Abstract
Background
Iron deficiency anemia (IDA) has been associated with altered cognitive, motor, and social-emotional outcomes in human infants. We recently reported that rats with chronic perinatal IDA, had altered regional brain iron, monoamines, and sensorimotor skill emergence during early development.
Objective
To examine the long-term consequences of chronic perinatal IDA on behavior, brain iron and monoamine systems after dietary iron treatment in rats.
Methods
Sixty dams were randomly assigned to iron-sufficient (CN) or low-iron (EID) diets during gestation and lactation. Thereafter, all offspring were fed the iron-sufficient diet, assessed for hematology and behavior after weaning and into adulthood and for brain measures as adults (regional brain iron, monoamines, dopamine and serotonin transporters, and dopamine receptor). Behavioral assessments included sensorimotor function, general activity, response to novelty, spatial alternation, and spatial water maze performance.
Results
Hematology and growth were similar for EID and CN rats by postnatal day 35. In adulthood, EID thalamic iron content was lower. Monoamines, dopamine transporter, and dopamine receptor concentrations did not differ from CN. EID serotonin transporter concentration was reduced in striatum and related regions. EID rats had persisting sensorimotor deficits (delayed vibrissae-evoked forelimb placing, longer sticker removal time, and more imperfect grooming chains), were more hesitant in novel settings, and had poorer spatial water maze performance than CN. General activity and spatial alternation were similar for EID and CN.
Conclusion
Rats that had chronic perinatal IDA showed behavioral impairments that suggest persistent striatal dopamine and hippocampal dysfunction despite normalization of hematology, growth and most brain measures.
Keywords: Iron deficiency anemia, Rat, Brain, Behavior, Dopamine, Serotonin
1. Introduction
Iron deficiency anemia (IDA), during infancy in humans, has been associated with poorer performance on mental and motor measures and behavioral alterations such as wary, hesitant behavior [1,2]. Longitudinal studies report cognitive and behavioral alterations persisted into childhood and adolescence despite iron treatment in infancy [1,3,4]. The basic neurobiological alterations that occur with iron deficiency early in life have not been fully delineated and cannot be directly studied in the human [5].
Investigators have explored the central nervous system effects of iron deficiency anemia (IDA) in rodent models [5] induced by pre- and post-weaning periods of dietary iron restriction [6–17]. To relate the studies in rodents to the human condition, it is important to recognize that the timing of birth relative to brain development is different between species. While there are important differences by brain region and system, a general framework is that the 10-day-old rat brain approximates that of the full-term human neonate and by weaning (postnatal day 21), rat brain development approximates that of the late toddler age child [18].
Iron deficiency anemia has been demonstrated to reduce brain iron. However, the pattern and degree of such changes appear to depend on the timing and severity of anemia suggesting that the processes of regional acquisition of iron are developmentally bound [5,13,17]. Brain iron deficiency has direct and indirect, and immediate and long-term effects that likely include processes of morphogenesis [15], cell growth and differentiation [19], cellular bioenergetics [20], biochemistry [5,15], myelin biology [9,16,21,22], and neurotransmitter systems. The dopamine (DA) system has been studied most extensively with regard to IDA [6,9,14,16,23–25].
Investigations of IDA and dopamine biology, brain iron, iron management proteins, and behavior have documented effects that depend on time of nutritional deficit and the time of iron treatment. When severe IDA was induced after weaning, extracellular striatal dopamine and its metabolites increased, dopamine receptors decreased in striatum and nucleus accumbens, and dopamine transporter densities decreased. In this model, dopamine receptor and brain iron concentrations were directly correlated, and alterations of iron regulatory proteins were observed [13,14,23–25]. In addition, rats made IDA after weaning had reduced activity (at baseline [16,23,26–28] and in response to cocaine administration [23]), evidenced fewer exploratory and stereotypic behaviors [16], and had poorer performance in a Y-swim maze [29]. Treatment with iron appeared to reverse some of the biochemical and behavioral findings with post-weaning IDA [5]. Studies of IDA induced in lactation have found reduced regional brain iron, altered iron regulatory proteins, and altered dopamine measures. Within several weeks of iron treatment at weaning, brain iron content was nearly normalized, and there were improvements in extracellular dopamine concentration and dopamine receptor densities that correlated with improvements in brain iron concentration. However, despite iron treatment, there were persistent alterations in dopamine system measures (receptors and transporters) in the terminal fields and related behaviors (exploration and stereotypes) [11–13,23]. Recent studies have explored even earlier periods of iron deficiency and iron treatment. With perinatal IDA and iron treatment by mid-lactation, altered performance was observed on a brief spatial learning task [8]. Persistent changes of iron regulatory proteins, hippocampal structure, and hippocampal metabolism have also been observed [19,20,30]. Thus, the effects of iron deficiency on brain and behavior and the reversibility of these effects appear to depend on the timing of IDA and iron treatment in rats.
In most animal studies of IDA, peripheral iron status has been severely reduced, by 60% or more, and significant growth retardation was often coincident (body weight reduced ~30%) [7,23–25]. Although severe IDA does occur in human infants, the more common condition world-wide is chronic iron deficiency that is not typically associated with severe reductions of iron status or growth [1]. We were interested in whether biologically meaningful alterations would be documented in rats that had chronic iron deficiency, with a less severe level of anemia than previous rodent studies.
The rodent model we developed [17] differed from previous investigations in three respects. First, the design was based on the concept of the chronic iron deficiency that human infants around the world often experience—from in utero development into toddler age (18–24 months), followed by improved iron status due to dietary factors [1]. Second, the model was designed to maintain a stable and less severe level of anemia during lactation, the developmental period generally equivalent to late gestation through toddler age in the human infant. Third, growth retardation was less than in previous models. The first report of this model documented the effects during the period of anemia. Hematologic measures were reduced by approximately 40% at both postnatal day (P)10 and P25. Brain weight was reduced by 5% at P10 and 10% at P25 and body weight was reduced by 15% at P10 and 25% at P25 for the IDA pups. Regional brain iron was lower for IDA pups at P25. Alterations in dopamine and serotonin metabolism were observed even earlier (P10). In addition, iron-deficient pups had reduced activity and delayed emergence of sensorimotor behaviors that specifically assessed for the integrity of the striatal dopamine system [17]. Thus, chronic perinatal IDA was associated with significant alterations of brain iron, dopamine and serotonin biology and specific behavioral deficits in rats during anemia.
This study examined the long-term effects of chronic perinatal IDA in rats using a comprehensive battery of behavioral tasks and assessments of brain iron and monoamine systems. The objective was to determine whether dietary iron rehabilitation would correct the regional brain iron, monoamine and behavioral alterations observed during lactation with this model. Based on the studies of more severe IDA in rodents and the human infancy studies, we predicted that iron treatment at weaning would not be sufficient to normalize all brain and behavioral measures. In particular, we hypothesized that behaviors involving striatal dopamine and hippocampal systems would show persistent deficits. This prediction was based on the fundamental behavioral deficits we observed in these iron-deficient pups during anemia and the persistent effects of early IDA on hippocampal anatomy and metabolism observed by Georgieff and colleagues [19,20].
