Abstract
We have previously identified the presence of Ras/Raf-independent constitutive activation of extracellular signal-regulated kinase (ERK) in the hairy cells (HCs) of hairy cell leukemia. The aim of the present study was to characterize the signaling components involved in this activation and their relationship to the reported activation of Rac1. We found that both Rac1 and ERK activation in HCs are downstream of active Src and protein kinase C (PKC). Inhibition with toxin B showed that Rac1 plays no role in ERK activation in HCs. However, toxin B inhibited p60src and the Rac1-GEF Vav, demonstrating a positive feedback/activation of p60src by Rac1. Treatment with specific small interfering RNA for various PKC isoforms, or with PKC isoform-specific inhibitors, demonstrated a central role for PKCε in the constitutive activation of Rac1 and ERK in HCs. PKCε and active ERK were mutually associated and co-localized with mitochondria in HCs. Furthermore, active PKCε was nitrated on tyrosine, pointing to a reactive oxygen species-dependent mechanism of activation. By being involved in activation of ERK and Rac1, PKCε plays roles in both the survival of HCs and in the cytoskeletal dynamics responsible for the distinctive morphology and tissue homing of these cells. Our study therefore describes novel aspects of signaling important for the pathogenesis of hairy cell leukemia.
Hairy cell leukemia (HCL) is unique among chronic B-cell leukemias in that the malignant cells show particularly pronounced features of activation.1 For example, the cortical cytoskeleton of the malignant hairy cells (HCs) is clearly in a state of active reorganization as indicated by the distinctive membrane ruffling and microvilli formation that gave the malignant cells their name.2 This cytoskeletal activity has been shown to involve phosphatidylinositol 3-kinase-independent, Src-dependent activation of the Rho GTPase Rac1.3
Recently, we have reported that HCs are characterized by the presence of constitutively active extracellular signal-regulated kinase (ERK) and that this activation does not depend on Ras→Raf interaction but rather on MEK1/2 activation by a still unclear route.4 We also showed that neither p38 MAP kinase nor c-Jun NH2-terminal kinase are constitutively active in HCs. As regards the mode of ERK activation in HCs, we demonstrated that Src and a protein kinase C (PKC) are involved in this process, since inhibitors of these kinases abolished the constitutive ERK phosphorylation. However, how the various signals identified in HCs are related to each other, how they are initiated, and how they are related to the activation of Rho GTPases remains unclear. The aims of the present study were to explore further the signals involved in the activation of both ERK and Rho GTPases, define their mutual relationships, and establish how different signals contribute to maintenance of the activated phenotype of HCs.
Materials and Methods
Materials
Ro32-0432, safingol, Gö6976, PKCα peptide, SU6656, and Clostridium difficile toxin B were from Calbiochem-Novabiochem (Nottingham, UK). PP1 (4-amino-5-[4-methylphenyl]-7-[t-butyl]pyrazolo[3,4-d]pyrimidine) was from Alexis Corporation (Nottingham, UK). Cytochalasin D, polyHEMA (poly[2-hydroxyethylmethacrylate]), myelin basic protein, phenylmethanesulfonyl fluoride (PMSF), leupeptin, cell culture grade bovine serum albumin, N-acetylcysteine, and ATP were from Sigma-Aldrich (Gillingham, UK). Aprotinin was from Bayer (Newbury, UK). Tissue culture media and Lymphoprep were from Gibco (Invitrogen, Paisley, UK). Protein G-Sepharose was from Zymed (Invitrogen). Reagents for protein determination were from Bio-Rad Laboratories (Hemel Hempstead, UK). Polyvinylidene difluoride (PVDF) (Immobilon-P) membrane from Millipore (Hatters Lane, UK), glutathione-Sepharose 4B beads, enhanced chemiluminescence reagent, [γ-32P]ATP (3000 Ci/mmol), and Hyperfilm from GE Health Care UK Ltd. (Chalfont St. Giles, UK) were also used. All other reagents used were of analytical grade. Small interfering RNA (siRNA) for PKCε, PKCδ, and nonspecific siRNA were purchased from either Dharmacon RNA Technologies (Perbio Science UK Ltd., Cramlington, UK) or from Qiagen (Crawley, UK).
Antibodies
Mouse monoclonal antibodies (mAbs) against Cdc42, p95Vav, phospho-ERK1/2, and rabbit polyclonal antibodies against Lyn, PKCε, PKCδ, MEK1/2, ERK1/2, and p60src were from Santa Cruz Biotechnology (Insight Biotechnology Ltd., Wembley, UK). Rabbit polyclonal antibodies against phospho-p60src, phospho-MEK, and phospho-Vav were from New England Biolabs (Hitchin, UK). mAb 327 against p60src was a gift from Dr. S. Feller (Cancer Research UK, Oxford, UK). mAbs against PKC isoforms were from Transduction Laboratories (Cowley, UK) and from Santa Cruz Biotechnology. Anti-β-actin mAb was from Sigma-Aldrich. Monoclonal anti-phosphotyrosine (PY20 mAb), anti-nitrotyrosine, and anti-Rac1 antibodies were from Upstate Biotechnology (Lake Placid, NY).
Patients, Cell Isolation, and Culture
Blood was obtained with informed consent and with the approval of the Liverpool Research Ethics Committee. All patients had typical HCL as determined by clinical presentation, morphology, tartrate-resistant acid phosphatase staining, and immunophenotype.5 B cells were isolated from peripheral blood and cultured as described previously.4 When inhibitors were used, control cells contained the equivalent amounts of cell culture grade dimethylsulfoxide (Sigma-Aldrich), the solvent used to dissolve all inhibitors, except toxin B, which was made up in distilled water. All inhibitors were used at the concentrations used in the reports referenced.
Cell Staining
Cell cytospins were stained with May-Grunwald-Giemsa (Sigma-Aldrich) and analyzed by light microscopy.
Cell Lysates and Western Blotting
Preparation of cell lysates, sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), and Western blotting were carried out as described previously.4 Intensity of the bands was analyzed by densitometry.
Rac1 and Cdc42 Pulldown Assay
Pulldowns of GTP-Rac1 and -Cdc42 from HC lysates were performed as described6,7 using a fusion protein consisting of glutathione S-transferase coupled to the Cdc42-/Rac1-binding domain (amino acids 59 to 272) of p21-activated protein kinase. Proteins bound to this fusion protein were isolated by affinity chromatography on glutathione-Sepharose 4B and analyzed by Western blotting.
