Abstract
The loss of hair cells resulting in a sensorineural hearing loss (SNHL) also leads to the secondary degeneration of spiral ganglion neurons (SGNs). The effectiveness of cochlear implantation in patients with a profound SNHL relies, in part, upon the survival of SGNs; therefore any therapy that can prevent or halt the loss of these neurons would be of potential clinical benefit. Previous research has shown that intracochlear infusion with neurotrophins can provide trophic support to SGNs in deafened guinea pigs. It remains to be determined whether this effect is seen in other species. After documenting the rate of SGN degeneration following ototoxic deafening, we investigated the trophic effects of exogenous brain-derived neurotrophic factor (BDNF) on rat SGNs. The left cochleae of profoundly deafened rats were implanted with a drug delivery system connected to a mini-osmotic pump. BDNF or artificial perilymph (AP) was infused for 28 days and the cochleae were then prepared for histology. Treatment with BDNF led to a statistically significant increase in SGN density and a highly significant increase in SGN soma area compared to AP-treated and untreated deafened cochleae. This work has demonstrated the trophic advantage of exogenous BDNF in the mature rat cochlea and provides confidence that SGN rescue following SNHL with exogenous BDNF may have clinical application.
Keywords: Neural degeneration, neurotrophin, deafness, ototoxicity, cochlear implant
Introduction
The loss of hair cells resulting in a sensorineural hearing loss (SNHL) also leads to the secondary degeneration of spiral ganglion neurons (SGNs; 1-4). The degeneration of SGNs appears to be due, at least in part, to the withdrawal of neurotrophic support from the hair cells within the organ of Corti (5-8).
Both brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3), together with their high affinity receptors (Trk B and Trk C respectively), are responsible for SGN survival and target innervation during development (9,5,6,10). Moreover, these neurotrophins are also thought to contribute to neuronal maintenance in the adult inner ear (5). In the absence of hair cells, the neuroprotective effects of chronically delivered exogenous BDNF or NT-3 on SGNs in vivo has been demonstrated (11-15).
Although both immature and adult rat SGNs have used to study the effects of neurotrophins on both survival and neurite sprouting in vitro (16,17), all previous in vivo studies of SGN rescue using neurotrophins have been performed in the deafened guinea pig. It is important, when evaluating the potential clinical application of neurotrophins, to establish whether they exhibit similar neuroprotective effects in other mammalian species. In the present study we show that BDNF delivered to the deafened rat cochlea over a 28-day period results in significant SGN rescue through all turns of the treated cochleae.
Materials and Methods
This study was divided into two parts. We first examined the rate of SGN loss following ototoxic deafening in the rat for periods of up to 10 weeks. This was performed in order to ensure that the duration of deafness used in the second phase of this work would result in a significant reduction in SGN density compared with normal hearing controls. Using the same deafening regime we then chronically delivered exogenous BDNF to the deafened cochleae and compared the degree of SGN rescue with deafened controls in which artificial perilymph (AP) had been delivered. All experiments were conducted in accordance with the Australian National Health and Medical Research Council’s animal experimentation guidelines and guidelines set by the Royal Victorian Eye and Ear Hospital’s Animal Research Ethics Committee (approval number 03/097).
A. Longitudinal deafening
Experimental animals
Hooded Wistar rats (200-300g) were used in this phase of the research. The animals were deafened (see below) for periods of two (four cochleae), four (four cochlea), six (five cochlea) or ten weeks (four cochlea), in order to examine the longitudinal effects of SNHL on SGNs in this species. Four cochleae from two normal hearing (NH) animals served as normal controls.
Assessment of Hearing Status
All animals had otoscopically normal tympanic membranes and normal hearing prior to deafening. Hearing status was determined using click-evoked auditory brainstem responses (ABR; threshold <46 decibels peak equivalent sound pressure level [dB p.e. SPL] re 20 μPa). ABR recording techniques have been described in detail previously (18). Briefly, animals were anaesthetized with a single intraperitoneal (i.p.) injection of 75mg/kg ketamine and 10mg/kg xylazine and placed on a heat pad (set at 37°C). Responses were recorded differentially using stainless-steel needle electrodes (vertex positive; neck negative; thorax ground). Computer generated 100 μs duration rarefaction clicks were presented to a loudspeaker placed 10 cm from the pinna of the test ear. The system was calibrated so that at this distance the maximum sound level at the pinna was 98 dB p.e. SPL. Click stimuli were presented at 33 per second, and the scalp recorded responses amplified by 105 and band-pass filtered (150 Hz-3 kHz). The output of the filter was fed to a 10 bit analogue-digital converter and sampled at 20 kHz for 12.5 ms following the stimulus onset. Five hundred responses were averaged for each recording and stored for subsequent analysis. A programmable attenuator controlled stimulus intensity; two recordings were made at each intensity. Threshold was defined as the smallest click amplitude required to evoke a peak-trough response amplitude of >0.25 μV for wave III of the ABR, i.e. within a latency window of 2.5-3.5 ms following stimulus onset for both responses (wave III was selected because it is the most robust in this recording configuration). All recordings were made in a sound attenuated, electrically shielded room. One week after deafening (see below) all deafened animals were again anaesthetized and ABRs recorded to confirm that they were severe-profoundly deaf (click-evoked ABR thresholds > 98 dB p.e. SPL).
