Abstract
Objective
To compare biobehavioral pain responses of preterm infants born at differing gestational ages (GAs) when pain was preceded by a rest period or by a series of routine nursing interventions.
Methods
In a randomized, within subjects, cross-over design, facial (Neonatal Facial Coding System), sleep/wake state and heart rate (HR) responses of 43 preterm infants [mean birth weight: 1303 g (range 590 g to 2345 g); mean GA at birth: 30 weeks (range 25 to 32)] were examined across 3 phases of blood collection (Baseline, Lance, and Recovery) under 2 conditions: pain after a 30-minute rest period versus pain after a series of routine nursing interventions (clustered care). Infant behavioral responses were coded from continuous bedside videotapes. HR was analyzed using custom physiologic signal processing software.
Results
Infants born at earlier GA (<30 wk) had equally intense facial responses during the Lance phase regardless of condition. However, later born infants (≥ 30 wk GA) showed heightened facial responses indicative of sensitized responses during blood collection when it was preceded by clustered care (P = 0.05). Moreover, later born infants had significantly lower facial (P = 0.05) and HR (P = 0.04) reactivity during Recovery when blood collection followed clustered care.
Discussion
Earlier born preterm infants showed heightened states of arousal and poor ability to modulate HR during Recovery when an invasive procedure was preceded by routine tactile nursing procedures. Alternatively, later born infants exhibited sensitized responses when clustered care preceded blood collection. Our findings support the importance of cue based individualized approaches to care.
Keywords: preterm infant, pain, clustered care
Preterm birth rates continue to rise in many industrialized countries due to new reproductive technologies, to advancing maternal age, and to advances in postdelivery neonatal care.1–3 However, from the therapeutic procedures intended to ensure their survival, preterm infants undergo repeated episodes of pain and stress. Recent studies indicate that noxious stimuli, such as skin breaking during heel lance, are transmitted directly to the somatosensory cortex as early as 25 weeks gestational age (GA) and that these responses are more pronounced in infants born at earlier GAs.4,5
Preterm infants are especially vulnerable to the effects of pain exposure.6 They are at risk for enhanced pain sensitivity because their developing central and peripheral nervous systems differ from those of adults. Converging evidence from animal and preterm infant studies suggests that early exposure to pain and stress induces long-term changes in pain sensitivity,7,8 may alter generalized stress-arousal systems,9–11 and may alter the developing brain.12
To help counter the adverse effects of early repetitive stressor exposure on developing neonates, many neonatal intensive care units (NICUs) have implemented developmental care models, the most commonly used one being the Newborn Individualized Developmental Care and Assessment program.13 These models direct care-givers to base the timing of interventions on infants’ individual cues, to reduce or eliminate unnecessary handling and environmental stressors, such as noise and bright lights, and to provide interventions, such as nonnutritive sucking, during intrusive or skin-breaking procedures.14
One suggested developmental care strategy to reduce stress is clustering care. The goal of clustering care is to allow infants to rest for long periods. In fact, a number of studies have shown that stable preterm infants slept more,15,16 weighed more, and had more rapid decline in the incidence of apnea17 when they were given rest periods. These benefits may be more apparent than real. The increased sleep reported in preterm infants after clustered interventions may be the result of increased energy expenditure.17 Furthermore, less stable preterm infants may find the clustering of procedures very stressful.18 In fact, a recent study has shown that preterm infant body movements indicative of stress were higher and oxygen saturations lower during clustered care than during blood collection.19
Most investigations of preterm infant pain responses have observed their reactions to a single event, usually heel lance during blood collection. Yet, in the clinical setting, blood collection may be clustered with other routine, nonskin breaking, care-giving tasks which may alter the pain experience. Indeed, in preterm infants, greater number of painful procedures experienced within the preceding 24 hours induced sensitized behavioral responses during subsequent endotracheal suctioning.20 However, this study did not examine the effects of tactile handling on subsequent pain responses, nor did the timing of the painful procedures mirror the “clustered” type of handling which is so common in NICUs.
In the only study to report the effects of clustered handling on subsequent pain responses, preterm infants who, before blood collection, experienced immobilization positioning, like that used during lumbar punctures, had higher heart rates (HRs), greater facial responses, and crying than infants who had not been handled before blood collection.21 Importantly, this study did not include infants born at early GAs, infants who are not only more vulnerable to the cumulative effects of handling, but also whose responses to pain and stress differ from those of later born infants.20,22–24
Therefore, the aims of our study were to compare preterm infant responses to procedural pain after a rest period with their responses to procedural pain after a series of routine interventions (clustered care) and to examine GA and sex differences in infant response patterns. To address the effects of maturity at birth, we compared responses of infants born <30 weeks GA with those born ≥ 30 weeks GA. We chose this GA cut-off for our grouping because over the past 5 years in our nursery, one of the largest tertiary units in Canada, 29–30 weeks seemed to be a cut-off for invasive intervention. More specifically, the majority of infants born at 29 weeks required intubation (57%); whereas, intubation rates for infants born at 30 weeks was only 43%.