Immunoprecipitation
Proteins were immunoprecipitated from HCs lysed with either radioimmunoprecipitation assay (RIPA) buffer [1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 25 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 25 mmol/L sodium pyrophosphate, 50 mmol/L sodium glycerophosphate, 50 mmol/L NaF, 2 mmol/L ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 2 mmol/L ethylenediamine tetraacetic acid (EDTA), 1 mmol/L Na3VO4, 10% glycerol, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 mmol/L PMSF] or NP-40 lysis buffer (1% Nonidet P-40, 20 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 2 mmol/L EDTA, 1 mmol/L Na3VO4, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 mmol/L PMSF) using antibodies that had been preadsorbed to protein G-Sepharose. The immunoprecipitates were washed three times with lysis buffer before analysis by Western blot or for further use in in vitro kinase assays.
In Vitro Kinase Assays
For immunoprecipitated PKCε, the protein G-Sepharose beads were transferred into kinase buffer (25 mmol/L HEPES, pH 7.5, 150 mmol/L NaCl, 25 mmol/L sodium glycerophosphate, 20 mmol/L MgCl2, and 1 mmol/L dithiothreitol). The kinase reaction was initiated with the addition of kinase buffer containing 10 μCi of [γ-32P]ATP and 1 μg of myelin basic protein as exogenous substrate. The kinase reaction was incubated for 30 minutes at 30°C and terminated with the addition of SDS-PAGE buffer. Proteins were separated by SDS-PAGE and transferred to PVDF membranes for analysis by Western blot and autoradiography.
Confocal Microscopy
HCs were prepared for confocal microscopy by centrifugation onto glass slides. The HCs were then fixed with methanol. In some experiments, MitoTracker Red was used to stain mitochondria using the procedure provided by the manufacturer (Cambrex Bio Science Wokingham, Ltd., Wokingham, UK). Primary anti-PKCε and -ERK antibodies were used at 1:10 dilution. Alexa-Fluor-488 and -555 second-layer antibodies (Invitrogen) were used at 1:50 dilution.
siRNA Knockdown of PKCε and δ in CLL Cells
Cell transfection with siRNA reagents was mediated with HiPerFect reagent (Qiagen) according to the manufacturer’s instructions. HCs (2 × 106) were subjected to three “hits” of siRNA specific for PKCδ, PKCε, or with nonspecific control siRNA (100 pmol) using 48-hour incubation time between each cycle of siRNA addition. Throughout this procedure HC viability was assessed by trypan blue exclusion, and in no case did cell viability drop below 90%. HCs were harvested at the end of the third cycle and subjected to SDS-PAGE and Western blot analysis.
Subcellular Fractionation of HCs
HCs were suspended in lysis buffer B (250 mmol/L sucrose, 5 mmol/L EDTA, 5 mmol/L EGTA, 10 mmol/L HEPES, pH 7.4, plus 1 mmol/L phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, and 10 μg/ml pepstatin) on ice and then lysed by passing the cells 15 times through a 25-gauge needle. The lysed cells were initially centrifuged at 750 × g for 10 minutes to sediment nuclei and other cellular debris. The supernatant was taken and centrifuged at 14,000 × g for 30 minutes to sediment the heavy membrane fraction. The supernatant was further centrifuged at 100,000 × g for 1.5 hours to separate the light membranes (pellet) from the cytosol (supernatant). Protein concentrations of each fraction were determined, and equal amounts of protein from each fraction were separated by SDS-PAGE and analyzed by Western blot.
Results
PKC Regulates Constitutive p60src Activity in HCs
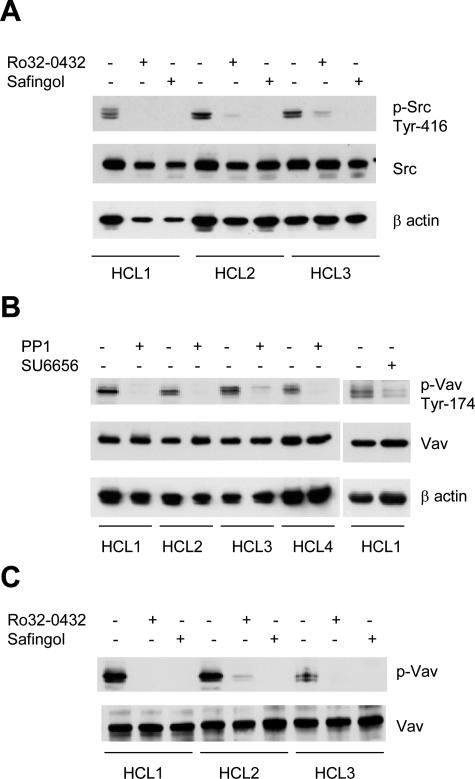
Src is constitutively active in HCs as revealed by phosphorylation at Tyr-416 demonstrated in both whole-cell lysates (Figure 1A) and in immunoprecipitates with p60src-specific mAb 327, an antibody that recognizes the active form of this enzyme8 (not shown). Because PKC activation induces disassembly of actin stress fibers and appearance of membrane ruffles via a Src-dependent pathway in other cells,9 it seemed important to examine whether PKC plays any role in Src activation in HCs. Thus, we treated HCs with Ro32-0432 and safingol, two general PKC inhibitors acting by different mechanisms, and showed that both inhibitors abrogated activation-dependent Src phosphorylation (Figure 1A). Ro32-0432 and safingol also abolished ERK activation,4 indicating a major role of PKC in the regulation of both Src and ERK activities.
Figure 1.
PKC regulates p60src activity in HCs; active p60src and PKC are required for tyrosine phosphorylation of Vav. Cells (107/ml) were incubated for 60 minutes with dimethylsulfoxide (control) or with 10 μmol/L Ro32-0432, safingol, PP1, or SU6656. A shows p60src in lysates of 106 cells Western blotted for activation-dependent Tyr-416 phosphorylation (top panel). The center and bottom panels show levels of p60src protein and β-actin as loading controls. In B, p60src was inhibited by treatment of HCs with PP1 or SU6656 and in C, by safingol or Ro32-0432. Then proteins in cell lysates (106 cells) were subjected to SDS-PAGE and immunoblotted with anti-phospho-Vav antibody (Tyr-174). Total Vav and β-actin were used as loading controls.
In HCs, Src and PKC Maintain Persistent Activation of Rac1
Because the Src substrate Vav is one of the guanine nucleotide exchange factors for Rac,10 we examined whether Vav and Rac are included among signals downstream of PKC and Src. Figure 1B shows that Vav in HCs is constitutively phosphorylated at Tyr-174, the site crucial for Rac activation by Vav,11 and that this phosphorylation was abolished in cells treated with the Src family kinase inhibitors PP1 and SU6656 (Figure 1B), as well as with PKC inhibitors either Ro32-0432 or safingol (Figure 1C). HC treatment with Gö6976, an inhibitor specific for classical PKCs, had no effect on either in vitro PKC kinase activity or Vav phosphorylation (not shown). Therefore, either a novel or an atypical PKC upstream of p60src regulates the activation of Vav in HCs.