2. Materials and methods
2.1. Diet design
The dietary protocol has been previously described [17]. Briefly, 60, 7-week-old Sprague Dawley female rats were obtained from Harlan, Sprague Dawley and fed a 40 mg/kg iron diet (TD89300, Harlan Teklad Nutritionals, Madison, WI) for 2 weeks prior to mating. Pregnant dams were randomly assigned to early iron deficiency (EID) and control (CN) groups at gestation day 7. Control dams continued the 40 mg/kg iron diet throughout gestation and lactation. EID group dams received a 4 mg/kg Fe diet (TD80936, Harlan Teklad Nutritionals, Madison, WI), from gestation day 7 to P7. EID group dams then received a 10 mg/kg iron diet (TD01094, Harlan Teklad Nutritionals, Madison, WI) from P7 to P20 to maintain a stable level of IDA and prevent significant growth faltering in the pups. At P20, all dams and pups received the 40 ppm iron diet. All pups continued on this diet after weaning at P23 until sacrifice. At P1–2, litters were culled to 10 pups per litter, retaining a balance of males and females as able. Litter mates remained together until P30. Thereafter, offspring were pair-housed by sex. Animals were housed in a temperature-controlled animal facility with a reversed, 12:12-h light/dark cycle. The experimental protocol was approved by the University Committee for the Care of Animals at the University of Michigan.
2.2. Growth and hematology
Body weight was measured at P35, P60, and at sacrifice (P120). Brain and liver were weighed at P120. Blood was assessed at P35 and P60 for hemoglobin and hematocrit and at P120 for hemoglobin, hematocrit, and serum iron by standard methods [17].
2.3. Behavioral assessments
The behavioral assessments were chosen to systematically assess hypotheses about effects on specific neurobiological systems likely to be affected by early IDA based on previous studies (e.g., striatal dopamine system, hippocampus). Behavioral testing was performed between 0900 and 1400 h (during the dark cycle), under red light. Individual rats from each litter received 2–3 behavioral tests. Testers were blind to diet group status at the time of testing.
Sensorimotor function. Upper extremity sensorimotor function was assessed with three tests sensitive to nigrostriatal dopamine tract impairments: vibrissae-evoked forelimb placing at P24, P27, P30 and P35 [31–34]; the sticker test at P35 and P70 [31], and naturalistic grooming sequences at P35 and P90 [35]. Rats with striatal injury have impaired forelimb placing and sticker removal from upper extremities [31,32]. The neostriatum controls the sequence of naturalistic or syntactic grooming, and lesions of the dorsolateral striatum or nigrostriatal projection neurons disrupt the syntactic pattern [35,36].
Vibrissae-stimulated forelimb placing. Each rat was assessed by stimulating the vibrissae on each side while holding at the torso with the ipsilateral upper extremity hanging free. The number of ipsilateral forelimb placements out of 10 trials per side was averaged for each animal. The results were averaged by litter and sex. The outcome measure was percent vibrissae-evoked placing by litter and sex at each age.
Sticker test. Small adhesive stickers were placed at the distal radial aspect of both upper extremities and rats were observed for 3-min. Time to first sticker touch, sticker removal, and the number of touches before removal (P70 only) were recorded. Time from first touch to sticker removal (removal time) was computed. Each rat had three, 3-min sticker trials in each of three settings: the home cage; the home cage with the cage top partially open (distraction); a new cage (clean bedding) with the top removed (novel). The first sticker trials were in the home cage for all rats. Distraction and novel cage settings followed immediately in a counter-balanced order across all rats. Inter-trial interval was less than 1 min. Different rats were assessed at P35 (20 CN [10 males], 17 EID [9 males]) and P70 (24 CN [12 males], 21 EID [11 males]). Measures for each upper extremity were averaged by rat for analysis. Later time to first touch might indicate delayed sensory recognition of the sticker. Increased time for removal or number of touches before removal might indicate poorer coordination of oral-extremity function or distraction from this effort.
Naturalistic grooming. Rats were individually videotaped from below with time-imprinting in a recording chamber for 1 h at P90. Number and duration of complete, incomplete, and imperfect chain grooming sequences were coded. Twenty CN (11 males), and 18 EID (9 males) were assessed.
Response to novelty. Emergence neophobia is a hippocampal-dependent behavior describing hesitancy to enter a novel environment [37]. We assessed emergence neophobia at P35–40. However, we first determined response to novel olfactory stimuli in the home cage to establish that EID rats could differentiate a novel from familiar odors [38].
Woodblock. The woodblock test was used to determine the basic ability of the rats to distinguish novel from familiar odors. Two wooden blocks were placed in the home cage overnight to become familiar for presence and take on the home cage odor. The next day, the blocks were removed for 1 h. At assessment, rats were singly placed into their home cage for two 3-min trials, which were counter-balanced across all rats. Inter-trial interval was 3 min. For one trial, the two familiar blocks were placed in the home cage. For the other trial, one familiar and one novel block (kept in another rat’s bedding overnight) were presented. Latency to first touch, number of touches, and duration of contact with each block were recorded. Rats that can distinguish the novel olfactory cue would be expected to contact the novel block sooner and remain in contact longer than with the familiar block in the home cage. Twenty CN rats (10 males) and 19 EID rats (10 males) were tested.
Emergence neophobia. Rats were singly placed into a container divided into three compartments (familiar, open and novel). The familiar and open areas contained a home-new bedding mix. The walls of the novel area were marked with black Xs and bedding from another rat was placed on the floor immediately before assessment. The animals had access to familiar and open areas for 22 h and adlib access to food and water. One hour before assessment, animals were contained in the open space. At assessment, both the familiar area and the novel area were opened simultaneously, allowing free access from the open space. Latency to enter the novel and familiar areas and duration in each area were determined for the next 10 min. Six CN (3 males) and 7 EID (5 males) rats were tested.
Activity. After 1–2 months of dietary iron rehabilitation, gross motor activity was assessed in two settings, open field [39] and an activity monitoring cage [7].
Open field. At P60, rats were singly placed in the center of a 1-m diameter open field for 3 min. The number of center, outer and total quadrant entries, rearing and freezing were recorded. Seventy-six CN (43 males) and 58 EID (26 males) rats were tested.
Activity monitoring cage. At P70, the rats were singly placed in 41 cm × 24 cm × 18 cm plastic cages. Activity was monitored for 1 h using infrared photocell emitters and detectors along the long axis. Lateral and horizontal (rearing) movements were detected by two beam breaks in succession and were counted for each 5-min interval. Twenty-one CN (11 males) and 16 EID (9 males) rats were assessed.