Deafening technique
Using the same anaesthetic protocol, each animal received an intravenous injection of the loop diuretic furosemide (175mg/kg) followed by gentamicin (350mg/kg) delivered subcutaneously (s.c.). Spiral ganglion neuron degeneration following gentamicin ototoxicity is considered secondary to the loss of hair cells rather than a direct insult to the SGN (19).
Cochlear pathology
At completion of the deafening period (2, 4, 6 or 10 weeks), each animal was euthanased with an overdose of sodium pentobarbitone and perfused intracardially with 100ml of 37°C, 0.9% saline solution (containing heparin, 1000 units/kg and 0.3ml of 10% sodium nitrite), followed by 100ml of 4°C paraformaldehyde in 0.1M phosphate buffer (pH 7.4). The cochleae were harvested, the oval and round windows opened to allow penetration of the fixative and the cochleae stored in fixative overnight. They were then decalcified in 4% ethylenediamine tetra-acetic acid (EDTA), embedded in Spurr’s resin and sectioned at a thickness of 2μm in the mid-modiolar plane.
Five randomly selected mid-modiolar sections per cochlea were stained with haematoxylin and eosin, and examined by light microscopy to determine SGN density within Rosenthal’s canal (RC) across all turns of the cochlea (lower basal turn [LBT], upper basal turn [UBT], lower middle turn [LMT], and upper middle turn [UMT]). This analysis covers the entire SGN population in the rat as SGNs in the UMT in this species innervate the most apical region of the cochlea. Area measurements of RC were made using NIH Image (http://rsb.info.nih.gov/nih-image/). The number of SGNs with a clear nuclear profile within this area was counted and SGN density within RC was calculated (neurons/mm2).
B. Chronic BDNF delivery
Experimental Animals
A total of 12 normal hearing Hooded Wistar rats (200-300g) were used as the experimental subjects. The animals were deafened as described above, and randomly divided into two treatment groups; BDNF (n=6) or AP (n=6). Their hearing status was confirmed both before and following deafening using the ABR techniques described above.
Osmotic Pump and Cannula Preparation
Under sterile conditions Alzet mini-osmotic pumps (model 2004) were loaded with either recombinant human BDNF (PeproTech), containing 0.1% rat albumin in 200μl of Ringer’s solution, giving a BDNF concentration of 5.4μg/ml (approximately 1.35μg per animal, allowing for 25% absorption) or AP (sterile Ringer’s solution). The pumps were then incubated in sterile saline at 37°C for 48 hours prior to implantation, in accordance with the manufacturer’s specifications. These pumps have a flow-rate of 0.25μl/hr and a capacity of 200μl, which is infused into the cochlea via a delivery cannula over a period of 28 days.
Delivery cannulae were prepared for cochlear infusion as described previously (20). Briefly, a 1.25cm length of polyimide tubing was inserted into the end of a 10cm length of PVC tubing and secured with silicon adhesive. Approximately 2mm of the polyimide tube extended from the PVC tube for insertion into the cochlea. Cannulae were sterilized using H2O2.
Surgery
Two weeks after deafening, animals in both the BDNF- and AP-treatment groups underwent implant surgery. Animals were anaesthetized with a single i.p. injection of 75mg/kg ketamine and 10mg/kg xylazine. Supplemental doses of ketamine/xylazine were administered every 20 mins, or upon observation of pedal or corneal reflexes. Local anaesthetic (Lignocaine, 4 mg) was injected s.c. along the incision line. Animals were also given systemic antibiotics (Baytril, 10mg/kg), electrolytes (10ml Hartmann’s solution) and analgesics (Rimadyl, 5mg/kg), all delivered s.c..
Under sterile conditions a postauricular incision was made and the left tympanic bulla was exposed. The bulla was opened and a fine probe was used to make a cochleostomy (∼0.2mm diameter) into the scala tympani of the basal turn just below the stapedial artery. The delivery tube was primed with either AP or BDNF and the tip of the cannula introduced into the cochleostomy until the PVC tubing was flush against the otic capsule. Crushed muscle was used to seal the opening in the bulla and Durelon cement fixed the cannula within the bulla cavity. The cannula was also secured at the skull using suture (Vetafil) and attached to the pump which was inserted into a subcutaneous pocket between the scapulae. The wound was closed with Vicryl sutures. The right cochlea of each animal served as an untreated deafened control (DC). At the end of the 28 day implantation period (i.e. a total deafening period of 6 weeks) each animal’s cochleae were harvested as described above.
SGN Density and Soma Area Measurements
SGN density measurements were performed using the same procedures as outlined in the Longitudinal deafening study described above. SGN soma area measurements were also made from the same five randomly selected sections as used in SGN density measurements. For soma area measurements, only those cells exhibiting a clear nucleolus were measured in order to minimize any variation in sampling. For each cochlea, 40 cells from each of the four cochlear regions were measured using NIH image. A single observer performed all area measurements blind.
Statistical Analysis
Results were expressed as mean and standard deviation, and statistical analysis was performed using a one-way ANOVA. Groups were compared using a pairwise multiple comparison procedure (Bonferroni t-test).