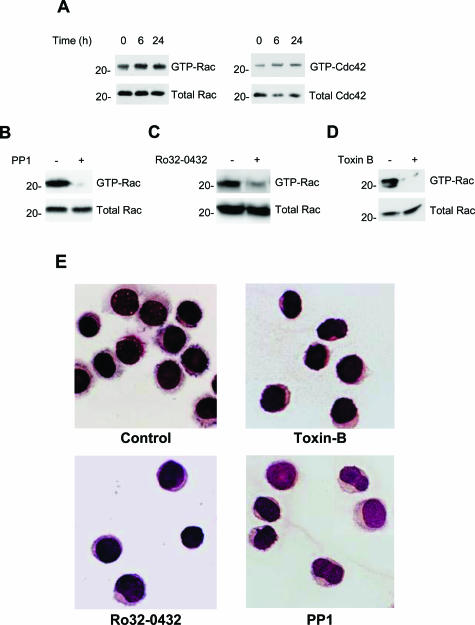
We then confirmed that both Rac1 and Cdc42 are activated in HCs.3 This activation did not change in cells cultured for 24 hours on a nonadherent surface in the absence of serum (Figure 2A), indicating that it is truly constitutive. Furthermore, both PP1 and Ro32-0432 abolished GTP loading of Rac1 (Figure 2, B and C), confirming that Src and PKC are indeed responsible for the activation of Rac1 in HCs.3 As expected, the Rho family GTPase inhibitor toxin B abolished the constitutive activation of Rac1 (Figure 2D).
Figure 2.
Rac1 and Cdc42 are constitutively active and control the morphological appearance of HCs. A–D: HCs (107 cells/ml) were cultured on polyHEMA-coated plates in the absence of serum and lysed at the indicated times. A: Proteins isolated by Rac1-pulldown were immunoblotted with anti-Rac1 or anti-Cdc42 antibodies and compared with the total Rac1 or Cdc42 contained in cell lysates. B: Rac1 pulldown of HCs treated with 10 μmol/L PP1 for 1 hour. C: Rac1 pulldown of HCs treated with 10 μmol/L Ro32-0432 for 1 hour. D: Rac1 pulldown of HCs treated with 20 ng/ml toxin B for 3 hours. In B–D, similar results were obtained using cells of another HCL case. E: Cytospins of control HCs or HCs treated for 3 hours with 20 ng/ml toxin B or for 1 hour with 10 μmol/L Ro32-0432 or with 10 μmol/L PP1. HCs were stained with May-Grunwald-Giemsa and examined with light microscopy.
The importance of PKC, Src, and Rac in maintaining HC phenotype was examined next. Figure 2E shows that membrane ruffles and microvilli were lost and cells became more rounded with a higher nuclear cytoplasmic ratio when HCs were treated with Ro32-0432, PP1, or toxin B.
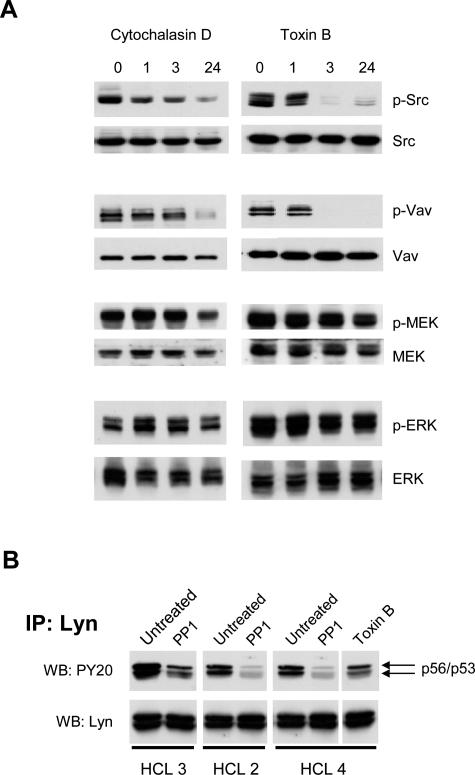
Src activation is accompanied by its translocation from the cytosol to the cell periphery, a process known to be actin dependent12 and potentially important for Src association with particular substrates. We therefore examined the importance of cytoskeletal integrity for Src-induced activation of Vav and Rac1. The incubation of HCs with the F-actin-disrupting agent cytochalasin D for up to 24 hours reduced the phosphorylation of Src and Vav but had a less pronounced effect on MEK or ERK phosphorylation (Figure 3A, left panels).
Figure 3.
The role of cytoskeleton in constitutive signaling in HCs. A: HCs were treated with 10 μmol/L cytochalasin D (left panels) or 20 ng/ml toxin B (right panels) for 1, 3, and 24 hours. These treatments did not affect HC viability. Phosphorylated Src, Vav, MEK1/2, and ERK1/2 were detected in cell lysates (106 cells) using antibodies against activated Src (Tyr-416), Vav (Tyr-174), MEK (Ser-217/221), and ERK (Tyr-204). Similar results were obtained with cells from two other HCL cases. B: Lyn was immunoprecipitated from HC lysates and the immunoprecipitated protein was Western blotted for phosphotyrosine and Lyn.
Collectively, these results indicate that Vav/Rac1 regulation depends on an active Src kinase and that cytoskeletal integrity is required for this regulation. We next used toxin B to inhibit Rac1 to establish whether the Rac→PAK→MEK→ERK pathway described in other cells13,14 is operative in HCs.
Inhibition of Rac1 Does Not Affect the MEK→ERK Pathway in HCs
The inhibition of Rac1 by toxin B had no effect on MEK or ERK phosphorylation (Figure 3A, right panels) despite pronounced inhibition of the phosphorylation of p60src and Vav. This shows that ERK activation in HCs does not involve the Rac→PAK→MEK pathway and depends on a PP1-sensitive Src family kinase4 other than p60src, most likely p56lyn (Figure 3B). Because we have already established that PKC is involved in the activation of ERK in HCs (this study and Ref. 4), we next used specific inhibitors to define which isoform(s) are involved and the pathway by which ERK is activated downstream of PKC.
PKCε Is Involved in the Constitutive Activation of ERK in HCs
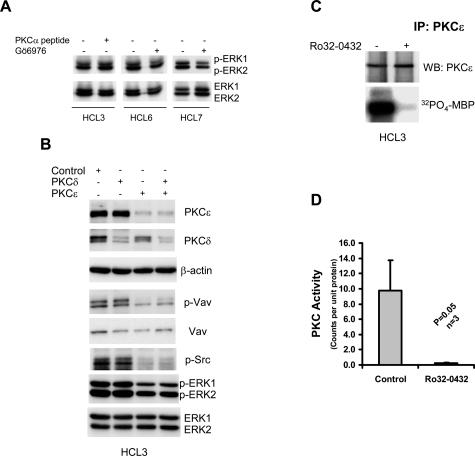
Neither chelation of intracellular Ca2+ (not shown) nor inhibition of classical PKCs by treating HCs with Gö6976 or PKCα inhibitor peptide (Figure 4A) affected constitutive activity of ERK. This indicates that classical PKC isoforms (α and β), despite being activated,4,15 are not involved in the constitutive activation of ERK in HCs.