Spatial alternation. This task has been demonstrated to be sensitive to alterations of prefrontal/frontal cortical dopaminergic and noradrenergic systems [40,41]. Animals were tested between P70 and P110 using a T-maze with a start box (10 cm × 10 cm) and two goal arms (10 cm × 30 cm). Animals had access to food for 2 h per day for 1 week prior to and during the period of testing (following the testing procedure each day). Animals were acclimated to the T-maze with 12 guided trials with a food reward. Animals were then given 12 trials per day. On the first trial, the goal arm chosen was rewarded. A correct response on the subsequent trials required that the rat enter the goal arm not chosen on the previous trial. Inter-trial interval was 1 min. Rats continued until reaching 80% correct choices on 2 of 3 consecutive days. Twenty-two (10 males) EID and 23 (11 males) CN rats were assessed.
Morris water maze. Performance on tasks of spatial learning and memory requires intact hippocampal and neocortical systems [42–45]. Rats were assessed at P35 in the water maze for place learning and memory for platform location.
Place learning. Rats had four, 1-min swim trials per day with a stationary, submerged platform in the middle of one quadrant. The point of pool entry (north, south, east or west) varied with each trial and between trial days. However, the points of entry remained consistent for a given trial day. Testing continued until rats reached criteria (latency to platform ≤ 20 s for at least three trials on 2 of 3 consecutive days) or they completed 10 trial days. If they did not meet criteria by 10 days, 11 was entered for the calculation of days to criteria. Other outcome measures were calculated as mean of four daily trials: latency to platform (s), swim speed, percent swim path in platform quadrant, and percent path by the wall of the pool (thigmotaxis).
Probe trial. Twenty-four hours after reaching criteria or 10 days total, rats had a single, 1-min swim trial with the platform removed (probe trial). All rats entered the pool at the west location. We assessed swim speed, percent path in the platform quadrant, percent thigmotaxis path and quadrant preference. Quadrant preference was calculated for percent path: [(platform quadrant [T] − quadrant 2) + (T − quadrant 3) + (T − quadrant 4)]/3 [45].
2.4. Tissue processing
Male and female pups from each litter were sacrificed at P120 to measure brain iron or monoamine content. After euthanasia with intraperitoneal pentobarbital (150 mg/kg), animals were perfused with phosphobuffered saline (pH 7.4) through the left ventricle and organs were rapidly removed. Eight brain regions were quickly dissected on ice for measurement of iron content: frontal cortex, caudate-putamen, hippocampus, thalamus, nucleus accumbens, pons, superficial cerebellum, and deep cerebellar nuclei. Brain regions were immediately placed in storage tubes and frozen at −80 °C. Half-brains were also reserved for autoradiography for monoamine studies and were frozen slowly in dry ice:isopentane slurry and then stored at −80 °C. Livers were rapidly removed, weighed, and immediately frozen at −80 °C. Liver non-heme iron was determined using published methods [17]. Blood samples for serum iron were centrifuged at 3000 × g at 4 °C for 15 min and sera were frozen at −80 °C.
2.5. Regional brain iron, monoamines and ligand binding
Brain regions were digested by published standard procedures and iron content was analyzed by atomic absorption spectrophotometry as previously described and reported as μg/g tissue [13,14]. Catecholamine analysis in striatum was conducted by HPLC as described previously [17]. Ligand binding for density of dopamine and serotonin transporters was performed on sections from reserved half-brains as previously reported [17]. The density of receptors and transporters were quantified for brain regions using NIH image (Bethesda, MD) as previously described [17].
2.6. Statistical analysis
The fundamental analyses were ANOVA with diet group or sex as the main effect variables and χ2 for categorical variables using SPSS. All biological data were examined for normal distributions and log transformed when necessary prior to ANOVA. Behavioral measures were analyzed using the mean scores for males and females by litter for vibrissae-evoked forelimb placing and for individuals for all other assessments. General linear modeling using SPSS and Proc Mix using SAS [46] were used to assess the contribution of other factors such as context of assessment. Interactions between main effects were examined with the level of significance set at p < 0.05. For behavioral and brain measures with effect sizes ranging from 1.5 to 3, 8–10 rats per group were sufficient to determine significant group differences.
3. Results
3.1. Growth and hematology (Table 1)
Table 1.
Growth, hematology and liver iron concentration at postnatal day 120
| Diet (n) | Body weight (g) | Brain weight (g)* | Liver weight (g)** | HGB (g/dl) | HCT (%) | Liver iron (μ |
|---|---|---|---|---|---|---|
| CN (89) (45M, 44F) | 365 ± 3 | 1.90 ± 0.02 (0.13 g/g) | 12.69 ± 0.61 (0.43 g/g) | 15.5 ± 0.4 | 40 ± 1 | 23.09 ± 2.65 |
| EID (67) (33M, 34F) | 363 ± 15 | 1.82 ± 0.3 (0.13 g/g) | 13.34 ± 0.78 (0.69 g/g) | 15.7 ± 0.5 | 41 ± 1 | 20.16 ± 1.67 |
Mean ± S.E.; values in parentheses are organ weight in g/g body weight. Body, brain and liver weights were significantly less for females p < 0.001 than males but there was no diet × sex interaction.
Absolute weight, p < 0.02.
Normalized liver weight, p < 0.01.
After 2 weeks of the iron-sufficient diet and thereafter, EID body weight and hematology did not differ from CN. Liver weights were also similar at sacrifice. At sacrifice, EID absolute brain weights were lower than CN. However, when normalized for body weight, brain weight did not differ significantly by diet group Table 1.
3.2. Behavior
3.2.1. Sensorimotor function
Vibrissae-evoked forelimb placing. As shown in Fig. 1, EID rats had reduced unilateral vibrissae-evoked forelimb placing after weaning as compared to CN rats. By P30 EID rats reached CN levels.
-
Sticker test. There were no significant differences for time to first sticker touch or removal between EID and CN rats on any trial and setting at P35. However, at P70 EID was worse than CN in each setting as summarized below.
Home cage. At P70, the sticker removal time (time from first touch to removal), was significantly longer for EID rats (shown in Fig. 2, averaged for all three trials). The absolute time that stickers were removed was also later for EID rats (average seconds for all three trials ± S.E.; EID: 30.7 ± 4.7 versus CN: 16.3 ± 2.9; F = 7.19, p ≤ 0.01). The average time of first sticker touch (shown in Fig. 2, averaged for all three trials) and the average number of touches before sticker removal did not differ significantly by diet group.
Distracting home cage. At P70, EID rats had more touches before sticker removal than CN rats on the first of the three sticker trials (EID: 1.4 ± 0.1 versus CN: 1.2 ± 0.6; F = 4.42, p ≤ 0.04). EID rats removed the stickers later than CN rats on the first trial (Trial 1 EID: 53.2 s ± 11.9 versus CN: 26.5 ± 6.5; F = 4.13, p < 0.05). The removal time (time from first sticker touch to removal) was also significantly longer for EID versus CN rats on the first trial (shown in Fig. 3). There was no significant difference for these measures on trials 2 and 3 in this setting. Time of first sticker touch did not differ significantly by diet group on any trial.
