Abstract
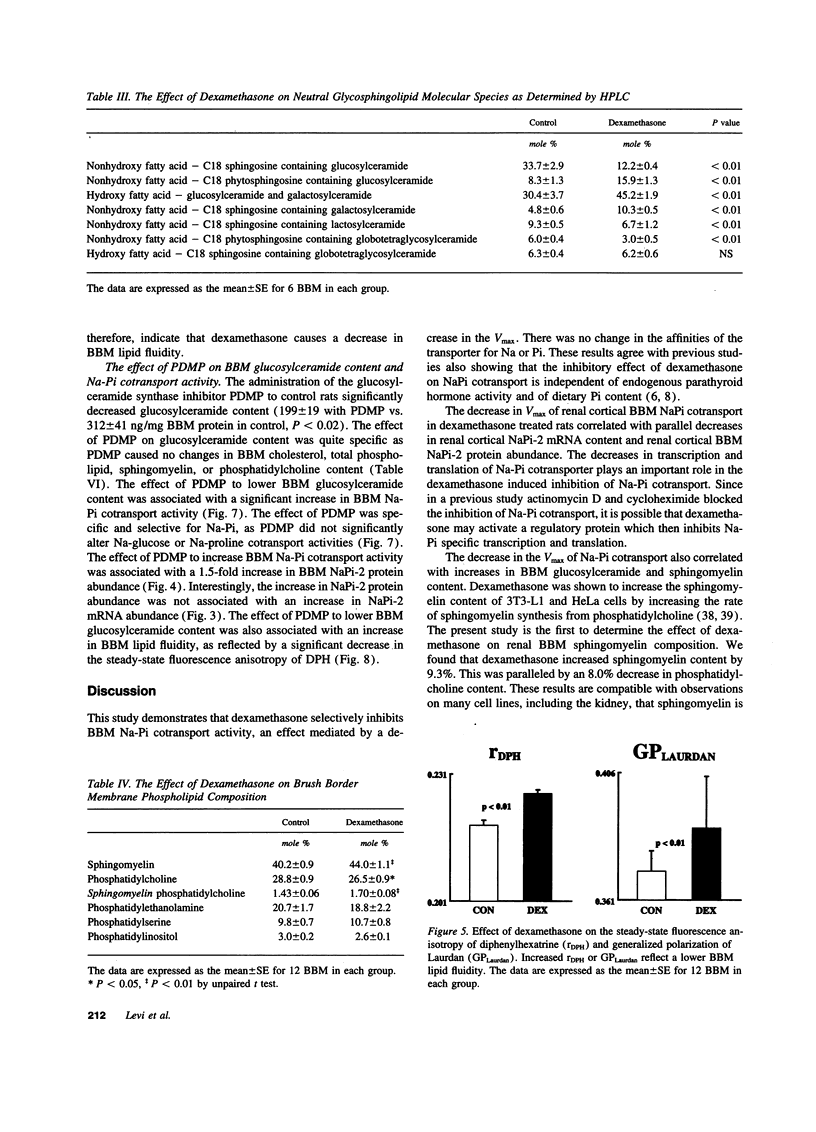
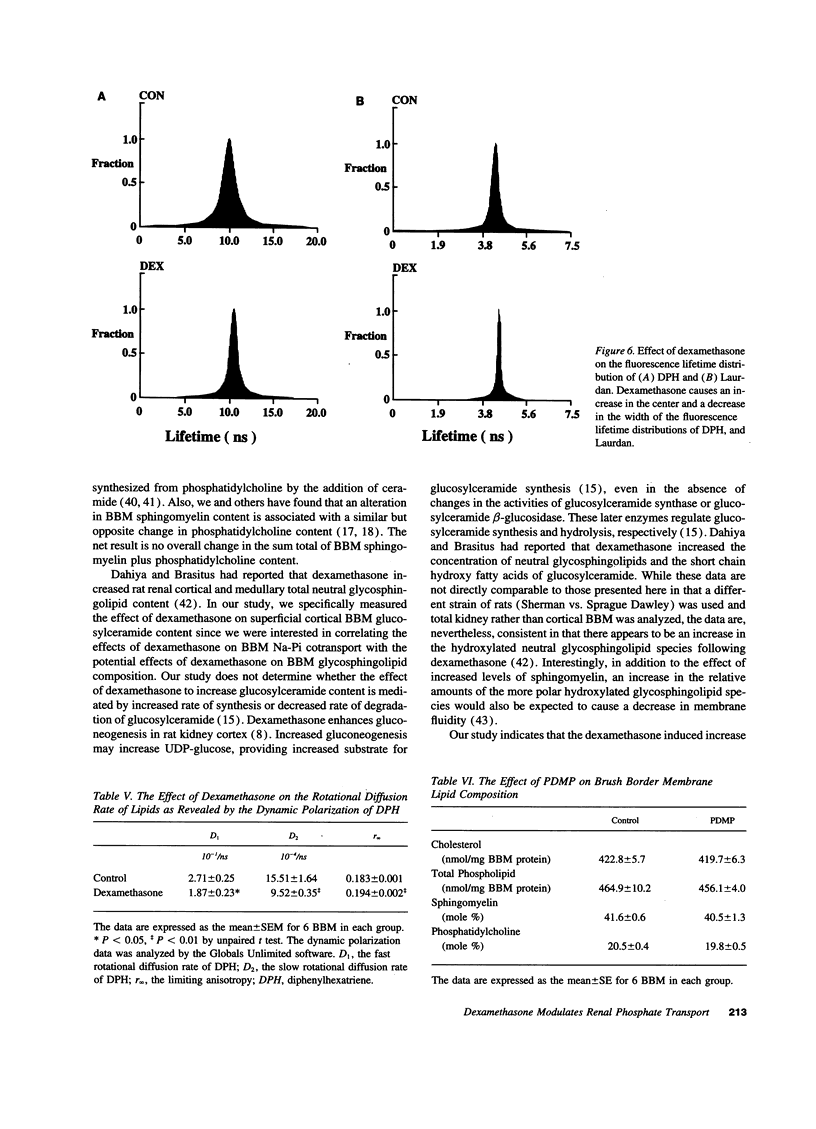
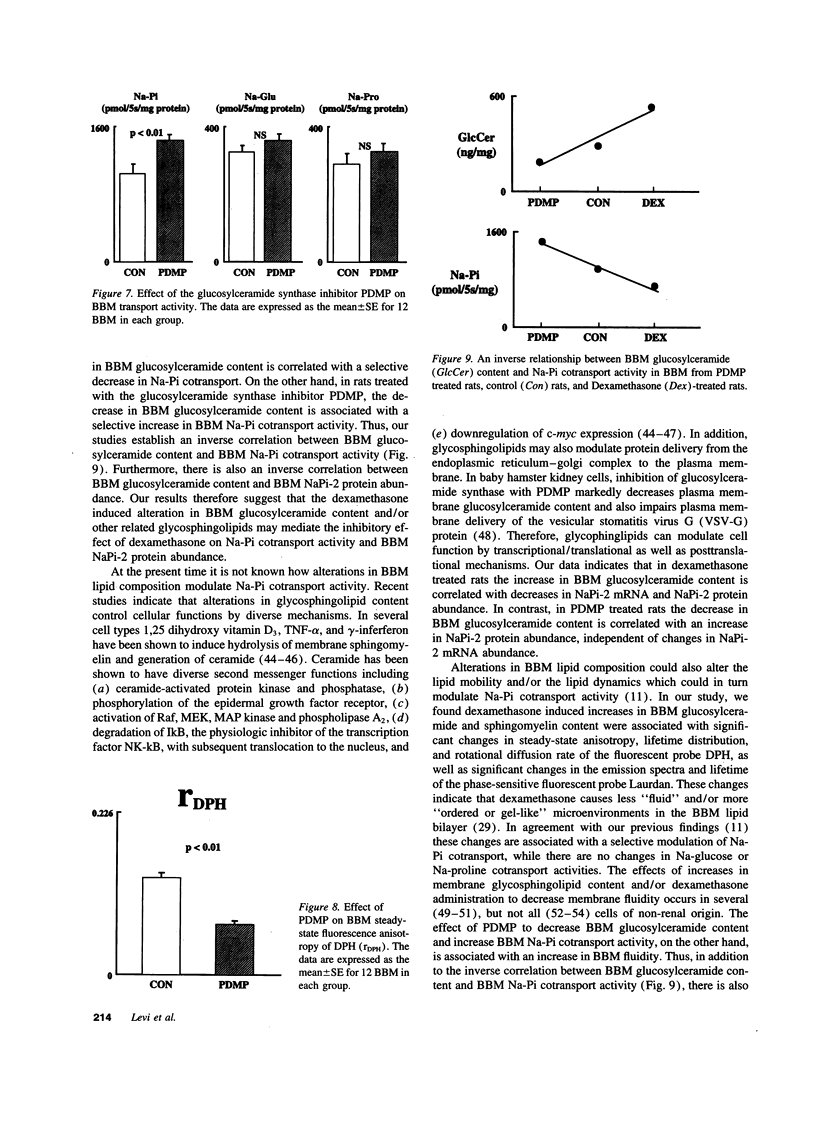
Glucocorticoids are important regulators of renal phosphate transport. This study investigates the role of alterations in renal brush border membrane (BBM) sodium gradient-dependent phosphate transport (Na-Pi cotransporter) mRNA and protein abundance in the dexamethasone induced inhibition of Na-Pi cotransport in the rat. Dexamethasone administration for 4 d caused a 1.5-fold increase in the Vmax of Na-Pi cotransport (1785 +/- 119 vs. 2759 +/- 375 pmol/5 s per mg BBM protein in control, P < 0.01), which was paralleled by a 2.5-fold decrease in the abundance of Na-Pi mRNA and Na-Pi protein. There was also a 1.7-fold increase in BBM glucosylceramide content (528 +/- 63 vs. 312 +/- 41 ng/mg BBM protein in control, P < 0.02). To determine whether the alteration in glucosylceramide content per se played a functional role in the decrease in Na-Pi cotransport, control rats were treated with the glucosylceramide synthase inhibitor, D-threo-1-phenyl-2-decanoyl-amino-3-morpholino-1-propanol (PDMP). The resultant 1.5-fold decrease in BBM glucosylceramide content (199 +/- 19 vs. 312 +/- 41 ng/mg BBM protein in control, P < 0.02) was associated with a 1.4-fold increase in Na-Pi cotransport activity (1422 +/- 73 vs. 1048 +/- 85 pmol/5 s per mg BBM protein in control, P < 0.01), and a 1.5-fold increase in BBM Na-Pi protein abundance. Thus, dexamethasone-induced inhibition of Na-Pi cotransport is associated with a decrease in BBM Na-Pi cotransporter abundance, and an increase in glucosylceramide. Since primary alteration in BBM glucosylceramide content per se directly and selectively modulates BBM Na-Pi cotransport activity and Na-Pi protein abundance, we propose that the increase in BBM glucosylceramide content plays an important role in mediating the inhibitory effect of dexamethasone on Na-Pi cotransport activity.
