Abstract
Antegrade, target-directed axonal regeneration is the explicit goal of nerve repair. However, aberrant and dysfunctional regrowth is commonly observed as well. At the site of surgical nerve coaptation axonal sprouts encounter fibrotic connective tissue rich in growth-inhibiting chondroitin sulfate proteoglycan that may contribute to misdirection of axonal regrowth. In the present study we tested the hypothesis that degradation of chondroitin sulfate proteoglycan by application of chondroitinase at the site of nerve repair can decrease aberrant axonal growth. Adult rats received bilateral sciatic nerve transection and end-to-end repair. One nerve was injected with chondroitinase ABC and the contralateral nerve treated with vehicle alone. After 28 weeks, retrograde axonal regeneration was assessed proximal to the repair by scoring neurofilament-immunopositive axons within the nerve (intrafascicular) and outside the nerve proper (extrafascicular). Intrafascicular retrograde axonal growth was equivalent in both control and chondroitinase treatment conditions. In contrast, chondroitinase treatment caused a pronounced (93%) reduction in extrafascicular retrograde axonal growth. The decrease in axon egress from the nerve was coincident with an increase in antegrade regeneration and improved recovery of motor function. Based on these findings we conclude that chondroitinase applied at the site of nerve transection repair averts dysfunctional extrafascicular retrograde axonal growth.
Keywords: chondroitinase, chondroitin sulfate proteoglycan, retrograde regeneration, nerve injury, nerve repair, peripheral nerve, morphometry
INTRODUCTION
In peripheral nerve crush injury (axonotmesis), where there is axotomy but the continuity of the nerve sheaths remain intact, axons regenerate within their original basal lamina tubes and excellent recovery can be expected. In contrast, axonal regrowth may be severely compromised after nerve transection (neurotmesis) wherein the success of surgical repair is highly dependent on reestablishing nerve continuity. Epineurial coaptation (neurorrhaphy) only grossly realigns nerve elements, therefore, the extent of regeneration is highly variable and, at best, partial recovery of function can be achieved (Dagum, 1998). For severed peripheral nerves to regenerate successfully, axonal sprouts emanating from the proximal stump first must traverse the repair site and then access a basal lamina tube in the distal nerve segment. Nerve transection and repair is associated with extensive and haphazard deposition of fibrotic connective tissue that may obstruct axonal regrowth. In addition, axonal sprouts emerging from the proximal stump after nerve coaptation are likely to first encounter a nonpermissive substratum rich in inhibitory chondroitin sulfate proteoglycan (CSPG) (Zuo et al., 1998; 2002). For these reasons, nerve disorganization and misdirection of axonal regrowth become a major problem and therapeutic concern.
Although antegrade, target-directed axonal regeneration is the explicit goal of nerve repair, aberrant growth is also commonly observed. Cajal (1928) first described the neuroma as a source of aberrant sprouting caused by the disruption of endoneurial tubes in a traumatic nerve injury. Axonal sprouts can become impeded within a disorganized mass of perineurial and Schwann cells to form a neuroma. Sprouts might also turn back and grow in a retrograde direction or, alternatively, they might escape through the repair site and grow in different directions outside the nerve proper. Neuroma and aberrant nerve regeneration have important therapeutic implications and are associated with dysaesthesia, paresthesia, the Tinel sign, chronic pain, and abnormal motor function (Wall and Gutnick, 1974; Scadding, 1981; Amir and Devor, 1993).
CSPGs are potent inhibitors of axonal growth throughout the nervous system (Fitch and Silver, 1997; Carulli et al., 2005). Inhibitory CSPGs are abundant in normal nerve and injury induces a rapid upregulation surrounding and distal to nerve trauma (Braunewell et al., 1995; Zuo et al., 1998; Rezajooi et al., 2004). We found that degradation of CSPG by application of chondroitinase improves axonal regeneration through neurotmetric lesions, indicating that inhibition of axonal growth by CSPG contributes to the relatively poor regeneration associated with end-to-end repair of transected peripheral nerves (Zuo et al., 2002). Based on these findings we postulate that inactivation of inhibitory CSPGs enhances the ability of axonal sprouts to cross nerve coaptations and gain access to the basal lamina tubes of the distal nerve, improving the outcome of peripheral nerve repair. In the present study we define and characterize intrafascicular and extrafascicular retrograde axonal regeneration. A powerful light microscopy, morphometric digital image analysis method was developed to score axons by neurofilament immunolabeling. These approaches were used to quantify antegrade and retrograde axonal regeneration and to test the effects of chondroitinase on aberrant axonal growth in a nerve transection repair model.
MATERIALS AND METHODS
Surgical Procedures
Nerve transection and direct repair
All surgical procedures were performed according to IACUC-approved protocols. Prior to transection the sciatic nerve was injected with either chondroitinase ABC (1 U in 2 μl) (affinity pure, Sigma Chemical Co, St. Louis, MO) or phosphate buffered saline (PBS) alone (vehicle control). Injections were made 1mm distal to the eventual site of nerve transection. This allowed chondroitinase to diffuse throughout the eventual injury site, minimized the concern of treatment leakage after transection and repair, and avoided needle damage to the proximal nerve (see Zuo et al., 2002). Young (180–200 g) adult female Sprague-Dawley rats (Harlan, Indianapolis, IN) were anesthetized with isoflourane. The sciatic nerve was exposed through a gluteal muscle-splitting incision and isolated free of underlying fascia and transected using serrated scissors. Each animal received bilateral transection injuries (chondroitinase treated and control nerves). The proximal and distal stumps were coapted by epineurial neurorrhaphy using 9–0 Ethilon sutures. Fibrin glue (fibrinogen and thrombin) was then applied to stabilize the union. The muscle was closed with 4–0 sutures, and the skin was sealed with wound clips. Animals were returned to standard housing.
Specimen preparation
Animals were deeply anesthetized and decapitate. Repaired nerves were excised and fixed by immersion in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.2) for 16 h at 4 °C. Nerves were equilibrated with PBS and cryoprotected by saturation with 30% sucrose in 0.1 M phosphate buffer for 2 days at 4 °C. Nerve segments were then trimmed according to suture landmarks and embedded for cryosectioning.
