Abstract
There is evidence that the developmental trajectory of cortisol secretion in preterm infants is altered, with elevated basal cortisol levels observed postnatally through at least 18 months corrected age (CA). This alteration is possibly due to neonatal pain-related stress. High cortisol levels might contribute to greater risk of impaired neurodevelopment. Since maternal factors are important for the regulation of infant stress responses, we investigated relationships between infant (neonatal pain-related stress, attention, cortisol) and maternal (stress, interactive behaviors) factors at age 8 months CA. We found that interactive maternal behaviors buffered the relationship between high neonatal pain-related stress exposure and poorer focused attention in mothers who self-reported low concurrent stress. Furthermore, in preterm infants exposed to high concurrent maternal stress and overwhelming interactive maternal behaviors, higher basal cortisol levels were associated with poor focused attention. Overall, these findings suggest that maternal factors can influence the cognitive resilience at 8 months of preterm infants exposed to early life stress.
Keywords: premature birth, pain, stress, maternal stress, maternal behaviors, infant attention
INTRODUCTION
Preterm infants born at a very low gestational age (VLGA,≤32 weeks) are exposed to stresses of the extra-uterine environment in the neonatal intensive unit (NICU) during a time of increased central nervous system vulnerability, immature regulation of the blood–brain barrier, and rapid development of the neuronal cytoarchitecture (Volpe, 2001). Repeated routine medical procedures administered in the NICU over a prolonged period induce early life stress in VLGA infants, which might contribute to vulnerability to negative developmental outcomes.
The majority of preterm infants show decreased attention, learning, and memory compared to full-term infants (e.g., Haley, Weinberg, & Grunau, 2006; Rose, Feldman, & Jankowski, 2001, 2002; Rose, Feldman, Jankowski, & Van Rossem, 2005; Ruff, 1986), and these deficits persist through childhood and adolescence (e.g., Anderson, Doyle, & the Victorian Infant Collaborative Study Group, 2004; Bhutta, Cleves, Casey, Craddock, & Anand, 2002; Grunau, Whitfield, & Davis, 2002; Grunau, Whitfield, & Fay, 2004b; Hack et al., 1994; Saigal, Hoult, Streiner, Stoskopf, & Rosenbaum, 2000). These poorer developmental outcomes may at least partially reflect alterations in the development of stress-sensitive systems. In children born extremely premature, we found heightened behavioral stress responses to cognitive challenge involving novelty both in the preschool years (Grunau, 2003) and in later childhood (Whitfield, Grunau, & Holsti, 1997), as well as a higher prevalence of anxiety-related behavior in adolescence (Grunau et al., 2004b; Levy-Shiff, Einat, Mogilner, Lerman, & Krikler, 1994), compared to full-term controls of comparable socio-demographic background. These observations of heightened stress and anxiety behavior in preterm populations are consistent with animal studies showing poor stress regulation in adult rats exposed to early life stress (Meaney, 2001). That is, in experimental animal models, exposure to early life stress is associated with enhanced hypothalamic-pituitary-adrenal (HPA) responsiveness to stressors, and reduced cognitive abilities in adulthood (for a review, see Meaney, 2001). These effects have been linked to reduced synaptogenesis and expression of hippocampal glucocorticoid receptor mRNA levels in juvenile and adult rats (Meaney, 2001). It remains unclear whether stress-related alterations in the HPA axis and cognitive function are detectable at an earlier developmental stage, that is, in infancy. Importantly, enrichment of the rearing environment of juvenile rats partially compensates for early life stress (Bredy, Humpartzoomian, Cain, & Meaney, 2003; Francis, Diorio, Plotsky, & Meaney, 2002) related to both hormonal and behavioral responsiveness. This raises the question of whether early caregiving environments in humans during and after the neonatal period may also buffer developmental difficulties induced by early stress exposure.
To our knowledge, studies in human infants born preterm have not examined whether or not stress during the neonatal period adversely affects cognitive function through altered HPA activity. Repeated pain exposure in neonatal rat pups has not resulted in heightened HPA activity at later ages (Anand, Coskun, Thrivikraman, Nemeroff, & Plotsky, 1999; Walker, Kudreikis, Sherrard, & Johnston, 2003). However, an increase in nurturing maternal behaviors (licking and grooming) following pup exposure to repeated neonatal pain appeared to prevent later elevation in HPA activity (Walker et al., 2003).