Figure 4.
The constitutive activation of ERK in HCs involves PKCε. A: Cell lysates were analyzed for the presence of phosphorylated ERK in HCs treated with 10 μmol/L PKCα-blocking peptide or with 100 nmol/L Gö6976 for 1 hour. This figure shows the results of one of three independent experiments using the PKCα-blocking peptide and two of five independent experiments using Gö6976. B: Lysates from HCs treated with control siRNA or with siRNA specific for PKCε and PKCδ were analyzed for the presence of phosphorylated Src, Vav, and ERK. Data in this figure represent one of three independent experiments with similar results. C: Immune complex kinase assay of PKCε immunoprecipitated from HC lysates performed in the presence or absence of Ro32-0432. D: A summary of the results presented in C, representing the data generated using three different HC cases. Statistical analysis was performed using a Student’s t-test.
Among the novel PKC isoforms, PKCδ and ε have been implicated in ERK activation in other cell types.16–20 We used siRNAs to knock down the expression of these two isoforms. Figure 4B shows that the treatment of HCs with siRNAs against PKCδ and ε reduced their respective protein levels by around 80%. Knockdown of PKCε reduced phosphorylation of ERK, Vav, and Src (Figure 4B), whereas knockdown of PKCδ had no effect on the phosphorylation of these proteins.
In our initial studies (not shown), ERK activation was also inhibited by rottlerin, an agent often used as a specific PKCδ inhibitor. However, the mechanism of inhibition was found to be an indirect consequence of the uncoupling of mitochondrial respiration from oxidative phosphorylation.21 This, together with the absence of an effect of PKCδ knockdown on ERK phosphorylation, makes it highly unlikely that PKCδ is involved in constitutive ERK activation in HCs.
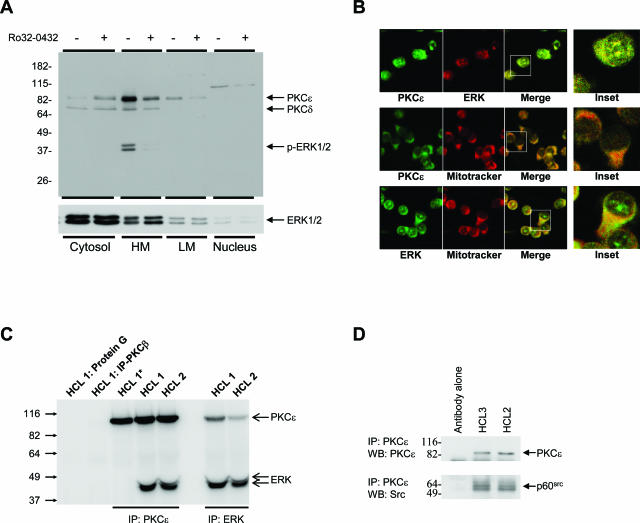
We next confirmed that PKCε is active in HCs. Figure 4, C and D, shows that significant kinase activity is immunoprecipitated from lysates of HCs using anti-PKCε antibodies. Furthermore, Figure 4, C and D, also shows that Ro32-0432 almost completely inhibited this immunoprecipitated PKCε activity. Similar results were observed in experiments using a second mAb that targeted a different epitope of PKCε (data not shown). Furthermore, Western blot analysis of HC cytosolic, heavy membrane, and light membrane subcellular fractions show that PKCε is primarily localized in heavy membrane fractions and not in HC cytosol (Figure 5A). Because membrane translocation is a paradigm of PKC activation,22,23 this further supports our observation that PKCε is active in HCs. Taken together, the above data demonstrate a role for active PKCε in the constitutive activation of ERK in HCs.
Figure 5.
PKCε co-localizes with ERK and p60src. A: HCs were fractionated into cytosolic, light membrane, heavy membrane (containing the mitochondria), and nuclear cell compartments by differential centrifugation. Equal protein amounts (10 μg) were separated by SDS-PAGE, and Western blots were probed with the indicated antibodies. Data in this figure represent one of three independent experiments with similar results. B: Confocal microscopy showing co-localization of PKCε and ERK and their association with mitochondria in HCs. Insets are magnifications of representative cells in the merged images. These images are representative of the malignant cells from two cases of HCL. C: Lysates of cells from two cases of HCL were immunoprecipitated with the indicated antibodies or with protein G-Sepharose and analyzed by Western blot for the presence of PKCε and ERK. The immune complexes in lane HCL 1* were washed with RIPA buffer before gel loading to disrupt the molecular association between PKCε and ERK. D: PKCε was isolated from cell lysates from two HC cases by immunoprecipitation and analyzed by Western blot for PKCε and p60src. C and D are representative of at least two experiments using cells from different HCL cases.
PKCε Co-Associates with and Activates ERK at the Mitochondrial Membrane
In other cell types, the cytoprotective effect of PKCε is mediated by its co-localization at the mitochondrial membrane with elements of the MAP kinase cascade.24,25 We investigated PKCε localization in HCs by subcellular fractionation. Figure 5A shows that PKCε was concentrated in the heavy membrane fraction, known to contain mitochondria. Moreover, ERK in the heavy membrane fraction was phosphorylated, although nonphosphorylated ERK was present in all fractions (Figure 5A). Inhibition of PKCε with Ro32-0432 abolished ERK phosphorylation in the heavy membrane fraction without affecting its localization there. These results strongly suggest that PKCε specifically co-associates with and activates ERK at the mitochondrial membrane. Indeed, confocal microscopy of HCs shows that PKCε and ERK are co-localized and are both associated with mitochondria (Figure 5B). Furthermore, ERK was detected in the immunoprecipitates of PKCε from HC lysates, and PKCε was detected in the immunoprecipitates of ERK from HC lysates (Figure 5C).