Immunocytochemistry
Cryosections (14 μm) were mounted on Superfrost Plus slides (Fisher) and heated at 50°C for 20 min. Embedding medium was removed by rinsing in PBS. Tissue sections were permeablized with 0.5% Triton X100 in PBS and then treated with 10% serum in PBS containing 0.1% Triton X100 (Blocking buffer) for 1 h at 37 °C. Primary antibodies diluted in Blocking buffer were applied to the sections and incubated overnight at 4 °C in a humidified chamber. Bound primary antibodies were labeled with swine anti-rabbit immunoglobulins (Dako, Carpinteria, CA) or goat anti-mouse immunoglobulins (Sigma) conjugated with fluorescein or rhodamine for 1 h at 37 °C in darkness. The anti-mouse secondary antibody was preadsorbed with rat serum prior to use. After thorough rinsing the immunolabeled sections were post-fixed for 10 min with 4% paraformaldehyde in 0.1 M phosphate buffer, rinsed and coverslipped in fluorophore-stabilizing mounting media. Regenerating axons were assessed by immunolabeling with polyclonal anti-GAP-43 IgG (2 μg/ml) (Ferguson and Muir, 2000) (NB300-143; Novus Biologicals, Littleton, CO). All axons were labeled by monoclonal anti-neurofilament IgG (4 μg/ml) (NAP4; Harris et al., 1993). Monoclonal anti-choline acetyl transferase (ChAT) (1:250) (MAB305; Chemicon International, Temecula CA) and polyclonal anti-calcitonin gene related protein (CGRP) (1:500) (Sigma, St. Louis, MO) antibodies were used to label cholinergic motor neurons and peptinergic sensory neurons, respectively. Schwann cells were labeled with polyclonal anti-S-100 antiserum (1:500) (Dako). Polyclonal anti-laminin (1:500) (NB300-144; Novus) and monoclonal anti-laminin (3 μg/ml) (2E8; Engvall et al., 1986) were used with and without pepsin antigen retrieval (Ekblom et al., 1982). Monoclonal antibodies C4S (1:300) (MAB2030; Chemicon) and C6S (1:300) (MAB2035; Chemicon) were used to label CSPG subtypes after digestion with chondroitinase as described previously (Zuo et al., 2002). Sudan black was used for histological staining of myelin (Gerrits et al., 1992).
Photomicrographs were captured using an Axioscope II (Carl Zeiss, Jena, Germany) equipped with a Spot II digital camera (Diagnostic Instruments, Sterling Heights, MI). Axon counting image analysis was performed with ImagePro Plus (Media Cybernetics, Inc., Silver Springs, MD). Fluorescence photographs were captured with a 40x objective. Images were flatten ten times (background-Dark, 20 pixels) and Best Fit brightness and contrast was applied. Area of interest (2500 μm2 frame) was selected and HiGauss Filter (7x7, Pass-1, strength-10) applied. Count range was set to 28-255 (gray level) with no filter and Watershed split at 2x used. Counts were obtained from 6 distinct areas of interest selected at random for each 40x micrograph, totaling 18 sample areas per nerve. Data were exported and analyzed using Excel (Microsoft, Redman, CA). Counts of intrafascicular axons (within the epineurial boundary of the nerve proper) were calculated from sampled axon densities extrapolated to the total area of nerve. Extrafascicular axons (those outside the nerve proper) were counted directly and were all inclusive. Photographic images were contrast-enhanced and sharpened (unsharp mask) for printing in Photoshop (Adobe Systems, San Jose, CA).
Behavioral testing of sciatic nerve function
Animals were acclimated to handling and test procedures prior to nerve injury and repair. Several tests of reflexive sciatic nerve function were conducted including thermal and tactile sensations, foot spread and foot grip strength. Response to heat was conducted on a hotplate analgesia meter (Columbus Instruments, Columbus, OH) set at 48 °C. Latency of initial response and the number of foot licks during a 180 second trial were recorded for each leg. Response to a tactile pain was conducted by pinching digits 4 and 5 with blunt forceps and scoring on a 3-point scale (no response, partial response, normal withdrawal with vocalization). Foot spread was assessed by rapidly raising the animal by the tail and scoring the extent of toe spreading on a 3-point scale. Foot grip strength was carried out on a platform fitted with a horizontal bar attached to a force meter (Imada, Inc. Northbrook, IL). The animal was pulled backwards on the platform, which it resisted by gripping the bar. The force meter recorded the maximum force (in grams) exerted until the grip was broken. Normal performance scores were obtained during the week prior to surgery and testing was conducted on a weekly schedule thereafter. Rats exhibiting digit autotomy were excluded.
RESULTS
Intrafascicular retrograde axonal growth
After nerve transection and repair, axon sprouts arise in the proximal stump and advance distally toward the coaptation. Some axons fail to traverse the coaptation, become misdirected, and grow in the opposite direction. This retrograde axonal regeneration was classified as intrafascicular and extrafascicular. Many axonal sprouts turn back and grow in association with established basal laminae and remain within the original perineurium (intrafascicular). On the other hand, other axonal sprouts deviate laterally at the repair site, exit the nerve proper and grow outside the epineurium (extrafascicular). Even 28 weeks after injury axonal sprouts remained active in and around the repair site and were readily visualized by GAP-43 immunolabeling (Fig. 1). Intrafascicular retrograde regeneration (IRR) was initiated near the axotomy and, in some instances, could be traced from its parent axon or observed as a growth cone growing proximal to the repair site. The reverse orientation of these growth cones was also inferred by the retrograde projection of their filopodia (Fig 1A). Quantitative assessment of IRR by directly counting GAP-43 immunolabeled axons was difficult because of the intermingling of retrograde sprouts with their proximal axons, and the latter were variably immunolabeled. However, IRR has been calculated as the difference between the number of axons in a normal nerve and the number of axons in a nerve several mm proximal to nerve repair (e.g., Mackinnon et al., 1991). Quantitative light microscopic image analysis of neurofilament-immunopositive profiles in transverse nerve sections detected 21,589 (±2,243) axons in the normal (uninjured) rat sciatic nerve at midthigh (Fig. 1B). Twenty-eight weeks after nerve transection and end-to-end repair the mean axon count 3 mm proximal to the repair was 28,257 (±1907), representing a 31% increase in axon counts compared to that for the normal nerve. This increase was mainly attributed to a distinct population of small axon clusters observed proximal to the repair site that were not found in normal nerves (Fig. 1C). Within the proximal nerve, these axon clusters were a distinguishable feature of IRR. The clusters of small caliber axons most likely represent multiple sprouts arising from axons growing as a regenerating unit (growing in the retrograde direction). These small axon clusters were previously described as retrograde regeneration by Bardosi (1989).