Novel cage. At P70, the time of first sticker touch was later for EID than CN rats on the first trial (EID: 91.7 s ± 10.9 versus CN: 62.9 ± 7.5; F = 4.98, p < 0.04) with a trend at trial 2 (EID: 40.3 s ± 9.6 versus CN: 21.5 ± 6.0; F = 2.91, p < 0.10). EID rats removed the stickers significantly later than CN rats on the first trial (EID: 112.0 s ± 10.8 versus CN: 79.5 ± 9.4, F = 5.34, p < 0.03). There was no significant difference for removal time by diet group on any trial. Removal time for the first trial is shown in Fig. 3.
Effect of diet and setting on sticker performance. The removal time for the first trial was compared across settings (home, distraction, novel; Fig. 3). There was a significant diet by setting interaction for removal time (EID > CN, F = 6.32, p < 0.02).
Naturalistic grooming. EID rats had significantly more imperfect chains (Table 2) and a trend for a longer duration for this chain type (EID: 10.6 ms ± 1.0 versus CN: 8.3 ± 0.6, F = 3.552, p = 0.07). The percent of chains that were classified as incomplete was significantly less for EID rats (Table 2).
Fig. 1.
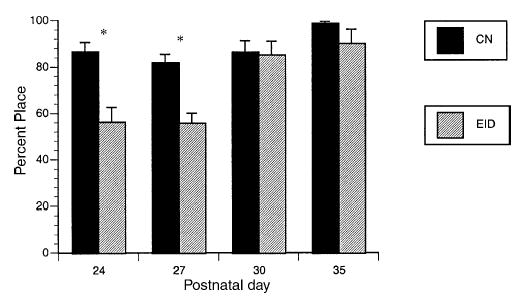
Vibrissae-evoked forelimb placing. Mean percent placing ± S.E.M. *p < 0.001.
Fig. 2.

Average sticker touch and removal time in the home cage by diet group. First sticker touch and removal time (time of removal − time of first touch). Mean seconds ± S.E.M. for three trials in the home cage. CN = 24, EID = 21. *p < 0.01.
Fig. 3.

Sticker removal time at first trial by setting. Removal time (time of removal − time of first touch) at the first trial in each setting. Mean seconds ± S.E.M. CN = 24, EID = 21. *Diet group: p < 0.05. Diet × setting interaction: p < 0.02.
Table 2.
Grooming chains: number and duration at postnatal day 90
| Number of chains
|
Percent of total chains
|
|||||
|---|---|---|---|---|---|---|
| Complete | Incomplete | Imperfect* | Complete | Incomplete* | Imperfect* | |
| CN | 1.9 ± 1.6 | 1.3 ± 0.3 | 1.0 ± 0.2 | 43.3 ± 8.5 | 29.7 ± 5.9 | 29.0 ± 5.7 |
| EID | 2.4 ± 2.2 | 0.6 ± 0.2 | 1.8 ± 0.4 | 43.3 ± 7.0 | 11.9 ± 3.7 | 45.7 ± 8.6 |
Number and duration of chains in 1-h by diet group at P35 and 90 (mean ± S.E.M.). n = 20 CN, 18 EID.
p < 0.05.
3.2.2. Response to novelty
Woodblock. In this test of ability to distinguish novel from familiar (in the home cage), all rats demonstrated a preference for the novel odor versus the familiar odor block. All rats showed significantly shorter latencies to first contact for the novel block (F = 54.34, p < 0.001), and a greater number of novel block touches (F = 4.79, p < 0.04), as compared to the familiar block. There was no significant difference for these variables by diet group and no diet by block type interaction.
Emergence neophobia. After 22 h of acclimation to familiar and open areas, the median time for rats to enter the novel area was 16 s. Five of 6 CN rats were at or below the median while 5 of the 7 EID rats were above (χ2, F = 3.9, p < 0.05). Over the entire 10 min, all rats spent the majority of the time in the novel area and there were no significant diet group differences (EID: 466.4 ± 72.2 versus CN: 438.2 ± 42.1, NS).
3.2.3. Activity
Open field. EID rats had a trend for less rearing in the open field (EID: 8.1 ± 0.5 versus CN: 9.2 ± 0.5, F = 3.67, p = 0.057). There were no other significant differences by diet group.
Activity monitoring cage. During the first 10 min in the activity box, EID rats had less rearing than CN rats (EID: 79.5 ± 8.6 versus CN: 106.1 ± 7.4, F = 6.33 p < 0.02) and a trend for fewer lateral movements (EID: 36.6 ± 4.1 versus CN: 43.1 ± 3.2, F = 3.82, p < 0.06). There were no significant differences by diet group when averaged over the whole hour.
3.2.4. Spatial alternation
Body weight declined to a similar degree for both experimental groups before and during testing (<5%). There was no significant difference by diet group or sex for days to criteria on this task. Control rats averaged 32.1 ± 3.8 days versus EID 32.3 ± 7.4 days to reach 80% correct responses on 2 of 3 consecutive days.
3.2.5. Water maze
Place learning. EID animals had poorer performance than CN rats on spatial learning trials. EID rats took significantly more days to reach criteria (EID: 9.5 ± 0.5 versus CN: 8.1 ± 0.4, F = 5.27, p < 0.03). Six of 12 EID rats met criteria by day 10 as compared to 16 of 19 CN rats. As shown in Fig. 4, latency to reach the platform was significantly longer for EID rats than CN rats at and after day 2. Path length to reach the platform was also significantly longer (repeated measures ANOVA for the first 7 days: F = 13.77, p = 0.001). The percent path in the platform quadrant was significantly less for EID rats on days 3–7 (Fig. 5). There was no significant difference by diet group for swim speed. Interestingly, EID rats demonstrated a greater percent of path length by the wall (thigmotaxis). Considering the first 7 days of training, EID rats averaged 65.1 ± 5.8% thigmotaxic path compared to 39.2 ± 3.62 for CN (F = 16.18, p < 0.001).
Probe trial. Considering all rats, there were no significant differences by diet group for percent path in the platform quadrant (EID: 42.9 ± 4.4 versus CN: 48.2 ± 3.8, F = 1.03, p = 0.32) or for quadrant preference (EID: 23.0 ± 5.9 versus CN: 31.0 ± 5.0, F = 1.03, p = 0.32). The EID rats continued to show significantly greater percent thigmotaxis path on the probe trial (EID: 28.0 ± 6.8 versus CN: 15.5 ± 1.8, F4.73 p < 0.04), and this was attributable to the rats that did not make criteria in place learning. In comparisons restricted to rats that made criteria, there was no significant difference for thigmotaxis on the probe trial by diet group.
Fig. 4.
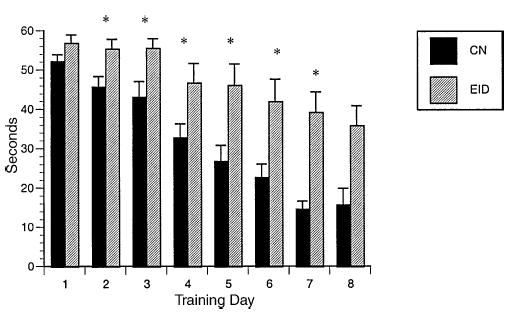
Water maze: latency to reach platform. Latency to reach platform in seconds (±S.E.M.) by diet group. CN = 19, EID = 12; day 8 CN = 8, EID = 10. *p < 0.05 days 2–8.
