Abstract
We examine here the outcome of viral encephalomyelitis [mouse hepatitis virus (MHV) A59, Theiler’s encephalomyelitis virus, and Coxsackievirus B3] in mice with autoantibodies to a central nervous system (CNS)-specific antigen, myelin oligodendrocyte glycoprotein, that usually develop no clinical disease. Morbidity and mortality of the acute viral CNS disease was augmented by the presence of the autoantibodies in all three viral infections. Transfer of serum containing the autoantibodies at the time of infection with MHV was sufficient to reproduce the exacerbated disease. The presence of the autoantibodies was found to result in increased infiltration of mononuclear cells into the brain. Early demyelination was severely augmented in brains and spinal cords of MHV-infected mice with CNS-specific autoantibodies. The antibody-mediated exacerbation was shown to be independent of the complement system but to require expression of Fc receptors, because it was observed in C′-3-deficient but not in Fc receptor-deficient mice. Our study illustrates the possibility that infections can lead to much more profound immunopathology in the presence of an otherwise latent autoimmune condition.
It is now well established in animal models that infectious organisms can trigger or augment immune responses to self-antigens, by mechanisms such as molecular mimicry or bystander activation (see review1). However, autoimmunity does not always result in clinically apparent disease, and cumulative infections by the same or unrelated agents may be required to unmask a pre-existing autoimmune process.1 Conversely, a viral infection could take a worsened course in autoimmune-prone individuals. It has been shown that infections can provoke relapses or worsen autoimmune conditions in animal models.2–4 This is consistent with reports showing that common infections augment the risk for or the severity of relapses in multiple sclerosis (MS) patients.5,6
The aforementioned animal reports all studied T-cell-mediated autoimmune responses, but autoantibodies also have an important pathogenic potential (see review7). In the central nervous system (CNS), autoantibodies to myelin components and to myelin oligodendrocyte glycoprotein (MOG) in particular can play a damaging role. MOG-specific antibodies have been shown to play a major role in the murine model of MS, experimental autoimmune encephalomyelitis (EAE),8 and have been clearly associated with demyelinating lesions in MS patients.9 They are thus likely to mediate some of the pathology, at least in the subset of patients with lesions presenting antibody depositions.10 However, the presence of antibodies specific for MOG or other CNS antigens is detected not only in MS patients but also in healthy subjects, albeit with a lower frequency,11 and it is thus likely that additional triggers may be required for disease development.
Litzenburger et al12 previously demonstrated that transgenic mice, in which the rearranged VDJ region of a MOG-specific monoclonal antibody H chain replaced the germline JH locus [anti-MOG immunoglobulin (Ig) “knock-in” mice, also referred to as THMOG mice in the present report], exhibited an exacerbated form of EAE. However, without additional autoantigenic immunization, the THMOG mice do not develop spontaneous clinical disease, despite the presence of autoreactive B cells and the release of MOG-specific IgG and IgM in the serum.12 Because the transgenic B cells in THMOG can undergo normal differentiation and maturation, autoantibodies of different classes and subclasses, membrane-associated or secreted, are generated. Thus, all of the possible interactions of the autoreactive Igs with the other effectors of the immune system can occur in these mice. Therefore, they constituted a good model to evaluate the hypothesis that a viral infection of the CNS would lead to worsened immunopathology and disease, if it were to be superimposed on an underlying humoral autoimmune condition.
The neurotropic strains of murine coronaviruses mouse hepatitis virus (MHV) A59, MHV JHM, and related mutants have provided an informative model for the pathogenesis of virus-induced encephalitis and demyelination (see reviews13,14). Mice infected with MHV A59 typically undergo a brief period of acute encephalomyelitis followed by an extended period of chronic demyelination. During the acute phase of the infection, the virus can be found in astrocytes, oligodendrocytes, and neurons.15 T cells are responsible for the initial control of the infection,16 but antibodies are required for complete clearance of the virus and prevention of re-emergence.17,18 During the second phase of the disease, macrophages19 but also CD4+20,21 and CD8+21 T cells have been shown to mediate the demyelination.
We report here that intracranial infection of THMOG mice with MHV A59 results in accelerated kinetics of onset, severity of clinical disease, and increased death in THMOG compared with wild-type mice. The exacerbation of the CNS disease was shown to be transferable by the autoantibodies and was observed in other models of viral encephalitis as well. Immunohistochemical examination of brain sections and fluorescence-activated cell sorting analysis of infiltrating cells in brains showed an overall increase of the number of mononuclear cells. Demyelination was found to be augmented in brains and spinal cords of mice with anti-MOG antibodies. Fc receptor-deficient mice were shown to be protected from the autoantibody-mediated enhanced pathology, indicating that the mechanism for the exacerbation involved Fc-mediated effects. Thus, autoantibodies specific for antigens in the immunologically privileged CNS in combination with local, virally induced inflammation and tissue destruction can lead to increased sensitivity of disease.
Materials and Methods
Mice
THMOG mice encode the rearranged cDNA of the pathogenic MOG-specific monoclonal antibody 8.18C5 in place of the germline JH locus.12 This results in MOG-binding Ig surface expression on about one-third of the B cells that can undergo normal class-switching and secretion and affinity maturation after encountering the antigen.12 C57Bl/6 control mice were obtained from The Scripps Research Institute breeding facility. CD4-KO and CD8-KO mice obtained from Dan Littman (Sloan-Kettering Institute, New York, NY) and C′-3-KO and inducible NO synthase (iNOS)-KO mice purchased from the Jackson Laboratory (Bar Harbor, ME) (all on a C57Bl/6 background) were bred at Scripps. Fcer1-KO mice (583-M, C57Bl/6 background) were purchased from Taconics Transgenics (Germantown, NY). All experiments were conducted in accordance with the Scripps Research Institute Institutional Animal Care and Use Committee guidelines.
