Abstract
Little is known about how the composition of stromal cells within the lung cancer microenvironment varies during tumor progression. We examined by immunohistochemistry each of six different stromal cell populations during the development of chemically induced primary lung cancer in mice. Blood vessels were seen even in microscopic lesions, and their numbers increased with tumor size. Neutrophils infiltrated the alveoli of tumor-bearing lungs and within the periphery of macroscopic adenomas and adenocarcinomas. The numbers of peritumoral lymphocytes and macrophages increased during oncogeny, but quantitative changes in mast cells and fibroblasts were not evident. Because macrophage depletion reduces tumor growth and these cells are thus important to tumorigenesis, we also investigated their phenotype. Pulmonary macrophages expressed arginase I (subtype M2) but not inducible nitric-oxide synthase in lungs with premalignant lesions, whereas macrophages in carcinoma-bearing lungs expressed inducible nitric-oxide synthase (subtype M1) but not arginase I. Local pulmonary stimuli did not seem responsible for this shift in macrophage activation state because monocytes still residing within the bone marrow adopted these expression patterns before entering the circulation, presumably in response to tumor-derived signals. These biochemical markers of macrophage activation states would have diagnostic and/or therapeutic value if analogous systemic shifts occur in humans.
Three major mesenchyme components associated with neoplastic tissue include 1) the vasculature, comprised of endothelial cells, pericytes, and smooth muscle cells; 2) extracellular matrix and fibroblasts, which provide latent signaling information; and 3) innate and adaptive immune cells, which generate free radicals and growth signals to modulate cell survival. Some of these cells normally reside in tissues, whereas others are newly recruited to the tumor microenvironment. Adjacent stromal cells and normal epithelial cells can cause cytostasis or induce the death of epithelial cells that undergo neoplastic transformation. In vitro studies demonstrated this phenomenon in normal fibroblasts,1 melanocytes,2 and mammary epithelial cells.3 However, surveillance by stromal cells is weakened in vivo in response to tumor-derived signals, and this encourages cancer development. For example, fibroblasts from normal prostate tissue can prevent the growth of prostatic carcinoma cells, but fibroblasts isolated from prostate tumors cannot.4 Advanced cancer cells stimulate nonneoplastic cells within the tumor niche to alter their transcriptional program to aid neoplastic growth.5 An excellent example of such complicity is the endothelium. In a healthy adult, proliferation-quiescent endothelial cells secrete signals that suppress the growth of adjacent epithelial cells. In contrast, tumor-associated endothelial cells release growth factors for tumor cells, and the tumor reciprocates by providing endothelial growth signals. In prostate cancer, infiltrating macrophages can convert androgen-receptor modulators from antagonists to agonists.6 Recent therapeutic strategies combine cytotoxic agents acting directly on proliferating tumor cells with anti-angiogenic or anti-inflammatory drugs that target supportive cells.7 Cisplatin or paclitaxol co-administered with exisulind inhibited the growth of lung cancer cell xenografts more potently than when either is given alone.8
Lung cancer is the most common cause of cancer death worldwide.9 Delineating key stromal cell types that regulate lung cancer growth and identifying those signals that control their switching from anti-neoplastic behavior, to cessation of surveillance, to their eventual complicity in tumor growth, hold therapeutic promise. Similar to other cancers,10 lung cancer risk is enhanced by chronic inflammatory diseases,11 and inflammation is further exacerbated by neoplastic destruction of pulmonary tissue and occlusion of nearby vasculature.12 Tumors secrete chemokines that direct bone marrow-derived monocytes (BDMCs) toward the tumor site and cytokines that prolong survival of these infiltrating cells.10,11,13 Tumor-associated macrophages (TAMs) then produce growth factors, angiogenic factors, proteases, and other cytokines that stimulate invasion and metastasis.14 As a result, lung cancer prognosis is inversely associated with the extent of pulmonary infiltration by BDMCs.15
The ability of macrophages to promote neoplasia may depend on their activation state. Macrophages classically activated by interferon (IFN)-γ, alone or along with lipopolysaccharide (LPS) and tumor necrosis factor (TNF-α), are called M1 macrophages and produce high levels of inducible nitric-oxide synthase (iNOS) and proinflammatory cytokines such as interleukin (IL)-12.16–19 Nitric oxide (NO) produced by M1 macrophages can combine with superoxide to produce cytotoxic peroxynitrite. Mouse macrophages can also be alternatively activated by the following stimuli: IL-4 plus IL-13, immune complexes, or toll-like receptor (TLR) ligands, each of which generates macrophage properties generally referred to as an M2 phenotype. M2 macrophages express arginase I and anti-inflammatory IL-10 and during wound healing promote tissue remodeling and angiogenesis.18–23 Arginase I catalyzes the formation of ornithine, which leads to synthesis of polyamines necessary to make DNA and stimulate cell division while depleting the arginine substrate needed to produce NO.23,24 The pathway by which arginine is metabolized in macrophages may influence cancer progression.25 Ovarian tumor cell lines can alternatively activate human and mouse monocytes in vitro.26 The data presented in this study demonstrate the ability of primary lung tumors to affect macrophage and monocyte activation states during tumorigenesis in vivo.
Macrophage activation states have not been examined during human lung cancer progression, but chemically induced primary lung tumors in mice provide a model in which all stages leading to the development of pulmonary adenocarcinomas can be investigated.5,27 Although this model of human adenocarcinoma dates back to the early part of last century, the stromal cell composition and degree to which inflammatory cells enter the lung tumor parenchyma, accumulate, and become activated have not been rigorously examined. Variations in lung tumor stromal composition might account in part for the contrasting fates of early lesions induced by different carcinogens. Although most hyperplastic foci induced by urethane disappear,28 the remaining microscopic lesions (or microadenomas) grow to macroscopic size.29–31 In contrast, most microadenomas induced by two-stage initiation/promotion carcinogenesis spontaneously regress.31 These behavioral variations are analogous to the small pulmonary nodules frequently observed on spiral computed tomography scanning of smokers and ex-smokers in which very few nodules progress to frank malignancy.32 Stage-dependent changes in tumor stroma may affect whether nodules advance, remain static, or regress.
Accordingly, we have begun to systematically evaluate mesenchymal cell types during primary lung tumor induction by urethane. Several cell types were quantified, and we determined whether they penetrate the tumor parenchyma, accumulate peritumorally, or reside elsewhere in the lungs. The activation states of macrophages during neoplastic progression were examined. Our most intriguing results include the following. Neoplastic cells at the tumor border are surrounded by neutrophils that take residence within the tumor periphery and by macrophages located immediately adjacent to the tumor. Macrophages change their activation state during the course of tumor development from M2 in early lesions to M1 in adenocarcinomas. This change occurs before BDMCs reach the lungs to extravasate into tumors rather than within the lungs in response to local pulmonary signals. Tumors therefore emit stimuli that change the BDMC phenotype from naïve to M2 to M1 as neoplasia progresses before BDMCs enter the circulation to infiltrate the lungs. Pharmacological interference with signals emanating from lung tumors that affect myeloid cells still in the bone marrow may retard neoplastic progression.
Materials and Methods
Urethane-Induced Tumor Model
A/J mice (male, 4 to 6 weeks of age) were purchased from the Jackson Laboratory (Bar Harbor, ME), housed on hardwood bedding with 12-hour light/dark cycles, and fed standard rodent diet (Harlan Teklad, Madison, WI) at the Center of Laboratory Animal Care in the University of Colorado Health Sciences Center. Mice were administered freshly prepared urethane (ethyl carbamate; Sigma, St. Louis, MO) as a single intraperitoneal injection of 1 mg of urethane/g body weight dissolved in sterile 0.9% NaCl, as previously described.33 Control mice were given a single saline intraperitoneal injection. Animals were sacrificed 3, 6, 9, 12, 16, 24, and 42 weeks after urethane exposure, and their lungs prepared for histological examination. Four to five mice were examined per time point.
Bronchoalveolar Lavage (BAL)
Control and urethane-treated mice were sacrificed by lethal intraperitoneal phenobarbital injection 16, 24, and 42 weeks after urethane treatment, as described previously.34 The trachea was cannulated and the lungs lavaged three times with 1 ml of phosphate-buffered saline (PBS) containing 0.6 mmol/L ethylenediaminetetraacetic acid. Inflammatory cell infiltration was determined by pooling lavaged samples from each animal, staining each cell type as indicated below, and counting cells using a hemocytometer. Differential cell counts based on cell morphology, as determined by Wright’s stain (University Hospital Clinical Laboratory, Denver, CO), classified the infiltrating cells as monocytes/macrophages, lymphocytes, neutrophils, or eosinophils.