Full text
PDF


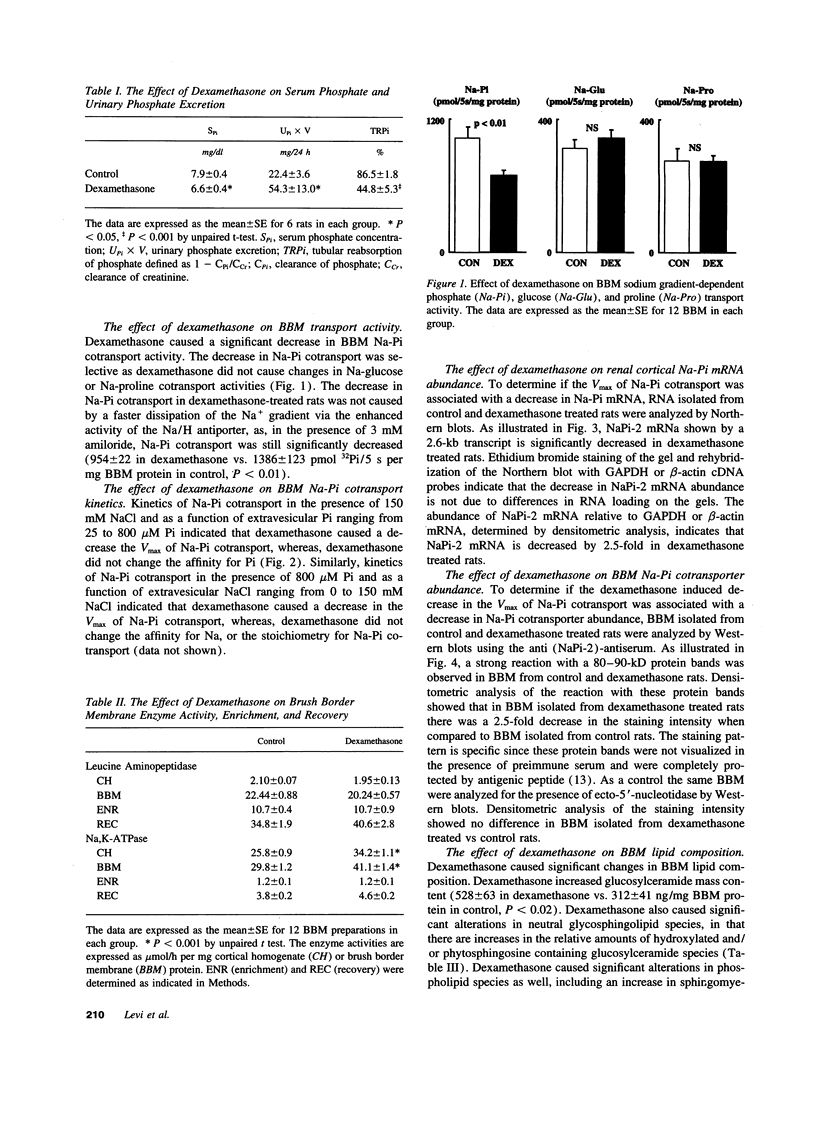
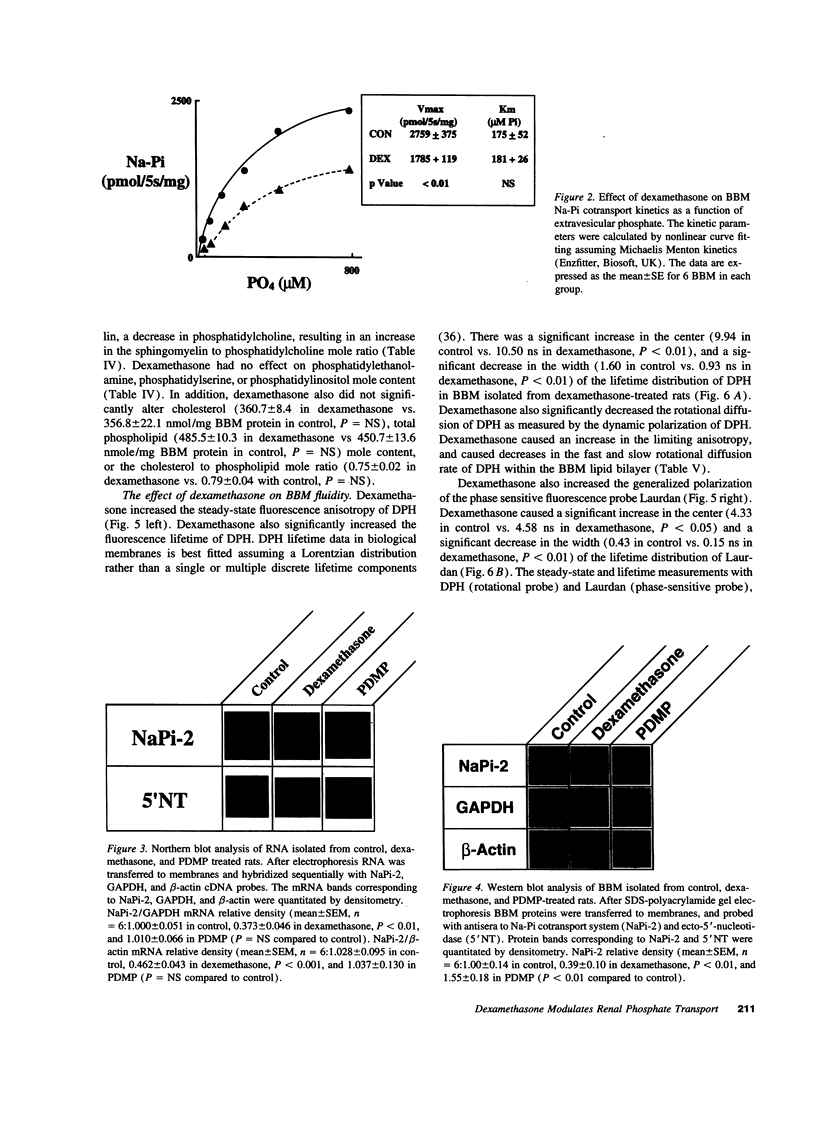


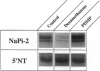
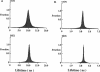
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- ANDERSON J., FOSTER J. B. The effect of cortisone on urinary phosphate excretion in man. Clin Sci. 1959 Aug;18:437–439. [PubMed] [Google Scholar]
- Allan D., Quinn P. Resynthesis of sphingomyelin from plasma-membrane phosphatidylcholine in BHK cells treated with Staphylococcus aureus sphingomyelinase. Biochem J. 1988 Sep 15;254(3):765–771. doi: 10.1042/bj2540765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Boross M., Kinsella J., Cheng L., Sacktor B. Glucocorticoids and metabolic acidosis-induced renal transports of inorganic phosphate, calcium, and NH4. Am J Physiol. 1986 May;250(5 Pt 2):F827–F833. doi: 10.1152/ajprenal.1986.250.5.F827. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Curatolo W. The physical properties of glycolipids. Biochim Biophys Acta. 1987 Jun 24;906(2):111–136. doi: 10.1016/0304-4157(87)90008-6. [DOI] [PubMed] [Google Scholar]
- Dahiya R., Brasitus T. A. Dexamethasone-induced alterations in the glycosphingolipids of rat kidney. Lipids. 1988 Sep;23(9):863–868. doi: 10.1007/BF02536206. [DOI] [PubMed] [Google Scholar]
- Dawson T. P., Gandhi R., Le Hir M., Kaissling B. Ecto-5'-nucleotidase: localization in rat kidney by light microscopic histochemical and immunohistochemical methods. J Histochem Cytochem. 1989 Jan;37(1):39–47. doi: 10.1177/37.1.2535703. [DOI] [PubMed] [Google Scholar]