Figure 1.
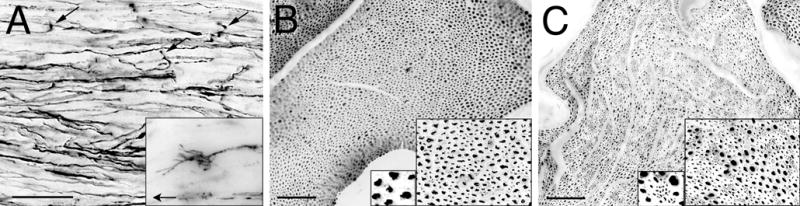
Intrafascicular retrograde regeneration (IRR) in rat sciatic nerve 28 weeks after transection and repair (direct epineurial neurorrhaphy). A) GAP-43 immunolabeling of regenerating axons in longitudinal section proximal to the repair site revealed many disoriented axons. Axonal sprouts were observed turning away from the repair site (arrows). Growth cones were found clearly oriented in the retrograde direction (inset; arrow points proximally). B) Transverse section of normal sciatic nerve showed a usual distribution of large and small diameter neurofilament immunolabeled axons. C) IRR was apparent proximal to the site of transection repair 28 weeks after injury by the appearance of clusters of small caliber, neurofilament-immunopositive axons (insets). Scale bars: A, 50 μm (inset: 4x); B and C, 100 μm (insets: 2x and 4x).
To evaluate axon numbers in nerve repair most previous studies used light microscopic counting of myelin profiles (toluidine blue staining of semi-thin sections). This method reveals less than 1/3 of the total axons in the normal sciatic nerve and, more so, may not accurately account for actively growing axons. Therefore, we tested several light microscopic axon scoring methods and developed an image analysis method for counting neurofilament-immunopositive axons (see Materials and Methods). This method is efficient and non-selective. A most comprehensive study (including light and electron microscopy) of fiber count in the rat sciatic nerve revealed approximately 27,000 axons at midthigh (Schmalbruch, 1986). By comparison our semi-automated, digital method detected approximately 21,600 axons at midthigh (80% of that detected by Schmalbruch). Furthermore, the neurofilament immunolabeling method was sensitive to normal and regenerating axons, myelinated and unmyelinated fibers, and discriminated individual small axon sprouts growing in clusters as regenerating units.
Extrafascicular retrograde axonal growth
Extrafascicular retrograde regeneration (ERR) emanated from the nerve coaptation and appeared as a distinct collection of GAP-43-positive axons found outside the epineurium (Fig. 2A). ERR was seen in transverse section as axon clusters running parallel to the nerve proximal to the site of repair (Fig. 2B). Small bundles of ERR axons were observed peeling away and invading neighboring tissues, especially muscle, where some appeared to synapse (Fig. 2C).
Figure 2.
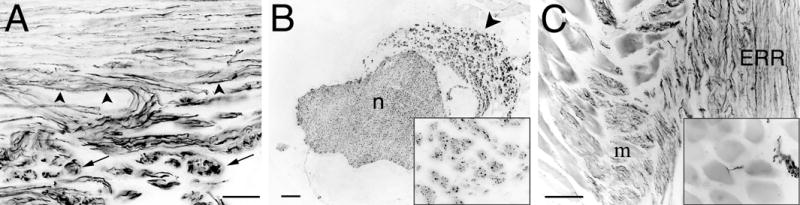
Extrafascicular retrograde regeneration (ERR) in rat sciatic nerve 28 weeks after transection and repair. A) At the suture line, ERR axons were evident as GAP-43 immunopositive axons extending outside the original nerve sheath (arrowheads). After exiting the nerve proper, ERR axons formed discrete clusters or minifascicles (arrows). B) Three mm proximal to the suture line, ERR was evident as neurofilament-immunopositive axons that formed an appendage of minifascicles (arrowhead) that ran anti-parallel to the nerve proper (n). Each minifascicle contained numerous small axons (inset). C) Some ERR minifascicles invaded and appeared to form synapses (inset) in adjacent muscle (m). Scale bars: A, 50 μm; B and C, 100 μm (insets: 4x).
ERR was accompanied by hyperplasia and the formation of supplementary nerve sheaths outside the nerve proper. ERR was readily distinguished by double-immunolabeling for GAP-43 (regenerating axons) and laminin (basal laminae) (Fig. 3A). We confirmed that the GAP-43 immunopositive ERR profiles were indeed axons by neurofilament immunolabeling (Fig. 3B).
Figure 3.
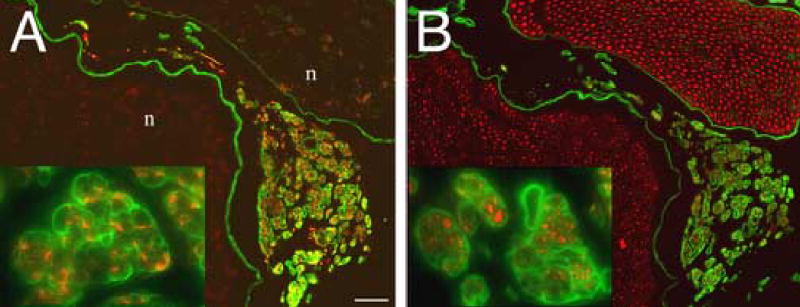
Definitive identification of ERR as GAP-43 immunoreactive axons within laminin labeled minifascicles. Transverse sections, 3mm proximal to the coaptation 28 weeks after nerve transection and repair, were double immunolabeled for (A) GAP-43 (growing axons, red) and laminin (basal laminae; green) or (B) neurofilament (all axons, red) and laminin (basal laminae, green). In (A), ERR minifascicles contain growing axons which express high levels of GAP-43 compared to unlabeled axons within the fascicles of the nerve proper (n). Laminin labeling revealed an intense immunoreactive basal laminae surrounding each ERR minifascicle (inset). In (B), a serial section from that in (A), neurofilament immunolabeling confirmed the identity of GAP-43 immunopositive profiles as axons and that only axons within ERR minifascicles express high levels of GAP-43. The lack of laminin labeling within the endoneurium of the nerve proper is an artifact of aldehyde fixation. As used here, laminin immunolabeling without antigen retrieval revealed the basal laminae of ERR minifascicles. Scale bars: A and B, 50 μm (insets: 4x).