Fig. 5.
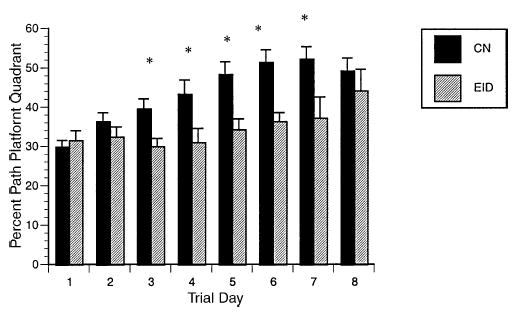
Water maze: percent path in platform quadrant. Percent path (cm) in the platform quadrant by diet group ± S.E.M. CN = 19, EID = 12; day 8 CN = 8, EID = 10. *p < 0.05 days 3–7.
3.3. Regional brain iron concentration (Table 3)
Table 3.
Brain iron concentration by region
| CX | DCN | HC | NA | Pons | SC | CPU | TH† | |
|---|---|---|---|---|---|---|---|---|
| CN | 18.69 ± 1.01 | 19.52 ± 1.43 | 20.70 ± 1.69 | 12.73 ± 0.69 | 11.58 ± 0.57 | 15.18 ± 0.56 | 13.48 ± 0.82 | 14.11 ± 0.57 |
| EID | 17.56 ± 0.76 | 21.73 ± 1.35 | 20.06 ± 1.81 | 13.64 ± 1.56 | 12.64 ± 0.47 | 14.66 ± 0.62 | 13.86 ± 1.19 | 12.74 ± 0.41 |
Mean ± S.E.M. μg/g tissue iron, CN n = 20, EID n = 18. No diet × sex interaction for any variable. CX, cortex; DCN, deep cerebellar nuclei; HC, hippocampus; NA, nucleus accumbens; SC, superficial cerebellum; CPU, caudate-putamen; TH, thalamus.
ANOVA by diet group: p < 0.06.
The EID rats did not differ from CN rats for regional brain iron at P120, with the exception of a marginally lower iron concentration in the thalamus. There was a significant effect of sex on iron concentration in the frontal cortex and deep cerebellar nuclei, but no diet by sex interaction for brain iron in any region (Table 3).
3.4. Regional monoamine, receptor, and transporter outcomes
At P120, monoamine concentrations in the striatum (MPHG [methoxyhydroxyphenyl glycol], norepinephrine, epinephrine, DOPAC [dihyroxyphenyl acetic acid], dopamine, HVA [homovanillic acid], 5-HIAA [5-hydroxyindole acetic acid], and serotonin) did not differ significantly by diet group (Table 4). There was a trend for reduced dopamine D2 receptor in the substantia nigra for EID as compared to CN rats (Table 5) but no effect of diet group on DAT density (Table 6). Serotonin transporter concentrations (SERT) were significantly greater for CN than EID rats in caudate-putamen and related regions (Table 7).
Table 4.
Monoamine concentration in striatum
| MHPG* | NE | EPI* | DOPAC* | DA* | HVA* | 5-HIAA* | |
|---|---|---|---|---|---|---|---|
| CN | 0.74 ± 0.13 | 0.07 ± 0.01 | 2.97 ± 0.75 | 1.40 ± 0.24 | 1.47 ± 0.36 | 0.76 ± 0.12 | 0.34 ± 0.08 |
| EID | 0.85 ± 0.13 | 0.08 ± 0.02 | 2.67 ± 0.75 | 1.62 ± 0.12 | 1.71 ± 0.40 | 0.79 ± 0.07 | 0.27 ± 0.08 |
Mean ± S.E.M. μg/g tissue; CN n = 15; EID n = 12. No significant differences by diet, and no diet × sex interaction for any variable. MHPG, methoxyhydroxyphenyl glycol; NE, norepinephrine; EPI, epinephrine; DOPAC, dihyroxyphenyl acetic acid; DA, dopamine; HVA, homovanillic acid; 5-HIAA, 5 hydroxyindole acetic acid.
ANOVA by sex: p < 0.02.
Table 5.
Dopamine D2 receptor concentration by region
| CPU | NA | OT | SN† | VTA | MN | |
|---|---|---|---|---|---|---|
| CN | 12.50 ± 0.46 | 10.00 ± 0.39 | 8.63 ± 0.28 | 4.43 ± 0.21 | 4.60 ± 0.20 | 4.28 ± 0.15 |
| EID | 11.35 ± 0.60 | 9.36 ± 0.50 | 8.16 ± 0.41 | 3.83 ± 0.27 | 4.28 ± 0.17 | 3.66 ± 0.47 |
Mean ± S.E.M. CN n = 21; EID n = 19. No significant differences by sex nor diet × sex interaction for any variable. CPU, caudate-putamen; NA, nucleus accumbens; OT, olfactory tubercle; SN, substantia nigra; VTA, ventral tegmentum; MN, tuberomammillary nucleus.
ANOVA by diet: p < 0.10.
Table 6.
Dopamine transporter concentration by region
| CPU | NA | OT | SN | |
|---|---|---|---|---|
| CN | 26.27 ± 1.63 | 17.90 ± 1.89 | 17.70 ± 1.76 | 10.39 ± 1.17 |
| EID | 26.34 ± 1.78 | 16.73 ± 1.91 | 15.69 ± 1.80 | 10.77 ± 1.40 |
Mean ± S.E.M. CN n = 22; EID n = 18. No significant differences by diet or sex, nor diet × sex interaction for any variable. CPU, caudate-putamen; NA, nucleus accumbens; OT, olfactory tubercle; SN, substantia nigra.
Table 7.
Serotonin transporter concentration by region
| CPU* | NA† | OT | SN | VN | LC | LPB | SUG | OPT | LTN* | VDM† | RTN* | ZI* | CX† | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CN | 9.06 ± 0.54 | 16.25 ± 0.62 | 23.15 ± 0.62 | 20.98 ± 0.49 | 13.03 ± 0.69 | 23.93 ± 1.31 | 16.88 ± 0.92 | 23.97 ± 0.85 | 24.49 ± 0.64 | 17.90 ± 0.57 | 21.68 ± 0.73 | 19.95 ± 0.53 | 22.72 ± 0.46 | 11.84 ± 0.48 |
| EID | 7.35 ± 0.47 | 14.68 ± 0.73 | 21.97 ± 0.79 | 20.51 ± 0.80 | 12.29 ± 0.90 | 22.06 ± 1.34 | 17.09 ± 0.95 | 22.21 ± 1.06 | 23.02 ± 0.81 | 15.51 ± 0.77 | 19.61 ± 0.77 | 17.96 ± 0.69 | 19.79 ± 0.49 | 10.60 ± 0.55 |
Mean ± S.E.M. &μg/g tissue CN n = 22; EID n = 18. No significant differences by sex and no diet × sex interaction for any variable. CPU, caudate-putamen; NA, nucleus accumbens; OT, olfactory tubercle; SN, substantia nigra; VN, vestibular nucleus; LC, locus ceruleus; LPB, lateral parabrachial; SUG, superficial gray layer; OPT, optic tract; LTN, laterodorsal thalamic nucleus; VDM, ventral dorsal medial thalamus; RTN, reticular thalamic nucleus; ZI, zona incerta; CX, cortex.