Viruses
Mouse hepatitis virus-attenuated neurotropic isolate A-59 was grown on delayed brain tumor (DBT) cells. Theiler’s murine encephalomyelitis virus (TMEV) Daniel’s strain obtained from Dr. D. McGavern (The Scripps Research Institute) was grown on BHK21 cells. Viral titers were determined by plaque assay on DBT cells. Coxsackievirus B3 was grown and titered as previously described.22 Pichinde virus stocks were grown and titrated on Vero cells.
Induction of CNS Immune Pathology
Isoflurane-anesthetized mice were inoculated with 30 μl containing the virus diluted in sterile phosphate-buffered saline (PBS), using a 27-gauge needle with no more than 2-mm penetration of the cranium just lateral of the midline. Signs of clinical CNS disease were monitored daily and scored using standards as described in Figure 1. Serum from THMOG mice was obtained by retroorbital bleeding or cardiac puncture and pooled to be used for transfer experiments. Recipient animals received 500 μl of the serum pool intraperitoneally at the time of the viral infection, whereas controls received the same volume of normal mouse serum or in later repeat experiments, PBS, because normal serum was found to have no measurable effect on the course of the infection.
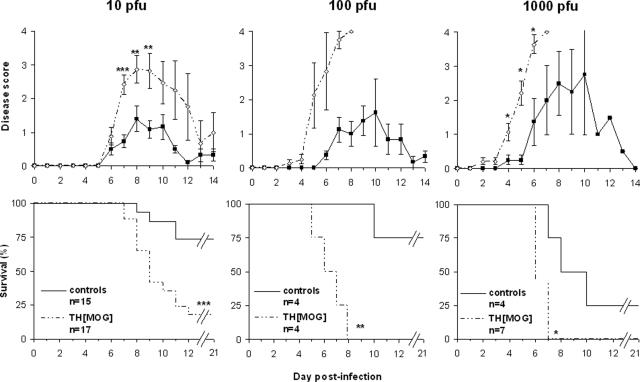
Figure 1.
The outcome of MHV A59 acute infection of the CNS is exacerbated in THMOG knock-in mice. Mice were observed and given a numerical score (top panels) for behavioral signs of encephalitis (0, no detectable sign of disease; 1, ruffled fur; 2, slightly hunched back and ruffled fur; 3, very hunched back and lethargy; and 4, death) daily during the acute infection phase in mice infected with increasing doses of virus (left to right). Data represent the average and standard errors for each group. The bottom panels show the survival of the animals in the same experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
Histology
Mice were euthanized with an overdose of chloral hydrate diluted in saline and perfused with 10 ml of 10% formalin. After fixation overnight, brains and spinal cord paraffin-embedded sections were stained by hematoxylin and eosin (H&E) (to evaluate infiltration) and luxol fast blue (for demyelination).
Flow Cytometry
Brains from PBS-perfused mice were mechanically disrupted between frosted slides. The lipid components and debris were removed after collagenase (Sigma C-6885) treatment and centrifugation through Percoll (Sigma). Brains of groups of five or six mice were pooled to obtain enough mononuclear cells to assay. Fc receptors were blocked (14-0161; eBiosciences, San Diego, CA), and cells were stained with antibodies directly coupled to fluorochromes. Data were acquired on a BD FACSCalibur. CD11b+ (for macrophages/microglia) or CD45+ (for the other cell types) cells were gated to analyze the expression of specific markers. All antibodies were obtained from eBiosciences.
Statistical Analysis
Significance was evaluated using Mann-Whitney (for day-by-day differences between clinical scores, viral titers or ratio of areas affected by demyelination), Fisher’s exact test (for incidence of disease in the autoimmune mice when there was none in the control group), or log-rank survival (for differences in mortality over entire experiments) tests using the GraphPad/Instat software.
Results
MHV-Induced Acute CNS Disease Is Exacerbated in THMOG Transgenic Mice
THMOG and C57Bl/6 mice were infected with three increasing doses of MHV, strain A59. Typical symptoms of encephalitis were observed in both groups and for each of the viral doses. However, clinical signs of CNS disease occurred earlier and were stronger in the THMOG mice compared with Bl6 controls (Figure 1, top). The three viral doses also resulted in an increased death rate in THMOG mice in comparison with controls (Figure 1, bottom).
The Enhanced Susceptibility to CNS Disease after MHV Infection Can Be Transferred by Anti-MOG Autoantibodies
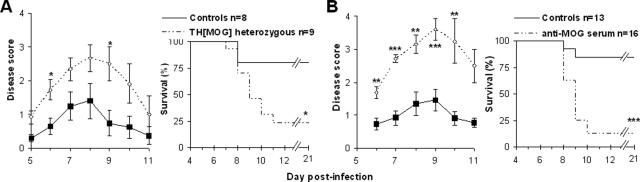
To rule out that the enhanced disease observed in the transgenic mice could have been caused by the absence of a functional antibody repertoire or any other possible genetic defect, we first examined the outcome of the CNS infection by MHV A59 in heterozygous THMOG mice obtained by backcrossing the transgenic mice with the parental C57Bl/6 strain. The acute encephalitis was also exacerbated in these mice compared with controls (Figure 2A), indicating that the aggravation of disease was probably due to the presence of CNS-specific B cells or autoantibodies.
Figure 2.
Autoantibodies account for the exacerbated viral encephalitis. A: Clinical disease and survival after intracranial (i.c.) inoculation of 10 PFU of MHV A59 in C57Bl/6 controls (black squares, plain lines) and THMOG heterozygous mice (open diamonds, dashed lines). B: Comparison of the viral encephalitis after injection of 10 PFU of MHV A59 in C57Bl/6 that received serum of THMOG mice intraperitoneally at the time of infection (open diamonds, dashed lines) or mock-treated controls (black squares, plain lines). *P < 0.05; **P < 0.01; ***P < 0.001.