PKCε Is Nitrated on Tyrosine
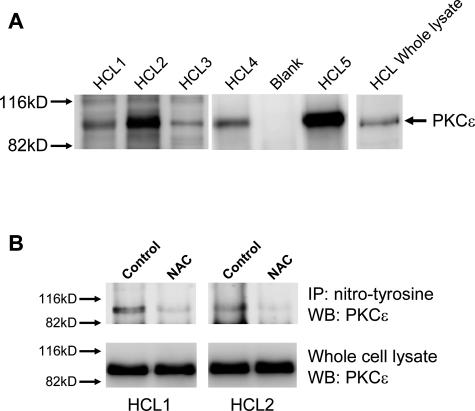
PKCε belongs to the novel family of PKC proteins and as such is activated by the presence of diacylglycerol.23 However, other factors such as NO-induced nitration of tyrosine are also known to activate this PKC.26,27 Protein nitration in cells is caused by high levels of NO and reactive oxygen species (ROS).28 Peroxynitrite (ONOO−) is a well-characterized nitrating species formed when NO reacts with superoxide anion,26 known to be produced during electron transport in the mitochondrial respiratory chain and, in HCs, also by the plasma membrane-associated NADPH oxidase NOX5.7 Because HCs express high levels of catalytically active inducible nitric-oxide synthase (iNOS),29 and because they also contain high amounts of NADPH oxidase-generated ROS,7 we investigated PKCε nitration in HCs. Experiments sequentially using specific antibodies against nitro-tyrosine for immunoprecipitation and against PKCε for Western blotting clearly showed that PKCε in HCs is nitrated on tyrosine (Figure 6). Furthermore, when HCs were treated with N-acetylcysteine (NAC) to reduce intracellular ROS, we found that the amount of PKCε that could be immunoprecipitated with anti-nitrotyrosine mAbs was clearly reduced (Figure 6B). These results suggest that PKCε is activated through a combination of NO generation and ROS production.
Figure 6.
PKCε is nitrated on tyrosine in HCs. HC lysates were immunoprecipitated with anti-nitrotyrosine antibodies, and Western blots were probed with anti-PKCε antibodies. A: Western blots of immunoprecipitated proteins from lysates of malignant cells from five cases of HCL. A whole-cell lysate was also run together with the immunoprecipitates to show equal position of anti-PKCε-reactive bands in both samples. B: Western blots of immunoprecipitated proteins from lysates of HCs incubated overnight in the presence or absence of 25 mmol/L NAC. Cell viability was similar in treated and untreated cells.
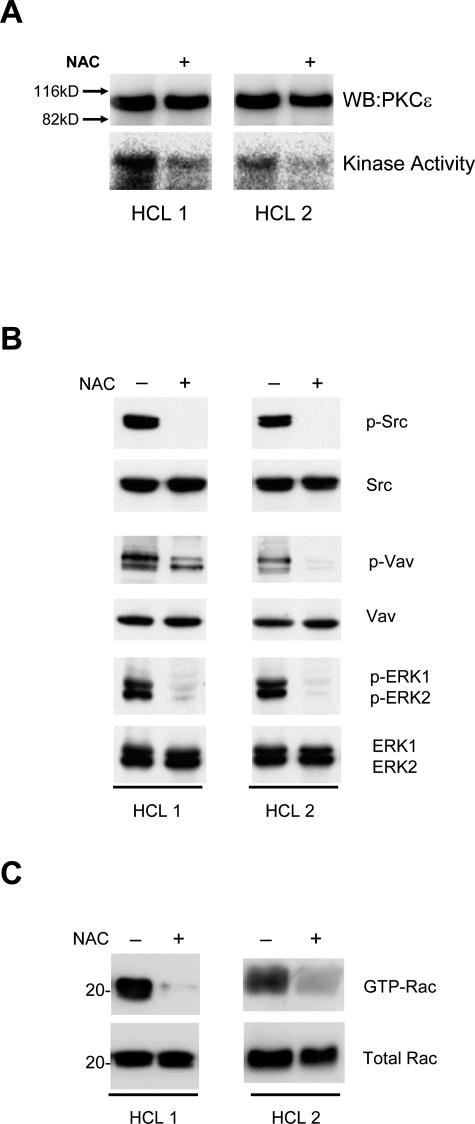
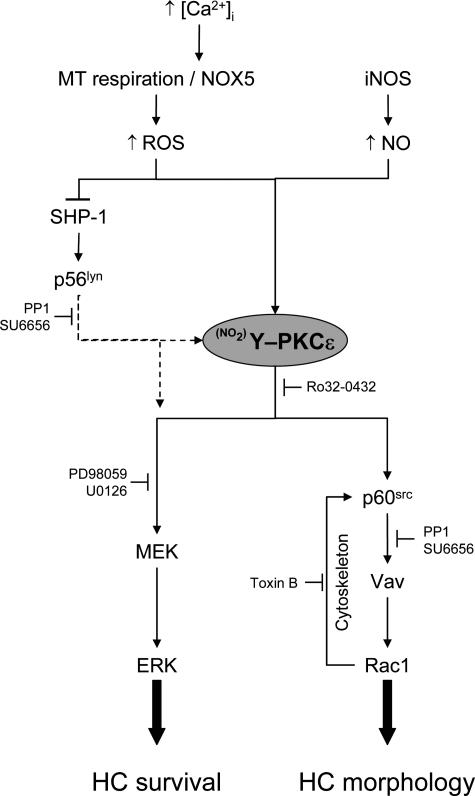
We next investigated the role of ROS in PKCε activation. Figure 7A shows that PKCε activity was inhibited when HCs were treated with NAC. Moreover, signals downstream of PKCε, including phosphorylation of Src, Vav, and ERK, and GTP loading of Rac1, were also inhibited (Figure 7, B and C). Taken together, these results demonstrate that ROS are involved in PKCε activation in HCs. Our findings, summarized in Figure 8, suggest a model where PKCε plays a central role downstream of ROS in the two partially independent signaling pathways responsible for the constitutive activation of HCs.
Figure 7.
NAC treatment inhibits PKCε and PKCε-dependent downstream signals in HCs. HCs from two HCL cases were incubated in the presence or absence of 25 mmol/L NAC for 1 hour. A: PKCε immunoprecipitated from these lysates was subjected to in vitro kinase assay. B: Whole-cell lysates of these HCs were also analyzed by Western blot for the presence of phosphorylated Src, Vav, and ERK. C: Rac1 pulldown from HC lysates from these two cases. The isolated proteins were immunoblotted with anti-Rac1 antibodies and compared with the total Rac1 in the whole-cell lysates. The figure shows that NAC inhibited PKCε activity as well as those of Src, Vav, ERK, and Rac1.
Figure 8.
Proposed pathways of constitutive signaling in hairy cells. This figure shows the schematic representation of the possible associations into two parallel pathways of active signaling components detected in HCs. PKCε plays a central role, controlling the activation of p60src, Rac1, and ERK. PKCε is itself activated through nitration of tyrosine residues by the presence of ROS and NO. ROS contribute to constitutive HC activation through potentiation of protein tyrosine kinase signaling by inhibiting the tyrosine phosphatase SHP-1.
Discussion
Here, we describe the signaling components involved in the previously reported PKC- and Src-dependent constitutive activation of ERK and Rac1 in HCs.3,4 We demonstrate that this constitutive activation of ERK and Rac1 involves two partially independent pathways that are stimulated by active PKCε.
ERK activation in malignant cells has been described previously and can originate from different sources. In acute myeloid leukemia,30 this was attributed to a combination of ERK overexpression and down-regulation of a dual-specificity phosphatase, PAC1.31 ERK overexpression was also observed in human breast cancer,32 whereas in melanoma, activation of ERK was attributed to Raf mutations and excessive cell stimulation by autocrine growth factors.33 Site-directed mutagenesis34 suggests that ERK mutation is another possible cause of its constitutive activation.