ANOVA by diet group: p ≤ 0.05.
diet group: p ≤ 0.10.
4. Discussion
This study examined the long-term consequences of chronic perinatal IDA in rats using a comprehensive battery of behavioral tasks and assessments of brain iron and monoamine systems. With the level and timing of IDA and iron treatment in this model, we observed that most measures of brain iron and monoamines were restored by adulthood. However, as reported in longitudinal studies of chronic iron deficiency in human infants, behavioral deficits persisted despite iron treatment and recovery of iron status measures [1,3,4].
There is growing evidence that IDA affects sensorimotor functions. We and others previously reported delayed emergence of forelimb placing with chronic perinatal IDA [17,47]. In the present study of long-term outcome, we observed delayed forelimb placing into the 4th week of life [32]. EID rats eventually attained this skill, and at 1 month of age no deficits were noted on the sticker task. However, 2–3 months later, sensorimotor deficits (sticker removal and naturalistic grooming) were detectable again. EID rats required significantly more time to remove forelimb stickers, and grooming was notable for more imperfect chains, characterized as having inserted or omitted events in the chain sequence. The findings suggest that these EID rats had difficulty successfully coordinating patterns of upper extremity motor movements. Damage to the striatum, neonatal dopamine depletion, and specifically, damage to the dorsolateral striatum alter sticker and syntactic grooming performance [31,35,48]. Thus, one explanation for our findings is that EID caused a fundamental alteration in dopamine nigrostriatal pathways or the feedback systems that affect these pathways. These sensorimotor deficits may help explain longitudinal findings of persistent motor and senorimotor impairments in children who had IDA during infancy [49,50].
Previous studies of rats and human infants showed that those with early IDA were more wary and hesitant [2,12]. In the present study, we observed similar behavioral differences. EID rats had less initial exploration in tasks assessing activity and emergence neophobia. Hesitance could have consequences on interaction with others and the progress and processes of learning [2]. The neurobiological basis of this behavior is not clear but dopaminergic, other neurotransmitter systems and potentially hypothalamic adrenal pituitary systems could play a role [51,52]. In addition, the timing of early iron deficiency may be important. In a recent study of pre- and postnatal iron deprivation in monkeys, behavioral differences depended on the timing. Prenatal iron deprivation was associated with “less fearful” and more impulsive behaviors, whereas postnatal deprivation was associated with “more tense” and withdrawn behaviors in testing environments [53]. The latter pattern resembles the behavior in studies of human IDA infants [2].
EID rats also had poorer place learning performance in the spatial water maze. This difference may relate to behavioral alterations coincident with, or apart from, learning per se. The EID rats showed more thigmotaxis, a pre-potent strategy whereby animals search the structure or the periphery for a route of escape [42,43]. The CN rats appeared to learn quickly that thigmotaxis was not a functional strategy, but the EID rats did not abandon this maladaptive strategy as readily. Deficits in hippocampal morphology may underlie the poorer performance and altered behavior of the EID rats. Morphologic and metabolic abnormalities in the hippocampus have been demonstrated with perinatal IDA [15,19,20]. However, the persistent thigmotaxis could also reflect increased emotionality and/or less cognitive flexibility in the context of the novel and mildly stressful task of place learning in the water maze [54]. The influence of the different behavioral responsiveness on tasks of cognitive functioning is relatively un-explored and warrants further investigation [2,11,12].
We previously reported that rats in this chronic perinatal IDA model had more brain iron deficiency as they progressed through lactation, with associated changes in dopamine transporter, serotonin transporter, and D2 receptor levels in the mesolimbic and nigrostriatal systems [17]. In the present report of long-term effects, we observed that several months of dietary iron “rescue” was sufficient to restore brain iron and most measures of dopamine and serotonin biology. These findings are similar to those in a previous study that used a shorter period of IDA during lactation and dietary recovery at weaning [13]. When severe IDA occurred post-weaning in other studies, iron repletion did not always reverse changes in monoamine concentrations, dopamine transporter and D2 receptor levels [16,24,55]. Thus, the timing of IDA and treatment appears to be important to brain iron and monoamine outcome. We observed that EID rats still had reduced serotonin transporter density in adulthood in a number of thalamic and striatal brain regions [17]. It is possible that this result involved compensatory mechanisms. Early injury in the striatal dopamine system has been associated with a compensatory increase in serotonergic innervation in the striatum and nucleus accumbens [56,57]. The mechanisms of this increase and the consequences behaviorally are not completely understood. However, if such mechanisms were activated with chronic perinatal IDA, it could result in a brief “compensation” period followed by long-term declines in serotonin transporter densities as observed in the current model.
One limitation in this study was that all animals underwent developmental testing every 3 days during lactation (beginning at P6) [17]. It is know that “handling” in the postnatal period alters stress responses later in life [58,59]. Thus, it is possible that our early postnatal evaluations affected the behavioral responses we observed in adulthood. It is also important to note that the level of iron deficiency anemia in this rodent model and the effects on growth were more significant than typically observed in human infants with chronic iron deficiency where hematologic indicators are typically reduced by 20% and there is little to no retardation of growth [2,17]. However, the growth retardation was less pronounced and the degree of brain iron deficiency was more moderate in this model than other rodent models of IDA during early development [17].
In conclusion, this study systematically assessed the adult behavioral phenotype of rats after dietary iron rehabilitation for chronic perinatal IDA. Despite recovery of brain iron and most measures of neurotransmitter function, behavioral deficits consistent with fundamental alterations of the striatal dopaminergic and hippocampal systems persisted. We observed differences in sensorimotor abilities, response to novel settings, and performance on a spatial learning task. Development is influenced by motor, sensory, social, and cognitive experiences. If these are impoverished or slowed by chronic perinatal IDA, this effect might underlie the deficits in functional behavioral outcomes we observed in adulthood. Using rodent models to understand the neurobiologic underpinnings (neurotransmitter, myelination, anatomy and metabolism) for these behavioral findings and how they change depending on the time and degree of IDA and iron treatment may help identify optimal management of chronic iron deficiency in humans.
Acknowledgments
The entire group of investigators participating in the Brain and Behavior in Early Iron Deficiency Program Project contributed to our thinking about brain and behavioral analyses and interpretation for this paper.
Footnotes
Supported by a program project grant from NIH (P01 HD39386, Brain and Behavior in Early Iron Deficiency, Betsy Lozoff, Principal Investigator) and R01 NS35088 (JB).