There is evidence that during early childhood, exposure to maternal distress concurrently or during infancy, or to stress-related situations such as low socioeconomic status is related to elevated cortisol levels in children born full-term (Essex, Klein, Cho, & Kalin, 2002; Lupien, King, Meaney, & McEwen, 2000, 2001). These high cortisol levels were associated with later externalizing and internalizing behavior problems (Essex et al., 2002), and poorer cognitive performance (Lupien et al., 2000, 2001). Moreover, maternal stress within the months following delivery has been found to predict more negative maternal attitudes and behaviors during interactive play with their infants (Crnic, Ragozin, Greenberg, Robinson, & Basham, 1983), and reduced maternal sensitivity and increased controlling maternal behavior during interactions with their 6-month old preterm infants (Muller-Nix et al., 2004). Since high quality of caregiving can buffer HPA secretion in children (reviewed by Gunnar, 1998), taken together, these findings suggest that maternal stress during infancy may affect HPA secretion in children through interference with the quality of interactive maternal behavior.
Most studies examining the HPA function in preterm infants during the neonatal period showed evidence of HPA downregulation, for example, lower basal cortisol levels (e.g., Grunau et al., 2005; Hingre, Gross, Hingre, Mayes, & Richman, 1994; Huysman, Hokken-Koelega, De Ridder, & Sauer, 2000; Lee, Rajagopalan, Berg, & Moshang, 1989; Ng et al., 2004; Watterberg, Gerdes, & Cook, 2001). These lower levels appear to persist to the early infancy period (Haley, Grunau, Weinberg, & Whitfield, 2004; Haley et al., 2006). In contrast, at 8 and 18 months corrected age (CA) for prematurity (CA), cortisol levels in the tiniest preterm infants were found to be higher than in full-term infants, suggesting a resetting of the HPA axis associated with extreme prematurity (Grunau, Weinberg, & Whitfield, 2004a; Grunau, Haley, Whitfield, & Weinberg, in press). These elevated levels were found to be linked to cumulative exposure to neonatal stress indexed by the number of skin-breaking procedures from birth to term, adjusted for initial illness severity and neonatal intravenous morphine exposure.
In the adult population, basal cortisol modulates cognition through an inverted U-shape curve. Specifically, too little or too much circulating cortisol levels can impair memory function. Very little is known about the relationship between cortisol and cognition in infants and young children. To our knowledge, only one study has investigated such relationship in young infants. At 3 months CA, moderate basal and reactive cortisol levels were associated with better immediate and delayed learning in both VLGA and full-term infants (Haley et al., 2006). Given the evidence that in preterm infants, the HPA axis might be downregulated during the neonatal and early infancy, and then, upregulated at 8 and 18 months CA, it is possible that the direction of the relationship between cognition and cortisol at 8 months CA would be different from that reported by Haley et al. (2006) at 3 months CA.
Infant attention is frequently used as a marker of cognition, since attentional processes predict cognitive outcomes in early childhood (e.g., Mirsky, 1996; Posner & Petersen, 1990; Posner & Raichle, 1994; Ruff & Rothbart, 1996). Focused attention during toy exploration in the middle of the first year of life is sensitive to differences between high-risk preterm and full-term infants (Ruff, 1988; Ruff, Lawson, Parrinello, & Weissberg, 1990; Ruff, McCarton, Kurtzberg, & Vaughan, 1984), and can predict cognitive abilities in 2, 3, and 4/5 years (Lawson & Ruff, 2004).
In the present study, we investigated the relationships among neonatal pain-related stress, cognitive indices (focused attention), and basal cortisol levels in VLGA infants at 8 months CA. Further, we investigated the potential role of maternal factors (parenting stress and interactive behaviors) in ameliorating possible adverse relationships among stress, cortisol, and focused attention. We hypothesized that maternal factors will modulate: (1) the relationship between neonatal pain-related stress and focused attention and (2) the relationship between cortisol and infant focused attention, at 8 months CA. In addition, we included a sample of full-term infants as a reference group for infant focused attention and maternal stress and behavior.