BDMC Preparation
BDMCs were removed from femurs by flushing sterile PBS through the bone marrow cavies of age-matched control mice and mice 16, 24, 32, and 42 weeks after urethane treatment. Cells were counted using a hemocytometer, 20,000 cells were cytospun onto a slide, and differential cell counts were determined as above.
Immunohistochemistry
As described previously,31 perfused lungs were fixed in formalin and after a series of ethanol rinses, embedded in paraffin, and cut into 4-μm sections using an 820 Spencer microtome (American Optical Corp., Del Mar, CA). They were rehydrated, and endogenous endoperoxidase activity inhibited with 3% H2O2 in methanol for 15 minutes. Antigens were retrieved using warm 100 mmol/L citrate buffer, pH 6.0, or digested with protease I (Ventana, Tucson, AZ) for 8 minutes in a humidified chamber. Five lung sections were examined per mouse with each of the following antibodies: goat polyclonal PECAM-1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at 1:200 dilution to stain endothelial cells, goat polyclonal matrix metalloproteinase 13 (MMP-13) antibody (Chemicon, Temecula, CA) at 1:350 dilution to stain fibroblasts, a polyclonal rat CD-45 (BD Pharmingen, San Diego, CA) antibody at 1:100 dilution to stain lymphocytes, polyclonal rabbit CD-15 (Sigma) antibody at 1:50 dilution to stain neutrophils, rat polyclonal F4/80 antibody (Caltag Laboratories, Burlingame, CA) at 1:100 to stain macrophages, goat polyclonal arginase I antibody (Santa Cruz Biotechnology) at 1:100 dilution to stain M2 macrophages, and rabbit polyclonal iNOS antibody (BD Pharmingen) at 1:1000 to stain M1 macrophages. Samples were treated with biotin-conjugated anti-goat, -rabbit, or -mouse IgG secondary antibody (Vector Laboratories, Burlingame, CA), followed by peroxidase-conjugated streptavidin tertiary antibody complex (Vector Laboratories). 3,3-Diaminobenzidine (Sigma) was the peroxidase substrate for antibody detection, and hematoxylin (Sigma) was used to counterstain. Collagen was detected by staining sections with Masson’s trichrome stain, which stains collagen blue, whereas the surrounding tissue is red. Mast cells were stained with toluidine blue (Sigma) in 1% sodium chloride for 3 minutes at room temperature. For all antibodies used, primary alone, secondary alone, and internal positive controls were performed to ensure staining specificity. Pictures were taken at all time points: 3, 6, 12, 16, 24, and 42 weeks after urethane injection and in age-matched controls, and the results included herein are those most characteristically observed that demonstrate change or lack of any change. The number of endothelial cells/mm2 was determined by double-blinded observers counting PECAM-1-positive cells in early lesions and advanced carcinomas.
Immunofluorescence
Twenty thousand cells from BAL and bone marrow aspirates were affixed onto slides with a Shandon Cytospin 3 and fixed in −20°C methanol for 2 minutes. Three slides per mouse were examined for both BAL and bone marrow samples. In these experiments, all samples were first blocked with avidin-biotin solution (Vector Laboratories) followed by exposure to the above primary and secondary antibodies. Fluorescent avidin conjugates (rhodamine, AMCA, and fluorescein) at 15 μg/ml concentrations were attached to the secondary antibodies (Vector Laboratories). A digital deconvolution microscopy imaging system attached to an Axioplan 2 EPI fluorescence upright microscope (Carl Zeiss, Thornwood, NY) was used to image fluorescent staining, and cells were viewed under a ×63 oil immersion lens (final magnification, ×630). The microscope is configured with different fluorescence cube sets, including, 4,6-diamidino-2-phenylindole, CY3, and fluorescein isothiocyanate, and color bright-field microscopy. The microscope, stage, filter wheels, and camera are controlled through Slidebook (version 4.1.0.11), a software interface (Intelligent Imaging Innovations, Denver, CO). Images were digitally captured with a Cooke Sensicam QE high resolution (1376 × 1024 resolution; Cooke Corporation, Romulus, MI), black and white, supercooled, charge-coupled device camera and assigned colors by the software interface.
Immunoblotting
Protein concentrations in each sample were determined by the method of Lowry and colleagues,35 and samples were mixed 1:1 with 2× sample loading buffer (100 mmol/L Tris, pH 6.8, 0.4% sodium dodecyl sulfate, 2% β-mercaptoethanol, 20% glycerol, and 0.3% pyronine Y). Immunoblotting of iNOS and arginase I proteins was performed by incubating membranes with primary antibodies overnight at 4°C using 1:1000 (iNOS) and 1:100 (arginase I) concentrations in blocking solution. Samples were then incubated with rabbit anti-mouse alkaline phosphatase secondary antibody (iNOS) or goat anti-mouse alkaline phosphatase secondary (arginase I) (1:30,000 dilution; Bio-Rad, Hercules, CA) for 1 hour. Protein bands were visualized by incubation with Immuno-Star chemiluminescent substrate (1:20,000 dilution; Santa Cruz Biotechnology) followed by exposure to CL-XPosure X-ray film (Pierce, Rockford, IL). The proteins were quantified by densitometry using Un-Scan-It software (Silk Scientific Corporation, Orem, UT). To confirm even protein loading of the gels, the membranes were stained with 0.1% Ponceau S (Fisher Biotech, Fair Lawn, NJ) in 5% acetic acid and stained for β-actin at 1:2500 (Sigma) for primary and 1:10,000 anti-mouse secondary (Santa Cruz Biotechnology).
Statistics
Data are presented as means ± SEM. Differences between groups were examined using Student’s unpaired t-test. One-way analysis of variance compared more than two groups, and posthoc Newman-Keuls tests identified differences between groups. P < 0.05 was considered to be significant.
Results
Sections from mouse lungs were examined throughout a time course of tumor development after urethane was administered. Age-matched controls were examined at each time point and displayed consistent phenotypes throughout the aging process. Early lesions appear in the lungs by 3 weeks and progress into macroscopic lesions by 9 weeks.31 Benign adenomas are visible by 16 weeks after urethane injection and maintain well-defined borders and structural organization through 24 weeks. Malignant adenocarcinomas 42 weeks after urethane were characterized by nuclear atypia, disorganization of the tumor parenchyma, and the formation of invasive, irregular tumor borders.
Stromal Cells Whose Number Does Not Change during Tumor Development
Mast Cells and Fibroblasts
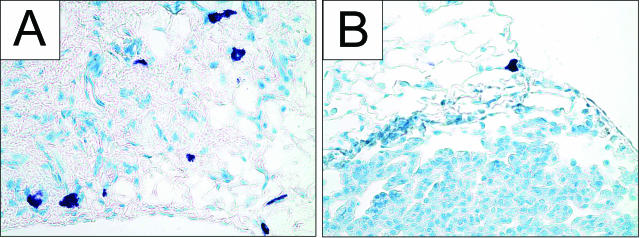
Two populations of stromal cells, the pulmonary mast cells and fibroblasts, did not demonstrate demographic or apparent qualitative changes during lung tumor progression. Resident mast cells stained with toluidine blue were observed in lungs obtained from naïve mice (Figure 1A) and in tumor-bearing lungs (Figure 1B). At all times examined, mast cells were located singly or in pairs along the lung periphery (Figure 1, A and B), but their numbers did not seem to change during neoplastic development and they were not found within tumors.
Figure 1.
Mast cells are present in control lungs and during tumor development. Mast cells stained with toluidine blue are purple. A: Control lungs show mast cells in the alveoli. B: Tumor development does not obviously affect the number or location of mast cells, and 24 weeks after urethane, the mast cells remain at the lung periphery and are not observed within the tumor parenchyma. Original magnifications, ×400.
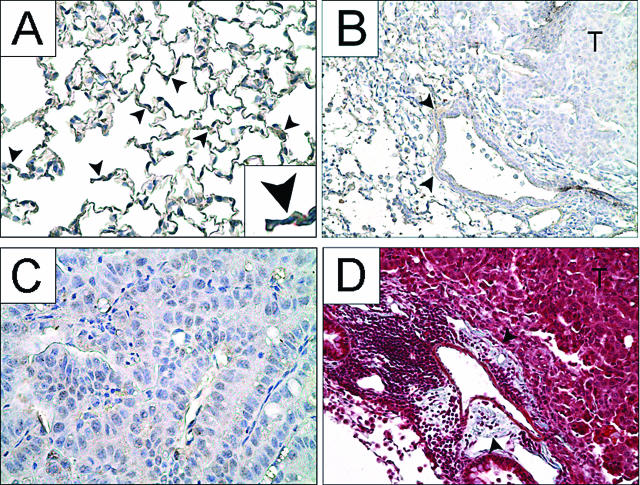
Fibroblasts surrounded large blood vessels and were located interstitially within the alveolar wall, consistent with normal lung histology (Figure 2A). Fibroblasts did not change in number, nor did they migrate into either early lesions or macroscopic tumors, as determined by immunostaining with MMP-13. Fibroblasts continued to be observed in the stroma and surrounding large blood vessels in tumor-bearing mice and thus acted as internal positive controls (Figure 2B). High-magnification images verified a lack of fibroblast staining within the tumor parenchyma (Figure 2C). To confirm these results, 42-week tumor sections were also stained with Masson’s trichrome (Figure 2D) to demonstrate that collagen was located in the same areas that stained positively for fibroblasts. No collagen was evident within the tumor parenchyma. Sections were also stained with a lung-specific pentachrome stain that differentially identifies elastic fibers, collagen and reticular fibers, ground substance, mucin, fibrin, and muscle. Peripheral alveolar tissue from tumor-bearing mice did not differ from those stained age-matched controls, nor were any collagen or reticular fibers within the tumor parenchyma (data not shown), confirming the results shown in Figure 2 obtained with MMP-13 and Masson’s trichrome staining.