The organization of extrafascicular axons and their associated sheaths was reminiscent of the minifascicles observed in nerve development and in nerve regeneration through acellular nerve grafts. Small clusters of axons were compartmentalized and encased in a laminin-rich sheath (Fig. 3). ERR minifascicles were easily distinguished from the established endoneurium within the nerve proper by a selective procedure for immunostaining laminin. Laminin immunolabeling of the endoneurial basal lamina in normal nerve was masked by aldehyde fixation and required antigen retrieval methods (e.g., pepsin pretreatment) for effective detection. In contrast, ERR minifascicles were intensely immunolabeled for laminin in fixed specimens without antigen retrieval, providing a reliable light microscopic means to distinguish ERR from any normal nerve branches. This was true for immunostaining using several monoclonal and polyclonal antibodies raised against laminin-1, but did not apply to unfixed tissues. The reason for this fixation-dependent, selective immunolabeling of endoneurial laminin remains unclear but might involve steric interactions that occur only in fully mature basal laminae. This selective immunolabeling is not exclusive to ERR and also was observed in association with regeneration distal to the nerve coaptation and within acellular nerve grafts (not shown). Related observations have been reported in other tissues (e.g., Ekblom et al., 1982; Mori et al., 1992).
The ultrastructure of ERR minifascicles has been described previously but little is known about the composition of the regenerative sheaths. Immunolabeling for laminin and the heparan sulfate proteoglycan, perlecan, were colocalized and indicated the presence of a Schwann cell basal lamina (Figs. 4A and B). We also examined the ERR minifascicular sheath for proteoglycans known to contribute to the structure and function of conventional nerve sheaths. First, two classes of chondroitin sulfate proteoglycans (CSPGs) were immunolabeled. Chondroitin-4-sulfate proteoglycan (CS4-PG) immunoreactivity was found surrounding the ERR minifascicles (Fig. 4C) while chondroitin-6-sulfate proteoglycan (CS6-PG) occupied the inner aspect of the minifascicles, more closely associated with the extraneural axons (Fig. 4D). Next, we examined the distributions of two specific CSPGs. NG2 immunolabeling circumscribed the ERR minifascicles, representing a subset of the CS4-PG distribution (Fig. 4E). Versican was restricted to the interior of the ERR sheaths and colocalized with CS6-PG immunolabeling (Fig. 4F). Therefore, versican was mainly colocalized with laminin and perlecan, most likely indicating the formation of a basal lamina-like structure closely associated with and partitioning the Schwann cell-axon units. NG2, on the other hand, occupied the region between and around the Schwann cell-axon units, suggesting deposition by perineurial cells. Overall, ERR minifascicles contained promoters and inhibitors of axonal regeneration in arrangements similar to that observed in antegrade regeneration within the endoneurium and Schwann cell basal lamina distal to nerve repair (Braunewell et al., 1995; Zuo et al., 1998; Martin et al., 2001; Morgenstern et al., 2003; Rezajooi et al., 2004). The functional significance of these arrangements and the separate compartmentation of CS4-PG (e.g., NG2) and CS6-PG (e.g., versican) in normal and regenerating peripheral nerve remains unclear and is presently under investigation.
Figure 4.
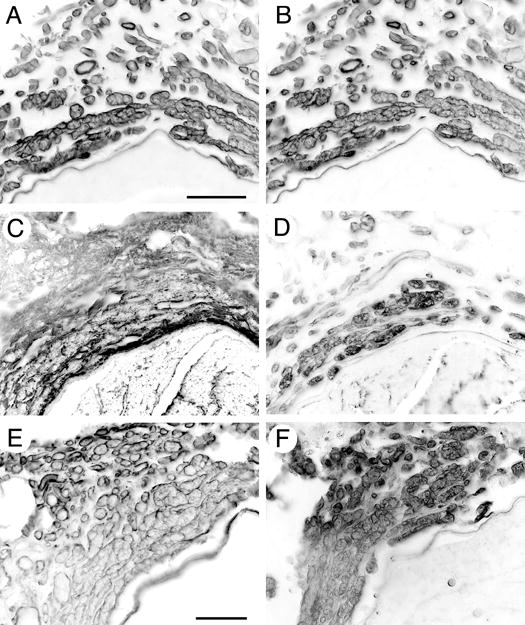
Characterization of ERR basal laminae components. Transverse sections of 28 week regenerated sciatic nerve 3mm proximal to the coaptation were immunolabeled for (A) laminin and (B) perlecan. Colocalization of perlecan and laminin is consistent with that in basal laminae in normal nerve. Chondroitin-4-sulfate proteoglycan (CS4-PG) immunoreactivity was found surrounding the ERR minifascicles (C) while chondroitin-6-sulfate proteoglycan (CS6-PG) occupied the inner aspect of the minifascicles, more closely associated with the extraneural axons (D). NG2 immunolabeling circumscribed the ERR minifascicles, representing a subset of the CS4-PG distribution (E). Versican was restricted to the interior of the ERR sheaths and colocalized with CS6-PG immunolabeling (F). Scale bars: A-D, 50 μm; E-F, 25μm.
ERR includes myelinated motor and sensory axons
Retrograde regeneration has been implicated as the cause of spontaneous discharges and hyperalgesia attributed to aberrant growth of unmyelinated sensory fibers. We investigated whether both motor and sensory neurons contributed to ERR. Sections of nerve proximal to the site of transection repair were immunostained for ChAT and CGRP to identify motor and sensory axons, respectively. Figure 5A shows numerous ERR axons were strongly immunoreactive for CGRP. Numerous ChAT immunopositive axons were found within each EER minifascicle as well (Fig. 5B). Many of the ERR minifascicles apparently contained both sensory and motor axons. Numerous S-100 immunopositive Schwann cells were closely associated with the ERR axons and surely contributed to their ensheathment (Fig. 5C). Also, numerous ERR axons were finely myelinated and both myelinated and unmyelinated axons were found within most ERR minifascicles (Fig. 5D). Based on these observations we concluded that ERR involves both sensory and motor axons that are ensheathed by differentiated Schwann cells.
Figure 5.