We then examined whether the transgenic B cells, the autoantibodies, or both were responsible for the increased CNS disease. We first transferred purified THMOG B cells to WT mice and observed some exacerbation of the CNS disease compared with controls receiving wild-type B cells, although to a much lesser extent than in the original THMOG animals (data not shown). However, when serum from uninfected THMOG mice was transferred to wild-type recipients at the time of infection with MHV, an exacerbation of CNS disease similar to the levels found in MHV-infected THMOG mice was observed (Figure 2B). In addition, transfer of Protein-A purified antibodies from the serum of THMOG resulted in a comparable exacerbation of MHV-induced encephalitis as the serum (data not shown). These studies clearly mapped the cause for enhancement of the virally induced CNS disease in THMOG mice to the anti-MOG autoantibodies.
Anti-MOG Antibodies Exacerbate Viral Encephalitis in Other Viral Models
We then tested the effects of the presence of the anti-MOG antibodies in other nonlethal viral models of CNS infection to determine whether viruses from different families and with different degrees of pathogenicity could be exacerbated by the presence of anti-MOG autoantibodies. Although no difference was observed in the clinically silent encephalitis induced by 105 plaque-forming units (PFU) of Pichinde virus, an exacerbation of the CNS disease by the autoantibodies was observed in mice infected with both TMEV and CV B3 (Table 1). C57Bl/6 are genetically resistant to TMEV infection, ie, the virus replicates but does not cause detectable inflammation or demyelination,23 and as expected, no behavioral signs of acute CNS disease were seen in the control mice. However, 44% of the THMOG heterozygous animals developed a clinically evident disease, and one-half of the sick animals died within the 1st week (Table 1). Likewise, no signs of disease could be observed in any of the 2-week-old control pups infected with 103 or 104 PFU CV B3, whereas 20 or 100% of the heterozygous autoimmune mice became sick, respectively (Table 1). This was reflected by a profound difference in the weights of the two groups when injected with 104 PFU CV B3: all of the pups initially gained weight at an equal rate (111 ± 3% in THMOG± versus 108 ± 4% in controls at 5 days after infection). The transgenic animals then started to grow at a slower rate and eventually lost weight, resulting in an average of 109 ± 2% of their starting weight at day 10 after infection, versus 137 ± 1% in the controls (P < 0.01, Mann-Whitney test). These experiments show that the adverse effects of pre-existing autoantibodies can have an impact on the outcome of a variety of viral encephalitis. They also show that exacerbation can occur over a large spectrum of clinical manifestations of encephalitis: Autoantibodies can transform a clinically silent infection into a symptomatic disease (TMEV and CV B3), transform a mild CNS pathology into a lethal one (MHV A59, 10 PFU), or accelerate the clinical course (MHV A59, 1000 PFU).
Table 1.
Outcome of Other Viral CNS Infections in Control and THMOG Heterozygous Mice
| Virus | Dose (PFU) | C57Bl/6 controls
|
THMOG +/−
|
||||
|---|---|---|---|---|---|---|---|
| Clinical signs
|
Mortality | Clinical signs
|
Mortality (days)‡ | ||||
| Incidence | Score | Incidence* | Score (days)† | ||||
| Pichinde | 10E5 | 0/4 | N.A. | 0/4 | 0/4 | N.A. | 0/4 |
| TMEV | 10E5 | 0/5 | N.A. | 0/5 | 4/9 | 0.83 ± 0.59 (5) | 2/9 (5, 7) |
| CV B3 | 10E3 | 0/5 | N.A. | 0/5 | 2/5 | 0.70 ± 0.19 (15) | 1/5 (16) |
| 10E4 | 0/5 | N.A. | 0/5 | 7/7§ | 1.5 ± 0.14 (9) | 0/7 | |
The viruses were injected i.c. at the indicated doses in 2-week-old (CV B3) or 6-week-old (Pichinde and TMEV) mice. The table summarizes the follow-up of the clinical status and mortality in control (C57Bl/6) and autoimmune (heterozygous THMOG) animals. N.A., not applicable.
Number of mice with detectable clinical disease over total number in each group.
Highest average clinical score for each group, and the day after infection when it was observed.
Number of deaths over total number of animals studied and timing of death.
P < 0.01, Fisher’s exact test.
Viral Replication of MHV in Mice with MOG-Specific Antibodies
To test whether viral replication was required to trigger disease in mice with CNS autoantibodies, we injected 5 × 105 PFU equivalent of UV-inactivated MHV A59, which did not cause any detectable clinical disease in homozygous THMOG mice over a 21-day period (n = 2; data not shown). Likewise, no sign of clinical disease was detected in such mice on infection by 100 PFU of MHV A59 via the intraperitoneal route (n = 2; data not shown), a dose that results in a clinically silent hepatitis with active viral replication for over a week in C57Bl/6 mice (R.B. and M.J.B., personal observations). Thus, neither the disruption of the blood brain barrier, viral antigens in the CNS, nor infection of a peripheral organ elicited enhanced immunopathology in THMOG mice, showing that active viral replication in the CNS was required.
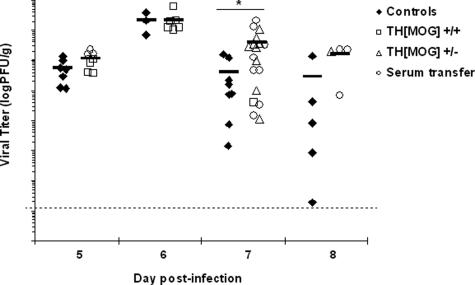
Viral titers in the brains of control C57Bl/6 mice and mice with autoantibodies (THMOG homo- and heterozygous mice and C57Bl/6 recipients of anti-MOG serum) were then compared at different time points. No significant difference was found in the titers in mice with autoantibodies compared with the controls from day 3 (data not shown) up to days 5 and 6 (Figure 3) after the infection, which indicates that the virus replicates initially at similar rates in all of the animals. However, although the titers started to decrease in both groups at day 7, the reduction of the viral load was less pronounced in mice with anti-MOG autoantibodies compared with controls (Figure 3). Thus, the ability to control the viral CNS infection is slightly impaired in the presence of the CNS autoantibodies.
Figure 3.