Figure 2.
Fibroblasts are not present within tumors during lung neoplasia. Fibroblasts stained for MMP-13 (brown) are in control lung alveoli (A) but not inside advanced tumors 42 weeks after urethane (B and C). Fibroblasts are present neither along the leading edge of malignant tumors nor within the tumor parenchyma, and their population size does not obviously increase during tumorigenesis (B and C). B: Black arrowheads indicate fibroblasts around a large blood vessel in the interstitium, an internal positive control. D: Similar sections stained with Masson’s trichrome indicate collagen (blue) localized to areas where fibroblasts are present (black arrowheads). T indicates tumor. Original magnifications: ×40 (B, D); ×400 (A); ×1000 (C); ×800 (inset).
Stromal Cells That Infiltrate Tumors
Neutrophils
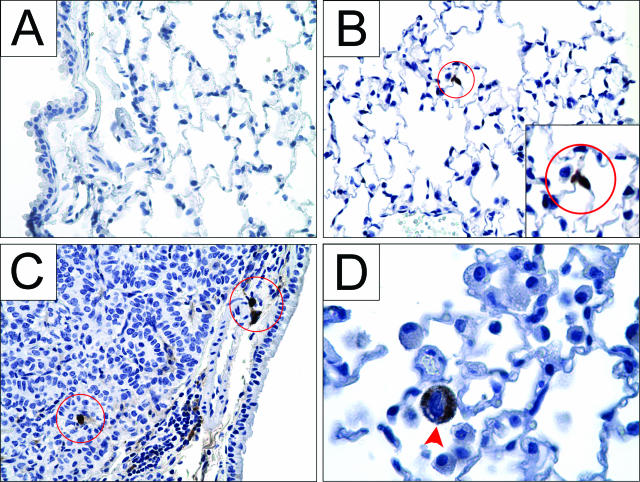
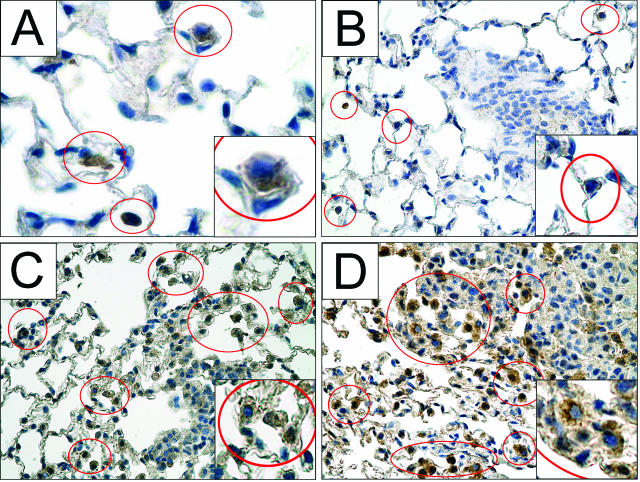
Neutrophils typically accumulate in lungs only during acute inflammation and were absent from control lungs (Figure 3A). To determine whether any neutrophils occupied tumor-bearing lungs, sections were stained with a neutrophil-specific antibody CD-1536 and characterized by polymorphic nuclear morphology (Figure 3D). Their presence was monitored throughout the 42-week course of tumor development. Three weeks after urethane administration, neutrophils were detected in the alveoli of lungs that contained microscopic neoplastic lesions, and the presence of neutrophils in the alveoli continued throughout tumor development (Figure 3, B–D). The number of alveolar neutrophils increases as tumors progress, as observed in tissue sections and quantified in BAL (see Figure 7). In adenomas of macroscopic size and in carcinomas (Figure 3C), neutrophils additionally collected just inside the edge of the tumor parenchyma but not more centrally. These results were consistent in each of several tumors examined. Because the life span of a neutrophil in normal lung tissue is typically only a few days, it is probable that recruiting signals are continuously provided by the developing tumor or other pulmonary cell types to maintain this tumor-associated neutrophil population.
Figure 3.
Neutrophils are present within the alveolar airspaces and within the tumor parenchyma during neoplastic development. A: Lungs from naïve healthy mice contain no neutrophils. Three weeks after urethane exposure, neutrophils stained for CD15 (brown) in the alveoli are indicated within red circles (B), and this continues as tumors develop to adenocarcinomas 42 weeks later (C). In more advanced tumors (C), more alveolar neutrophils appear compared with the 3-week lesion (B). D: A neutrophil at higher magnification shows polymorphic nuclear morphology. Original magnifications: ×400 (A–C); ×1000 (D); ×800 (inset).
Figure 7.
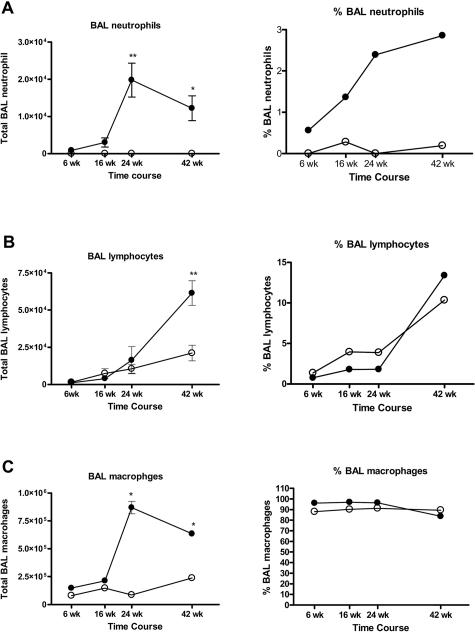
BAL leukocytes increase during tumor progression. BAL neutrophils (A) and macrophages (C) increase significantly 24 and 42 weeks after urethane injection compared with age-matched controls, whereas BAL lymphocytes increase significantly at 42 weeks after urethane injection (B). The percentage of the BAL cell population consisting of neutrophils and lymphocytes increases as tumors develop (A and B), and the percentage of macrophages decreases slightly (C). Mean ± SEM, n = 5; *P < 0.01 and **P < 0.001 compared with age-matched controls.
Endothelial Cells
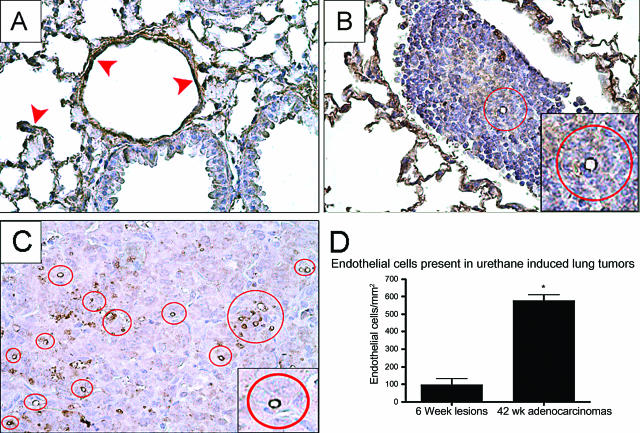
The well-documented process of angiogenesis allows tumors to grow beyond a diameter of 1 mm.37 Endothelial cells were stained with PECAM-1 (CD-31), a specific marker that stains both endothelial cells located in the alveoli and those within tumors (Figure 4, A–C).38 In control lungs, endothelial cell staining in alveoli and lining pulmonary vessels was used as an internal positive control (Figure 4A). We observed blood vessels even within the microscopic lesions that developed 6 weeks after urethane injection (Figure 4B). The number of endothelial cells within the tumor parenchyma and the microvessels they comprise increase proportionally as tumors grow (Figure 4D). Advanced tumors contained many well-defined microvessels (Figure 4C).
Figure 4.