Results
A. Longitudinal Deafening Study
Co-administration of gentamicin and furosemide caused a complete loss of both IHCs and OHCs in all turns, although the structure of the organ of Corti initially remained largely intact (Fig. 1). The structure of the organ of Corti did, however, degenerate - usually between 6 and 10 weeks following the onset of deafness. SGNs exhibited evidence of degeneration from 2 weeks after deafening; a reduction in SGN density (Fig. 2), and changes to the soma, including the appearance of vacuoles, hyperpigmented nuclei and cell shrinkage, became apparent at this time. SGN loss was less extensive in the apical than in the basal regions of the cochlea.
Figure 1.
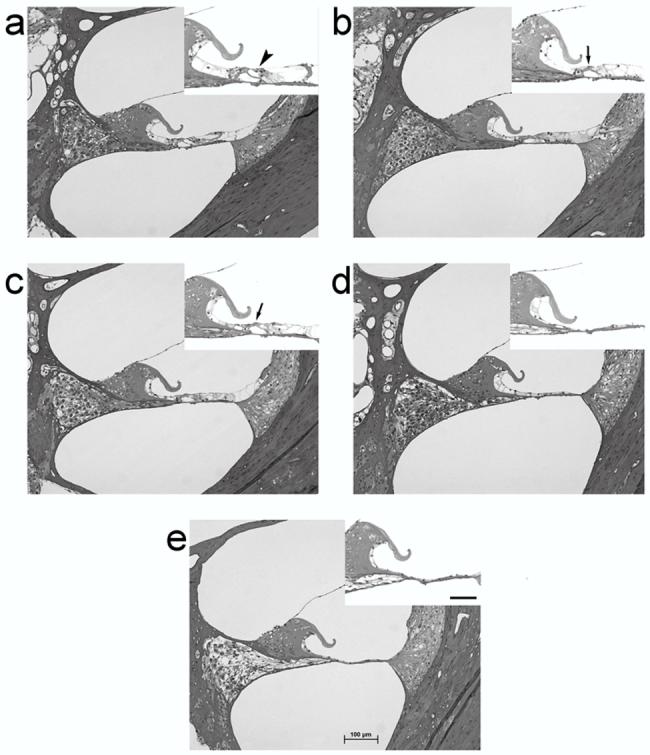
Photomicrographs of the UBT in: (a) normal hearing; and (b) 2 week; (c) 4 week; (d) 6 week; (e) and 10 week deafened rat cochleae. Note the absence of hair cells in the deafened cochleae, and the gradual degeneration of peripheral processes and SGNs. Scale bar = 100 μm. The inset in each micrograph illustrates the organ of Corti/basilar membrane at higher magnification. The arrowhead in the normal cochlea (a) illustrates the presence of outer hair cells. Arrows in the 2 and 4 week deafened cohorts (b & C) illustrate the structure of the organ of Corti in cochleae where the hair cells have undergone degeneration. In cochleae deafened for 6 and 10 weeks (d & e) the structure of the organ of Corti has completely degenerated. (Scale bar = 50 μm).
Figure 2.
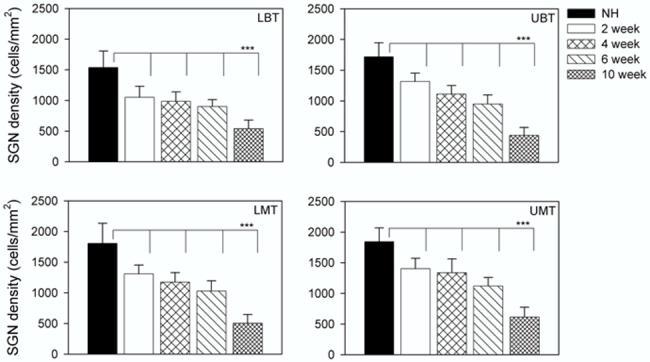
SGN density (cells/mm2) as a function of duration of deafness. The plot illustrates the mean (+ 1 standard deviation) for each cohort across the four cochlear turns examined. All deafened cohorts exhibited a statistically significant reduction in cell density compared with the NH controls across each cochlear region (all P<0.001; Table 1).
SGN density in normal hearing animals was significantly greater than that seen in all deafened groups for all four cochlear turns (p<0.001, Bonferroni t-test). While there was evidence of ongoing neural degeneration as a function of duration of deafness, there was not always a significant loss of SGNs from one time point to the next (Fig. 2; Table 1). There was, however, a statistically significant difference between the 10 week deafened animals and all other groups (Table 1).
Table 1.
Multiple pairwise comparison (Bonferroni t-test) of SGN density between normal hearing controls (NH) and animals deafened for 2, 4, 6 and 10 weeks
| LBT | UBT | LMT | UMT | |||||
|---|---|---|---|---|---|---|---|---|
| t | P | t | P | t | P | t | P | |
| NH vs 2 wk | 8.32 | <0.001 | 7.94 | <0.001 | 7.84 | <0.001 | 7.53 | <0.001 |
| NH vs 4 wk | 8.75 | <0.001 | 11.99 | <0.001 | 9.97 | <0.001 | 8.65 | <0.001 |
| NH vs 6 wk | 10.91 | <0.001 | 16.07 | <0.001 | 12.99 | <0.001 | 13.04 | <0.001 |
| NH vs 10 wk | 13.95 | <0.001 | 25.28 | <0.001 | 20.62 | <0.001 | 21.03 | <0.001 |
| 2 wk vs 4 wk | 1.07 | NS | 4.05 | 0.001 | 2.13 | NS | 1.12 | NS |
| 4 wk vs 6 wk | 1.35 | NS | 3.38 | 0.01 | 2.48 | NS | 3.92 | 0.002 |
| 6 wk vs 10 wk | 5.04 | <0.001 | 10.63 | <0.001 | 8.75 | <0.001 | 9.14 | <0.001 |
Note: LBT, lower basal turn; UBT, upper basal turn; LMT, lower middle turn; UMT, upper middle turn.