We have previously demonstrated constitutive activation of ERK in HCs4 that could not be attributed to any of the above mechanisms. Thus, ERK was not overexpressed, Raf kinase activity was either absent or low, and Raf inhibitors did not affect ERK activation. Blocking autocrine growth factors (basic fibroblast growth factor, vascular endothelial growth factor, and tumor necrosis factor-α) in HCs also did not have an effect (data not shown). Moreover, ERK itself was not mutated because its activity was completely abolished by upstream inhibition of MEK1/2. This effect of MEK inhibitors also excluded ERK activation by previously reported MEK-independent pathways.35
The activation of MEK by PAK, an effector of Rac1 and Cdc42,13,14 is another possible pathway of ERK activation in HCs, because these two GTPases are known to be active in these cells.3 Here, we confirm the presence of GTP-Rac1 in HCs and the involvement of p60src in its activation. Moreover, we show that the likely mechanism of Rac1 activation by p60src involves phosphorylation/activation of Vav. However, Rac1 inhibition with toxin B had no effect on the constitutive activities of either MEK or ERK. Clearly, therefore, the Rac1→PAK→MEK pathway is not responsible for the constitutive activation of ERK in HCs.
To explore further the previously described involvement of PKC in ERK activation,4 we first confirmed that this activation is inhibited in cells treated with the general PKC inhibitors safingol and Ro32-0432. However, Gö6976 (an inhibitor of classical PKCs) had no such effect, suggesting involvement of novel PKC isoforms in the ERK activation. Specific knockdown of PKCδ with siRNA had no effect on ERK, Src, or Vav phosphorylation. In contrast, siRNA-mediated knockdown of PKCε resulted in a clear reduction in the activities of ERK, Src, and Vav. When we combined PKCε and δ siRNAs to knock down both proteins, no additional effects were observed, suggesting that PKCδ had no role to play in the activation pathways leading to active Rac and ERK. When these experiments were repeated using different PKCε and PKCδ siRNA constructs, similar results were observed, indicating that the reductions in phosphorylation of ERK, Src, and Vav were due to a reduction in PKCε protein and not to off-target effects of the siRNA used. Taken together, these results strongly implicate PKCε in the activation of ERK and Rac in HCs.
Both PKCδ and PKCε can be involved in ERK activation at several levels of the classical MAP kinase cascade,16,17,36–38 including activation through a direct interaction with MEK.20,37 Because we have previously shown that Ras and Raf are not involved in the constitutive ERK activation in HCs,4 we conclude that PKCε acts at the level of MEK.
That PKCε is also involved in the activation of Rac1 in HCs is suggested by the reduction of phosphorylation of p60src and Vav and a reduction in GTP-Rac1 when cells were treated with siRNA specific for PKCε or with Ro32-0432. This finding in HCs is supported by observations in endothelial cells, indicating that association of active PKCε with Src in a functional signaling module results in Src activation.39,40 Indeed, we could demonstrate co-immunoprecipitation of PKCε and p60src from HC lysates (Figure 5D). Active Rac1 also has a role to play, because when we inhibited Rac1 with toxin B this also resulted in the reduction of p60src and Vav phosphorylation. We postulated that this dependence of Src and Vav phosphorylation on Rac1 might involve an effect of this GTPase on the cytoskeleton. Rac1 regulates cytoskeletal dynamics,41,42 and p60src is known to bind to certain types of cytoskeletal structures.12 This binding, in turn, could further promote Src activation or protect the kinase from inhibitors. This proposition is supported by our demonstration that actin depolymerization by cytochalasin D led to a pronounced reduction in the phosphorylation of both p60src and Vav.
Our previous work using PP1 demonstrated that not only PKC but also Src kinases are involved in ERK activation. However, in contrast to PP1, toxin B did not affect ERK activation despite strong inhibition of p60src. This suggested the involvement of another PP1-sensitive Src family kinase(s) in the ERK activation pathway. Our preliminary experiments suggest the involvement of Lyn in this pathway, because activation of this kinase was strongly inhibited by PP1 but not toxin B (Figure 3B).
The central role of PKCε in the signaling pathways leading to ERK and Rac left us with the question of what activates this PKC isozyme. We show in immune complex kinase assays that PKCε is indeed active in HCs. As a novel PKC isoform, PKCε can be activated by diacylglycerol23; however, other factors are known to influence its activity.26,43 In the present study, we could not demonstrate a constitutively active phospholipase C required for diacylglycerol generation (not shown); therefore, we focused on tyrosine nitration as an alternative mechanism of PKCε activation.26 This was an attractive proposition, because our previous work demonstrated that HCs produce large amounts of ROS7 and because others have shown that HCs express also high levels of active iNOS.29 Both NADPH oxidase-generated superoxide anion and NO together produce ONOO− as a major agent responsible for the nitration of tyrosine residues in proteins.28 Our demonstration that tyrosine nitrosylation and kinase activity of PKCε are reduced when HCs are treated with NAC shows that nitration of tyrosine residues is actively taking place in HCs and is likely to be the source of PKCε activation in these cells. Moreover, subcellular fractionation suggested that the process of nitration is taking place on mitochondria where ROS are products of oxidative phosphorylation associated with mitochondrial respiration.
In our initial experiments, we had indication that PKCδ may also be involved in ERK activation in HCs. Although we could not affect ERK activation by PKCδ knockdown, rottlerin (an inhibitor thought to be specific for this PKC isoform) did inhibit ERK activation in HCs. However, because rottlerin is known to uncouple oxidative phosphorylation from mitochondrial respiration,21 we believe that the effect of this inhibitor was due to an interference with the ROS production required for PKCε nitrosylation on the mitochondrial membrane rather than to the inhibition of PKCδ.
Apart from mitochondrial respiration, ROS production in HCs involves also the newly described presence of NOX5 in the plasma membrane of these cells.7 In the presence of previously described high levels of intracellular Ca2+,44 this oxidase produces the bulk of ROS found in HCs leading to pronounced inhibition of the protein tyrosine phosphatase SHP-1.7 The resulting potentiation of protein tyrosine kinase-dependent signals4 contributes to the persistent activation state of HCs.
In conclusion, the present study demonstrates a central role of PKCε in the constitutive activation of ERK and Rac1 in HCs. PKCε is activated by high levels of the ROS and NO produced in these cells. NOX5-generated ROS also potentiate and maintain Src family kinase-dependent signals by inhibiting SHP-1. PKCε can bind and directly activate p60src40 and also maintain p60src activity through a Rac1-dependent feedback mechanism involving p60src interaction with cytoskeleton.12 Another Src family kinase, most likely p56lyn, appears to be involved at some stage in the process of PKCε-dependent ERK activation. Taken together, our data support a model of coordinated constitutive activation of Rac and ERK in HCs (Figure 8). This activation could explain both the unique morphology and the previously reported prolonged survival of HCs, and strongly implicates activation of PKCε and Src family kinases in the malignant transformation of these cells.