METHODS
Participants
One hundred three infants born at VLGA ≤32 weeks and 55 healthy full-term infants born at ≥38 weeks gestation participated in this study. Preterm infants were recruited from the neonatal intensive care unit (NICU) at Children’s and Women’s Health Centre of British Columbia (C&W), which is the major tertiary neonatal unit in British Columbia. Full-term infants were recruited from the full-term nursery at the same institution. All infants were recruited between 2001 and 2004 by one neonatal research nurse and were assessed in a laboratory setting at the Child and Family Research Institute at 8 months CA. The participants in this study are part of a larger cohort involved in a longitudinal study (Grunau et al., under review). Mother and infant demographic characteristics are presented in Table 1.
Table 1.
Demographic Characteristics (Mean ±SD)
| Preterm | Full-Term | p-Value | |
|---|---|---|---|
| Birthweight (g) | |||
| LOW parenting stress | 1,369.8 (445.1) | 3,590.8 (590.1) | .389 |
| HIGH parenting stress | 1,205.3 (503.7) | 3,599.2 (533.9) | |
| Overall | 1,282.7 (481.4) | 3,594.9 (555.6) | .001a |
| Gestational age (weeks) | |||
| LOW parenting stress | 29.6 (2.6) | 40.1 (1.0) | .528 |
| HIGH parenting stress | 29.0 (2.7) | 40.1 (1.0) | |
| Overall | 29.3 (2.7) | 40.1 (1.0) | .001a |
| Number of skin-breaking procedures | |||
| LOW parenting stress | 110.5 (99.2) | NA | .455 |
| HIGH parenting stress | 129.0 (92.2) | NA | |
| Overall | 120.1 (95.5) | NA | NA |
| Illness severity (SNAP-II Day 1) | |||
| LOW parenting stress | 9.2 (10.3) | NA | .025b |
| HIGH parenting stress | 15.8 (11.9) | NA | |
| Overall | 12.6 (11.5) | NA | NA |
| Days of mechanical ventilation | |||
| LOW parenting stress | 10.3 (16.7) | NA | .291 |
| HIGH parenting stress | 15.1 (21.2) | NA | |
| Overall | 12.8 (19.2) | NA | NA |
| Days on postnatal glucocorticoids | |||
| LOW parenting stress (n = 1) | 11.0 (.0) | NA | .06 |
| HIGH parenting stress (n = 5) | 3.6 (2.5) | NA | |
| Overall (n = 6) | 4.8 (3.8) | NA | NA |
| i.v. Morphine (mg/kg × days) | |||
| LOW parenting stress | .4 (1.1) | NA | .006b |
| HIGH parenting stress | 3.1 (6.3) | NA | |
| Overall | 1.8 (4.8) | NA | NA |
| Total PSI score | |||
| LOW parenting stress | 194.1 (11.9) | 179.7 (19.2) | .001b |
| HIGH parenting stress | 242.6 (23.8) | 229.3 (12.0) | |
| Overall | 219.7 (30.9) | 203.9 (29.7) | .001c |
| Child domain PSI score | |||
| LOW parenting stress | 84.8 (10.0) | 77.4 (11.5) | <.001b |
| HIGH parenting stress | 108.3 (13.6) | 101.7 (9.8) | |
| Overall | 97.2 (16.8) | 89.2 (16.2) | .00 7c |
| Parent Domain PSI score | |||
| LOW parenting stress | 109.3 (10.5) | 103.3 (9.74) | <.001b |
| HIGH parenting stress | 134.3 (15.2) | 127.7 (11.8) | |
| Overall | 122.5 (18.1) | 115.2 (16.3) | .013c |
| Maternal behavior | |||
| LOW parenting stress | .2 (.9) | −.2 (1.0) | .505 |
| HIGH parenting stress | .03 (1.1) | −.3 (1.0) | |
| Overall | .1 (1.0) | −.2 (1.0) | .109 |
| Maternal age (years) | |||
| LOW parenting stress | 34.0 (4.4) | 35.1 (4.5) | .032d |
| HIGH parenting stress | 31.1 (6.0) | 33.7 (3.4) | |
| Overall | 32.5 (5.5) | 34.4 (4.0) | .064a |
| Maternal education (years) | |||
| LOW parenting stress | 15.9 (2.8) | 17.9 (3.20 | .006d |
| HIGH parenting stress | 14.3 (2.7) | 16.5 (2.7) | |
| Overall | 15.0 (3.0) | 17.2 (3.0) | <.001a |
| Attention | |||
| LOW parenting stress | 9.9 (3.3) | 11.8 (3.2) | .426 |
| HIGH parenting stress | 10.6 (3.7) | 9.8 (3.5) | |
| Overall | 10.3 (3.5) | 10.8 (3.5) | .398 |
| Basal cortisol concentrations (mg/dL) | |||
| LOW parenting stress | .2 (.2) | .3 (.3) | .258 |
| HIGH parenting stress | .2 (.2) | .2 (.1) | |
| Overall | .2 (.2) | .2 (.2) | .722 |
| Time of testing (min since midnight) | |||
| LOW parenting stress | 641.3 (86.0) | 637.6 (105.3) | .820 |
| HIGH parenting stress | 624.3 (103.8) | 658.8 (113.7) | |