B. BDNF Study
Animal Recovery
Eleven of the twelve implanted animals made an uneventful recovery from surgery. In contrast, one animal from the AP group exhibited a significant decline in weight and was withdrawn from the study.
Delivery system patency
Post mortem examination showed that in all cases, the polyimide tip of the cannula had remained within the cochlea throughout the infusion period. Examination of the delivery system showed that all connections had remained intact. All delivery systems were found to be patent and no pump contained residual fluid.
SGN survival
The deafening technique resulted in complete loss of hair cells throughout all cochlear turns of the BDNF-treated, AP-treated and DC cochleae. Representative photomicrographs showing the typical cochlear histology from the UBT of each of the treatment groups are illustrated in Fig. 3. SGNs in DC showed evidence of degeneration including shrunken somata, hyperpigmented nuclei and the presence of large vacuoles (Fig. 4). The SGNs in deafened AP-treated cochleae exhibited a pathological response similar to the DC cochleae (Fig. 4). Moreover, the extent and profile of the SGN loss in these cochleae were similar to the untreated DC (Fig. 5).
Figure 3.
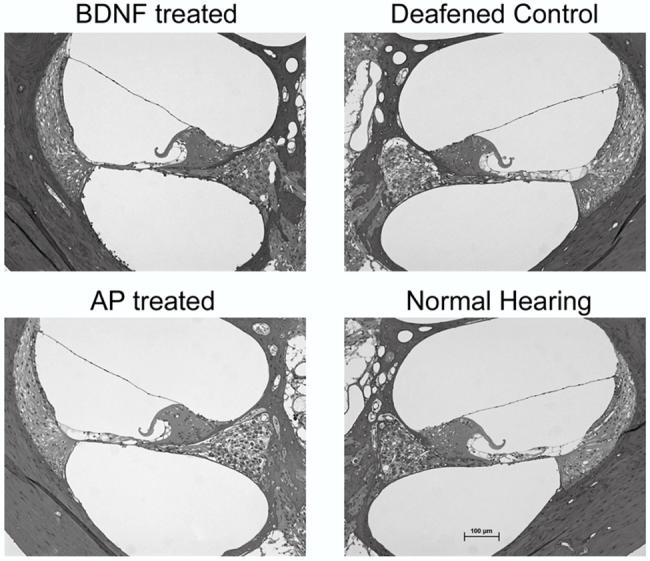
Representative photomicrographs of the UBT in deafened BDNF-treated animals, untreated DC, deafened AP-treated controls and NH cochleae. Note that all deafened cochleae were deafened for a period of 6 weeks. Scale bar = 100 μm
Figure 4.
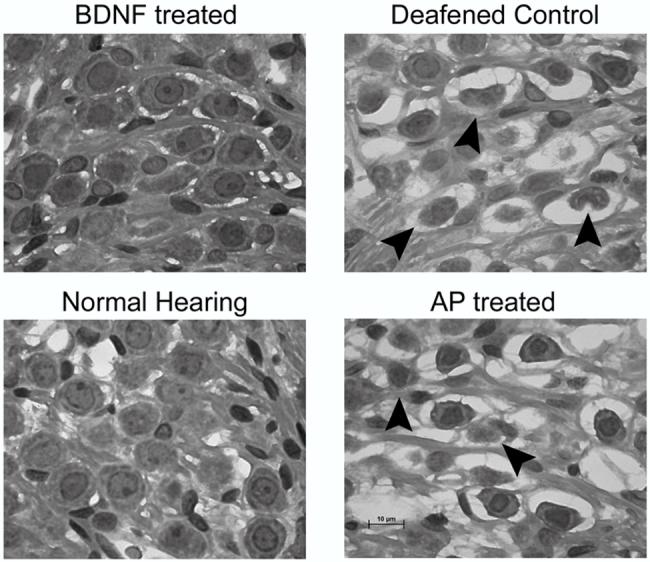
Higher power photomicrographs illustrating representative SGN morphology in each of the groups studied. Arrowheads illustrate a number of SGNs that are in the process of degeneration. Scale bar = 10 μm
Figure 5.