References
- 1.Grantham-McGregor S, Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr. 2001;131:649S–68S. doi: 10.1093/jn/131.2.649S. [DOI] [PubMed] [Google Scholar]
- 2.Lozoff B, Brittenham GM, Wolf AW, McClish DK, Kuhnert PM, Jimenez E, et al. Iron deficiency anemia and iron therapy: effects on infant developmental test performance. Pediatrics. 1987;79:981–95. [PubMed] [Google Scholar]
- 3.Lozoff B, Jimenez E, Wolf AW. Long-term developmental outcome of infants with iron deficiency. N Engl J Med. 1991;325:687–94. doi: 10.1056/NEJM199109053251004. [DOI] [PubMed] [Google Scholar]
- 4.Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:E51. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- 5.Beard JL, Connor JR. Iron status and neural functioning. Ann Rev Nutr. 2003;23:41–58. doi: 10.1146/annurev.nutr.23.020102.075739. [DOI] [PubMed] [Google Scholar]
- 6.Youdim MBH, Ben-Shachar D, Yehuda S. Putative biological mechanisms of the effect of iron deficiency on brain biochemistry and behavior. Am J Clin Nutr. 1989;50:607–17. doi: 10.1093/ajcn/50.3.607. [DOI] [PubMed] [Google Scholar]
- 7.Pinero D, Jones B, Beard JL. Variations in dietary iron alter behavior in developing rats. J Nutr. 2001;131:311–8. doi: 10.1093/jn/131.2.311. [DOI] [PubMed] [Google Scholar]
- 8.Felt BT, Lozoff B. Brain iron and behavior of rats are not normalized by treatment of iron deficiency anemia during early development. J Nutr. 1996;126:693–701. doi: 10.1093/jn/126.3.693. [DOI] [PubMed] [Google Scholar]
- 9.Kwik-Uribe CL, Gietzen D, German JB, Golub MS, Keen CL. Chronic marginal iron intakes during early development in mice result in persistent changes in dopamine metabolism and myelin composition. J Nutr. 2000;130:2821–30. doi: 10.1093/jn/130.11.2821. [DOI] [PubMed] [Google Scholar]
- 10.Weinberg J, Dallman PR, Levine S. Iron deficiency during early development in the rat: behavioral and physiological consequences. Pharmacol Biochem Behav. 1980;12:493–502. doi: 10.1016/0091-3057(80)90179-3. [DOI] [PubMed] [Google Scholar]
- 11.Findlay E, Reid RL, Ng KT, Armstrong SM. The effect of iron deficiency during development on passive avoidance learning in the adult rat. Physiol Behav. 1981;27:1089–96. doi: 10.1016/0031-9384(81)90375-9. [DOI] [PubMed] [Google Scholar]
- 12.Weinberg J, Levine S, Dallman PR. Long-term consequences of early iron deficiency in the rat. Pharmacol Biochem Behav. 1979;11:631–8. doi: 10.1016/0091-3057(79)90254-5. [DOI] [PubMed] [Google Scholar]
- 13.Pinero DJ, Li NQ, Connor JR, Beard JL. Alterations in brain iron metabolism in response to dietary iron changes. J Nutr. 2000;130:254–63. doi: 10.1093/jn/130.2.254. [DOI] [PubMed] [Google Scholar]
- 14.Erikson KM, Jones BC, Hess EJ, Zhang Q, Beard JL. Iron deficiency decreases dopamine D1 and D2 receptors in rat brain. Pharmacol Biochem Behav. 2001;69:409–18. doi: 10.1016/s0091-3057(01)00563-9. [DOI] [PubMed] [Google Scholar]
- 15.Rao R, Tkac I, Townsend EL, Gruetter R, Georgieff MK. Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus. J Nutr. 2003;133:3215–21. doi: 10.1093/jn/133.10.3215. [DOI] [PubMed] [Google Scholar]
- 16.Beard JL, Erikson KM, Jones BC. Neurobehavioral analysis of developmental iron deficiency in rats. Behav Brain Res. 2002;134:517–24. doi: 10.1016/s0166-4328(02)00092-x. [DOI] [PubMed] [Google Scholar]
- 17.Beard JL, Felt B, Schallert T, Burhans M, Connor JR, Georgieff MK. Moderate iron deficiency in young rats: alterations in biology and behavior; 2005 [in revision]. [DOI] [PubMed]
- 18.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 19.Jorgenson LA, Wobken JD, Georgieff MK. Perinatal iron deficiency alters apical dendritic growth in hippocampal CA1 pyramidal neurons. Dev Neurosci. 2003;25:412–20. doi: 10.1159/000075667. [DOI] [PubMed] [Google Scholar]
- 20.DeUngria M, Rao R, Wobken JD, Luciana M, Nelson CA, Georgieff MK. Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr Res. 2000;48:169–76. doi: 10.1203/00006450-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Ortiz E, Pasquini JM, Thompson K, Felt B, Butkus G, Beard J, et al. Effect of manipulation of iron storage, transport, or availability on myelin composition and brain iron content in three different animal models. J Neurosci Res. 2004;77:681–9. doi: 10.1002/jnr.20207. [DOI] [PubMed] [Google Scholar]
- 22.Yu GS, Steinkirchner TM, Rao GA, Larkin EC. Effect of prenatal iron deficiency on myelination in rat pups. Am J Pathol. 1986;125:620–4. [PMC free article] [PubMed] [Google Scholar]
- 23.Erikson K, Jones B, Beard JL. Iron deficiency alters dopamine transporter functioning in rat striatum. J Nutr. 2000;130:2831–7. doi: 10.1093/jn/130.11.2831. [DOI] [PubMed] [Google Scholar]
- 24.Nelson C, Erikson K, Pinero DJ, Beard JL. In vivo dopamine metabolism is altered in iron-deficient anemic rats. J Nutr. 1997;127:2282–8. doi: 10.1093/jn/127.12.2282. [DOI] [PubMed] [Google Scholar]
- 25.Beard JL, Chen Q, Connor JR, Jones BC. Altered monoamine metabolism in caudate nucleus on iron deficient rats. Pharmacol Biochem Behav. 1994;48:621–4. doi: 10.1016/0091-3057(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 26.Glover J, Jacobs A. Activity pattern of iron-deficient rats. BMJ. 1972;2:627–8. doi: 10.1136/bmj.2.5814.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edgerton VR, Bryant SL, Gillespie CA, Gardner GW. Iron deficiency anemia and physical performance and activity in rats. J Nutr. 1972;102:381–400. doi: 10.1093/jn/102.3.381. [DOI] [PubMed] [Google Scholar]
- 28.Hunt JR, Zito CA, Erjavec J, Johson LK. Severe or marginal iron deficiency affects spontaneous physical activity in rats. Am J Clin Nutr. 1994;59:413–8. doi: 10.1093/ajcn/59.2.413. [DOI] [PubMed] [Google Scholar]
- 29.Yehuda S, Youdim MBH, Mostofsky DI. Brain iron deficiency causes reduced learning capacity in rats. Pharmacol Biochem Behav. 1986;25:141–4. doi: 10.1016/0091-3057(86)90244-3. [DOI] [PubMed] [Google Scholar]
- 30.Siddappa AJ, Rao R, Wobken JD, Casperson K, Liebold EA, Connor J, et al. Iron deficiency alters iron regulatory protein and iron transport protein expression in the perinatal rat brain. Pediatr Res. 2003;53:800–7. doi: 10.1203/01.PDR.0000058922.67035.D5. [DOI] [PubMed] [Google Scholar]