- Dudeja P. K., Dahiya R., Brown M. D., Brasitus T. A. Dexamethasone influences the lipid fluidity, lipid composition and glycosphingolipid glycosyltransferase activities of rat proximal-small-intestinal Golgi membranes. Biochem J. 1988 Jul 15;253(2):401–408. doi: 10.1042/bj2530401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudeja P. K., Foster E. S., Brasitus T. A. Modulation of rat distal colonic brush-border membrane Na+-H+ exchange by dexamethasone: role of lipid fluidity. Biochim Biophys Acta. 1987 Dec 11;905(2):485–493. doi: 10.1016/0005-2736(87)90478-0. [DOI] [PubMed] [Google Scholar]
- Esko J. D., Raetz C. R. Mutants of Chinese hamster ovary cells with altered membrane phospholipid composition. Replacement of phosphatidylinositol by phosphatidylglycerol in a myo-inositol auxotroph. J Biol Chem. 1980 May 25;255(10):4474–4480. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fiorini R., Valentino M., Wang S., Glaser M., Gratton E. Fluorescence lifetime distributions of 1,6-diphenyl-1,3,5-hexatriene in phospholipid vesicles. Biochemistry. 1987 Jun 30;26(13):3864–3870. doi: 10.1021/bi00387a019. [DOI] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiberg J. M., Kinsella J., Sacktor B. Glucocorticoids increase the Na+-H+ exchange and decrease the Na+ gradient-dependent phosphate-uptake systems in renal brush border membrane vesicles. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4932–4936. doi: 10.1073/pnas.79.16.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick A., Durasin I., Neuweg M. Glucocorticoid-induced inhibition of the reabsorption of inorganic phosphate in the proximal tubule in the absence of parathyroid hormone. Adv Exp Med Biol. 1984;178:81–86. doi: 10.1007/978-1-4684-4808-5_13. [DOI] [PubMed] [Google Scholar]