MATERIALS AND METHODS
Participants
The study sample comprised 43 preterm neonates (19 female, 24 male) born ≤ 32 completed weeks GA [mean 30 weeks (range 25 to 32)], admitted to a major regional level-III NICU. Infants with a major congenital anomaly, significant intraventricular hemorrhage (IVH grade III), and/or parenchymal brain injury (IVH grade IV and/or periventricular leukomalacia), as well as infants who had received analgesics or sedatives within 72 hours of the targeted study session, were excluded. All infants were tested at 32 weeks ( ± 7 d) postconceptional age. Thirty-three infants were appropriate for GA, 8 were small for GA, and 2 were large for GA. GA was defined using very early maternal ultrasound.25 More than 80% of mothers had an obstetric ultrasound. For those who did not have an early ultrasound, obstetric dates were established by a combination of time from last menstrual period corroborated by data from ultrasound examinations later in the pregnancy, and by clinical characteristics of the baby at delivery. GPOWER was used to calculate the sample size estimate.26 Using conventional parameters for sample size estimates (5% false positive rate and a power of 80%, 2 sided), and a significance level of 0.05, sample size calculations were carried out on the basis of the Neonatal Facial Coding Systems (NFCS) during blood collection at 31 to 33 weeks post conceptional age.27,28 Using the most conservative estimate, 15 infants were needed to detect differences between each phase of blood collection. To examine sex and GA differences, the sample size was increased to 43.
Procedures
The infants were recruited by a NICU research nurse, and written informed consent was obtained from the mother according to a protocol approved by the Clinical Research Ethics Board of the University of British Columbia. Using a within subjects cross over design in random order, each infant was tested on 2 occasions on separate days. On 1 day, a cluster of nursing procedures preceded blood collection [Clustered Care followed by Pain (CCP)]. For the CCP condition, a rest interval of 20 minutes was necessary between the clustered care and the blood collection because our nursery follows a developmental care model which structures care so that infants are not exposed to prolonged periods of handling. Therefore, we did not alter the normal pattern of care for research purposes. On the other day, the blood collection was preceded by an uninterrupted period of rest of at least 30 minutes [rest followed by pain (RP)]. The order of RP and CCP was randomly assigned to infants when they were entered into the study.
Each infant was lying in the isolette undisturbed for a minimum of 30 minutes before the recording. Videotaping and physiologic recordings were carried out continuously. HR data were collected by attaching the leads from the bedside monitor to a custom-designed computer data acquisition system. A camera was positioned for close-up on the face and was attached to a custom made recording setup on a moveable cart which included a 9″ video monitor. The signal was fed directly to a VCR and a time code was imprinted automatically. Each study phase was marked with an inaudible event cue signal recorded simultaneously on the videotape and on the physiologic acquisition systems. A research technician set up the video camera and the VCR, operated the computerized cardiac data acquisition system and marked each event.
A single research nurse carried out clustered nursing procedures (clustered care) in the following set order: changing the diaper, measuring the abdominal girth, taking the axillary temperature, and cleaning the mouth with gauze and sterile water. Blood collection (pain) after heel warming was carried out by a laboratory technician who cleansed the heel, applied a lancet, and squeezed the heel to collect blood. For this study, infant responses across the 3 phases of blood collection (Baseline, Lance, and Recovery) were analyzed to compare biobehavioral responses to pain either after rest or after clustered care.
Measures
Infant State
Infant sleep/wake state was coded according to the NIDCAP protocol: 1 = deep sleep, 2 = light sleep, 3 = drowsy, 4 = quiet awake, 5 = active awake, 6 = highly aroused/crying, and 7 = prolonged respiratory pause >8 seconds.29 The predominant state over the first 2-minute period was coded for each phase; the most frequently observed state during each phase was used for data analysis. The state coding was performed by 1 of the coauthors (LH) who is NIDCAP certified. Reliability for the NIDCAP was initially established during the certification process.30 In addition, a randomly selected sample of 5% of NIDCAP video segments from the study was coded to evaluate reliability. NIDCAP reliability was calculated by determining % agreement of occurrence (both coders indicating the presence or absence of a behavior) within each 2-minute time segment. Interrater agreement was 87%.