Acknowledgments
We are grateful to Professor Alan Hall (University College London, London, UK) for the gift of the plasmid clone pGEX-PAK and to Dr. Stephen Feller (Cancer Research UK, Oxford, UK) for the provision of mAb 327.
Footnotes
Address reprint requests to Dr. Joseph R. Slupsky, Department of Haematology, University of Liverpool, Third Floor Duncan Building, Daulby St., Liverpool, UK L69 3GA. E-mail: jslupsky@liverpool.ac.uk.
Supported by grants from the Leukaemia Research Fund UK (to J.C.C., M.Z., and J.R.S.).
J.R.S. and A.S.K. contributed equally to this work.
References
- Cawley JC, Zuzel M, Caligaris-Cappio F. Tallman MS, Polliack A, editors. Amsterdam: Harwood Academic Press,; Biology of the Hairy Cell. 2000:pp 9–18. [Google Scholar]
- Burthem J, Cawley JC. London: Springer-Verlag,; Hairy-Cell Leukaemia. 1996 [Google Scholar]
- Zhang X, Machii T, Matsumura I, Ezoe S, Kawasaki A, Tanaka H, Ueda S, Sugahara H, Shibayama H, Mizuki M, Kanakura Y. Constitutively activated Rho guanosine triphosphatases regulate the growth and morphology of hairy cells. Int J Hematol. 2003;77:263–273. doi: 10.1007/BF02983784. [DOI] [PubMed] [Google Scholar]
- Kamiguti AS, Harris RJ, Slupsky JR, Baker P, Cawley J, Zuzel M. Regulation of hairy-cell survival through constitutive activation of mitogen-activated protein kinase pathways. Oncogene. 2003;22:2272–2284. doi: 10.1038/sj.onc.1206398. [DOI] [PubMed] [Google Scholar]
- Jaffe ES, Harris NL, Stein H, Vardiman JW. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press,; World Health Organisation Classification of Tumours. 2001 [Google Scholar]
- Benard V, Bohl BP, Bokoch GM. Characterization of Rac and Cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- Kamiguti AS, Serrander L, Lin K, Harris RJ, Cawley JC, Allsup DJ, Slupsky JR, Krause K-H, Zuzel M. Expression and activity of NOX5 in the circulating malignant B cells of hairy cell leukemia. J Immunol. 2005;175:8424–8430. doi: 10.4049/jimmunol.175.12.8424. [DOI] [PubMed] [Google Scholar]
- Kawakatsu H, Sakai T, Takagaki Y, Shinoda Y, Saito M, Owada MK, Yano J. A new monoclonal antibody which selectively recognizes the active form of src tyrosine kinase. J Biol Chem. 1996;271:5680–5685. doi: 10.1074/jbc.271.10.5680. [DOI] [PubMed] [Google Scholar]
- Brandt D, Gimona M, Hillmann M, Haller H, Mischak H. Protein kinase C induces actin reorganization via a Src- and Rho-dependent pathway. J Biol Chem. 2002;277:20903–20910. doi: 10.1074/jbc.M200946200. [DOI] [PubMed] [Google Scholar]
- Cantrell D. Lymphocyte signalling: a coordinating role for Vav? Curr Biol. 1998;8:R535–R538. doi: 10.1016/s0960-9822(07)00341-7. [DOI] [PubMed] [Google Scholar]
- Han J, Luby-Phelps K, Das B, Shu X, Xia Y, Mosteller RD, Krishna UM, Falck JR, White MA, Broek D. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- Fincham VJ, Unlu M, Brunton VG, Pitts JD, Wyke JA, Frame MC. Translocation of Src kinase to the cell periphery is mediated by the actin cytoskeleton under the control of the Rho family of small G proteins. J Cell Biol. 1996;135:1551–1564. doi: 10.1083/jcb.135.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost JA, Steen H, Shapiro P, Lewis T, Ahn N, Shaw PE, Cobb MH. Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. EMBO J. 1997;16:6426–6438. doi: 10.1093/emboj/16.21.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost JA, Xu S, Hutchison MR, Marcus S, Cobb MH. Actions of Rho family small G proteins and p21-activated protein kinases on mitogen-activated protein kinase family members. Mol Cell Biol. 1996;16:3707–3713. doi: 10.1128/mcb.16.7.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams ST, Lakum T, Lin K, Jones GM, Treweeke AT, Farahani M, Hughes M, Zuzel M, Slupsky JR: B cell receptor signalling in chronic lymphocytic leukaemia cells is regulated by overexpressed active protein kinase Cβ. Blood 2006 (epub September 26, 2006; DOI 10.1182/blood-2006-03-012021) [DOI] [PubMed] [Google Scholar]
- Ping P, Zhang J, Cao X, Li RCX, Kong D, Tang X-L, Qiu Y, Manchikalapudi S, Auchampach JA, Black RG, Bolli R. PKC-dependent activation of p44/p42 MAPKs during myocardial ischemia-reperfusion in conscious rabbits. Am J Physiol. 1999;276:H1468–H1481. doi: 10.1152/ajpheart.1999.276.5.H1468. [DOI] [PubMed] [Google Scholar]
- Ding M, Huang C, Lu Y, Bowman L, Castranova V, Vallyathan V. Involvement of protein kinase C in crystalline silica-induced activation of the MAP kinase and AP-1 pathway. Am J Physiol. 2006;290:L291–L297. doi: 10.1152/ajplung.00053.2005. [DOI] [PubMed] [Google Scholar]
- Heidkamp MC, Bayer AL, Martin JL, Samarel AM. Differential activation of mitogen-activated protein kinase cascades and apoptosis by protein kinase Cε and δ in neonatal rat ventricular myocytes. Circ Res. 2001;89:882–890. doi: 10.1161/hh2201.099434. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Hirai S, Osada S, Suzuki A, Mizuno K, Ohno S. Protein kinase C activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J Biol Chem. 1996;271:23512–23519. doi: 10.1074/jbc.271.38.23512. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Foster DA, Rosner MR. Protein kinase Cδ mediates neurogenic but not mitogenic activation of mitogen-activated protein kinase in neuronal cells. Mol Cell Biol. 1999;19:4209–4218. doi: 10.1128/mcb.19.6.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltoff SP. Rottlerin is a mitochondrial uncoupler that decreases cellular ATP levels and indirectly blocks protein kinase Cδ tyrosine phosphorylation. J Biol Chem. 2001;276:37986–37992. doi: 10.1074/jbc.M105073200. [DOI] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- da Rocha AB, Mans DRA, Regner A, Schwartsmann G. Targeting protein kinase C: new therapeutic opportunities against high-grade malignant gliomas? Oncologist. 2002;7:17–33. doi: 10.1634/theoncologist.7-1-17. [DOI] [PubMed] [Google Scholar]