Infectious virus levels in the brain. Brains obtained from control mice (black diamonds) or animals with autoantibodies (white squares, homozygous THMOG; white triangles, heterozygous THMOG; white circles, wild-type recipients of THMOG serum) infected with 10 PFU of MHV A59 were obtained at various time points after inoculation. Viral titers from homogenized half-brains were determined by plaque assay. Data are pooled from six independent experiments. Each point represents an individual animal, and black lines represent the average for each group. *P < 0.05.
To exclude any systemic defect on the cytotoxic immune response to MHV in the MOG-Ig mice, we compared the frequencies of virus-specific CD8+ T cells in the spleen of controls and recipients of anti-MOG serum by enzyme-linked immunospot (ELISPOT) assays, which were performed as described previously.24 We found no significant difference in the frequencies of purified CD8+ cells that responded to the immunodominant MHV A59 epitope S598-605, which is located in the viral envelope protein, at day 5 or 7 after infection (data not shown). Thus, the autoantibodies did not alter the systemic antiviral cytotoxic immune response.
Infiltration Is Augmented in Mice with Anti-MOG Autoantibodies
To study the mechanisms responsible for the autoantibody-mediated augmentation of viral encephalitis, we chose a model that results in severe outcome in a majority of the autoimmune mice while sparing most of the control animals, and the following experiments have thus all been performed with 10 PFU of MHV A59.
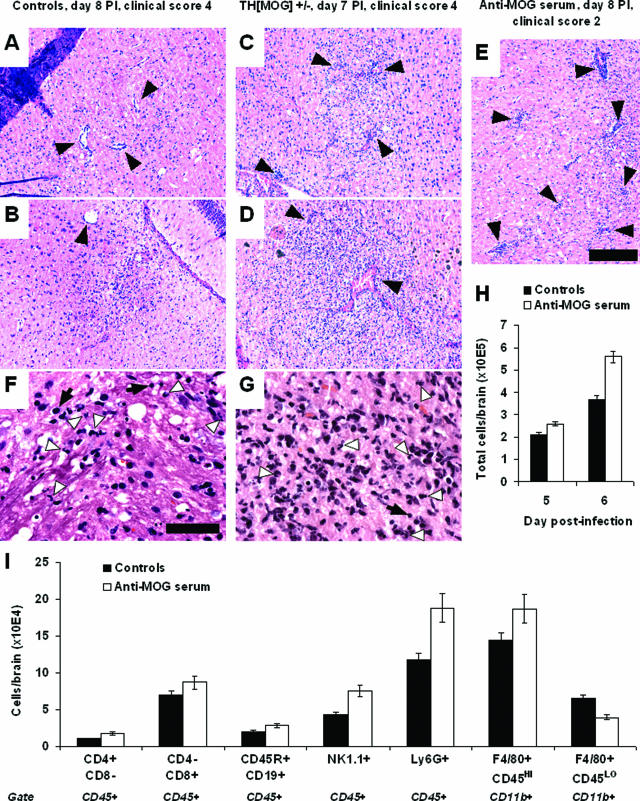
H&E-stained brain and spinal cord sections obtained from controls and mice with autoantibodies were examined for the presence of infiltrating cells. The degree of infiltration appeared to be augmented in mice with autoantibodies compared with control animals at days 7 to 9 after infection. Both the frequency of perivascular cuffing and the number of infiltrating cells within inflammation areas were higher in mice with autoantibodies compared with controls (Figure 4, A–E). Morphologically, the infiltrates appeared to be composed of mixed inflammatory cell populations, with numerous neutrophils being seen both in the controls and in the mice with autoantibodies (Figure 4, F–G). To determine more precisely the nature of the infiltrating cells, we performed flow cytometry experiments on mononuclear cells purified from infected brains. Enumeration of the purified cells from individual brains confirmed the increased cellularity in the brains of mice with autoantibodies (Figure 4H). The presence of the autoantibodies resulted in no significant differences in the numbers of T or B lymphocytes (Figure 4I). On the contrary, the frequency of NK cells and blood-borne macrophages was somewhat more elevated in mice with autoantibodies (Figure 4I). Altogether, these results show that brain infiltration of lymphocytes, and in particular cells bearing Fc receptors, is augmented by the presence of anti-MOG antibodies during MHV A59 encephalitis.
Figure 4.
Infiltration is augmented in the presence of anti-MOG antibodies. A–E: Representative H&E-stained sections from brains of MHV A59-infected mice. Moderate inflammation, with some perivascular cuffing (black arrows), was detected in the brains of control mice (A and B, both obtained from different moribund animals 8 days after the infection). The degree of infiltration was augmented in all mice with autoantibodies (E, recipient of anti-MOG serum at day 8, clinical score 2) or earlier in the course of infection (C and D, obtained from moribund mice at day 7 after infection). Black bar = 0.2 mm. F and G: Close-up pictures from sections A and D, respectively, showing mixed inflammatory cell infiltrates. White arrowheads indicate some individual polymorphonuclear cells, which also can be found in large cluster in G. Some cells with lymphocyte morphology (black arrows) can also be seen in infiltrates in both groups of mice. Black bar = 0.05 mm. H: Total numbers of mononuclear cells purified from individual brains collected from control (black bars) or recipient of anti-MOG serum (open bars) mice 5 or 6 days after infection with MHV A59. Average and SE, n = 6 (day 50) or 13/14 (day 6) per group. I: Brains from control mice (average clinical score, 0.56 ± 0.12, n = 8) and recipients of anti-MOG serum (1.78 ± 0.18, n = 7) were collected 6 days after MHV A59 infection. Mononuclear cells were purified from each individual brain, counted, pooled by group, and stained for flow cytometry analysis. The graph shows the average number of T lymphocytes (CD4+CD8-cd45+ and CD4-CD8+cd45+), B lymphocytes (B220+CD19+CD45+), NK cells (NK1.1+CD45+), neutrophils (Ly6G+CD45+), blood-borne macrophages (CD11b+F4/80+CD45HIGH), and resident microglia (CD11b+F4/80+CD45LOW) per animal. Similar results were obtained in additional independent experiments performed at days 5, 6, and 7 after infection.