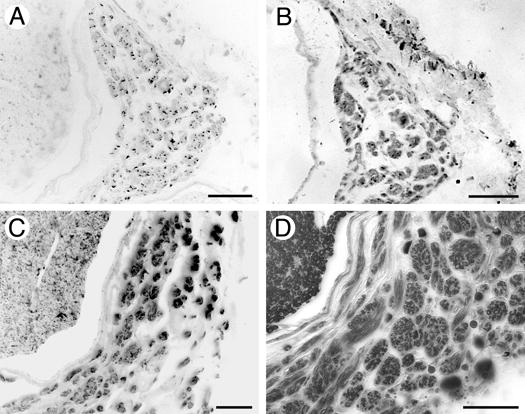
Identification of myelinated motor and sensory axons within ERR minifascicles. Transverse sections of 28 week regenerated sciatic nerve 3mm proximal to the coaptation were immunolabeled for the specific sensory and motor axon markers, CGRP (A) and ChAT (B), respectively. ERR minifascicles contain both sensory and motor axons. The identification of S100 immunopositive Schwann Cells (C) and myelination with Sudan Black (D) indicates that many axons within the ERR minifascicles are well organized and ensheathed by differentiated Schwann cells. Scale bars: A-D, μm.
Chondroitinase treatment decreases extrafascicular retrograde regeneration
Growth inhibiting CSPGs are abundant in the peripheral nerve and are upregulated after injury. In previous studies we found that degradation of CSPGs by application of chondroitinase at the site of nerve transection repair enhances axonal regeneration. Here, we tested the hypothesis that retrograde regeneration is decreased by application of chondroitinase in nerve repair. Adult rats received bilateral sciatic nerve transection and end-to-end repair (neurorrhaphy). One nerve was injected with chondroitinase ABC and the contralateral nerve treated with vehicle alone (control). After 28 weeks, IRR and ERR were assessed 3mm proximal to the repair site by scoring neurofilament-immunopositive axons within the nerve and outside the nerve proper, respectively. As described above, the mean axon count for normal sciatic nerves was 21,589 (±2,243). The axon count 3mm proximal to nerve transection repair in the control condition (transection repair with vehicle injection) increased to 28,257 (±1907) (Fig. 6A), indicating approximately 31% of these axons were growing in a retrograde direction (IRR) (P < 0.02). For repaired nerves injected with chondroitinase the mean axon count was 30,107 (±1,658) and not significantly different than the control condition (P < 0.24). These results indicate that chondroitinase treatment applied at the site of nerve transection repair did not affect IRR axon sprouting. Overall, no morphological differences were evident within the proximal nerves between the control and chondroitinase conditions.
Figure 6.
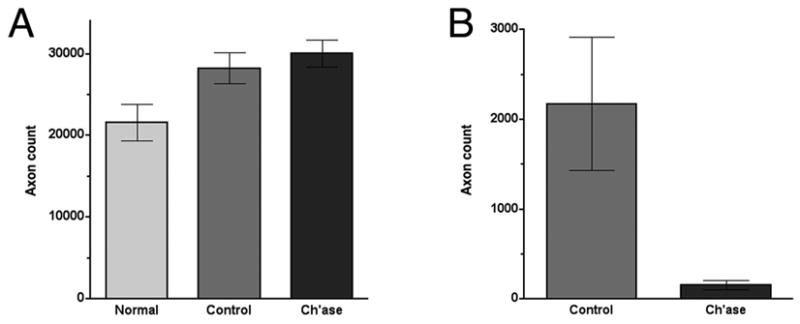
Chondroitinase treatment decreases extrafascicular retrograde regeneration. Adult rats received bilateral sciatic nerve transection and end-to-end repair (neurorrhaphy). One nerve was injected with chondroitinase ABC and the contralateral nerve treated with vehicle alone (control). After 28 weeks, neurofilament-immunopositive axons were counted 3mm proximal to the repair site. (A) Intrafascicular retrograde regeneration (IRR) was evident by an increase in axon count within repaired nerves when compared to normal nerves. Results indicate approximately 25% of the axons found within the nerve proper proximal to the repair were attributed to retrograde axonal growth. IRR was not altered significantly by chondroitinase treatment applied at the site of nerve transection repair. (B) Extrafasciclular retrograde regeneration (ERR) was scored by direct count of neurofilament-immunopositive axons found outside the nerve proper 3mm proximal to the repair. For repaired nerves injected with chondroitinase the mean number of ERR axons was a reduced significantly (P < 0.01, t-test). Data represent the means (±SE) of 9 nerves in each condition.
In contrast to IRR, chondroitinase treatment had a pronounced effect on retrograde axonal growth found outside the nerve proper. In the control condition the mean number of ERR axons was 2,176 ±740. For repaired nerves injected with chondroitinase the mean number of ERR axons was a mere 153 ±51 (P < 0.01) (Fig. 6B). The marked reduction in ERR resulting from chondroitinase treatment was observed for all animals (n=9, subject to each condition in bilateral repair). As expected, along with the reduction in ERR axons in the chondroitinase condition there was a corresponding decrease in the formation of extraneural minifascicles and total cellularity associated with ERR.
Chondroitinase treatment improves axonal regeneration after nerve transection repair
Our previous short-term, morphometric studies provide strong evidence that degradation of CSPG by in vivo application of chondroitinase enhances the ability of axonal sprouts to cross nerve coaptations after nerve repair and gain access to the basal lamina tubes of the distal nerve (Zuo et al., 2002). In the present study we tested the effects of chondroitinase treatment on long-term (up to 28 weeks) axonal growth and recovery of sciatic nerve function after transection repair. In the same subjects, axons were counted 5mm distal to nerve repair (antegrade regeneration). At this location in normal (uninjured) nerves the mean axon count was 22,319 (±1,823) (Fig. 7A). For repaired nerves in the control condition (vehicle alone) the mean axon count was 28,947 (±641). For repaired nerves injected with chondroitinase the mean axon count was 31,393 (±1,308), and represented a statistically significant difference (P < 0.05) compared to the control condition. These findings indicate that chondroitinase treatment resulted in a persistent increase in distal axonal regeneration.
Figure 7.