The number of endothelial cells within the tumor parenchyma increases during neoplastic development. Endothelial cells stained for PECAM-1 (brown) are indicated within red circles within tumors. A: Control lungs demonstrate endothelial staining in the alveoli and in large vessels demonstrated by red arrowheads. B: As early as 6 weeks after urethane exposure, endothelial cells comprising blood vessels are observed within early lesions. C: The number of vessels increases during tumor development, with many seen within the tumor parenchyma in 42-week adenocarcinomas. D: Endothelial cell counts within the tumor parenchyma comparing early lesions to adenocarcinomas show a significant increase in positively stained cells during tumor development. *P < 0.005. Original magnifications: ×400 (A–C); ×800 (insets).
Stromal Cells Whose Number Increases but Do Not Enter the Tumor Parenchyma
Lymphocytes
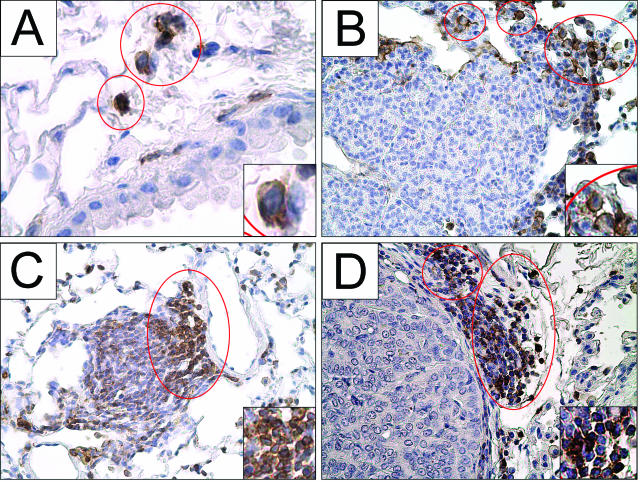
Few lymphocytes identified by positive CD45 staining were apparent in normal lungs outside of the bronchus-associated lymphatic tissue (BALT) (Figure 5A). Pulmonary lymphocytes localize as single cells in the alveoli (Figure 5A), in small clusters bordering the tumors (Figure 5, B and C), and in larger aggregates (Figure 5D) at all stages of lung tumor development. Lymphocytic aggregates were adjacent to the tumor (Figure 5D) or close to larger airways, where they comprise the BALT.39,40 The sizes of these aggregates close to the tumor border increased dramatically in malignant tumors (Figure 5D), and the overall size of the pulmonary lymphocyte populations increased, as estimated in BAL samples (Figure 7B).
Figure 5.
The number of lymphocytes in uninvolved lung tissue increases during tumor formation. Lymphocytes stained with CD45 (brown) are enclosed within red circles. During tumor development, lymphocytes accumulate near tumors but not within the tumor parenchyma. Few lymphocytes were observed in control lungs (A), but lymphocytes become more abundant 9 weeks after urethane (B). Their numbers near adenomas at 16 weeks continue to increase (C) and appear as large aggregates adjacent to adenocarcinomas 42 weeks after urethane injection (D). Original magnifications: ×400 (A–D); ×800 (insets).
TAMs
Resident macrophages stained with anti-F4/80 in normal lungs are located in the alveolar interstitia and in airways (Figure 6A). Macrophages adjacent to tumors were observed starting 3 weeks after urethane injection (Figure 6B). TAMs migrate up to the leading edge of the growing tumor but do not infiltrate into the tumor parenchyma (Figure 6, C and D). The number of these peritumoral macrophages and those in uninvolved lung tissue farther away from the tumor (Figure 6, B–D) increased as tumors developed.
Figure 6.
Macrophages accumulate peritumorally as tumors progress. Representative macrophages stained with F4/80 (brown) are enclosed by red circles. During tumor development, more macrophages appear that are adjacent to but not within the tumor parenchyma. A: Interstitial and alveolar macrophages are seen in control lungs from naïve mice. Macrophages accumulate near early lesions 3 weeks after urethane (B) and continue to accumulate near 24-week adenomas (C) and 42-week adenocarcinomas (D). Original magnifications: ×1000 (A); ×400 (B–D); ×800 (insets).
Quantitation of Inflammatory Leukocytes in BAL Fluid
Estimates of the numbers of neutrophils, lymphocytes, and macrophages in tumor-bearing lungs were made using BAL samples harvested 6, 16, 24, and 42 weeks after urethane injection and from age-matched naïve control mice. Cells were differentiated by nuclear morphology after Wright’s staining. The number of BAL neutrophils detected in naïve mice was very small (Figure 7A), and this population began to grow early after urethane injection to became statistically significantly elevated compared with lungs of age-matched controls by 24 and 42 weeks (Figure 7A). Neutrophils from naïve mice typically comprise <1% of the BAL cell population, and this percentage rose 30-fold in malignant tumor-bearing mice (Figure 7A).
The lymphocyte population increased linearly with age in naïve mice with ∼1500 cells/mouse observed at 14 weeks and 21,000 cells/mouse at 50 weeks (Figure 7B). Lymphocyte numbers in urethane-treated mice increased above that in age-matched naïve controls 42 weeks after urethane (Figure 7B). Lymphocytes comprise 2% of the BAL cell populations in mice with adenomas and 13% in mice with adenocarcinomas (Figure 7B). The percentage of BAL cells that are lymphocytes was 10% in naive 50-week mice (Figure 7B).
The numbers of macrophages from naïve mice changed with age in untreated control mice, ranging from 8 × 104 cells/mouse in 14-week mice to 2.4 × 105 cells in 50-week mice. Macrophages typically comprised >90% of the total BAL cell population (Figure 7C). At 6 and 16 weeks after urethane treatment, the differences in macrophage numbers compared with age-matched controls were not significant. By 24 and 42 weeks, however, when mice had macroscopic adenomas and adenocarcinomas, respectively, significantly more BAL macrophages were detected relative to age-matched controls and reached a peak of 8.7 × 105 macrophages per mouse (Figure 7C). The percentage of BAL cells identified as macrophages decreased to 84% in mice bearing malignant tumors, mainly because of the increased size of the lymphocyte population (Figure 7C).
Temporal Changes in TAM Activation States
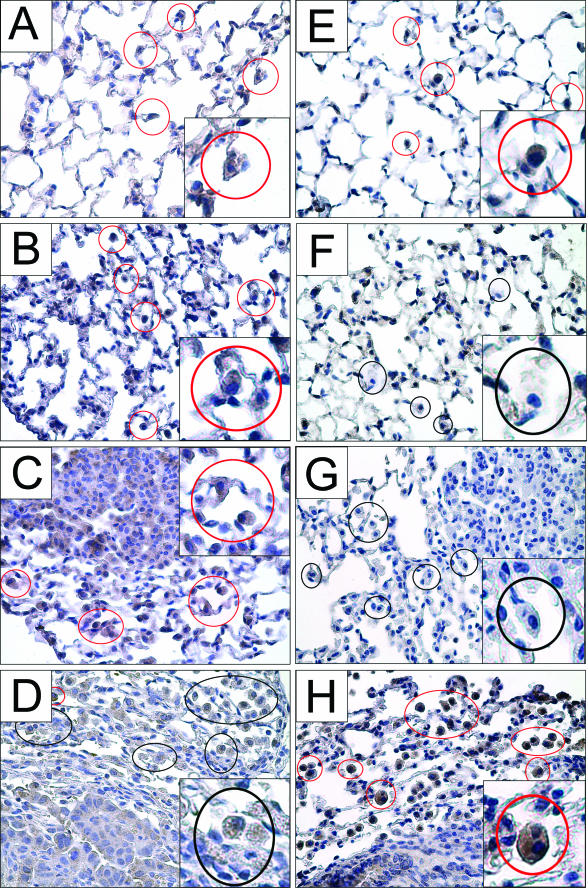
Classical M1 activation of mouse macrophages can be phenotypically characterized by iNOS expression and alternative M2 activation by arginase I expression.18,21 Lung sections stained with arginase I or iNOS displayed a temporal dependence on the stage of tumor development. Resident macrophages in control lungs from naïve mice contained both arginase I and iNOS (Figure 8, A and E). Slight arginase I staining was seen consistently in the tumors at all stages of development. In early lesions 3 weeks after urethane exposure, TAMs stained more intensely for arginase I than in untreated mice (Figure 8B), but iNOS expression diminished (Figure 8F). Arginase I was expressed in macrophages near the tumor and throughout lungs bearing early lesions (Figure 8B), and iNOS staining remained negligible (Figure 8F). This differential staining pattern also occurred in mice bearing large adenomas (Figure 8, C and G). In malignant tumors 42 weeks after urethane injection, the activation state of TAM was reversed; most TAMs no longer expressed arginase I (Figure 8D) but now expressed iNOS more intensely (Figure 8H). Thus, iNOS expression in lung macrophages is initially down-modulated as lung neoplasia begins and then re-expressed when tumors have progressed to malignancy. Arginase I expression ceases when tumors become malignant. This exclusivity of arginase I versus iNOS expression applied to all pulmonary macrophages, not just that population near the tumor.