Anti-MOG Antibodies Augment Demyelination in MHV Infection
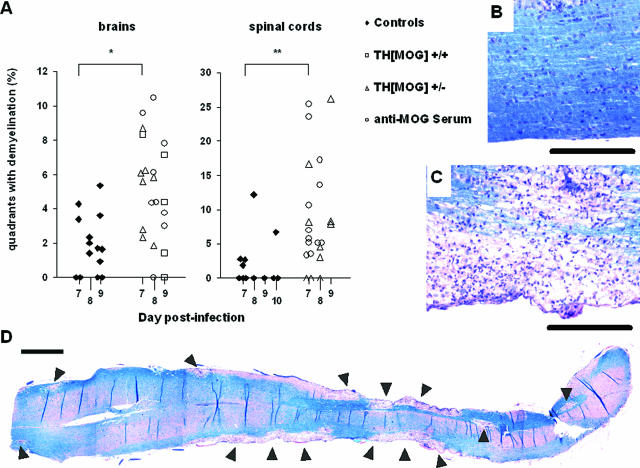
Both MHV A59 and anti-MOG antibodies (in EAE models) can provoke demyelination on their own. To evaluate the eventual additive effects of their joint presence in the CNS, we examined sections from brains and spinal cords of MHV-infected autoimmune and control mice for the presence of demyelinating lesions at 7 to 9 days after infection. The vast majority of the demyelinating areas were found in the central white matter of the cerebellum, in the brainstem, and in the region surrounding the third ventricle, both in controls and in mice with autoantibodies, but the extent of the lesions was found to be augmented in the latter group. In wild-type mice brains, small lesions were detected in a majority of the animals, but the mice with autoantibodies displayed lesions that were both more widespread in the tissue and larger in size (Figure 5A, left panel). This difference was even more pronounced in spinal cords, with only a small subset of the control mice displaying lesions at 7 to 10 days after infection [Figure 5, A (right panel) and B], whereas most animals with autoantibodies showed heavy demyelination in coronal (Figure 5A, right panel) and longitudinal (Figure 5, C and D) sections. These results show that demyelination plays a role in the enhancement of the CNS disease in our model.
Figure 5.
Demyelinating lesions in the brains and spinal cords of MHV-A59-infected mice. Luxol Fast Blue-stained tissue sections from control and autoimmune mice were examined for demyelination. A: Quantification of demyelinating lesions in control (black diamonds) and autoimmune (empty symbols) mice. A square grid was applied to sagittal brain sections (left) and coronal spinal cord sections (right), and results are expressed as the percentage of the total quadrants displaying lesions in each individual animal. Differences were statistically significant at day 7 for both organs. *P < 0.05. **P < 0.01. B and C: Representative pictures of spinal cords (longitudinal sections) from a control animal (B) and a C57Bl/6 recipient of anti-MOG serum (C) both obtained at day 8 after infection with 10 PFU of MHV A59. Note the massive infiltration in the lesion in C. Black bars = 0.2 mm. D: The reconstituted picture shows demyelinating lesions (arrowheads) spreading over the entire length of a spinal cord longitudinal section, obtained from the same animal as in C. It is representative of mice with autoantibodies at 7 to 9 days after infection, whereas all of the control mice in this study had little or no lesions in the spinal cord. Black bar = 1 mm.
The Autoantibody-Mediated Exacerbation of the CNS Disease Is T-Cell-Complement- and iNOS-Independent
To investigate the possible involvement of different effectors that have been associated with demyelination and CNS pathology in other models, we compared the outcome of CNS infection by MHV A59 in the presence or absence of anti-MOG antibodies in different KO mouse models. In the absence of either the CD4+ or the CD8+ T cells, control of the MHV A59 infection of the CNS is impaired. The mortality was found to be much higher for the control group in CD8 KO mice than what we had observed in WT C57Bl/6 mice. However, an exacerbation of the disease in the presence of autoantibodies was still observed in the absence of CD4 or CD8, both at the clinical (data not shown) and survival (Table 2) levels. Likewise, the exacerbation of CNS disease was still evident in the absence of a functional complement system in C′-3 KO mice (Table 2). Last, in mice deficient in iNOS, all of the controls survived, whereas all of the mice transferred with anti-MOG serum succumbed to the infection (Table 2). These results show that neither CD4 T cells, CD8 T cells, complement, nor iNOS alone is responsible for the autoantibody-mediated pathology.
Table 2.
Autoantibodies Exacerbate MHV A59 Encephalitis in KO Mice Models
| Mock transfer | Anti-MOG serum transfer | |
|---|---|---|
| CD4 KO | 2/12 | 9/13* |
| CD8 KO | 6/10 | 9/10† |
| C′-3 KO | 3/11 | 8/11* |
| iNOS KO | 0/6 | 6/6‡ |
The mortality of CD4 KO, iNOS KO, and C′-3 KO mice infected with 10 PFU of MHV A59 was significantly augmented in the presence of anti-MOG antibodies. The absence of CD8+ cells results in higher fatalities in the control group than in wild-type mice, but some exacerbation was still observed upon transfer of autoimmune serum.
P < 0.05.
P = 0.063.
P < 0.001.
Fc Receptors Are Required for Enhanced CNS Disease in MHV-Infected MOG-Ig Mice
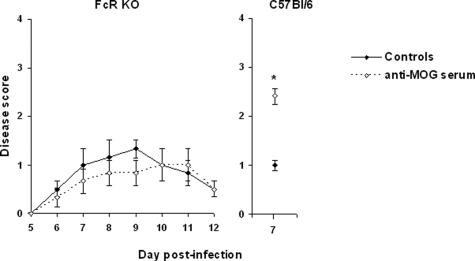
To determine whether the pathogenic effects of the autoantibodies required Fc receptors, we compared the outcome of the viral encephalitis in the presence or absence of the CNS-specific autoantibodies in mice deficient in Fc receptors FcγRI, FcγRIII, and FcεR. As shown on Figure 6, the clinical status of both groups was found to be similar, whereas MHV A59-infected wild-type C57Bl/6 mice showed the expected exacerbation when transferred with the serum from the same pool. The experiment in the Fc receptor-deficient mice was repeated with two more mice per group, and as the absence of exacerbation was confirmed on day 7 after infection (clinical scores of 1.0 and 2.0 versus 1.0 and 1.0 for the controls and the mice transferred with autoimmune serum, respectively), the animals were sacrificed to obtain histological data. Sections from brains and spinal cords showed no difference in the level of infiltration or demyelination between the controls and the recipients of the anti-MOG serum (data not shown). These experiments clearly demonstrate that cells bearing Fc receptors are involved in the augmented morbidity and mortality observed in MHV-infected mice in the presence of the anti-MOG antibodies.