| Overall | 632.3 (95.7) | 647.9 (108.5) | .550 |
| Time since last wake (min since midnight) | |||
| LOW parenting stress | 115.1 (134.6) | 102.4 (59.9) | .280 |
| HIGH parenting stress | 105.2 (116.0) | 69.0 (49.7) | |
| Overall | 109.8 (124.4) | 86.2 (57.0) | .297 |
Data are presented for preterm and full-term infants in the LOWand HIGH parenting stress groups, and overall. There were n = 40 PRETERM-LOW, n = 44 PRETERM-HIGH, n = 19 FULL-TERM LOW, and n = 18 FULL-TERM HIGH mother–infants dyads, unless specified otherwise. p-values reflect differences between low and high stress groups (first row) and between preterm and full-term infants (second row). No interaction effects were observed.
Preterm < full-term.
LOW parenting stress <HIGH parenting stress.
Preterm >full-term.
LOW parenting stress >HIGH parenting stress.
Procedures
Informed consent was obtained from each mother at recruitment when the child was in early infancy, and again prior to participation in the visit at 8 months CA, in accordance with the requirements of the Clinical Research Ethics Board of University of British Columbia (UBC) and the Research Review Committee at C&W. Upon arrival at the laboratory, the mother–infant dyad was given several minutes to settle and get acquainted with the testing environment. They were then presented with the following tasks: 6-min toy exploration play session for assessment of the quality of focused attention, followed by a 5-min interactive free play session during which maternal behaviors were assessed using criteria set by Crnic et al. (1983). Saliva samples for basal cortisol assay were collected shortly after arrival in the lab while the infant was seated on his/her mother’s lap. Since feeding and blood contamination due to teething can interfere with the accuracy of salivary cortisol assays (Kivlighan, Granger, Schwartz, Nelson, & Curran, 2004; Schwartz, Granger, Susman, Gunnar, & Laird, 1998), saliva samples were collected at least 25 min after feeding, and we verified that there had been no traces of blood from teething in 24 hr prior to the start of experimental session. The HPA axis is known to follow a circadian rhythm that is apparent as early as the 3rd month of life under normal conditions (Antonini, Jorge, & Moreira, 2000; de Weerth, Zijl, & Buitelaar, 2003; Gunnar, 1992; Larson, White, Cochran, Donzella, & Gunnar, 1998). Therefore, as much as possible, experimental sessions took place in the morning, between 8:30 a.m. and 1:30 p.m., which is an appropriate time of day for optimal infant cognitive performance. Five preterm and three full-term infants were seen between 1:30 p.m. and 3:00 p.m. Since cortisol can decrease transiently during naptime (Watamura, Sebanc, & Gunnar, 2002), the time of saliva collection and time since waking were included as covariates in data analyses of infant cortisol.
Measures
Infant Measures
Medical chart review
A neonatal research nurse conducted medical and nursing chart review that included, but was not limited to, birthweight, gestational age, illness severity (Score for Neonatal Acute Physiology II, SNAP-II, on postnatal Days 1) and infant medications. The total cumulative number of skin-breaking procedures from birth to term (39 weeks and 6 days) was used as an index of neonatal pain-related stress. These procedures included, but were not restricted to, heel lance, intramuscular injections, chest tube insertion, and central line insertion. Cumulative neonatal morphine exposure was indexed as the average daily dose of intravenous morphine adjusted for daily weight, multiplied by the number of days of intravenous morphine administration. Knowing that exposure to postnatal glucocorticoids can affect the HPA axis of juvenile offspring (Burlet et al., 2005), we also recorded the number of days of exposure to postnatal glucocorticoids for each preterm infant. Overall, six infants received glucocorticoids postnatally.
