopez C, Gane P, Le van Kim C, Cartron JP, Colin Y, Ripoche P (2005) Human Rhesus B and Rhesus C glycoproteins: properties of facilitated ammonium transport in recombinant kidney cells. Biochem J 391 33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
Note Added in Proof
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.