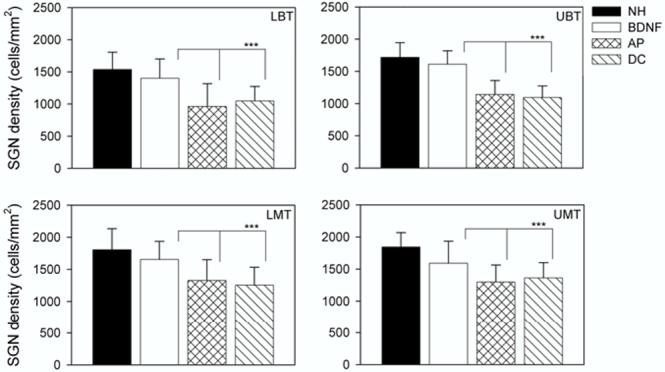
SGN density (cells/mm2) as a function of treatment group. The plot illustrates the mean (+ 1 standard deviation) for each cohort across the four cochlear turns examined. All deafened cohorts (BDNF; AP and DC) had been deafened for a total period of 6 weeks. There was no statistically significant reduction in cell density for the BDNF cohort compared with the NH controls across the basal-most three cochlear regions, although there was a significant reduction for the BDNF cohort in the UMT (Table 2). Both the NH control and BDNF groups exhibited statistically significant increases in SGN density compared to the AP and DC cohorts across all turns examined (Table 2).
In contrast, deafened BDNF-treated cochleae exhibited a SGN population similar in both histological appearance and neural density to those of NH controls. The SGN population included well-defined cell bodies, clear nuclei and prominent nucleoli (Fig. 4). Furthermore, SGNs in the BDNF-treated cochleae were clearly larger and more numerous than those in the other deafened cohorts.
Finally, the extent of SGN loss in deafened cochleae decreased towards the apical region with the most significant loss seen in the basal turn (Fig. 5). There was a clear trophic advantage in all turns of cochleae treated with BDNF, although this effect was more prominent in the basal region of the cochlea.
Statistical Analyses
There was a statistically significant difference between BDNF-treated cochleae and all other deafened cochleae across all four cochlear turns (Fig. 5; Table 2). There was also a statistically significant difference between NH animals and the untreated deafened and AP-treated cochleae. There was no statistically significant difference between the AP-treated cochleae and untreated DC cochleae. Importantly, there was no statistically significant difference in SGN density between BDNF-treated and NH control cochleae in the most basal three turns, although BDNF-treated cochleae did exhibit a significant reduction in SGN density compared to NH cochleae in the UMT (Table 2).
Table 2.
Multiple pairwise comparison (Bonferroni t-test) of SGN density between normal hearing controls and 6 week deafened animals treated with BDNF, AP or deafened untreated controls
| LBT | UBT | LMT | UMT | |||||
|---|---|---|---|---|---|---|---|---|
| t | P | t | P | t | P | t | P | |
| NH vs BDNF | 1.54 | NS | 1.78 | NS | 1.84 | NS | 3.36 | 0.01 |
| BDNF vs AP | 5.21 | <0.001 | 8.35 | <0.001 | 4.34 | <0.001 | 4.08 | <0.001 |
| BDNF vs DC | 3.99 | <0.001 | 9.17 | <0.001 | 5.29 | <0.001 | 3.18 | 0.019 |
| NH vs AP | 5.53 | <0.001 | 9.26 | <0.001 | 5.68 | <0.001 | 6.96 | <0.001 |
| AP vs DC | 1.01 | NS | 0.78 | NS | 0.92 | NS | 0.87 | NS |
Note: NH, normal hearing; BDNF, deafened & BDNF treated; AP, deafened and AP treated; DC, deafened, untreated controls.
Soma Area Measurements
Both DC and deafened AP-treated cochleae exhibited significant reductions in SGN soma area throughout the cochlea compared with BDNF-treated and NH control cochleae (Fig. 6; Table 3).
Figure 6.
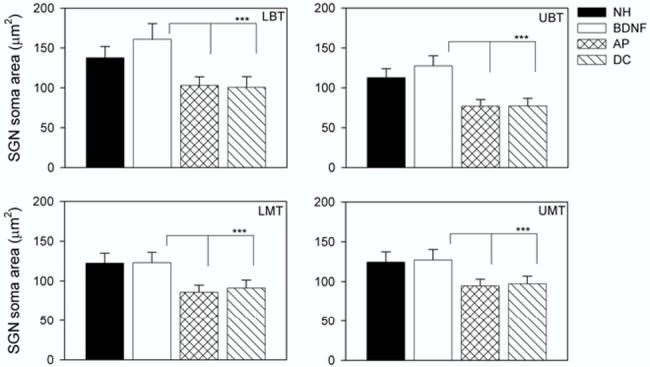
SGN soma area (μm2) as a function of treatment group. The plot illustrates the mean (+ 1 standard deviation) for each cohort across the four cochlear turns examined. All deafened cohorts (BDNF; AP and DC) had been deafened for a total period of 6 weeks. While there was a statistically significant increase in soma area for the BDNF cohort compared with the NH controls in the two basal-most cochlear turns, there was no significant difference in the two populations in LMT and UMT (Table 3). Both the NH control and BDNF groups exhibited statistically significant increases in SGN soma area compared to the AP and DC cohorts across all turns examined (all P<0.001; Table 3).
Table 3.