- Frick A., Durasin I. Proximal tubular reabsorption of inorganic phosphate in adrenalectomized rats. Pflugers Arch. 1980 Jun;385(3):189–192. doi: 10.1007/BF00647456. [DOI] [PubMed] [Google Scholar]
- Goins B., Masserini M., Barisas B. G., Freire E. Lateral diffusion of ganglioside GM1 in phospholipid bilayer membranes. Biophys J. 1986 Apr;49(4):849–856. doi: 10.1016/S0006-3495(86)83714-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun Y. A. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994 Feb 4;269(5):3125–3128. [PubMed] [Google Scholar]
- Johnston D., Matthews E. R., Melnykovych G. Glucocorticoid effects on lipid metabolism in HeLa cells: inhibition of cholesterol synthesis and increased sphingomyelin synthesis. Endocrinology. 1980 Nov;107(5):1482–1488. doi: 10.1210/endo-107-5-1482. [DOI] [PubMed] [Google Scholar]
- Keating K. M., Roess D. A., Peacock J. S., Barisas B. G. Glucocorticoid effects on membrane lipid mobility during differentiation of murine B lymphocytes. Biochim Biophys Acta. 1985 Aug 30;846(2):305–312. doi: 10.1016/0167-4889(85)90078-3. [DOI] [PubMed] [Google Scholar]
- Kinsella J. L. Action of glucocorticoids on proximal tubule transport systems. Semin Nephrol. 1990 Jul;10(4):330–338. [PubMed] [Google Scholar]
- Kiss C., Balázs M., Kéri-Fülöp I. Dexamethasone decreases membrane fluidity of leukemia cells. Leuk Res. 1990;14(3):221–225. doi: 10.1016/0145-2126(90)90129-w. [DOI] [PubMed] [Google Scholar]
- Kolesnick R., Golde D. W. The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell. 1994 May 6;77(3):325–328. doi: 10.1016/0092-8674(94)90147-3. [DOI] [PubMed] [Google Scholar]