Facial Activity: Neonatal Facial Coding System
The NFCS is a reliable, well validated behavioral pain measure used widely in studies of preterm infants.24,31,32 The entire NFCS was coded for the following 3 periods: Baseline = 20 seconds before the first contact by the laboratory technician, Lance = 20 seconds immediately after the heel lancing, and Recovery = 20 seconds after the last contact of the laboratory technician. The average time for the Lance phases for each condition (CCP and RP) was 4.9 minutes (SD 2.5) and was not significantly different between procedures (t = 0.08, P = ns). Coding was carried out by a trained coder who was blind to the purpose and design of the study, and to all medical information about the infants. All 9 facial actions (brow lowering, eyes squeezed shut, deepening of the nasolabial furrow, open mouth, vertical mouth stretch, horizontal mouth stretch, taut tongue, chin quiver, and tongue protrusion) were coded as occurred/did not occur for each 2-second segment during 20 seconds for each phase of blood collection. The frequency of each NFCS face action was examined, and those occurring less than 5% after heel lance were dropped; tongue protrusion and chin quiver were excluded due to low occurrence. The 7 remaining actions were summed across each time segment and also across each event for statistical analysis. Reliability coding was carried out on a subset of 20% of the sample with a reliability coefficient of 0.88.28
HR
Continuous electrocardiographic (ECG) activity was recorded from a single lead of surface ECG (lead II), and was digitally sampled at 360 Hz off-line using a specially adapted computer acquisition system. Custom physiologic signal processing software was used to acquire, process, and analyze HR.33 R waves were detected from the sampled ECG and were used to form a smoothed instantaneous 4-Hz time series as described previously.34 Mean HR was calculated for a 2-minute epoch for each study phase (Baseline, Lance, and Recovery).35 However, because there is risk for gross motor artifacts in response to the heel lance, and because of the biphasic nature of HR responses to sudden stimuli, the 2-minute epoch sampled for the “Lance” phase began 20 seconds after the skin breaking event.36–38
Infant Characteristics
Information was collected by prospective chart review from birth to each test day including, but not limited to the following: birth weight, GA at birth, Apgar score at 1 minute, illness severity using the Score for Neonatal Acute Physiology (SNAP-II),39 head ultrasound scan results, daily opioid and other analgesic and sedative exposure, number and types of invasive skin breaking procedures, respiratory support, and type and time of last handling just before blood collection. Cumulative procedural pain was operationalized as the sum of every skin breaking procedure from birth to the first assessment day (eg, heel lance, intramuscular injection, chest tube insertion, central line insertion). Each attempt at a procedure was included; thus, the sum reflected all skin breaks. Although it is recognized that procedures differ in pain intensity, in the absence of an empirical basis for assigning weights to every procedure, counting is used as a “marker” of infant acute pain in the NICU.22,40 Total intravenous (IV) morphine exposure was calculated from birth to the first test day by multiplying the average daily dose of IV morphine, adjusted for daily weight, by the number of days of IV morphine, as we have done previously.22 For example, if an infant received an average dose of 0.39 mg/kg body weight for 24 treatment days, the morphine score was 9.36 (mg/kg).
Data Analysis
Sleep-wake states were analyzed using nonparametric tests (Kruskall-Wallis and Friedman). Then, continuous measures (facial activity and HR) were examined using repeated measures analysis of covariance to compare pain responses after rest or after clustered care (RP vs. CCP), with illness severity on day 1 (SNAP score) as a covariate and sex and GA group at birth (group 1: infants born <30 wk GA; group 2: infants born ≥ 30 wk GA) as between subjects factors. Statistically significant analysis of covariance was followed by planned comparisons to identify differences between specific phases within each observation. Repeated measures data were examined for sphericity; when present, Greenhouse-Geisser ε values were used to determine significance. The significance level for each test was set at P ≤ 0.05.
RESULTS
First, 4 infants required handling within the 30-minute rest period before the blood collection in the RP group. The handling ranged from very mild tactile stimulation to secure ECG leads to repositioning 1 infant whose oxygen saturations dropped below clinically acceptable levels. We compared these infants’ facial and HR responses with the 39 infants who did not require handling. The exclusion of these 4 infants did not alter our results. The mean rest time before the blood collection in the remaining 39 infants in the RP group was 103 minutes (SD 46 min).
Next, HR data were examined for outliers, and 1 infant with exceptionally high Baseline HR (who was on dexamethasone treatment) was excluded from this study. In addition, 2 other infants’ HR data were excluded due to poor signal quality; however, their behavioral data were included in the analyses. The face of 1 infant was obscured during 1 Recovery phase, but all the other facial data for this infant were included. Thus, 42 infants were included in the behavioral data analyses, and 40 infants in the HR data analyses. Infant characteristics of the study group are presented in Table 1. In addition, sleep/wake state, facial and HR responses during Baseline for the RP and CCP procedures were compared. Although Baseline facial actions and HRs did not differ between procedures for the group as a whole, infants born <30 weeks GA were in active sleep during the Baseline phase for the RP procedure, but in deep sleep for the CCP procedure (z = − 2.1, P = 0.03). When we reanalyzed the data including Baseline state as a covariate, our findings were not altered. Finally, no sex differences were found in any of the analyses.
TABLE 1.
Participant Characteristics (N = 43)
| Mean ± SD | Median | Min | Max | |
|---|---|---|---|---|
| Birth weight | 1302 ± 405 | — | 590 | 2345 |
| Gestational age at birth | 29.7 ± 2.0 | — | 25.1 | 32.7 |
| Postnatal age on first testing day (d) | 16.5 | — | 3 | 50 |
| APGAR (5-min) | 8.5 ± 1.0 | — | 6 | 10 |
| Illness severity day 1 (SNAP-II)* | 11.1 ± 9.2 | — | 0 | 34 |
| Intravenous morphine exposure (mg/kg) | 0.08 | — | 0 | 0.63 |
| Days on ventilator† | — | 5.5 ± 9 | 0 | 38 |
| Other respiratory support† | — | 8.8 ± 8.8 | 0 | 32 |
| Invasive procedures since birth† | — | 61 ± 42 | 7 | 157 |
Score for Neonatal Acute Physiology.
From birth to first testing day.