- 31.Schallert T, Petrie BF, Whishaw IQ. Neonatal dopamine depletion: spared and unspared sensorimotor and attentional disorders and effects of further depletion in adulthood. Psychobiology. 1989;17:386–96. [Google Scholar]
- 32.Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–87. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 33.Schallert T, Woodlee MT. Brain-dependent movements and cerebral-spinal connections: key targets of cellular and behavioral enrichment in CNS injury models. J Rehab Res Dev. 2003;40:9–17. doi: 10.1682/jrrd.2003.08.0009. [DOI] [PubMed] [Google Scholar]
- 34.Felt BT, Schallert T, Shao J, Liu Y, Li X, Barks JD. Early appearance of functional deficits after neonatal excitotoxic and hypoxic-ischemic injury: fragile recovery after development and role of the NMDA receptor. Dev Neurosci. 2002;24:418–25. doi: 10.1159/000069053. [DOI] [PubMed] [Google Scholar]
- 35.Aldridge JW, Berridge KC. Coding of serial order by neostriatal neurons: a “natural action” approach to movement sequence. J Neurosci. 1998;18:2777–87. doi: 10.1523/JNEUROSCI.18-07-02777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cromwell HC, Berridge KC. Implementation of action sequences by a neostriatal site: a lesion mapping study of grooming syntax. J Neurosci. 1996;16:3444–58. doi: 10.1523/JNEUROSCI.16-10-03444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maren S, Patel K, Thompson RF, Mitchell D. Individual differences in emergence neophobia predict magnitude of perforant-path long-term potentiation (LTP) and plasma corticosterone levels in rats. Psychobiology. 1993;21:2–10. [Google Scholar]
- 38.Spinetta MJ, Woodlee MT, Hester NW, Heymann JC, Feinberg LM, Rajagopalan KN, et al. A simple and sensitive odor recognition test for rats and mice detects retrograde amnesia caused by ethanol or other drugs that interfere with memory consolidation. Washington, DC: Society for Neuroscience; 2005.
- 39.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 40.Alber SA, Strupp BJ. An in-depth analysis of lead effects in a delayed spatial alternation task: assessment of mnemonic effects, side bias, and proactive interference. Neurotoxicol Teratol. 1996;18:3–15. doi: 10.1016/0892-0362(95)02026-8. [DOI] [PubMed] [Google Scholar]
- 41.Hilson J, Strupp B. Analyses of response patterns clarify lead effects in ilfactory reversal and extradimensional shift tasks: assessment of inhibitory control, associative ability and memory. Behav Neurosci. 1997;111:532–42. doi: 10.1037//0735-7044.111.3.532. [DOI] [PubMed] [Google Scholar]
- 42.Day LB, Schallert T. Anticholinergic effects on acquisition of place learning in the morris water task: spatial mapping deficit or inability to inhibit nonplace strategies? Behav Neurosci. 1996;110:998–1005. doi: 10.1037//0735-7044.110.5.998. [DOI] [PubMed] [Google Scholar]
- 43.Day LB, Weisend M, Sutherland RJ, Schallert T. The hippocampus is not necessary for a place response but may be necessary for pliancy. Behav Neurosci. 1999;113:914–24. doi: 10.1037//0735-7044.113.5.914. [DOI] [PubMed] [Google Scholar]
- 44.Lindner MD, Schallert T. Aging and atropine effects on spatial navigation in the Morris water task. Behav Neurosci. 1988;102:621–34. doi: 10.1037//0735-7044.102.5.621. [DOI] [PubMed] [Google Scholar]
- 45.Whishaw IQ. Posterior neocortical (visual cortex) lesions in the rat impair matching-to-place navigation in a swimming pool: a reevaluation of cortical contributions to spatial behavior using a new assessment of spatial versus non-spatial behavior. Behav Brain Res. 2004;155:177–84. doi: 10.1016/j.bbr.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 46.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS system for mixed models. Cary, NC: SAS Institute; 1996.
- 47.Beard JL. Personal communication.
- 48.Schallert T, Upchurch M, Lobaugh N, Farrar SB, Spirduso WW, Gilliam P, et al. Tactile extinction: distinguishing between sensorimotor and motor asymmetries in rats with unilateral nigrostriatal damage. Pharmacol Biochem Behav. 1982;16:455–62. doi: 10.1016/0091-3057(82)90452-x. [DOI] [PubMed] [Google Scholar]
- 49.Beard J. Personal communication.
- 50.Lozoff B, Smith J, Liberzon T, Angulo-Barroso A, Calatroni A, Jimenez E. Longitudinal analysis of cognitive and motor effects of iron deficiency in infancy. APA plenary presentation Pediatr Res. 2004;55:23A. [Google Scholar]
- 51.Eseh R, Zimmerberg B. Age-dependent effects of gestational and lactational iron deficiency on anxiety behavior in rats. Behav Brain Res. 2005;164:214–21. doi: 10.1016/j.bbr.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 52.Fox NA, Bell MA, Jones NA. Individual differences in response to stress and cerebral asymmetry. Dev Neuropsychol. 1992;8:161–84. [Google Scholar]
- 53.Golub MS, Hogrefe CE, Germann SL, Capitanio JL, Lozoff B. Behavioral consequences of developmental iron deficiency in infant rhesus monkeys. Neurotoxicol Teratol. 2005;28:3–17. doi: 10.1016/j.ntt.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav. 1989;31:959–62. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- 55.Erikson K, Pinero DJ, Connor JR, Beard JL. Regional brain iron, ferritin and transferrin concentrations during iron deficiency and iron repletion in developing rats. J Nutr. 1997;127:2030–8. doi: 10.1093/jn/127.10.2030. [DOI] [PubMed] [Google Scholar]
- 56.Zhang K, Davids E, Tarazi FI, Baldessarini RJ. Serotonin transporter binding increases in caudate-putamen and nucleus accumbens after neonatal 6-hydroxydopamine lesions in rats: implications for motor hyperactivity. Brain Res. 2002;137:135–8. doi: 10.1016/s0165-3806(02)00436-4. [DOI] [PubMed] [Google Scholar]
- 57.Avale ME, Nemirovsky SI, Raisman-Vozari R, Rubinstein M. Elevated serotonin is involved in hyperactivity but not in the paradoxical effect of amphetamine in mice neonatally lesioned with 6-hydroxydopamine. J Neurosci Res. 2004;78:289–96. doi: 10.1002/jnr.20245. [DOI] [PubMed] [Google Scholar]
- 58.Levine S. Maternal and environmental influences on the adrenocortical response to stress in weanling rats. Science. 1967;156:258–60. doi: 10.1126/science.156.3772.258. [DOI] [PubMed] [Google Scholar]
- 59.Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. PNAS. 1998;95:5335–40. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]