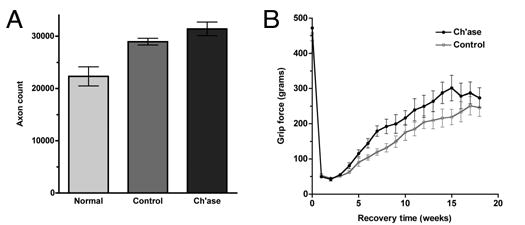
Chondroitinase treatment improves axonal regeneration after nerve transection repair. The effects of chondroitinase treatment was tested on long-term (up to 28 weeks) axonal growth and recovery of sciatic nerve function after transection repair. (A) Antegrade neurofilament-immunopositive axons were counted 5mm distal to nerve repair. Regenerated nerves contained more axons (including collaterals) than normal (uninjured) nerves. The mean axon count in repaired nerves injected with chondroitinase was significantly greater than those in the control condition (vehicle alone) (P < 0.05, t-test). (B) Recovery of motor function in the hind limbs was assessed by measuring grip strength using a force meter apparatus. Preoperative grip strength was approximately 460 g in both test groups and this dropped to near 50 g in the first weeks after nerve transection and repair. Average grip strength increased more rapidly and attained a higher maximum for repaired nerves treated with chondroitinase (62% recovery of preoperative grip force) compared to the control condition (54% recovery) (P < 0.01, Likelihood ratio test).
Several tests of sciatic nerve function were conducted including thermal and tactile sensations, reflexive foot spread and foot grip strength. Using a combined measures index (not shown), subjects in both conditions recovered approximately 50% of their original sensory and motor functions and approached an apparent maximal recovery by 18 weeks after nerve repair. The foot grip strength assessment was the most quantitative and discriminating test used and the only to detect a significant difference between control and chondroitinase treatment conditions (Fig. 7B). Preoperative grip strength was approximately 460 g in both test groups and this dropped to near 50 g in the first weeks after nerve transection and repair. Average grip strength increased more rapidly and attained a higher maximum for repaired nerves treated with chondroitinase compared to the control condition (P < 0.01). A 62% recovery of preoperative grip force was attained with chondroitinase treatment compared to 54% recovery with vehicle alone.
DISCUSSION
After nerve injury the distal segment of severed axons degenerate and are cleared from the path of the regenerating axons. As nerve regeneration proceeds the number of axons distal to the site of nerve repair increases transiently above that found originally and then gradually decreases toward normal levels (Jenq and Coggeshall, 1985; Mackinnon et al., 1991). This regenerative pattern is consistent with the observation that individual nerve fibers sprout several collaterals that grow in small clusters or “regenerating units” distal to the repair site (Morris et al., 1972). As axons reinnervate targets, sprouts that fail to make functional connections may degenerate or be pruned away (Brown and Booth, 1983; Brushart, 1993). Interestingly, a similar regenerative pattern occurs proximal to nerve repair because regenerating sprouts might fail to traverse the repair site, turn and grow in the retrograde direction. Retrograde regeneration has been estimated to increase the proximal fiber count by 40–50% in the first several months after nerve repair (Aitken, 1949; Scadding and Thomas, 1983; Mackinnon et al., 1991). Axonal sprouts which grow in the retrograde direction originate from the proximal stump near the suture line, can extend considerable distances along the parent nerve and may even backtrack to proximal branches, before innervating novel targets (Scadding and Thomas, 1983; Leis et al., 2003). Retrograde axonal regeneration can be classified as intrafascicular and extrafascicular. Intrafascicular axonal sprouts are those that turn back and grow within the original perineurial sheath. Extrafascicular axonal sprouts deviate laterally, exit at the repair site, and grow outside of the nerve proper. In species larger than rodents with multifascicular nerves, extrafascicular axonal regeneration may also occur in the interfascicular connective tissue (Spector et al., 2000).
For both antegrade and retrograde regeneration inappropriate reinnervation is associated with neuropathy, including loss of and abnormal function. Considerable progress has been made in developing methods to enhance nerve regeneration by induction of growth-promoting signaling pathways and reduction of growth inhibiting signals. Although these interventions can increase axonal regeneration, recent evidence indicates that appropriate target reinnervation remains a therapeutic challenge (English et al., 2005). The present study was performed to examine the long-term outcome of chondroitinase treatment on axonal growth and recovery of function. Our treatment scheme involved a single injection of chondroitinase at the time and site of nerve repair. This application method was shown to thoroughly degrade CSPG throughout the nerve several mm proximal and distal to the injection (Zuo et al., 2002). Chondroitinase activity is short-lived in vivo; so too is the depletion of CSPGs which are rapidly expressed in response to injury (Zuo et al., 1998). After 28 weeks of regeneration, a striking observation was the abundance of GAP-43 expression, indicating continuing axonal sprouting and growth. GAP-43 immunopositive sprouts with growth cones were found growing in all directions within the neuroma at the suture line. In addition, bundles of actively growing axons projected not only distally, but proximally as well. In general our observations confirm previous reports that proximal fiber counts increase after injury and represent axonal sprouts growing in an inappropriate retrograde direction (Aitken, 1949; Scadding and Thomas, 1983; Mackinnon et al., 1991). Our observations at 28 weeks after repair approximate the period of peak fiber counts in the time course study by Mackinnon et al. (1991). The mechanisms that lead to retrograde growth have not been studied directly. However, because there is a strong propensity for antegrade regeneration it is likely that misdirected growth results from the inability of axonal sprouts to navigate the repair site and access the promotive substratum in the distal nerve. At 28 weeks after repair, nerve continuity was clearly established yet axonal sprouting and retrograde growth persisted in the neuroma. This might explain why chondroitinase applied at the time of injury had no observable effect on IRR over time. Additional study is required to determine if application of chondroitinase after nerve continuity has been reestablished may be beneficial to the cessation of retrograde sprouting and the resolution of neuroma.