Figure 8.
Macrophages display an M2 phenotype, characterized by arginase I expression, early during tumor growth, but express iNOS, which is characteristic of an M1 phenotype, in adenocarcinomas. A–D: Macrophages stained with arginase I (brown), a marker of alternative activation, are enclosed by red circles. Macrophages that do not stain for arginase I are enclosed by black circles. Arginase-containing macrophages are present in control lungs of naïve mice (A), 3 weeks after urethane (B), and near macroscopic adenomas (C). D: When malignant tumors are present, most macrophages do not stain for arginase I. E–G: Macrophages stained with iNOS (brown staining) are enclosed by red circles; macrophages that do not stain for iNOS are indicated by black circles. E: iNOS-expressing macrophages are detected in control lungs from naïve mice. At 3 weeks after urethane (F) and during the development of adenomas (G), macrophages did not stain for iNOS. H: Macrophages near malignant tumors, however, display positive iNOS staining. Original magnifications: ×400 (A–H); ×800 (insets).
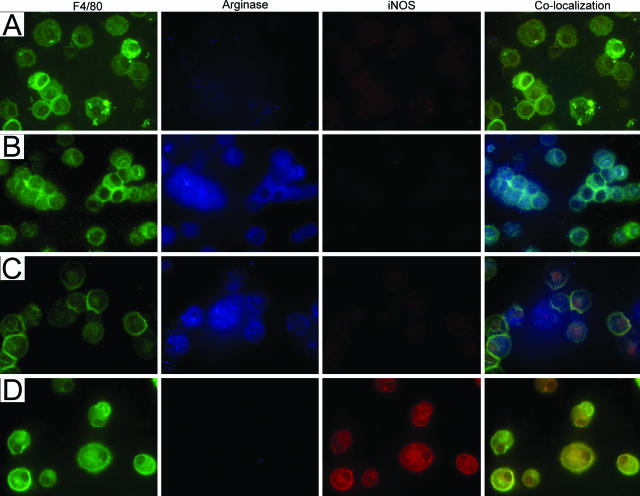
Differential expression of arginase I and iNOS by macrophages as a function of tumor progression can be observed even more dramatically using macrophages isolated from BAL fluid and examined by immunofluorescence. Macrophages (green) isolated from naïve age-matched control mice exhibited faint staining of both arginase I (blue) and iNOS (red). Co-localization shows that the same cells stain for all three proteins (Figure 9A). BAL macrophages from mice bearing early microscopic lesions and adenomas positively stained for arginase I but not for iNOS (Figure 9, B and C), whereas BAL macrophages obtained from malignant tumor-bearing mice stained positively for iNOS but not for arginase I (Figure 9D).
Figure 9.
Pulmonary macrophages isolated from BAL exhibit M1 or M2 activation states when fluorescently stained with arginase I or iNOS. Macrophages were identified with anti-F4/80 (column 1, green), arginase I (column 2, blue), and iNOS (column 3, red). Marker co-localization is shown in column 4. A: BAL macrophages isolated from naïve mice exhibit faint staining for both arginase I and iNOS. Macrophages isolated from mice 6 weeks (B) and 16 weeks (C) after urethane positively stain for arginase but not for iNOS. D: Macrophages from mice with malignant tumors (42 weeks) stain positively for iNOS but not for arginase. Original magnifications, ×630.
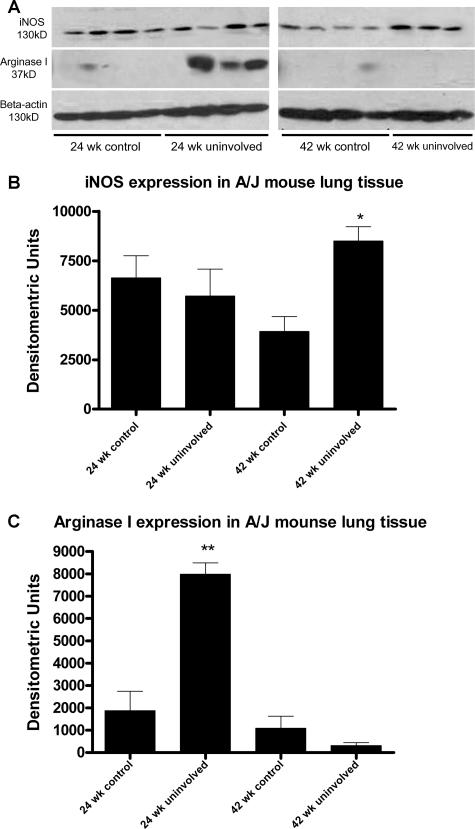
These activation state changes in macrophage populations are also shown by subjecting extracts prepared from control lungs versus uninvolved lung (ie, the tissue that surrounds tumors) to immunoblotting. Control and uninvolved lung tissue from mice 24 and 42 weeks after injection (saline and urethane, respectively) were probed with antibodies specific for iNOS and arginase I (Figure 10). Immunoblots showed no differences in iNOS expression between lungs 24 weeks after urethane injection and age-matched uninvolved lungs. iNOS expression in uninvolved lungs 42 weeks after injection significantly increased above control lungs, which corresponds with the increased size of the macrophage population at this time point (Figure 8C) and the positive iNOS staining observed with immunocytochemistry (Figures 8 and 9). The high level of iNOS at all time points is attributable to other lung cells that also express iNOS, such as endothelial and Clara cells.41 Arginase I expression is significantly higher in uninvolved lung tissue 24 weeks after urethane injection compared with age-matched control lung extracts or 42-week uninvolved lung tissue (Figure 10). This correlates with the increased macrophage numbers 24 weeks after urethane injection and the positive arginase I staining seen by immunocytochemistry (Figures 8 and 9).
Figure 10.
Immunoblotting of iNOS and arginase I in control and uninvolved lung tissue during tumorigenesis. A and B: iNOS is expressed in age-matched controls and uninvolved lung tissue from tumor-bearing mice but increases in uninvolved tissue 42 weeks after urethane injection. A and C: Arginase I expression is significantly greater in uninvolved lung tissue at 24 weeks compared with age-matched controls but decreases in uninvolved lung tissue at 42 weeks after urethane injection. β-Actin was used as a loading control for the gel. Densitometry was performed on immunoblots to generate graphs. *P < 0.05, **P < 0.001.
BDMCs
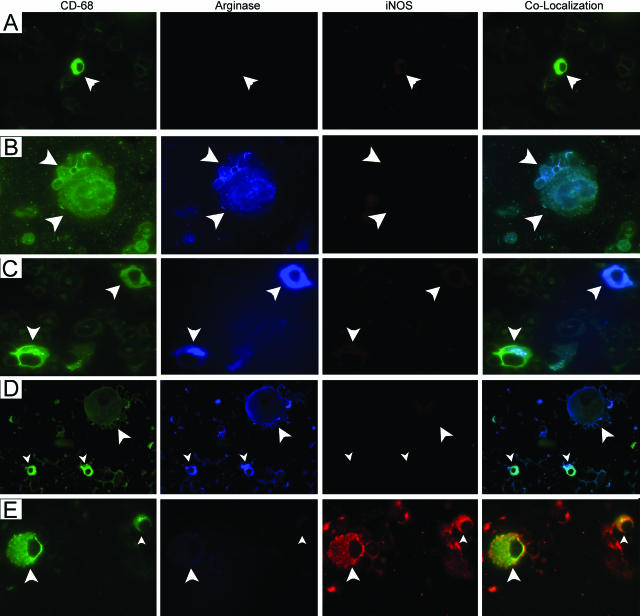
Because of the novelty of this observation that stage of lung tumor progression affects the activation state of pulmonary macrophages (Figures 8 and 9), we examined this phenomenon further. Because all of the macrophages exhibited these changes, not simply that subset in physical contact with the tumor, we hypothesized that monocytes arrived at the lungs already activated, ie, that tumor-derived signals acted on BDMCs still in the bone marrow. To test this hypothesis, BDMCs from naïve mice were stained with anti-CD68 to readily distinguish them from other hematopoietic cells; murine pulmonary macrophages stain poorly with anti-CD68, but we found them to stain strongly with anti-F4/80. BDMCs from control mice expressed neither arginase I nor iNOS, a result confirmed by co-localization (Figure 11A). At 6 and 24 weeks after urethane, harvested BDMCs stained positively for arginase I but did not co-stain for iNOS (Figure 11, B and C). This mirrors the M2 activation state of pulmonary macrophages at these periods of tumor development. BDMCs prepared from mice 42 weeks after urethane administration no longer express arginase I but now display significant iNOS staining (Figure 11D), again reflecting the activation state observed in pulmonary macrophages from malignant tumor-bearing mice (Figure 9). Because macrophages have not been reported to leave the lungs and return to the bone marrow, these results imply that tumors release soluble factors that systemically travel to the bone marrow to differentially activate monocyte populations, and the nature of these factors varies according to the state of progression of tumors in the lungs. Arginase I was induced in BDMCs by signals emanating from microscopic lesions induced 6 weeks after urethane, whereas iNOS is expressed in BDMCs obtained from carcinoma-bearing mice.