Multiple pairwise comparison (Bonferroni t-test) of SGN soma area between normal hearing controls and 6 week deafened animals treated with BDNF, AP or deafened untreated controls
| LBT | UBT | LMT | UMT | |||||
|---|---|---|---|---|---|---|---|---|
| t | P | t | P | t | P | t | P | |
| BDNF vs NH | 15.22 | <0.001 | 14.09 | <0.001 | 0.51 | NS | 2.34 | NS |
| BDNF vs AP | 38.69 | <0.001 | 48.97 | <0.001 | 34.45 | <0.001 | 30.42 | <0.001 |
| BDNF vs DC | 43.42 | <0.001 | 56.41 | <0.001 | 31.93 | <0.001 | 32.04 | <0.001 |
| NH vs AP | 23.65 | <0.001 | 33.54 | <0.001 | 33.30 | <0.001 | 27.54 | <0.001 |
Note: NH, normal hearing; BDNF, deafened & BDNF treated; AP, deafened and AP treated; DC, deafened, untreated controls.
Discussion
The present study reports significant rescue of SGNs in the deaf rat cochlea following chronic exogenous delivery of BDNF compared with AP-treated or untreated DC cochleae. The most extensive rescue of SGNs was evident in the basal region of the cochlea, although significant cell rescue did occur throughout all turns of the BDNF treated cochleae. This trophic advantage also resulted in a significant increase in SGN soma area compared with AP-treated or untreated DC cochleae. This is the first time, to our knowledge, that in vivo SGN rescue has been demonstrated in the rat - all previous studies of chronic exogenous neurotrophin delivery in the cochlea have been conducted using the guinea pig.
We also examined the longitudinal response of SGNs to gentamicin/furosemide-induced deafness in the rat. The profile of the SGN loss was similar to that seen in studies of other species, however the rate of SGN degeneration appeared to be slower in the rat than that of other rodents such as guinea pigs in which ∼50% SGN loss is evident four weeks following deafening via co-administration of an aminoglycoside and loop diuretic (14).
Exogenous application of BDNF in the rat cochlea rescues SGNs
BDNF-treated cochleae exhibited total loss of hair cells in all turns, consistent with untreated deafened cochleae. However, unlike DC or deafened AP-treated cochleae, the BDNF-treated cohort showed few signs of SGN degeneration when compared with NH control cochleae. Indeed, there was no statistically significant difference in SGN density between BDNF-treated and NH controls throughout most of the cochleae (LBT, UBT and LMT). Moreover, BDNF treatment produced a significant trophic effect on SGNs in all cochlear turns compared to untreated DC and AP-treated cochleae.
A significant reduction in SGN density, evident in the UMT of the BDNF cohort compared with NH controls, presumably reflects a reduction in the concentration of BDNF towards the apex of the cochlea, as the delivery cannula was located in the LBT. However, we cannot rule out the possibility that in the mature cochlea, basal SGNs are more sensitive to BDNF rescue compared with neurons located in more apical regions of the cochlea.
BDNF-treated cochleae also exhibited SGNs with large soma areas. In fact, in the basal region of the cochlea their somata were significantly larger than those in NH control cochleae. More apicalward the soma area of BDNF treated cochleae were similar to normal controls. While this observation must be interpreted cautiously due to the smaller number of control (n=4) versus BDNF treated cochleae (n=6), such an increase in SGN soma area associated with exogenous BDNF treatment is consistent with similar results using guinea pigs (21). Although the mechanisms underlying this increase in soma area remain unclear, studies of striatal projection neurons have also demonstrated that exogenous BDNF results in an increase in soma area when associated with neural rescue (22).
The present finding, that BDNF exerts a trophic effect on rat SGNs, demonstrates that this effect is not restricted to the guinea pig. This gives us confidence that the trophic effects of BDNF may be evident across a number of mammalian species, and that the use of this neurotrophin may have clinical applications in the future.
Safety and Clinical Implications
Since a broad objective of work in our laboratory is to develop therapies that may benefit cochlear implant patients, it is important to consider the possible clinical applications of neurotrophin therapy. The availability and functionality of SGNs are important factors that govern the benefits that patients can derive from cochlear implants. The number and integrity of these cells are considered to be of importance for the successful restoration of hearing for speech recognition (23,24). Therefore, any agent that can protect or regenerate SGNs would be of great clinical benefit.
Neurotrophins have been shown, by this study and others, to act as survival factors for mature SGNs in animal models of SNHL (12-15). Other research has demonstrated that neurotrophins and electrical stimulation act in synergy to prevent SGN degeneration (25,21). Taken together, this work suggests that neurotrophins may have clinical application when combined with cochlear implants. However, further research is required before neurotrophins can be used clinically. First, a number of in vitro studies have demonstrated synergistic effects on both SGN rescue and neuritogenesis when several growth factors are combined in treatment (16,26,27,17). In contrast, there is no evidence of a synergistic effect in the few in vivo studies that have combined growth factors (12). This discrepancy may be related to age and/or species effects, however, it is important that the full potential of combining growth factors is explored prior to any clinical application of neurotrophins for rescue of mature SGNs.