Sleep/Wake State
The infants were either in a drowsy state (RP n = 24, CCP n = 21) or were highly aroused/crying (RP n = 16, CCP n = 18) during the Lance. Sleep/wake states changed significantly across phases of blood collection (Baseline, Lance, and Recovery) during both the RP (χ2 = 43.5, P<0.0001) and CCP (χ2 = 48.8, P<0.0001) conditions, with shifts to greater arousal during Lance. We found no difference in sleep/wake states between the RP and CCP conditions during Lance or Recovery phases, nor were there sex or GA effects.
HR
After controlling for early illness severity and between groups differences in sex, for the infants as a whole, mean HR increased significantly from Baseline to Lance and decreased during Recovery for both the RP (F = 13.7, P<0.0001) and CCP (F = 25.1, P<0.0001) conditions. In addition, there remained a GA effect which explained 19% of the variance (F = 4.8, P<0.01). More specifically, infants born ≥ 30 weeks GA had a lower mean HR during the Recovery phase when blood collection occurred after clustered care (F = 4.1, P<0.04). To examine change in mean HR, the following formula was applied to generate a change score (HRchange): Lance HR-Baseline HR/Baseline HR (which takes into account the law of initial values).41 No significant differences in HRchange between the RP and CCP conditions, sex, or GA effects were found.
Facial Activity
Facial activity increased significantly from Baseline to Lance and decreased during Recovery for both the RP (F = 10.1, P<0.0001) and CCP (F = 21.6, P<0.0001) conditions. When facial responses during the Lance phase of the RP and CCP were compared, significantly more facial activity during Lance phase for the CCP procedure (t = 2.1, P<0.05) was found. That is, infants showed greater facial responses during blood collection after they had experienced clustered care. As with the HR changes, after controlling for early illness and sex, a GA effect remained (F = 4.3, P<0.04), an effect which explained 10% of the variance. This facial heightened response was observed only in the infants who were born ≥30 weeks GA (Fig. 1, Table 2). Although infants born <30 weeks GA did show a slight increase in facial activity while experiencing the Lance during the CCP procedure, this finding was not statistically significant.
FIGURE 1.
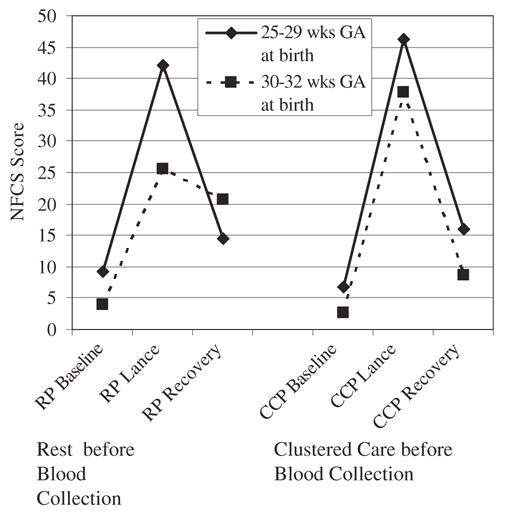
Facial responses during phases of blood collection (Baseline, Heel Lance, and Recovery) during the 2 procedures [rest before blood collection (RP) or clustered care before blood collection (CCP)] comparing infants 25 to 29 weeks and 30 to 32 weeks gestational age at birth.
TABLE 2.
Facial (Neonatal Facial Coding System Total Scores) and Heart Rate (Mean ± SD) Responses to Phases of Blood Collection (Baseline, Heel Lance, and Recovery) Before or After Clustered Care by GA Group
| Facial Activity
|
Mean HR
|
|||
|---|---|---|---|---|
| 25–29 wk GA at Birth | 30–32 wk GA at Birth | 25–29 wk GA at Birth | 30–32 wk GA at Birth | |
| Clustered care before blood collection | ||||
| Baseline* | 6.7 ± 7.6 | 2.6 ± 6.4 | 158.3 ± 10.2 | 148.1 ± 10.8 |
| Heel lance/squeeze | 46.2 ± 21.0 | 37.8 ± 23.3 | 182.9 ± 14.3 | 168.8 ± 13.8 |
| Recovery | 16.0 ± 22.6 | 8.6 ± 15.9 | 160.7 ± 15.4 | 151.0 ± 14.4† |
| Rest before blood collection | ||||
| Baseline* | 9.3 ± 9.1 | 3.9 ± 5.5 | 160.7 ± 11.5 | 152.6 ± 9.9 |
| Heel lance/squeeze | 42.1 ± 17.9 | 25.6 ± 19.6† | 183.5 ± 14.1 | 167.4 ± 10.1 |
| Recovery | 14.5 ± 17.9 | 20.6 ± 24.4† | 157.7 ± 14.1 | 158.7 ± 14.6 |
Baseline recording after undisturbed 30 min period.
P<0.05.
We also found GA differences in facial responses during the Recovery phase. Infants born ≥30 weeks GA had significantly lower facial responses during Recovery than earlier born infants when clustered care preceded blood collection (t = −2.1, P<0.05) (Fig. 1, Table 2). Infants born <30 weeks showed no differences in facial activity during the Recovery between the 2 blood collection procedures. No sex effects were found for either GA group.