- Baines CP, Zhang J, Wang G-W, Zheng Y-T, Xiu JX, Cardwell EM, Bolli R, Ping P. Mitochondrial PKCε and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCε-MAPK interactions and differential MAPK activation in PKCε-induced cardioprotection. Circ Res. 2002;90:390–397. doi: 10.1161/01.res.0000012702.90501.8d. [DOI] [PubMed] [Google Scholar]
- Lips DJ, Bueno OF, Wilkins BJ, Purcell NH, Kaiser RA, Lorenz JN, Voisin L, Saba-El-Leil MK, Meloche S, Pouyssegur J, Pages G, De Windt LJ, Doevendans PA, Molkentin JD. MEK1-ERK2 signaling pathway protects myocardium from ischemic injury in vivo. Circulation. 2004;109:1938–1941. doi: 10.1161/01.CIR.0000127126.73759.23. [DOI] [PubMed] [Google Scholar]
- Balafanova Z, Bolli R, Zhang J, Zheng Y, Pass JM, Bhatnagar A, Tang X-L, Wang O, Cardwell E, Ping P. Nitric oxide (NO) induces nitration of protein kinase Cε (PKCε), facilitating PKCε translocation via enhanced PKCε-RACK2 interactions: a novel mechanism of NO-triggered activation of PKCε. J Biol Chem. 2002;277:15021–15027. doi: 10.1074/jbc.M112451200. [DOI] [PubMed] [Google Scholar]
- Abou-Mohamed G, Johnson JA, Jin L, El-Remessy AB, Do K, Kaesemeyer WH, Caldwell RB, Caldwell RW. Roles of superoxide, peroxynitrite, and protein kinase C in the development of tolerance to nitroglycerin. J Pharmacol Exp Ther. 2004;308:289–299. doi: 10.1124/jpet.103.056119. [DOI] [PubMed] [Google Scholar]
- Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci USA. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman V, Zhao H, Fourneau J-M, Marconi A, Dugas N, Sigaux F, Kolb J-P. Expression of a functional inducible nitric oxide synthase in hairy cell leukemia and ESKOL cell line. Leukemia. 2000;14:696–705. doi: 10.1038/sj.leu.2401702. [DOI] [PubMed] [Google Scholar]
- Towatari M, Lida H, Tanimoto M, Iwata H, Hamaguchi M, Saito H. Constitutive activation of mitogen-activated protein kinase pathway in acute leukemia cells. Leukemia. 1997;11:479–484. doi: 10.1038/sj.leu.2400617. [DOI] [PubMed] [Google Scholar]
- Kim S-C, Hahn J-S, Min Y-H, Yoo N-C, Ko Y-W, Lee W-J. Constitutive activation of extracellular signal-regulated kinase in human acute leukemias: combined role of activation of MEK, hyperexpression of extracellular signal-regulated kinase, and downregulation of a phosphatase, PAC1. Blood. 1999;93:3893–3899. [PubMed] [Google Scholar]
- Sivaraman VS, Wang H-y, Nuovo GJ, Malbon CC. Hyperexpression of mitogen-activated protein kinase in human breast cancer. J Clin Invest. 1997;99:1478–1483. doi: 10.1172/JCI119309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyamoorthy K, Li G, Gerrero MR, Brose MS, Volpe P, Weber BL, van Belle P, Elder DE, Herlyn M. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res. 2003;63:756–759. [PubMed] [Google Scholar]
- Emrick MA, Hoofnagle AN, Miller AS, Eyck LFT, Ahn NG. Constitutive activation of extracellular signal-regulated kinase 2 by synergistic point mutations. J Biol Chem. 2001;276:46469–46479. doi: 10.1074/jbc.M107708200. [DOI] [PubMed] [Google Scholar]
- Grammer TC, Blenis J. Evidence for MEK-independent pathways regulating the prolonged activation of the ERK-MAP kinases. Oncogene. 1997;14:1635–1642. doi: 10.1038/sj.onc.1201000. [DOI] [PubMed] [Google Scholar]
- Jackson DN, Foster DA. The enigmatic protein kinase Cδ: complex roles in cell proliferation and survival. FASEB J. 2004;18:627–636. doi: 10.1096/fj.03-0979rev. [DOI] [PubMed] [Google Scholar]
- Besson A, Davey A, Robbins SM, Yong VW. Differential activation of ERKs to focal adhesions by PKCε is required for PMA-induced adhesion and migration of human glioma cells. Oncogene. 2001;20:7398–7407. doi: 10.1038/sj.onc.1204899. [DOI] [PubMed] [Google Scholar]
- Xuan Y-T, Guo Y, Zhu Y, Wang O-L, Rokosh G, Messing RO, Bolli R. Role of the protein kinase C-ε-Raf-1-MEK-1/2-p44/42 MAPK signaling cascade in the activation of signal transducers and activators of transcription 1 and 3 and induction of cyclooxygenase-2 after ischemic preconditioning. Circulation. 2005;112:1971–1978. doi: 10.1161/CIRCULATIONAHA.105.561522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Vondriska TM, Wang G-W, Klein JB, Cao X, Zhang J, Kang YJ, D’Souza S, Ping P. Molecular conformation dictates signaling module formation: example of PKCε and Src tyrosine kinase. Am J Physiol. 2002;282:H1166–H1171. doi: 10.1152/ajpheart.00830.2001. [DOI] [PubMed] [Google Scholar]
- Vondriska TM, Zhang J, Song C, Tang X-L, Cao X, Baines CP, Pass JM, Wang S, Bolli R, Ping P. Protein kinase Cε-Src modules direct signal transduction in nitric oxide-induced cardioprotection: complex formation as a means for cardioprotective signaling. Circ Res. 2001;88:1306–1313. doi: 10.1161/hh1201.092994. [DOI] [PubMed] [Google Scholar]
- Ridley AJ. Rho family proteins and regulation of the actin cytoskeleton. Prog Mol Subcell Biol. 1999;22:1–22. doi: 10.1007/978-3-642-58591-3_1. [DOI] [PubMed] [Google Scholar]
- Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol. 1997;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- Prekeris R, Hernandez RM, Mayhew MW, White MK, Terrian DM. Molecular analysis of the interactions between protein kinase C-ε and filamentous actin. J Biol Chem. 1998;273:26790–26798. doi: 10.1074/jbc.273.41.26790. [DOI] [PubMed] [Google Scholar]
- Génot E, Bismuth G, Degos L, Sigaux F, Wietzerbin J. Interferon-α downregulates the abnormal intracytoplasmic free calcium concentration of tumor cells in hairy cell leukemia. Blood. 1992;80:2060–2065. [PubMed] [Google Scholar]