Second, the long-term safety and effectiveness of neurotrophins must be addressed. It has recently been shown that removal of neurotrophin treatment leads to an accelerated decline in SGN survival (14). This suggests that neurotrophins may need to be delivered long-term in order to be effective in the cochlea. The long-term delivery of neurotrophins into the cochlea is a major safety issue, particularly when exogenous neurotrophins are delivered at concentrations significantly above endogenous levels, as would be expected with the use of pumps. Under some circumstances neurotrophins are known to be neurotoxic as a result, for example, of free-radical induced necrosis (28). The strategy for application of neurotrophins in the inner ear is therefore a major issue that needs to be considered. The standard delivery technique in experimental studies is to cannulate the scala tympani and infuse via an osmotic pump (29). While osmotic pumps are effective in these studies, their finite lifespan, the potential for introducing infection into the inner ear, and the delivery of neurotrophins at concentrations well above physiological levels, make them unsuitable for long-term clinical use. A number of alternative delivery methods, including the use of viral vectors (25) and cell-based therapies (30), are currently being investigated. These techniques have the potential to provide long-term delivery of neurotrophins in a clinical setting.
Finally, because the scala tympani is connected to the cerebrospinal fluid via the cochlear aqueduct, future studies will also need to investigate the long-term safety implications of exogenous neurotrophin delivery in both the cochlea and the central nervous system.
Conclusion
We have shown that BDNF induces significant rescue of SGNs in the deafened rat cochlea. This finding demonstrates that the neurotrophic advantage that has previously been described in the guinea pig is also evident in the rat, and provides an important step in the development of neurotrophin therapy as a clinical intervention for cochlear implant patients.
Acknowledgements
We gratefully acknowledge the important contributions made by our colleagues; Dr. Patricia Hurley for advice on surgical techniques; Dr. Lisa Gillespie and Dr. Natalie Rickard for advice during the study and for critically reading a draft of this manuscript; Stephanie Epp, Anne Coco and Jenny Hardman for expert research assistance; Dr. Sue Peirce and Elisa Borg for veterinary advice and animal maintenance; and Maria Clarke and Prue Nielsen for histological support. This work was funded by the National Institutes of Health through the NIDCD (NO1-DC-3-1005), and the Faculty of Medicine, The University of Melbourne.
References
- 1.Leake PA, Hradek GT. Cochlear pathology of long term neomycin induced deafness in cats. Hear Res. 1988;33:11–33. doi: 10.1016/0378-5955(88)90018-4. [DOI] [PubMed] [Google Scholar]
- 2.Nadol JB., Jr. Degeneration of cochlear neurons as seen in the spiral ganglion of man. Hear Res. 1990;49:141–54. doi: 10.1016/0378-5955(90)90101-t. [DOI] [PubMed] [Google Scholar]
- 3.Shepherd RK, Hardie NA. Deafness induced changes in the auditory pathway: Implications for cochlear implants. Audiology & Neuro Otology. 2001;6:305–18. doi: 10.1159/000046843. [DOI] [PubMed] [Google Scholar]
- 4.Felix H, Pollak A, Gleeson M, Johnsson LG. Degeneration pattern of human first-order cochlear neurons. Adv Otorhinolaryngol. 2002;59:116–23. doi: 10.1159/000059249. [DOI] [PubMed] [Google Scholar]
- 5.Ylikoski J, Pirvola U, Moshnyakov M, et al. Expression patterns of neurotrophin and their receptor mRNAs in the rat inner ear. Hear Res. 1993;65:69–78. doi: 10.1016/0378-5955(93)90202-c. [DOI] [PubMed] [Google Scholar]
- 6.Schecterson LC, Bothwell M. Neurotrophin and neurotrophin receptor mRNA expression in developing inner ear. Hear Res. 1994;73:92–100. doi: 10.1016/0378-5955(94)90286-0. [DOI] [PubMed] [Google Scholar]
- 7.Wheeler EF, Bothwell M, Schecterson LC, von Bartheld CS. Expression of BDNF and NT-3 mRNA in hair cells of the organ of Corti: quantitative analysis in developing rats. Hear Res. 1994;73:46–56. doi: 10.1016/0378-5955(94)90281-x. [DOI] [PubMed] [Google Scholar]
- 8.Ernfors P, Van De Water T, Loring J, Jaenisch R. Complementary roles of BDNF and NT-3 in vestibular and auditory development. Neuron. 1995;14:1153–64. doi: 10.1016/0896-6273(95)90263-5. [DOI] [PubMed] [Google Scholar]
- 9.Lefebvre PP, Weber T, Rigo JM, et al. Peripheral and central target-derived trophic factor(s) effects on auditory neurons. Hear Res. 1992;58:185–92. doi: 10.1016/0378-5955(92)90127-9. [DOI] [PubMed] [Google Scholar]
- 10.Fritzsch B, Pirvola U, Ylikoski J. Making and breaking the innervation of the ear: neurotrophic support during ear development and its clinical implications. Cell & Tissue Research. 1999;295:369–82. doi: 10.1007/s004410051244. [DOI] [PubMed] [Google Scholar]