DISCUSSION
This is the first study to examine the effects of clustered nursing care on biobehavioral pain responses in preterm infants born at early GAs in the NICU. Unlike the procedure examined previously,21 the clustered nursing procedures examined in this study are tasks that preterm infants undergo many times a day, ones that are usually considered innocuous. However, growing evidence suggests that routine tactile procedures may be at least as stressful for preterm infants as painful procedures.19,42
In this study, the infants showed increased arousal in their sleep/wake states during the Lance of both procedures, but no differences in levels of arousal if the infants had experienced clustered care before the Lance. Our findings are in contrast to Porter et al,21 who found that infants who had been handled before blood collection showed less active sleep and more crying; however, our study included less mature infants who displayed a more limited range of states during both procedures.
As is found typically, along with the shifts in sleep/wake states, the infants had a concomitant increase in HR during blood collection regardless of the preceding condition.21,32,35 However, in contrast to others, we found that the magnitude of HR change was not greater if the infants had been handled before the blood collection.21 Our findings may differ from those of Porter and colleagues because the rest period in our study was twice as long; the 20 minutes of rest between the procedures may have provided enough time for stabilization of the HR and sleep/wake states; in effect, the rest period may have acted as a wash out period. Alternatively, this lack of difference may be due to the tendency for preterm infants to have a high Baseline HR and a decreased ability to mount an autonomic response to a painful event when compared with healthier and more mature infants.43,44 Finally, infants born at later GAs (≥30 wk GA) had a lower mean HR during the Recovery phase when blood collection was preceded by clustered care. This lower HR during the Recovery may indicate better capacity to modulate their autonomic responses by returning to Baseline more quickly.
We were required to include a 20-minute rest period between the clustered care and the blood collection in accordance with the standard of care in our nursery, one which directs that caregivers avoid prolonged handling of the infants. Even with this long rest period, and after controlling for differences in Baseline state,28,45,46 infants born at earlier GAs had equally intense facial responses regardless of whether they had experienced clustered care or rest before the blood collection. Moreover, when the blood collection was preceded by a rest period of at least 30 minutes (in this case, the average rest period was 103 min), the earlier born infants had intense facial responses. Thus, it has yet to be determined what length of time between procedures is optimal for these infants. Indeed, we speculate that the pattern of responses exhibited by the earlier born infants (ie, equally intense reactions to pain irrespective of prior handling) may be due to “presensitization” from their greater cumulative exposure to painful and stressful procedures since birth.20 In effect, these infants reach a “ceiling” of responsiveness and are unable to modulate an effective recovery. Over time, these high levels of arousal may contribute to altered stress systems in this population,10,47 effects which may be mediated partly through reduced expression of genes which are protective against neuronal cell death.48
Infants born at later GAs (≥30 wk) showed “sensitized” facial responses similar to those observed in other studies when clustered care preceded the blood collection.21 Preterm infants are at risk for enhanced pain sensitivity because their developing central and peripheral nervous systems differ from those of adults. For example, preterm infants below 35 weeks postconceptual age show altered peripheral nociceptive sensitization, exhibiting lowered thresholds to tactile stimulation (sensitization); their thresholds decrease further (primary hyperalgesia) after repeated pain exposure.49,50 In addition, preterm infants experience allodynia (pain arising from previously innocuous stimulation) as a result of central sensitization.51 This finding is significant for the preterm infant who, as a result of central sensitization, may perceive nonpainful events, such as diaper changing, as painful.
Previous research examining the effects of cumulative pain exposure in preterm infants has found that greater pain exposure since birth is associated with dampened biobehavioral responses (eg, Refs. 22, 23). However, the important distinction between this study and the others is that not only did we analyze our results using GA as a continuous variable, but our results were analyzed to discern differences in infants born at earlier versus later GAs.
Although in other epidemiologic and experimental pain studies using human and animal models, studies which have reported sex-correlated differences in pain related behaviors (eg, Refs. 52–56), we did not find sex-related differences in pain responses in this sample. Few studies of pain responses in infants have included analyses of sex differences. One of 2 studies in term born infants has shown that female infants expressed greater facial responses than male infants to capillary puncture.57 However, male infants had shorter time to cry and to show facial responses to heel lance than did female infants.28 To the best of our knowledge, only one other study has examined sex differences in pain responses in preterm infants in the NICU and, as in the current study, no sex effects were found.35 Given the limited information on differences in pain responses in male and female infants, further research is needed in this area.
Our study has several limitations. Our sample was heterogeneous; that is, our GA groups included appropriate for gestational age and small for gestational age infants. Infants born small for GA are exposed to prenatal stress which may alter their central nervous system and subsequent development.58 Moreover, we chose a measure of early illness severity (SNAP day 1) to control for one of many possible perinatal/neonatal differences between infants born at earlier and later GAs. Determining the relative contributions of immaturity, prenatal stress exposure, cumulative pain exposure, and illness severity on biobehavioral pain responses in preterm infants in the NICU remains diffcult because these variables are highly intercorrelated. Furthermore, although errors in estimating GA can, in some instances, be more than 2 weeks, with the high rate of early ultrasound determination of GA in this study, we would expect errors in attribution of GA to contribute minimally to the results or our conclusions of this study. Finally, we analyzed a short period of reactivity relative to the length of the procedures (20 s periods out of 5 min). Our intention was to capture the most immediate response to the blood collection procedures. Studies should be done to determine how the patterns of responses change over the length of the procedure.