Figure 6.
Anti-MOG antibodies do not exacerbate encephalitis in Fc receptor-deficient mice. Although all mice showed clinical signs of CNS infection, the intensity of MHV-induced disease was not affected by the transfer of anti-MOG autoantibodies in mice deficient in Fc receptors, and all of the mice survived the experiment in both groups (left, n = 3/group). MHV A59-infected wild-type mice transferred with the same serum pool displayed the normal exacerbation at the time they were sacrificed for a flow cytometry experiment (right, n = 6/group). *P < 0.05. The experiment was repeated as indicated in the main text section, confirming the absence of antibody-mediated pathology in the Fc receptor-deficient mice.
Discussion
We show here that the moderate CNS disease after infection of mouse CNS by MHV A59 is dramatically aggravated by the pre-existence of a humoral autoimmune response against a CNS antigen (MOG). This finding is not limited to this single virus, because we found that both TMEV and CV B3, used in conditions in which the control animals develop no disease at all, also trigger pathology in mice with autoantibodies. Because the three viruses have low or no genetic relation, this indicates that the mechanism responsible for the augmented disease is very unlikely to involve cross-reactivity between the self-antigen and the pathogen. More likely, the inflammatory milieu generated by the infections allows the entry of the autoantibody in the otherwise secluded CNS compartment and attracts other immune mediators that can contribute to the immunopathology.
We found that infiltration of mononuclear cells in the brain after MHV infection was augmented by the presence of autoantibodies affecting all infiltrating cell types. In particular, an augmentation of the frequency of neutrophils and blood-borne macrophages was detected in both the recipients of anti-MOG serum transfer and the heterozygous THMOG mice compared with the controls.
Anti-MOG antibodies, and 8.18C5 in particular, have been shown to augment demyelination in the EAE model.9,11,12 Likewise, we have shown here that both the incidence and the intensity of early demyelination were augmented in the brain and spinal cord of mice with anti-MOG autoantibodies. The localization of the areas affected in the brain was found to be similar in the control mice and in the animals with autoantibodies. Interestingly, a similar pattern was described in experiments performed by transfusing a MOG-specific monoclonal antibody along with MOG-specific T cells in rats.25 These histopathological patterns are reminiscent of the multifocal lesions throughout the brain and spinal cord found in EAE in animal models and in acute disseminated encephalomyelitis in humans.
Our results show that neither CD4+ nor CD8+ T cells alone are responsible for the aggravated disease, although both of these T-cell subsets have been shown to be the important effectors responsible for demyelination in CNS infection by MHV.20,21 The increased pathology observed in our autoimmune mice after viral infection thus results from a distinct autoantibody-mediated mechanism rather than from exacerbation of the damage caused directly by the virus or by the antiviral immune response. The augmented disease is transferred by serum containing the autoantibodies and is Fc receptor-mediated. In further support of this hypothesis, viral replication was similar in controls and mice with autoantibodies during the first 6 days of the infection and was only significantly higher in the autoimmune mice on day 7 after infection. ELISPOT and intracellular cytokine data indicated, however, that the viral-specific cytotoxic response was not significantly impaired in those mice, neither at the systemic nor at the local level.
Complement deposition has been found in lesions in MS patients,10 and the demyelination potential of myelin-specific antibodies has been shown to correlate with complement-binding ability.26 The experiments performed in C′-3 KO mice indicate that in our model, complement has little or no involvement in the autoantibody-mediated pathology. Another possible source of CNS damage can be NO,27 which has been shown to be involved in MS lesions.28 However, NO plays little role in MHV-induced demyelination, even though it can damage neurons during the infection,29,30 and iNOS-deficient mice have been reported to have a better survival rate than wild-type animals.29 In accordance with this literature, we found control iNOS KO mice to survive the infection better than the C57Bl/6 animals. However, our experiments showed that iNOS was not involved in the antibody-mediated pathology, because all of the KO mice that were transfused with autoimmune serum succumbed to the infection. On the contrary, we found that a deficiency in cellular Fc receptors FcγRI and FcγRIII was sufficient to abrogate completely the autoantibody-mediated pathology at both clinical and histological levels. This indicates that neutrophils, macrophages, or NK cells are responsible for the autoantibody-mediated pathology. Based on these results and on the characteristics of the mononuclear infiltration in the autoimmune mice, we propose that cells of the monocyte/macrophage/microglia lineage, neutrophils, or NK cells are responsible for the autoantibody generated damage, through a mechanism that does not involve complement or iNOS release.