- LARON Z., CRAWFORD J. D., KLEIN R. Phosphaturic effect of cortisone in normal and parathyroidectomized rats. Proc Soc Exp Biol Med. 1957 Dec;96(3):649–651. doi: 10.3181/00379727-96-23566. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levi M., Arar M., Kaissling B., Murer H., Biber J. Low-Pi diet increases the abundance of an apical protein in rat proximal-tubular S3 segments. Pflugers Arch. 1994 Jan;426(1-2):5–11. doi: 10.1007/BF00374664. [DOI] [PubMed] [Google Scholar]
- Levi M., Baird B. M., Wilson P. V. Cholesterol modulates rat renal brush border membrane phosphate transport. J Clin Invest. 1990 Jan;85(1):231–237. doi: 10.1172/JCI114417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi M. Heterogeneity of Pi transport by BBM from superficial and juxtamedullary cortex of rat. Am J Physiol. 1990 Jun;258(6 Pt 2):F1616–F1624. doi: 10.1152/ajprenal.1990.258.6.F1616. [DOI] [PubMed] [Google Scholar]
- Levi M., Jameson D. M., van der Meer B. W. Role of BBM lipid composition and fluidity in impaired renal Pi transport in aged rat. Am J Physiol. 1989 Jan;256(1 Pt 2):F85–F94. doi: 10.1152/ajprenal.1989.256.1.F85. [DOI] [PubMed] [Google Scholar]
- Levi M., Wilson P. V., Cooper O. J., Gratton E. Lipid phases in renal brush border membranes revealed by Laurdan fluorescence. Photochem Photobiol. 1993 Mar;57(3):420–425. doi: 10.1111/j.1751-1097.1993.tb02312.x. [DOI] [PubMed] [Google Scholar]
- Liscovitch M., Cantley L. C. Lipid second messengers. Cell. 1994 May 6;77(3):329–334. doi: 10.1016/0092-8674(94)90148-1. [DOI] [PubMed] [Google Scholar]