In conclusion, preterm infants are particularly vulnerable to the effects of repeated episodes of handling whether they are skin breaking or not. Infants born at earlier GAs showed heightened arousal and reduced ability to modulate their responses during recovery when they experience clustered care before a painful procedure. Even though more mature preterm infants were able to recover from clustered procedures more effectively, tactile handling before blood collection induced sensitized behavioral responses. Although clustering care, common in NICUs, provides infants with longer rest periods, our findings, and those of others, show that well-paced care can produce significant stress responses even in later born preterm infants. Accordingly, models of care should base the timing of rest periods and of handling upon infants’ individualized cues. In this way, we can minimize potentially deleterious stress responses.
Acknowledgments
The authors thank the staffand families of the Special Care Nursery at B.C. Children’s Hospital for their participation in this study, Colleen Fitzgerald, study coordinator, Gisella Gosse and Adi Amir for data collection, and Colleen Jantzen for carrying out reliability NFCS coding, all of whom are staff of the Early Human Experience Unit of the Centre for Community Child Health Research, Child and Family Research Institute.
Footnotes
National Institutes of Health grant HD39783 (REG), Canadian Institutes of Health Research grant MOP42469 (REG), a Canadian Child Health Clinician Scientist Career Award (L.H.), a Senior Scholar Award from the Michael Smith Foundation for Health Research (REG), and a Faculty of Odontology and Medicine and Oskarsfonden Foundation Fellowship, Umeå University, Sweden (V.L.).
References
- 1.Health Canada. The Canadian Perinatal Health Report. 2003 URL: www.hc.sc.gc.ca/pphb-dgspsp/rhs-ssg/index.html.
- 2.Lumley J. Defining the problem: the epidemiology of preterm birth. BJOG. 2003;110(suppl 20):3–7. [PubMed] [Google Scholar]
- 3.Effer SB, Moutquin J-M, Farine D, et al. Neonatal survival rates in 860 singleton live births at 24 and 25 weeks gestational age. A Canadian multicentre study. BJOG. 2002;109:740–745. doi: 10.1111/j.1471-0528.2002.01067.x. [DOI] [PubMed] [Google Scholar]
- 4.Slater R, Cantarella A, Gallella S, et al. Cortical pain responses in human infants. J Neurosci. 2006;26:3662–3666. doi: 10.1523/JNEUROSCI.0348-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartocci M, Bergzvist LL, Lagercrantz H, et al. Pain activates cortical areas in the preterm newborn brain. Pain. 2006;122:109–117. doi: 10.1016/j.pain.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Grunau R. Early pain in preterm infants. A model of long-term effects. Clin Perinatol. 2002;29:373–394. doi: 10.1016/s0095-5108(02)00012-x. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6:507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- 8.Grunau RE, Oberlander TF, Whitfield M, et al. Pain reactivity in former extremely low birth weight infants at corrected age 8 months compared with term born controls. Infant Behav Dev. 2001;24:31–55. [Google Scholar]
- 9.Grunau RE. Self-regulation and behavior in preterm children: effects of early pain. In: McGrath PJ, Finley GA, editors. Pediatric Pain: Biological and Social Context, Progress in Pain Research and Management. Vol. 26. Seattle: IASP Press; 2003. pp. 23–55. [Google Scholar]
- 10.Grunau RE, Weinberg J, Whitfield MF. Neonatal procedural pain and preterm infant cortisol response to novelty at 8 months. Pediatrics. 2004;114:e77–e84. doi: 10.1542/peds.114.1.e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grunau RE, Holsti L, Peters JWB. Long term effects of infant pain. Sem Fetal Neonatal Med. 2006;11:268–275. doi: 10.1016/j.siny.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Bhutta AT, Anand KJS. Vulnerability of the developing brain. Neuronal mechanisms. Clin Perinatol. 2002;29:357–372. doi: 10.1016/s0095-5108(02)00011-8. [DOI] [PubMed] [Google Scholar]
- 13.Als H. A synactive model of neonatal behavioral organization: framework for the assessment of neurodevelopmental development in the premature infant and for support of infants and parents in the neonatal intensive care unit. In: Sweeney JK, editor. The High-Risk Neonate: Developmental Therapy Perspectives. 6. Binghamton, New York: The Haworth Press; 1986. pp. 3–55. [Google Scholar]
- 14.Warren I. Facilitating infant adaptation: the nursery environment. Semin Neonatol. 2002;7:459–467. doi: 10.1053/siny.2002.0151. [DOI] [PubMed] [Google Scholar]
- 15.Holditch-Davis D, Barhan LN, O’Hale A, et al. Effect of standard rest periods on convalescent preterm infants. J Obstetr Gynecol Neonat Nurs. 1995;24:424–432. doi: 10.1111/j.1552-6909.1995.tb02499.x. [DOI] [PubMed] [Google Scholar]