Figure 11.
BDMCs exhibit M1 or M2 activation states. A: BDMCs (white arrowheads) from naive and tumor-bearing mice were stained with markers for monocytes (CD68, green), arginase I (blue), and iNOS (red). BDMCs from control mice do not stain for either arginase I or iNOS. BDMCs from mice 6 (B), 16 (C), and 24 (D) weeks after urethane positively stain for arginase but not for iNOS. E: BDMCs isolated from the femurs of mice with malignant tumors (42 weeks) stain positively for iNOS but not for arginase I. Original magnification, ×630.
Discussion
The changes observed in several stromal cell populations during urethane-induced lung tumorigenesis lead us to conclude that the developing tumor directs and alters the phenotypes of cells within its surrounding niche, presumably to foster a more favorable growth environment. Mast cells and fibroblasts exhibited no apparent quantitative changes (Table 1). Mast cells reside within tissues where they complete their maturation from bone marrow precursors, and they contain secretory granules released as chemical signals during acute inflammation.42,43 Mast cells up-regulate angiogenic factors such as vascular endothelial growth factor, basic fibroblast growth factor, IFN-α, and IL-8, associated with enhanced growth and invasion in several human cancers, including those in the cervix, lungs, epidermis, and breast.44–49 Mast cells invade into hyperplasias and the leading edge of squamous cell carcinomas of the epidermis.44 In contrast to one report of mast cell accumulation along the border of human lung adenocarcinomas, which positively associated with tumor microvessel density, we found no such localization of mast cells in the chemically induced mouse lung tumors examined herein.
Table 1.
Summary of Lung Tumor Stromal Cells and Their Relationship with the Tumor during Cancer Progression
| Stromal cell composition after urethane induction of mouse lung tumors
| ||
|---|---|---|
| Cells entering the tumor: | Cells accumulating near the tumor: | Cells that do not change in number: |
| Endothelial cells | Lymphocytes | Mast cells |
| Neutrophils | Macrophages | Fibroblasts |
A second stromal cell type whose population size did not change during lung neoplasia was fibroblasts. This is in contrast to several other cancers, including melanoma, breast, colon, and prostate, and in advanced mouse lung tumors arising in K-ras/p53 knockin mice.50 In these systems, fibroblasts infiltrate into tumors and aid tumor development.13,51–53 Human pulmonary adenocarcinomas are often physically associated with diffuse scarring, but this may be a desmoplastic reaction to the tumor, ie, a fibrotic response to pulmonary damage caused by the tumor rather than the fibroblasts being instrumental in tumor development.12 Because fibroblasts play such an important role in other cancers,51 we interpret our fibroblast data cautiously. Unlike neutrophils, lymphocytes, macrophages, and endothelial cells (Table 1), the number of pulmonary fibroblasts does not dramatically change during the development of urethane-induced lung tumors in mice. We did not assess, however, their phenotype other than to note their deposition of collagen and other structural components, and they may express different gene products than fibroblasts in control lungs.
The numbers of endothelial cells and neutrophils within the tumor parenchyma increased as malignancy progressed (Table 1). Endothelial cells are present early during tumor development when microscopic lesions appear 6 weeks after urethane administration. The importance of angiogenesis in nourishing lung tumor growth in mice was recently demonstrated by a study in which the anti-angiogenic activity of the dietary flavanone, silibinin, seemed to inhibit tumor growth.38
Neutrophils were the only innate immune cell that extravasated across tumor-associated vessels into the tumor; macrophages and lymphocytes remained outside of the tumor parenchyma. Different concentrations of cell type-specific adhesion molecules in tumor vasculature may have influenced this differential communication between endothelial cells and discrete leukocyte populations. Neutrophils appeared in the alveoli as soon as early lesions were detected and throughout malignant progression. Neutrophils were also found just within the border of macroscopically visible tumors but not more centrally. This continuous presence of neutrophils inside tumors was surprising because of the short tissue half-life of this granulocyte. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) are expressed by some human pulmonary adenocarcinomas, and these cytokines prolong alveolar neutrophil survival.54 With neutrophils on the inside edge of the tumors while peritumoral macrophages are just adjacent to the tumor border, interactions of these leukocytes with tumor cells at the tumor periphery may help direct the epithelial-to-mesenchymal transition (EMT) that precedes invasion. Neutrophils and TAMs communicate with tumor cells by paracrine signaling to facilitate angiogenesis, extracellular matrix breakdown, and metastasis.55–57 Neutrophils release vascular endothelial growth factor to promote release of G-CSF from tumor cells, which stimulates angiogenesis.57 Neutrophils were the major leukocyte infiltrate found when the fibrosarcoma MN-11 cell line was grown subcutaneously in C57BL/6 mice.58 However, the only report we found in the literature of neutrophils infiltrating into lung tumors was in the genetically driven mutant Kras codon 12 model of lung cancer.59 Urethane, in contrast induces codon 61 Kras mutations.60
Lymphocyte and macrophage populations increased during neoplastic progression, but neither cell type penetrated within the tumor parenchyma (Table 1). Pulmonary lymphocytes are present in naïve mice and throughout tumor development as small aggregates near the tumor and in large groups in BALT, similar to the cuffs of lymphocytes previously observed.29 Mouse lung tumors with a papillary growth pattern are more likely to display lymphocyte infiltrates than tumors with a solid morphology,39 and the number of pulmonary lymphocytes increased as tumors became malignant. Tumor-infiltrating regulatory T cells have been found in non-small cell lung cancer patients, and many of the lymphocytes associated with bronchoalveolar carcinomas were B cells.61 Elucidation of the specific subpopulations of lymphocytes in the mouse model and how lymphocyte composition changes during progression would be informative. Adaptive immunity protects against the growth of chemically induced lung tumors in mice. Tumor extracts protect naïve mice against lung tumor growth,62 and disarming adaptive immune responsiveness by depleting lymphocytes increases lung tumorigenesis.63 Both the major histocompatibility locus required for antigen presentation64 and other loci that regulate the activity of antigen-presenting cells65 regulate lung tumor susceptibility. Consistent with this requirement for antigen processing to defend against lung tumorigenesis, the TAP-1 gene is deleted in a cell line derived from a mouse lung tumor, interfering with the transport of antigens to the cell surface.66 A yeast-based vaccine against mutant Kras, the mutation that initiates mouse lung tumorigenesis,60 activates dendritic cells to cause regression of those lung tumors.67
Pulmonary macrophages from naïve mice express arginase and iNOS, probably in response to local activation, because BDMCs from these mice expressed neither arginase I nor iNOS. When microscopic lesions appeared, lung macrophages lost their M1 (iNOS-expressing) phenotype and begin to immunostain exclusively for arginase I. This M2 phenotype continues until tumors become malignant, at which time M1 macrophages are observed. Macrophage activation state determines which cytokines these cells secrete and may regulate tumor development,68,69 for example, by influencing the Th1 and Th2 lymphocyte response. M1 macrophages cause lymphocytes to increase IFN-γ production, whereas M2 macrophages induce lymphocytic production of TGF-β.21 High numbers of pulmonary TAMs in the stroma are associated with poor prognosis in lung cancer patients.15,70 Gene expression profiling of M1 and M2 macrophages show many distinct differences in membrane receptors, cytokines, and effector molecules. M1 macrophages express TLR2, TRL4, CD16, CED32, CD62, CD80, CD86, IL12, IL23, TNF-α, IL-1, IL-6, IL1 R1, and NO, whereas M2 macrophages express Scavenger receptors A and B, CD163, mannose receptor, CD14, CD23, IL-10, IL-1rα, and polyamines.17 M1 macrophages act as potent proinflammatory cells that kill microorganisms and tumor cells, whereas M2 macrophages temper the Th2 inflammatory response and promote angiogenesis and tissue remodeling.17,69 This temporal association of macrophage state with particular stages of tumor development probably reflects the fact that M1 and M2 macrophages differentially influence different neoplastic behaviors, eg, growth versus invasiveness.