- Magagnin S., Werner A., Markovich D., Sorribas V., Stange G., Biber J., Murer H. Expression cloning of human and rat renal cortex Na/Pi cotransport. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5979–5983. doi: 10.1073/pnas.90.13.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluer R. H., Ullman M. D., Jungalwala F. B. High-performance liquid chromatography of membrane lipids: glycosphingolipids and phospholipids. Methods Enzymol. 1989;172:538–575. doi: 10.1016/s0076-6879(89)72033-4. [DOI] [PubMed] [Google Scholar]
- Nelson D. H., Murray D. K. Dexamethasone increases the synthesis of sphingomyelin in 3T3-L1 cell membranes. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6690–6692. doi: 10.1073/pnas.79.21.6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noronha-Blob L., Sacktor B. Inhibition by glucocorticoids of phosphate transport in primary cultured renal cells. J Biol Chem. 1986 Feb 15;261(5):2164–2169. [PubMed] [Google Scholar]
- Parasassi T., De Stasio G., Ravagnan G., Rusch R. M., Gratton E. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys J. 1991 Jul;60(1):179–189. doi: 10.1016/S0006-3495(91)82041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poujeol P., Vandewalle A. Phosphate uptake by proximal cells isolated from rabbit kidney: role of dexamethasone. Am J Physiol. 1985 Jul;249(1 Pt 2):F74–F83. doi: 10.1152/ajprenal.1985.249.1.F74. [DOI] [PubMed] [Google Scholar]
- ROBERTS K. E., PITTS R. F. The effects of cortisone and desoxycorticosterone on the renal tubular reabsorption of phosphate and the excretion of titratable acid and potassium in dogs. Endocrinology. 1953 Mar;52(3):324–330. doi: 10.1210/endo-52-3-324. [DOI] [PubMed] [Google Scholar]
- Rosenwald A. G., Machamer C. E., Pagano R. E. Effects of a sphingolipid synthesis inhibitor on membrane transport through the secretory pathway. Biochemistry. 1992 Apr 14;31(14):3581–3590. doi: 10.1021/bi00129a005. [DOI] [PubMed] [Google Scholar]