- 16.Symanski ME, Hayes MJ, Akilesh MK. Patterns of premature newborns’ sleep-wake states before and after nursing interventions on the night shift. J Obstetr Gynecol Neonat Nurs. 2002;31:305–313. doi: 10.1111/j.1552-6909.2002.tb00052.x. [DOI] [PubMed] [Google Scholar]
- 17.Torres C, Holditch-Davis D, O’Hale A, et al. Effect of standard rest periods on apnea and weight gain in preterm infants. Neonat Netw. 1997;16:35–43. [PubMed] [Google Scholar]
- 18.Peters KL. Does routine nursing care complicate the physiologic status of the premature neonate with respiratory distress syndrome? J Perinat Neonatal Nurs. 1992;6:67–84. doi: 10.1097/00005237-199209000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Holsti L, Grunau RE, Oberlander TF, et al. Body movements, an additional important factor in discriminating pain from stress in preterm infants. Clin J Pain. 2005;21:491–498. doi: 10.1097/01.ajp.0000146163.30776.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grunau RVE, Holsti L, Whitfield MF, et al. Are twitches, startles and other body movements pain indicators in extremely low birth weight infants? Clin J Pain. 2000;16:37–45. doi: 10.1097/00002508-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Porter FL, Wolf CM, Miller JP. The effect of handling and immobilization on the response to acute pain in newborn infants. Pediatrics. 1998;102:1383–1389. doi: 10.1542/peds.102.6.1383. [DOI] [PubMed] [Google Scholar]
- 22.Grunau RE, Oberlander TF, Whitfield MF, et al. Demographic and therapeutic determinants of pain reactivity in very low birth weight neonates at 32 weeks postconceptional age. Pediatrics. 2001;107:105–112. doi: 10.1542/peds.107.1.105. [DOI] [PubMed] [Google Scholar]
- 23.Johnston CC, Stevens BJ. Experience in a neonatal intensive care unit affects pain response. Pediatrics. 1996;98:925–903. [PubMed] [Google Scholar]
- 24.Grunau RE, Holsti L, Haley D, et al. Neonatal procedural pain exposure predicts lower cortisol and behavioral reactivity in preterm infants in the NICU. Pain. 2005;113:293–300. doi: 10.1016/j.pain.2004.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Synnes AR, Ling EWY, Whitfield MF, et al. Perinatal outcomes of a large cohort of extremely low gestational age infants (23–28 completed weeks) J Pediatr. 1994;125:952–960. doi: 10.1016/s0022-3476(05)82015-3. [DOI] [PubMed] [Google Scholar]
- 26.Faul F, Erdfelder E. GPOWER: a priori-, post hoc-, and compromise power analyses for MS-DOS (Computer program) Bonn Germany: Bonn University; 1998. [Google Scholar]
- 27.Craig KD, Whitfield MF, Grunau RV, et al. Pain in the preterm neonate: behavioral and physiological indices. Pain. 1993;52:287–299. doi: 10.1016/0304-3959(93)90162-I. [DOI] [PubMed] [Google Scholar]
- 28.Grunau RV, Craig KD. Pain expression in neonates: facial action and cry. Pain. 1987;28:395–410. doi: 10.1016/0304-3959(87)90073-X. [DOI] [PubMed] [Google Scholar]
- 29.Als H. Manual for the naturalistic observation of newborn behavior (preterm and fullterm) Boston: The Children’s Hospital; 1995. [Google Scholar]
- 30.Pressler JL, Hepworth JT. A quantitative use of the NIDCAP tool. Clin Nurs Res. 2002;11:89–102. doi: 10.1177/105477380201100107. [DOI] [PubMed] [Google Scholar]
- 31.Lindh V, Wiklund U, Sandman P-O, et al. Assessment of acute pain in preterm infants by evaluation of facial expression and frequency domain analysis of heart rate variability. Early Hum Dev. 1997;48:131–142. doi: 10.1016/s0378-3782(96)01851-8. [DOI] [PubMed] [Google Scholar]
- 32.Grunau RE, Oberlander T, Holsti L, et al. Bedside application of the Neonatal Facial Coding System in pain assessment of premature neonates. Pain. 1998;76:277–286. doi: 10.1016/S0304-3959(98)00046-3. [DOI] [PubMed] [Google Scholar]
- 33.HR View Software. Brighton MA: Boston Medical Technologies; 1996. [Google Scholar]
- 34.Berger RD, Saul P, Cohen RJ. Transfer function analysis of autonomic regulation: canine atrial rate response. Am J Physiol. 1989;256:H142–H152. doi: 10.1152/ajpheart.1989.256.1.H142. [DOI] [PubMed] [Google Scholar]
- 35.Holsti L, Grunau RE, Oberlander TF, et al. Specific NIDCAP® movements help identify acute pain in preterm infants in the NICU. Pediatrics. 2004;114:65–72. doi: 10.1542/peds.114.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oberlander T, Saul JP. Methodological considerations for the use of heart rate variability as a measure of pain reactivity in vulnerable infants. Clin Perinatol. 2002;29:427–443. doi: 10.1016/s0095-5108(02)00013-1. [DOI] [PubMed] [Google Scholar]
- 37.Lindh V, Wiklund U, Hakansson S. Heel lancing in term new-born infants: an evaluation of pain by frequency domain analysis of heart rate variability. Pain. 1999;80:143–148. doi: 10.1016/s0304-3959(98)00215-2. [DOI] [PubMed] [Google Scholar]