By examining the activation state of BDMCs, we deduced that tumor cells influenced immature inflammatory cells before recruitment to the tumor site. These tumor-induced qualitative changes in the bone marrow during cancer progression, as opposed to altered rates of hematopoietic cell production are novel findings. Naïve BDMCs express neither arginase I nor iNOS. By the time that microadenomas are visible in the lungs, BDMCs have begun to synthesize arginase I but not iNOS, indicating an M2 phenotype. BDMCs retain M2 activation through the benign lung tumor stage, but when primary adenocarcinomas form, the BDMCs no longer express arginase I but now express iNOS. These temporal switches in BDMC activation mirror those in the TAM population. Because M1 and M2 phenotypes have been induced in the MHS-mouse alveolar macrophage cell line with IFN-γ and IL-4/IL-13, respectively,71 we hypothesize that these cytokines may be what tumors secrete to alter BDMC phenotype. Tumor activation of BDMCs before their maturation and recruitment to the lungs may present an opportunity for novel therapeutic screening and intervention if similar behaviors occur during human neoplasia. Once BDMCs leave the bone marrow to circulate as peripheral blood mononuclear cells, they could be isolated and assayed for their M1 or M2 phenotype in patients at high risk of developing lung cancer. Alternatively, these activation state changes in peripheral blood mononuclear cells could be used as an intermediate end point to monitor therapeutic efficacy.
Acknowledgments
We thank Drs. Lori Dwyer-Nield and Laura K. Zerbe for helpful discussions and critical reading of the manuscript, Paul O’Donnell for technical assistance, and Jason M. Fritz for participating in the blinded counting of cells.
Footnotes
Address reprint requests to Alvin M. Malkinson, University of Colorado at Denver and Health Sciences Center, Department of Pharmaceutical Sciences, Box C238, East Ninth Ave., Denver, CO 80262. E-mail: al.malkinson@uchsc.edu.
Supported by National Institutes of Health grants CA33497 and CA96133.
References
- Stoker MG, Shearer M, O’Neill C. Growth inhibition of polyoma-transformed cells by contact with static normal fibroblasts. J Cell Sci. 1966;1:297–310. doi: 10.1242/jcs.1.3.297. [DOI] [PubMed] [Google Scholar]
- Resnicoff M, Coppola D, Sell C, Rubin R, Ferrone S, Baserga R. Growth inhibition of human melanoma cells in nude mice by antisense strategies to the type 1 insulin-like growth factor receptor. Cancer Res. 1994;54:4848–4850. [PubMed] [Google Scholar]
- Quarrie LH, Pitts JD, Finbow ME. Interactions between normal mammary epithelial cells and mammary tumour cells in a model system. Cell Prolif. 1999;32:351–361. doi: 10.1111/j.1365-2184.1999.tb01353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkinson AM. Role of inflammation in mouse lung tumorigenesis: a review. Exp Lung Res. 2005;31:57–82. doi: 10.1080/01902140490495020. [DOI] [PubMed] [Google Scholar]
- Zhu P, Baek SH, Bourk EM, Ohgi KA, Garcia-Bassets I, Sanjo H, Akira S, Kotol PF, Glass CK, Rosenfeld MG, Rose DW. Macrophage/cancer cell interactions mediate hormone resistance by a nuclear receptor derepression pathway. Cell. 2006;124:615–629. doi: 10.1016/j.cell.2005.12.032. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- Soriano AF, Helfrich B, Chan DC, Heasley LE, Bunn PA, Jr, Chou TC. Synergistic effects of new chemopreventive agents and conventional cytotoxic agents against human lung cancer cell lines. Cancer Res. 1999;59:6178–6184. [PubMed] [Google Scholar]
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- Ballaz S, Mulshine JL. The potential contributions of chronic inflammation to lung carcinogenesis. Clin Lung Cancer. 2003;5:46–62. doi: 10.3816/CLC.2003.n.021. [DOI] [PubMed] [Google Scholar]
- Hammar SP. Common neoplasms. Dail DH, Hammar SP, Colby TV, editors. New York: Springer-Verlag,; Pulmonary Pathology Tumors. 1995:pp 1–156. [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, Schioppa T, Saccani A, Allavena P, Sica A. Infiltration of tumours by macrophages and dendritic cells: tumour-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Novartis Found Symp. 2004;256:137–145. [PubMed] [Google Scholar]
- Johnson SK, Kerr KM, Chapman AD, Kennedy MM, King G, Cockburn JS, Jeffrey RR. Immune cell infiltrates and prognosis in primary carcinoma of the lung. Lung Cancer. 2000;27:27–35. doi: 10.1016/s0169-5002(99)00095-1. [DOI] [PubMed] [Google Scholar]
- Ma J, Chen T, Mandelin J, Ceponis A, Miller NE, Hukkanen M, Ma GF, Konttinen YT. Regulation of macrophage activation. Cell Mol Life Sci. 2003;60:2334–2346. doi: 10.1007/s00018-003-3020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- Mantovani A. Cancer: inflammation by remote control. Nature. 2005;435:752–753. doi: 10.1038/435752a. [DOI] [PubMed] [Google Scholar]
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- Morris SM, Jr, Kepka-Lenhart D, Chen LC. Differential regulation of arginases and inducible nitric oxide synthase in murine macrophage cells. Am J Physiol. 1998;275:E740–E747. doi: 10.1152/ajpendo.1998.275.5.E740. [DOI] [PubMed] [Google Scholar]
- Kepka-Lenhart D, Mistry SK, Wu G, Morris SM., Jr Arginase I: a limiting factor for nitric oxide and polyamine synthesis by activated macrophages? Am J Physiol. 2000;279:R2237–R2242. doi: 10.1152/ajpregu.2000.279.6.R2237. [DOI] [PubMed] [Google Scholar]
- Li H, Meininger CJ, Hawker JR, Jr, Haynes TE, Kepka-Lenhart D, Mistry SK, Morris SM, Jr, Wu G. Regulatory role of arginase I and II in nitric oxide, polyamine, and proline syntheses in endothelial cells. Am J Physiol. 2001;280:E75–E82. doi: 10.1152/ajpendo.2001.280.1.E75. [DOI] [PubMed] [Google Scholar]
- Mills CD, Shearer J, Evans R, Caldwell MD. Macrophage arginine metabolism and the inhibition or stimulation of cancer. J Immunol. 1992;149:2709–2714. [PubMed] [Google Scholar]
- Hagemann T, Wilson J, Burke F, Kulbe H, Li NF, Pluddemann A, Charles K, Gordon S, Balkwill FR. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J Immunol. 2006;176:5023–5032. doi: 10.4049/jimmunol.176.8.5023. [DOI] [PubMed] [Google Scholar]
- Malkinson AM. Primary lung tumors in mice as an aid for understanding, preventing, and treating human adenocarcinoma of the lung. Lung Cancer. 2001;32:265–279. doi: 10.1016/s0169-5002(00)00232-4. [DOI] [PubMed] [Google Scholar]
- Shimkin MB, Polissar MJ. Some quantitative observations on the induction and growth of primary pulmonary tumors in strain A mice receiving urethan. J Natl Cancer Inst. 1955;16:75–97. [PubMed] [Google Scholar]
- Shimkin MB, Sasaki T, McDonough M, Baserga R, Thatcher D, Wieder R. Relation of thymidine index to pulmonary tumor response in mice receiving urethan and other carcinogens. Cancer Res. 1969;29:994–998. [PubMed] [Google Scholar]
- Thaete LG, Beer DG, Malkinson AM. Genetic variation in the proliferation of murine pulmonary type II cells: basal rates and alterations following urethan treatment. Cancer Res. 1986;46:5335–5338. [PubMed] [Google Scholar]
- O’Donnell EP, Zerbe LK, Dwyer-Nield LD, Kisley LR, Malkinson AM. Quantitative analysis of early chemically-induced pulmonary lesions in mice of varying susceptibilities to lung tumorigenesis. Cancer Lett. 2006;241:197–202. doi: 10.1016/j.canlet.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Swensen SJ, Jett JR, Sloan JA, Midthun DE, Hartman TE, Sykes AM, Aughenbaugh GL, Zink FE, Hillman SL, Noetzel GR, Marks RS, Clayton AC, Pairolero PC. Screening for lung cancer with low-dose spiral computed tomography. Am J Respir Crit Care Med. 2002;165:508–513. doi: 10.1164/ajrccm.165.4.2107006. [DOI] [PubMed] [Google Scholar]
- Malkinson AM, Beer DS. Major effect on susceptibility to urethane-induced pulmonary adenoma by a single gene in BALB/cBy mice. J Natl Cancer Inst. 1983;70:931–936. [PubMed] [Google Scholar]