The most conspicuous manifestation of retrograde regeneration was ERR, which appeared as bundles of regenerating axon units growing outside the epineurial sheath. Axonal regeneration after nerve repair is complicated by the misalignment of proximal and distal elements. Nerve continuity is further challenged by swelling and axoplasmic outflow (mushrooming) which interferes with accurate coaptation and realignment. In addition, inhibitory CSPG is rapidly upregulated at the injury site and NG2-expressing cells are prevalent throughout the connective tissue neuroma around the surgical coaptation (Morganstern et al., 2003). These features likely explain the diversion of axonal sprouts at the suture line leading to aberrant growth outside the nerve proper. A role for inhibitory CSPG in the initiation of extraneural growth was confirmed by the finding that chondroitinase applied at the time of injury and repair markedly reduced ERR. We postulate that degradation of inhibitory CSPG resolves a major molecular barrier to axonal growth that results from structural discontinuity and muddling of promotive and inhibitory compartments. Chondroitinase creates a more permissive nerve substratum and likely allows axonal sprouts greater latitude in their effort to locate and access basal laminae tubes in the distal nerve. This is consistent with our previous findings that chondroitinase enhances nerve regeneration in end-to-end nerve repair and nerve grafting (Zuo et al., 2002, Krekoski et al., 2001). Furthermore, axonal regeneration is a staggered process that will expose growth cones to an evolving molecular milieu (Witzel et al., 2005). An important attribute of chondroitinase treatment is a decrease in the latency of nerve regeneration (Krekoski et al., 2001). Therefore, because inhibitory CSPG is upregulated after injury, more axonal growth can occur before inhibition is reinstated and fibrosis ensues. In combination, the affects of chondroitinase improve antegrade axonal growth and nerve continuity which, in turn, reduces axonal disorientation and ERR. This may be especially important in the initial stages of nerve repair before the outer nerve sheaths have mended, presumably preventing the egress of axons thereafter. The reduction in axonal egress at the suture line by chondroitinase treatment may be even more efficacious in cumbersome repair of larger, multifascicular human nerves and in nerve grafting.
Thus far we have only examined the impact of a single chondroitinase injection made at the time of nerve injury and repair. It is clear that the process of nerve regeneration and remodeling continues for at least two years in the rodent (Mackinnon et al., 1991). Therefore, the early effects of chondroitinase treatment are likely to be overshadowed by natural processes over time. Nevertheless, in the present 28 week study there was a significant increase in the number of regenerated axons distal to repair in chondroitinase treated nerves compared to control nerves. Interestingly, the average increase in distal axon counts approximated the reduction in ERR axons resulting from chondroitinase treatment. Although we have no way of knowing if these outcomes are related directly, it is reasonable to assume that a reduction in axons lost at the suture line will increase the amount of intraneural axonal regeneration. Furthermore, the increase in antegrade regeneration was associated with better recovery of motor function. The most marked improvement in grip strength observed in the chondroitinase condition was attributed to a rapid rate of recovery in the first two months after repair. This is consistent with the conclusion that a single injection of chondroitinase at the time of nerve repair improves early axonal regeneration. Future study is required to determine if additional benefits in nerve regeneration and recovery of function can be realized by a temporal series of chondroitinase treatments.
It is generally accepted that at least some supernumerary axonal regeneration is eventually pruned back in favor of fibers that effectively reinnervate target tissues. On the other hand, misdirected axonal growth can still innervate targets and therefore become permanent. Although there is little, if any, direct anatomical evidence that retrograde regeneration contributes to actual recovery of function, clinical reports implicate retrograde axonal growth in a variety of a paradoxical reinnervation patterns observed after injury (Sunderland, 1968; Leis et al., 2003). In the present study ERR axons were found to invade adjacent muscle and therefore may contribute to abnormal motor function. Extrafascicular regeneration contributes to neuroma formation and retrograde nerve regeneration is associated with dysesthesia, paresthesia, and chronic pain (Wall and Gutnick, 1974; Scadding, 1981; Amir and Devor, 1993). Therefore, in addition to improving the chances for appropriate reinnervation of distal targets, aversion of ERR by application of chondroitinase at nerve coaptations may also have important therapeutic potential for attenuating neuropathy associated with nerve repair.
Acknowledgments
Funded by a grant from the National Institutes of Health (NS37901). The authors thank Jonathan Young and Cheryl Richie for excellent technical assistance.
References
- Aitken JT. The effect of peripheral connexions on the maturation of regenerating nerve fibres. Journal of Anatomy. 1949;83:32–43. [PMC free article] [PubMed] [Google Scholar]
- Amir R, Devor M. Ongoing activity in neuroma afferents bearing retrograde sprouts. Brain Res. 1993;630:283–288. doi: 10.1016/0006-8993(93)90667-c. [DOI] [PubMed] [Google Scholar]
- Bardosi A. Schwann cell recruitment from intact nerve fibers. Exp Neurol. 1989;103:123–134. doi: 10.1016/0014-4886(89)90073-3. [DOI] [PubMed] [Google Scholar]
- Braunewell KH, Martini R, LeBaron R, Kresse H, Faissner A, Schmitz B, Schachner M. Up-regulation of a chondroitin sulphate epitope during regeneration of mouse sciatic nerve: Evidence that the immunoreactive molecules are related to the chondroitin sulphate proteoglycans decorin and versican. Eur J Neurosci. 1995;7:792–804. doi: 10.1111/j.1460-9568.1995.tb00682.x. [DOI] [PubMed] [Google Scholar]
- Brown MC, Booth CM. Postnatal development of the adult pattern of motor axon distribution in rat muscle. Nature. 1983;304:741–742. doi: 10.1038/304741a0. [DOI] [PubMed] [Google Scholar]