The existence of cross-reactivity between antibodies binding to viral and self-antigens has been extensively investigated, but only a few reports, to our knowledge, have focused on the in vivo interplay of noncross-reactive autoantibodies and viral infections. The γ-herpesvirus γHV-68 has been shown to provoke relapses of experimental autoimmune arthritis in mice.31 Lactate dehydrogenase-elevating virus and MHV each resulted in dramatic exacerbation of the pathology and mortality in a mouse model of antibody-triggered autoimmune hemolytic anemia.32 Likewise, both lactate dehydrogenase-elevating virus and MHV infections also provoke a severe thrombocytopenia in mice treated with anti-platelet antibodies at otherwise nonpathogenic doses.33 To our knowledge, the present report constitutes the first description of a similar phenomenon in the CNS compartment in an animal model. Our findings are consistent with previous studies performed with human patients, which suggest a correlation between the presence of autoantibodies in the cerebrospinal fluid (CSF) and the outcome of encephalitis. Desai et al34 reported that fatal outcome in a cohort of 72 patients with Japanese encephalitis virus infection was significantly associated to the presence of CSF autoantibodies, against neurofilament proteins in particular. Likewise, Matsui et al35 found that in a group of 14 patients with viral encephalitis of undefined etiology, three of the four patients with CNS autoantibodies were among the total of five individuals with an unfavorable outcome. Both of these studies were performed with patients enrolled at the time that pathology was declared, making it impossible to determine whether the autoantibodies existed before the CNS infection or if they appeared as a consequence of the encephalitis. Likewise, there was no way to determine whether the autoantibodies had a direct role in the unfavorable outcomes or were merely an associated marker. However, in view of the results obtained in the present study, it is very possible that the autoimmune status of those patients had an adverse effect on the evolution of their CNS disease.
In conclusion, our study strengthens the hypothesis that in individuals with pre-existing autoantibodies, with or without clinical autoimmune disease, certain viruses can have a heightened pathogenic potential. It reinforces the concept that pathogens that are unrelated to the initial development of the autoimmune condition may cause exacerbation episodes by modifying the local or systemic environment. The model of an autoimmune “fertile field” proposes that autoreactive T cells are first generated by some infections through molecular mimicry or bystander mechanisms but may remain nonpathogenic for extended periods and that unrelated viruses or bacteria may be responsible for triggering the actual autoimmune pathology.1,4 In this model, Koch’s postulates (one disease/one pathogen) are not strictly respected, which may explain why it has been so difficult to associate a single organism to a given autoimmune disease.
Acknowledgments
We are very grateful to Dr. Kent Osborn for his help with the analysis of the morphological analysis of the infiltrates. We also thank Dr. Jason Botten for advice in designing the ELISPOT assays and for useful discussion.
Footnotes
Address reprint requests to Matthias G. von Herrath, La Jolla Institute of Allergy and Immunology, Immune Regulation Lab, 9420 Athena Circle, La Jolla, CA 92037. E-mail: matthias@liai.org.
Related Commentary on page 436
Supported by National Institutes of Health grants U19 AI51973, P01 AI058105, DK51091, AI44451, and JDRF 1-2002-726 to M.G.V.H. and AI25913 and AI43103 to M.J.B.
References
- von Herrath MG, Fujinami RS, Whitton JL. Microorganisms and autoimmunity: making the barren field fertile? Nat Rev Microbiol. 2003;1:151–157. doi: 10.1038/nrmicro754. [DOI] [PubMed] [Google Scholar]
- Christen U, Edelmann KH, McGavern DB, Wolfe T, Coon B, Teague MK, Miller SD, Oldstone MB, von Herrath MG. A viral epitope that mimics a self antigen can accelerate but not initiate autoimmune diabetes. J Clin Invest. 2004;114:1290–1298. doi: 10.1172/JCI22557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panoutsakopoulou V, Sanchirico ME, Huster KM, Jansson M, Granucci F, Shim DJ, Wucherpfennig KW, Cantor H. Analysis of the relationship between viral infection and autoimmune disease. Immunity. 2001;15:137–147. doi: 10.1016/s1074-7613(01)00172-8. [DOI] [PubMed] [Google Scholar]
- McCoy L, Tsunoda I, Fujinami RS. Multiple sclerosis and virus induced immune responses: autoimmunity can be primed by molecular mimicry and augmented by bystander activation. Autoimmunity. 2006;39:9–19. doi: 10.1080/08916930500484799. [DOI] [PubMed] [Google Scholar]
- Buljevac D, Flach HZ, Hop WC, Hijdra D, Laman JD, Savelkoul HF, van Der Meche FG, van Doorn PA, Hintzen RQ. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain. 2002;125:952–960. doi: 10.1093/brain/awf098. [DOI] [PubMed] [Google Scholar]
- Andersen O, Lygner PE, Bergstrom T, Andersson M, Vahlne A. Viral infections trigger multiple sclerosis relapses: a prospective seroepidemiological study. J Neurol. 1993;240:417–422. doi: 10.1007/BF00867354. [DOI] [PubMed] [Google Scholar]
- Martin F, Chan AC. B cell immunobiology in disease: evolving concepts from the clinic. Annu Rev Immunol. 2006;24:467–496. doi: 10.1146/annurev.immunol.24.021605.090517. [DOI] [PubMed] [Google Scholar]
- Lyons JA, Ramsbottom MJ, Cross AH. Critical role of antigen-specific antibody in experimental autoimmune encephalomyelitis induced by recombinant myelin oligodendrocyte glycoprotein. Eur J Immunol. 2002;32:1905–1913. doi: 10.1002/1521-4141(200207)32:7<1905::AID-IMMU1905>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Genain CP, Cannella B, Hauser SL, Raine CS. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med. 1999;5:170–175. doi: 10.1038/5532. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Cross AH, Trotter JL, Lyons J. B cells and antibodies in CNS demyelinating disease. J Neuroimmunol. 2001;112:1–14. doi: 10.1016/s0165-5728(00)00409-4. [DOI] [PubMed] [Google Scholar]