- 11.Ernfors P, Duan ML, ElShamy WM, Canlon B. Protection of auditory neurons from aminoglycoside toxicity by neurotrophin-3. Nat Med. 1996;2:463–7. doi: 10.1038/nm0496-463. [DOI] [PubMed] [Google Scholar]
- 12.Staecker H, Kopke R, Malgrange B, Lefebvre P, Van de Water TR. NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cells. Neuroreport. 1996;7:889–94. doi: 10.1097/00001756-199603220-00011. [DOI] [PubMed] [Google Scholar]
- 13.Miller JM, Chi DH, O’Keeffe LJ, et al. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int J Dev Neurosci. 1997;15:631–43. doi: 10.1016/s0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- 14.Gillespie LN, Clark GM, Bartlett PF, Marzella PL. BDNF-induced survival of auditory neurons in vivo: Cessation of treatment leads to an accelerated loss of survival effects. J Neurosci Res. 2003;71:785–90. doi: 10.1002/jnr.10542. [DOI] [PubMed] [Google Scholar]
- 15.Gillespie LN, Clark GM, Marzella PL. Delayed neurotrophin treatment supports auditory neuron survival in deaf guinea pigs. Neuroreport. 2004;15:1121–5. doi: 10.1097/00001756-200405190-00008. [DOI] [PubMed] [Google Scholar]
- 16.Lefebvre PP, Malgrange B, Staecker H, et al. Neurotrophins affect survival and neuritogenesis by adult injured auditory neurons in vitro. Neuroreport. 1994;5:865–8. doi: 10.1097/00001756-199404000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Marzella PL, Gillespie LN, Clark GM, Bartlett PF, Kilpatrick TJ. The neurotrophins act synergistically with LIF and members of the TGF-beta superfamily to promote the survival of spiral ganglia neurons in vitro. Hear Res. 1999;138:73–80. doi: 10.1016/s0378-5955(99)00152-5. [DOI] [PubMed] [Google Scholar]
- 18.Hardie NA, Shepherd RK. Sensorineural hearing loss during development: morphological and physiological response of the cochlea and auditory brainstem. Hear Res. 1999;128:147–65. doi: 10.1016/s0378-5955(98)00209-3. [DOI] [PubMed] [Google Scholar]
- 19.Imamura SI, Adams JC. Changes in Cytochemistry of Sensory and Nonsensory Cells in Gentamicin-Treated Cochleas. J Assoc Res Otolaryngol. 2003 doi: 10.1007/s10162-002-2037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown JN, Miller JM, Altschuler RA, Nuttall AL. Osmotic pump implant for chronic infusion of drugs into the inner ear. Hear Res. 1993;70:167–72. doi: 10.1016/0378-5955(93)90155-t. [DOI] [PubMed] [Google Scholar]
- 21.Shepherd RK, Serruto A, Epp SB, Crook JM. Protective effects of electrical stimulation and neurotrophin delivery on auditory neurons in vivo: Implications for cochlear implants. Assoc. Res. Otolaryngol. Daytona Beach, Fl. 2003:770. [Google Scholar]
- 22.Perez-Navarro E, Alberch J, Neveu I, Arenas E. Brain-derived neurotrophic factor, neurotrophin-3 and neurotrophin-4/5 differentially regulate the phenotype and prevent degenerative changes in striatal projection neurons after excitotoxicity in vivo. Neuroscience. 1999;91:1257–64. doi: 10.1016/s0306-4522(98)00723-4. [DOI] [PubMed] [Google Scholar]
- 23.Nadol JB, Jr., Young YS, Glynn RJ. Survival of spiral ganglion cells in profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol. 1989;98:411–6. doi: 10.1177/000348948909800602. [DOI] [PubMed] [Google Scholar]
- 24.Gantz BJ, Woodworth GG, Knutson JF, Abbas PJ, Tyler RS. Multivariate predictors of audiological success with multichannel cochlear implants. Ann Otol Rhinol Laryngol. 1993;102:909–16. doi: 10.1177/000348949310201201. [DOI] [PubMed] [Google Scholar]
- 25.Kanzaki S, Stover T, Kawamoto K, et al. Glial cell line-derived neurotrophic factor and chronic electrical stimulation prevent VIII cranial nerve degeneration following denervation. J Comp Neurol. 2002;454:350–60. doi: 10.1002/cne.10480. [DOI] [PubMed] [Google Scholar]
- 26.Mou K, Hunsberger CL, Cleary JM, Davis RL. Synergistic effects of BDNF and NT-3 on postnatal spiral ganglion neurons. J Comp Neurol. 1997;386:529–39. [PubMed] [Google Scholar]
- 27.Marzella PL, Clark GM, Shepherd RK, Bartlett PF, Kilpatrick TJ. Synergy between TGF-b3 and NT-3 to promote the survival of spiral ganglia neurones in vitro. Neuroscience Letters. 1998;240:77–80. doi: 10.1016/s0304-3940(97)00928-2. [DOI] [PubMed] [Google Scholar]
- 28.McDonald JW, Stefovska VG, Liu XZ, et al. Neurotrophin potentiation of iron-induced spinal cord injury. Neuroscience. 2002;115:931–9. doi: 10.1016/s0306-4522(02)00342-1. [DOI] [PubMed] [Google Scholar]
- 29.Prieskorn DM, Miller JM. Technical report: chronic and acute intracochlear infusion in rodents. Hear Res. 2000;140:212–5. doi: 10.1016/s0378-5955(99)00193-8. [DOI] [PubMed] [Google Scholar]
- 30.Andrew J. Rehabilitation of the deafened auditory nerve with Schwann cell transplantation. Hons. University of Melbourne; Melbourne: 2003. Thesis Department of Otolaryngology. [Google Scholar]