- Brushart TM. Motor axons preferentially reinnervate motor pathways. J Neurosci. 1993;13:2730–2738. doi: 10.1523/JNEUROSCI.13-06-02730.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carulli D, Laabs T, Geller HM, Fawcett JW. Chondroitin sulfate proteoglycans in neural development and regeneration. Curr Opin Neurobiol. 2005;15:116–120. doi: 10.1016/j.conb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Dagum AB. Peripheral nerve regeneration, repair, and grafting. J Hand Ther. 1998;11:111–117. doi: 10.1016/s0894-1130(98)80007-0. [DOI] [PubMed] [Google Scholar]
- Ekblom P, Miettinen M, Rapola J, Foidart JM. Demonstration of laminin, a basement membrane glycoprotein, in routinely processed formalin-fixed human tissues. Histochemistry. 1982;75:301–307. doi: 10.1007/BF00496733. [DOI] [PubMed] [Google Scholar]
- English AW. Enhancing axon regeneration in peripheral nerves also increases functionally inappropriate reinnervation of targets. J Comp Neurol. 2005;490:427–441. doi: 10.1002/cne.20678. [DOI] [PubMed] [Google Scholar]
- Engvall E, Davis GE, Dickerson K, Ruoslahti E, Varon S, Manthorpe M. Mapping of domains in human laminin using monoclonal antibodies: localization of the neurite-promoting site. J Cell Biol. 1986;103:2457–2465. doi: 10.1083/jcb.103.6.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson TA, Muir D. MMP-2 and MMP-9 increase the neurite-promoting potential of Schwann cell basal laminae and are upregulated in degenerated nerve. Mol Cell Neurosci. 2000;16:157–167. doi: 10.1006/mcne.2000.0859. [DOI] [PubMed] [Google Scholar]
- Fitch MT, Silver J. Glial cell extracellular matrix: Boundaries for axon growth in development and regeneration. Cell Tissue Res. 1997;290:379–384. doi: 10.1007/s004410050944. [DOI] [PubMed] [Google Scholar]
- Gerrits PO, Brekelmans-Bartels M, Mast L, Gravenmade EJ, Horobin RW, Holstege G. Staining myelin and myelin-like degradation products in the spinal cords of chronic experimental allergic encephalomyelitis (Cr-EAE) rats using Sudan black B staining of glycol methacrylate-embedded material. J Neurosci Methods. 1992;45:99–105. doi: 10.1016/0165-0270(92)90047-h. [DOI] [PubMed] [Google Scholar]
- Harris J, Moreno S, Shaw G, Mugnaini E. Unusual neurofilament composition in cerebellar unipolar brush neurons. J Neurocytol. 1993;22:1039–1059. doi: 10.1007/BF01235748. [DOI] [PubMed] [Google Scholar]
- Jenq CB, Coggeshall RE. Numbers of regenerating axons in parent and tributary peripheral nerves in the rat. Brain Res. 1985;326:27–40. doi: 10.1016/0006-8993(85)91381-2. [DOI] [PubMed] [Google Scholar]
- Krekoski CA, Neubauer D, Zuo J, Muir D. Axonal regeneration into acellular nerve grafts is enhanced by degradation of chondroitin sulfate proteoglycan. J Neurosci. 2001;21:6206–6213. doi: 10.1523/JNEUROSCI.21-16-06206.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis AA, Lancon JA, Stokic DS. Retrograde regeneration following neurotmesis of the ulnar nerve. Muscle Nerve. 2003;28:512–514. doi: 10.1002/mus.10444. [DOI] [PubMed] [Google Scholar]
- Mackinnon SE, Dellon AL, O'Brien JP. Changes in nerve fiber numbers distal to a nerve repair in the rat sciatic nerve model. Muscle Nerve. 1991;14:1116–1122. doi: 10.1002/mus.880141113. [DOI] [PubMed] [Google Scholar]
- Martin S, Levine AK, Chen ZJ, Ughrin Y, Levine JM. Deposition of the NG2 proteoglycan at nodes of ranvier in the peripheral nervous system. J Neurosci. 2001;21:8119–8128. doi: 10.1523/JNEUROSCI.21-20-08119.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern DA, Asher RA, Naidu M, Carlstedt T, Levine JM, Fawcett JW. Expression and glycanation of the NG2 proteoglycan in developing, adult, and damaged peripheral nerve. Mol Cell Neurosci. 2003;24:787–802. doi: 10.1016/s1044-7431(03)00245-8. [DOI] [PubMed] [Google Scholar]
- Mori S, Sternberger NH, Herman MM, Sternberger LA. Variability of laminin immunoreactivity in human autopsy brain. Histochemistry. 1992;97:237–241. doi: 10.1007/BF00267633. [DOI] [PubMed] [Google Scholar]
- Morris JH, Hudson AR, Weddell G. A study of degeneration and regeneration in the divided rat sciatic nerve based on electron microscopy. II. The development of the "regenerating unit". Z Zellforsch Mikrosk Anat. 1972;124:103–130. [PubMed] [Google Scholar]
- Ramon y Cajal S. Degeneration and regeneration of the nervous system. Oxford; London: 1928. [Google Scholar]
- Rezajooi K, Pavlides M, Winterbottom J, Stallcup WB, Hamlyn PJ, Lieberman AR, Anderson PN. NG2 proteoglycan expression in the peripheral nervous system: upregulation following injury and comparison with CNS lesions. Mol Cell Neurosci. 2004;25:572–584. doi: 10.1016/j.mcn.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Scadding JW. Development of ongoing activity, mechanosensitivity, and adrenaline sensitivity in severed peripheral nerve axons. Exp Neurol. 1981;73:345–364. doi: 10.1016/0014-4886(81)90271-5. [DOI] [PubMed] [Google Scholar]
- Scadding JW, Thomas PK. Retrograde growth of myelinated fibres in experimental neuromas. J Anat. 1983;136 (Pt 4):793–799. [PMC free article] [PubMed] [Google Scholar]
- Schmalbruch H. Fiber composition of the rat sciatic nerve. Anat Rec. 1986;215:71–81. doi: 10.1002/ar.1092150111. [DOI] [PubMed] [Google Scholar]
- Spector JG, Lee P, Derby A. Rabbit facial nerve regeneration in autologous nerve grafts after antecedent injury. Laryngoscope. 2000;110:660–667. doi: 10.1097/00005537-200004000-00023. [DOI] [PubMed] [Google Scholar]
- Sunderland S. Nerves and nerve injuries. Williams & Wilkins; Baltimore: 1968. pp. 180–193. [Google Scholar]
- Wall PD, Gutnick M. Properties of afferent nerve impulses originating from a neuroma. Nature. 1974;248:740–743. doi: 10.1038/248740a0. [DOI] [PubMed] [Google Scholar]
- Witzel C, Rohde C, Brushart TM. Pathway sampling by regenerating peripheral axons. J Comp Neurol. 2005;485:183–190. doi: 10.1002/cne.20436. [DOI] [PubMed] [Google Scholar]
- Zuo J, Hernandez YJ, Muir D. Chondroitin sulfate proteoglycan with neurite-inhibiting activity is up-regulated following peripheral nerve injury. J Neurobiol. 1998;34:41–54. [PubMed] [Google Scholar]
- Zuo J, Neubauer D, Graham J, Krekoski CA, Ferguson TA, Muir D. Regeneration of axons after nerve transection repair is enhanced by degradation of chondroitin sulfate proteoglycan. Exp Neurol. 2002;176:221–228. doi: 10.1006/exnr.2002.7922. [DOI] [PubMed] [Google Scholar]