- 38.Lindh V, Wiklund U, Blomquist HK, et al. EMLA cream and oral glucose for immunization pain in three-month-old infants. Pain. 2003;104:381–388. doi: 10.1016/s0304-3959(03)00046-0. [DOI] [PubMed] [Google Scholar]
- 39.Richardson DK, Corcoran JD, Esobar GJ, et al. SNAP-II and SNAPPE-II: simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138:92–100. doi: 10.1067/mpd.2001.109608. [DOI] [PubMed] [Google Scholar]
- 40.Porter FL, Wolf CM, Miller JP. Procedural pain in newborn infants: the influence of intensity and development. Pediatrics. 1999;104:e13. doi: 10.1542/peds.104.1.e13. [DOI] [PubMed] [Google Scholar]
- 41.Lacey JI. The evaluation of autonomic responses: towards a general solution. Ann N Y Acad Sci. 1956;67:125–163. doi: 10.1111/j.1749-6632.1956.tb46040.x. [DOI] [PubMed] [Google Scholar]
- 42.Hellerud BC, Storm H. Skin conductance and behaviour during sensory stimulation of preterm and term infants. Early Hum Dev. 2002;20:35–46. doi: 10.1016/s0378-3782(02)00070-1. [DOI] [PubMed] [Google Scholar]
- 43.Lindh V. Procedural Pain and Distress in Infants. Alleviation of Acute Pain Assessed by Heart Rate Variability and Behavioral Measures. Umeä, Sweden: Umeä University; 2002. [Google Scholar]
- 44.Oberlander T, Saul JP. Methodological considerations for the use of heart rate variability as a measure of pain reactivity in vulnerable infants. Clin Perinatol. 2002;29:427–443. doi: 10.1016/s0095-5108(02)00013-1. [DOI] [PubMed] [Google Scholar]
- 45.Stevens B, Johnston CC, Horton L. Factors that influence the behavioral pain responses of premature infants. Pain. 1994;59:101–109. doi: 10.1016/0304-3959(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 46.Johnston CC, Stevens BJ, Franck LS, et al. Factors explaining lack of response to heel stick in preterm newborns. J Obstet Gynecol Neonatal Nurs. 1999;28:587–594. doi: 10.1111/j.1552-6909.1999.tb02167.x. [DOI] [PubMed] [Google Scholar]
- 47.Grunau RE, Weinberg J, Whitfield MF. Neonatal procedural pain and preterm infant cortisol response to novelty at 8 months. Pediatr. 2004;114:e77–e84. doi: 10.1542/peds.114.1.e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barr GA, Gao R, Wang S, et al. Micrarray analysis of gene expression following formalin test in the infant rat. Pain. 2005;177:6–18. doi: 10.1016/j.pain.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 49.Fitzgerald M, Millard C, McIntosh N. Hyperalgesia in premature infants. Lancet. 1988;1:292. doi: 10.1016/s0140-6736(88)90365-0. [DOI] [PubMed] [Google Scholar]
- 50.Fitzgerald M, Shaw A, McIntosh N. Postnatal development of the cutaneous flexor reflex: comparative study of preterm infants and newborn rat pups. Dev Med Child Neurol. 1988;30:520–526. doi: 10.1111/j.1469-8749.1988.tb04779.x. [DOI] [PubMed] [Google Scholar]
- 51.Fitzgerald M, Millard C, McIntosh N. Cutaneous hypersensitivity following peripheral tissue damage in newborn infants and its reversal with topical anaesthesia. Pain. 1989;39:31–36. doi: 10.1016/0304-3959(89)90172-3. [DOI] [PubMed] [Google Scholar]
- 52.Riley JL, III, Robinson ME, Wise E, et al. Sex differences in perception of noxious experimental stimuli: a meta-analsysis. Pain. 1998;74:181–187. doi: 10.1016/s0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- 53.Unruh AM. Gender variation in clinical pain experience. Pain. 1996;65:123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 54.Myers CD, Riley JL, Robinson ME. Psychosocial contributions to sex-correlated differences in pain. Clin J Pain. 2003;19:225–232. doi: 10.1097/00002508-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Bhutta AT, Rovnaghi C, Simpson PM, et al. Interactions of inflammatory pain and morphine in infant rats. Long-term behavioral effects. Physiol Behav. 2001;73:51–58. doi: 10.1016/s0031-9384(01)00432-2. [DOI] [PubMed] [Google Scholar]
- 56.Grunau RVE, Whitfield MF, Petrie JA. Pain sensitivity and temperament in extremely-low-birth-weight premature toddlers and preterm and full-term controls. Pain. 1994;58:341–346. doi: 10.1016/0304-3959(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 57.Guinsburg R, de Araujo Peres C, Branco de Almeida MF, et al. Differences in pain expression between male and female newborn infants. Pain. 2000;85:127–133. doi: 10.1016/s0304-3959(99)00258-4. [DOI] [PubMed] [Google Scholar]
- 58.Gutbrod T, Wolke D, Soehne B, et al. Effects of gestation and birth weight on the growth and development of very low birth weight small for gestational age infants: a matched group comparison. Arch Dis Child Fetal Neonatal Ed. 2000;82:F208–F214. doi: 10.1136/fn.82.3.F208. [DOI] [PMC free article] [PubMed] [Google Scholar]