- Litzenburger T, Fassler R, Bauer J, Lassmann H, Linington C, Wekerle H, Iglesias A. B lymphocytes producing demyelinating autoantibodies: development and function in gene-targeted transgenic mice. J Exp Med. 1998;188:169–180. doi: 10.1084/jem.188.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane TE, Buchmeier MJ. Murine coronavirus infection: a paradigm for virus-induced demyelinating disease. Trends Microbiol. 1997;5:9–14. doi: 10.1016/S0966-842X(97)81768-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews AE, Weiss SR, Paterson Y. Murine hepatitis virus: a model for virus-induced CNS demyelination. J Neurovirol. 2002;8:76–85. doi: 10.1080/13550280290049534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Perlman S. Spread of a neurotropic coronavirus to spinal cord white matter via neurons and astrocytes. J Virol. 1995;69:633–641. doi: 10.1128/jvi.69.2.633-641.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombold JL, Sutherland RM, Lavi E, Paterson Y, Weiss SR. Mouse hepatitis virus A59-induced demyelination can occur in the absence of CD8+ T cells. Microb Pathog. 1995;18:211–221. doi: 10.1016/S0882-4010(95)90058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann CC, Ramakrishna C, Kornacki M, Stohlman SA. Impaired T cell immunity in B cell-deficient mice following viral central nervous system infection. J Immunol. 2001;167:1575–1583. doi: 10.4049/jimmunol.167.3.1575. [DOI] [PubMed] [Google Scholar]
- Dandekar AA, Jacobsen G, Waldschmidt TJ, Perlman S. Antibody-mediated protection against cytotoxic T-cell escape in coronavirus-induced demyelination. J Virol. 2003;77:11867–11874. doi: 10.1128/JVI.77.22.11867-11874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GF, Perlman S. Macrophage infiltration, but not apoptosis, is correlated with immune-mediated demyelination following murine infection with a neurotropic coronavirus. J Virol. 1999;73:8771–8780. doi: 10.1128/jvi.73.10.8771-8780.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane TE, Liu MT, Chen BP, Asensio VC, Samawi RM, Paoletti AD, Campbell IL, Kunkel SL, Fox HS, Buchmeier MJ. A central role for CD4(+) T cells and RANTES in virus-induced central nervous system inflammation and demyelination. J Virol. 2000;74:1415–1424. doi: 10.1128/jvi.74.3.1415-1424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GF, Dandekar AA, Pewe L, Perlman S. CD4 and CD8 T cells have redundant but not identical roles in virus-induced demyelination. J Immunol. 2000;165:2278–2286. doi: 10.4049/jimmunol.165.4.2278. [DOI] [PubMed] [Google Scholar]
- Feuer R, Mena I, Pagarigan RR, Harkins S, Hassett DE, Whitton JL. Coxsackievirus B3 and the neonatal CNS: the roles of stem cells, developing neurons, and apoptosis in infection, viral dissemination, and disease. Am J Pathol. 2003;163:1379–1393. doi: 10.1016/S0002-9440(10)63496-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Leibowitz J, David CS. Susceptibility to Theiler’s virus-induced demyelination: mapping of the gene within the H-2D region. J Exp Med. 1986;163:620–631. doi: 10.1084/jem.163.3.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botten J, Alexander J, Pasquetto V, Barrowman P, Ting J, Peters B, Southwood S, Stewart B, Rodriguez-Carreno MP, Mothe B, Whitton JL, Sette A, Buchmeier MJ. Identification of protective Lassa virus epitopes that are restricted by HLA-A2. J Virol. 2006;80:8351–8361. doi: 10.1128/JVI.00896-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeson AP, Piddlesden S, Morgan BP, Reynolds R. The distribution of inflammatory demyelinated lesions in the central nervous system of rats with antibody-augmented demyelinating experimental allergic encephalomyelitis. Exp Neurol. 1994;129:299–310. doi: 10.1006/exnr.1994.1172. [DOI] [PubMed] [Google Scholar]
- Piddlesden SJ, Lassmann H, Zimprich F, Morgan BP, Linington C. The demyelinating potential of antibodies to myelin oligodendrocyte glycoprotein is related to their ability to fix complement. Am J Pathol. 1993;143:555–564. [PMC free article] [PubMed] [Google Scholar]
- Brown GC, Bal-Price A. Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria. Mol Neurobiol. 2003;27:325–355. doi: 10.1385/MN:27:3:325. [DOI] [PubMed] [Google Scholar]
- Liu JS, Zhao ML, Brosnan CF, Lee SC. Expression of inducible nitric oxide synthase and nitrotyrosine in multiple sclerosis lesions. Am J Pathol. 2001;158:2057–2066. doi: 10.1016/S0002-9440(10)64677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BP, Lane TE. Lack of nitric oxide synthase type 2 (NOS2) results in reduced neuronal apoptosis and mortality following mouse hepatitis virus infection of the central nervous system. J Neurovirol. 2002;8:58–63. doi: 10.1080/135502802317247820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GF, Pewe L, Perlman S. Coronavirus-induced demyelination occurs in the absence of inducible nitric oxide synthase. J Virol. 2000;74:7683–7686. doi: 10.1128/jvi.74.16.7683-7686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarilin DA, Valiando J, Posnett DN. A mouse herpesvirus induces relapse of experimental autoimmune arthritis by infection of the inflammatory target tissue. J Immunol. 2004;173:5238–5246. doi: 10.4049/jimmunol.173.8.5238. [DOI] [PubMed] [Google Scholar]
- Meite M, Leonard S, Idrissi ME, Izui S, Masson PL, Coutelier JP. Exacerbation of autoantibody-mediated hemolytic anemia by viral infection. J Virol. 2000;74:6045–6049. doi: 10.1128/jvi.74.13.6045-6049.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musaji A, Cormont F, Thirion G, Cambiaso CL, Coutelier JP. Exacerbation of autoantibody-mediated thrombocytopenic purpura by infection with mouse viruses. Blood. 2004;104:2102–2106. doi: 10.1182/blood-2004-01-0310. [DOI] [PubMed] [Google Scholar]
- Desai A, Ravi V, Guru SC, Shankar SK, Kaliaperumal VG, Chandramuki A, Gourie-Devi M. Detection of autoantibodies to neural antigens in the CSF of Japanese encephalitis patients and correlation of findings with the outcome. J Neurol Sci. 1994;122:109–116. doi: 10.1016/0022-510x(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Matsui M, Tanaka K, Nagumo F, Kuroda Y. Central nervous system immunity associated with clinical outcome in acute encephalitis. J Neurol Sci. 2004;227:139–147. doi: 10.1016/j.jns.2004.09.022. [DOI] [PubMed] [Google Scholar]