- Bauer AK, Dwyer-Nield LD, Hankin JA, Murphy RC, Malkinson AM. The lung tumor promoter, butylated hydroxytoluene (BHT), causes chronic inflammation in promotion-sensitive BALB/cByJ mice but not in promotion-resistant CXB4 mice. Toxicology. 2001;169:1–15. doi: 10.1016/s0300-483x(01)00475-9. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosenbough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Hogg N, MacDonald S, Slusarenko M, Beverley PC. Monoclonal antibodies specific for human monocytes, granulocytes and endothelium. Immunology. 1984;53:753–767. [PMC free article] [PubMed] [Google Scholar]
- Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- Singh RP, Deep G, Chittezhath M, Kaur M, Dwyer-Nield LD, Malkinson AM, Agarwal R. Effect of silibinin on the growth and progression of primary lung tumors in mice. J Natl Cancer Inst. 2006;98:846–855. doi: 10.1093/jnci/djj231. [DOI] [PubMed] [Google Scholar]
- Beer DG, Malkinson AM. Genetic influence on type 2 or Clara cell origin of pulmonary adenomas in urethan-treated mice. J Natl Cancer Inst. 1985;75:963–969. doi: 10.1093/jnci/75.5.963. [DOI] [PubMed] [Google Scholar]
- Richeldi L, Franchi A, Rovatti E. Lymphocytes. Crystal RG, West JB, Barnes PJ, Weible ER, editors. Philadelphia: Lippincott,; The Lung, Scientific Foundations. 1997:pp 803–820. [Google Scholar]
- Kisley LR, Barrett BS, Bauer AK, Dwyer-Nield LD, Barthel B, Meyer AM, Thompson DC, Malkinson AM. Genetic ablation of inducible nitric oxide synthase decreases mouse lung tumorigenesis. Cancer Res. 2002;62:6850–6856. [PubMed] [Google Scholar]
- Galli SJ. New concepts about the mast cell. N Engl J Med. 1993;328:257–265. doi: 10.1056/NEJM199301283280408. [DOI] [PubMed] [Google Scholar]
- Wedemeyer J, Tsai M, Galli SJ. Roles of mast cells and basophils in innate and acquired immunity. Curr Opin Immunol. 2000;12:624–631. doi: 10.1016/s0952-7915(00)00154-0. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shijubo N, Kojima H, Nagata M, Ohchi T, Suzuki A, Abe S, Sato N. Tumor angiogenesis of non-small cell lung cancer. Microsc Res Tech. 2003;60:186–198. doi: 10.1002/jemt.10257. [DOI] [PubMed] [Google Scholar]
- Kankkunen JP, Harvima IT, Naukkarinen A. Quantitative analysis of tryptase and chymase containing mast cells in benign and malignant breast lesions. Int J Cancer. 1997;72:385–388. doi: 10.1002/(sici)1097-0215(19970729)72:3<385::aid-ijc1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Lachter J, Stein M, Lichtig C, Eidelman S, Munichor M. Mast cells in colorectal neoplasias and premalignant disorders. Dis Colon Rectum. 1995;38:290–293. doi: 10.1007/BF02055605. [DOI] [PubMed] [Google Scholar]
- Imada A, Shijubo N, Kojima H, Abe S. Mast cells correlate with angiogenesis and poor outcome in stage I lung adenocarcinoma. Eur Respir J. 2000;15:1087–1093. doi: 10.1034/j.1399-3003.2000.01517.x. [DOI] [PubMed] [Google Scholar]
- Benítez-Bribiesca L, Wong A, Utrera D, Castellanos E. The role of mast cell tryptase in neoangiogenesis of premalignant and malignant lesions of the uterine cervix. J Histochem Cytochem. 2001;49:1061–1062. doi: 10.1177/002215540104900816. [DOI] [PubMed] [Google Scholar]
- Jackson EL, Olive KP, Tuveson DA, Bronson R, Crowley D, Brown M, Jacks T. The differential effects of mutant p53 alleles on advanced murine lung cancer. Cancer Res. 2005;65:10280–10288. doi: 10.1158/0008-5472.CAN-05-2193. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyden B. The myofibroblast: a study of normal, reactive and neoplastic tissues, with an emphasis on ultrastructure. Part 1—normal and reactive cells. J Submicrosc Cytol Pathol. 2005;37:109–204. [PubMed] [Google Scholar]
- Wislez M, Fleury-Feith J, Rabbe N, Moreau J, Cesari D, Milleron B, Mayaud C, Antoine M, Soler P, Cadranel J. Tumor-derived granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor prolong the survival of neutrophils infiltrating bronchoalveolar subtype pulmonary adenocarcinoma. Am J Pathol. 2001;159:1423–1433. doi: 10.1016/S0002-9440(10)62529-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin EY, Pollard JW. Role of infiltrated leucocytes in tumour growth and spread. Br J Cancer. 2004;90:2053–2058. doi: 10.1038/sj.bjc.6601705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, Graf T, Pollard JW, Segall J, Condeelis J. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- Ohki Y, Heissig B, Sato Y, Akiyama H, Zhu Z, Hicklin DJ, Shimada K, Ogawa H, Daida H, Hattori K, Ohsaka A. Granulocyte colony-stimulating factor promotes neovascularization by releasing vascular endothelial growth factor from neutrophils. FASEB J. 2005;19:2005–2007. doi: 10.1096/fj.04-3496fje. [DOI] [PubMed] [Google Scholar]
- Sandhu JK, Privora HF, Wenckebach G, Birnboim HC. Neutrophils, nitric oxide synthase, and mutations in the mutatect murine tumor model. Am J Pathol. 2000;156:509–518. doi: 10.1016/S0002-9440(10)64755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wislez M, Fujimoto N, Izzo JG, Hanna AE, Cody DD, Langley RR, Tang H, Burdick MD, Sato M, Minna JD, Mao L, Wistuba I, Strieter RM, Kurie JM. High expression of ligands for chemokine receptor CXCR2 in alveolar epithelial neoplasia induced by oncogenic kras. Cancer Res. 2006;66:4198–4207. doi: 10.1158/0008-5472.CAN-05-3842. [DOI] [PubMed] [Google Scholar]
- You M, Candrian U, Maronpot RR, Stoner GD, Anderson MW. Activation of the Ki-ras protooncogene in spontaneously occurring and chemically induced lung tumors of the strain A mouse. Proc Natl Acad Sci USA. 1989;86:3070–3074. doi: 10.1073/pnas.86.9.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axiotis CA, Jennings TA. Observations on bronchiolo-alveolar carcinomas with special emphasis on localized lesions. A clinicopathological, ultrastructural, and immunohistochemical study of 11 cases. Am J Surg Pathol. 1988;12:918–931. doi: 10.1097/00000478-198812000-00003. [DOI] [PubMed] [Google Scholar]
- Prehn RT. Specific isoantigenicities among chemically induced tumors. Ann NY Acad Sci. 1962;101:107–113. [Google Scholar]
- Trainin N, Linker-Israeli M, Small M, Boiato-Chen L. Enhancement of lung adenoma formation by neonatal thymectomy in mice treated with 7,12-dimethylbenz(a) anthracene or urethan. Int J Cancer. 1967;2:326–336. doi: 10.1002/ijc.2910020407. [DOI] [PubMed] [Google Scholar]
- Festing MF, Yang A, Malkinson AM. At least four genes and sex are associated with susceptibility to urethane-induced pulmonary adenomas in mice. Genet Res. 1994;64:99–106. doi: 10.1017/s0016672300032705. [DOI] [PubMed] [Google Scholar]
- Fijneman RJ, Vos M, Berkhof J, Demant P, Kraal G. Genetic analysis of macrophage characteristics as a tool to identify tumor susceptibility genes: mapping of three macrophage-associated risk inflammatory factors, marif1, marif2, and marif3. Cancer Res. 2004;64:3458–3464. doi: 10.1158/0008-5472.CAN-03-3767. [DOI] [PubMed] [Google Scholar]
- Gabathuler R, Reid G, Kolaitis G, Driscoll J, Jefferies WA. Comparison of cell lines deficient in antigen presentation reveals a functional role for TAP-1 alone in antigen processing. J Exp Med. 1994;180:1415–1425. doi: 10.1084/jem.180.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Bellgrau D, Dwyer-Nield LD, Malkinson AM, Duke RC, Rodell TC, Franzusoff A. Mutation-selective tumor remission with Ras-targeted, whole yeast-based immunotherapy. Cancer Res. 2004;64:5084–5088. doi: 10.1158/0008-5472.CAN-04-1487. [DOI] [PubMed] [Google Scholar]
- Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A. Tumour-associated macrophages as a prototypic type II polarised phagocyte population: role in tumour progression. Eur J Cancer. 2004;40:1660–1667. doi: 10.1016/j.ejca.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Wislez M, Spencer ML, Izzo JG, Juroske DM, Balhara K, Cody DD, Price RE, Hittelman WN, Wistuba II, Kurie JM. Inhibition of mammalian target of rapamycin reverses alveolar epithelial neoplasia induced by oncogenic K-ras. Cancer Res. 2005;65:3226–3235. doi: 10.1158/0008-5472.CAN-04-4420. [DOI] [PubMed] [Google Scholar]
- Redente EF, Malkinson AM. Quantitative changes in stromal composition during mouse lung tumorigenesis. Proc Am Thor Soc. 2005;2:A741. [Google Scholar]