- Schütze S., Potthoff K., Machleidt T., Berkovic D., Wiegmann K., Krönke M. TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced "acidic" sphingomyelin breakdown. Cell. 1992 Nov 27;71(5):765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- Shayman J. A., Radin N. S. Structure and function of renal glycosphingolipids. Am J Physiol. 1991 Mar;260(3 Pt 2):F291–F302. doi: 10.1152/ajprenal.1991.260.3.F291. [DOI] [PubMed] [Google Scholar]
- Tsui Z. C., Hou W. H., Yang L., Zhu Z. M. Effect of a cell differentiation inducer, ganglioside GM3, on the neutral glycosphingolipid composition and cell membrane fluidity of a human promyelocytic leukemia cell line HL-60. In Vivo. 1990 May-Jun;4(3):205–208. [PubMed] [Google Scholar]
- Turner S. T., Kiebzak G. M., Dousa T. P. Mechanism of glucocorticoid effect on renal transport of phosphate. Am J Physiol. 1982 Nov;243(5):C227–C236. doi: 10.1152/ajpcell.1982.243.5.C227. [DOI] [PubMed] [Google Scholar]
- Voelker D. R., Kennedy E. P. Cellular and enzymic synthesis of sphingomyelin. Biochemistry. 1982 May 25;21(11):2753–2759. doi: 10.1021/bi00540a027. [DOI] [PubMed] [Google Scholar]
- Webster S. K., Haramati A., Knox F. G. Effect of dexamethasone on segmental phosphate reabsorption in phosphate-deprived rats. Am J Physiol. 1986 Oct;251(4 Pt 2):F576–F580. doi: 10.1152/ajprenal.1986.251.4.F576. [DOI] [PubMed] [Google Scholar]
- Zador I. Z., Deshmukh G. D., Kunkel R., Johnson K., Radin N. S., Shayman J. A. A role for glycosphingolipid accumulation in the renal hypertrophy of streptozotocin-induced diabetes mellitus. J Clin Invest. 1993 Mar;91(3):797–803. doi: 10.1172/JCI116299. [DOI] [PMC free article] [PubMed] [Google Scholar]