Focused attention
The quality of focused attention during toy exploration was scored from videotape following the methods of Lawson and Ruff (2001). The toy exploration session comprised presenting four different toys consecutively for 90 s per toy. Toys were chosen to be age-appropriate, made of washable plastic, varied in color and texture, and were presented to the infant in a standard order. Quality of focused attention is defined as the global quality of visual examination while the infant was not banging or mouthing the toys, using a five-point rating scale, ranging from 1 (little or no investment of energy in object exploration) to 5 (high levels of sustained and concentration attention). This global rating of focused attention during toy exploration in preterm infants has been shown to predict cognitive outcome later in childhood (Lawson & Ruff, 2004). With this paradigm, two blinded coders independently rated the global quality of focused attention in all infants. If the two coders did not agree, they re-rated the video independently and if agreement was reached, then this second rating was retained. If disagreement remained and the difference between scores was less than one point, then the mean score was used. Differences beyond one point were resolved by a third independent coder (from personal communications with Dr. Lawson). The quality of infant focused attention was averaged across the four toys to yield one single score of focused attention.
Maternal Measures
Demographic characteristics
Maternal characteristics at delivery, including the mother’s age and years of education, were collected through a questionnaire. Antenatal glucocorticoids, which are given to mothers as a preventive measure against premature delivery may affect the infant’s basal (Ashwood et al., 2006; Kajantie et al., 2004) and reactive (Glover, Miles, Matta, Modi, & Stevenson, 2005) HPA secretion in early infancy (before 4 months of age). Very little is known about long-term changes. Therefore, we have also collected information regarding maternal exposure to antenatal glucocorticoids (n = 8).
Interactive behaviors
Interactive maternal behaviors during the play session were recorded on videotape, and later scored by blinded coders on a scale from 1 (poor) to 5 (high) for Gratification, Affect, Sensitivity, and Organization, according to the criteria of Crnic et al. (1983). Inter-rater reliability was carried out on 25% of infants. Weighted kappa using agreement within one scale point was .95, 1.0, .79, and .94 for Gratification, Affect, Sensitivity, and Organization, respectively. To reduce the number of variables, we combined the four subscales of maternal behaviors (Gratification, Affect, Sensitivity, and Organization) into a single factor score using principal component analysis.
Parenting stress
Mothers completed the long-form Parenting Stress Index (PSI), a 101-item questionnaire (Abidin, 1995) which provides parenting stress scores on the Parent Domain, Child Domain, Total Stress, and Defensive Responding. Low scores (<24) on Defensive Responding are viewed as under-reporting (Abidin, 1995), therefore infants whose mothers scored below 24 were excluded from analysis, leaving 84 preterm (43 boys, 46 girls) and 37 full-term (20 boys and 17 girls) mother–infant dyads available for study. Using median split of the Total PSI scores (median evaluated at 220), mother–infant dyads were divided a posteriori into low (PRETERM-LOW, n = 40; 20 boys, 20 girls; FULL-TERM LOW, n = 19, 10 boys, 9 girls) and high (PRETERM-HIGH, n = 44; 20 boys, 24 girls; FULL-TERM HIGH, n = 18, 11 boys, 7 girls) parenting stress groups.
That sample size, knowing that 10 participants per predictor variable are required to perform linear regression analysis, was appropriate to examine the relationship between the quality of focused attention (dependent variable) and neonatal pain-related stress (adjusted for cumulative intravenous morphine exposure and initial illness severity) and maternal interactive behavior (independent variables) within each of the PRETERM-LOWand PRETERM-HIGH parenting stress groups.
Infant Cortisol Analysis
Saliva was collected using cotton dental rolls, extracted by needleless syringes into vials, and stored at −20°C until assayed in Dr. Weinberg’s laboratory at the UBC. Salivary cortisol was assayed using the Salimetrics High Sensitivity Salivary Cortisol Enzyme Immunoassay kit (Salimetrics LLC, Philadelphia, PA). Intra and interassay coefficients of variability were 3.04 and 5.65%, respectively.
Statistical Analysis
The cohort of full-term infants was used as a reference group for both infant (quality of focused attention) and maternal (parenting stress, interactive behaviors) characteristics. Given that preterm boys have poorer developmental outcomes than preterm girls (e.g., Hindmarsh, O’Callaghan, Mohay, & Rogers, 2000), we have included sex of the infant as a grouping variable in our analyses. Comparisons of effects of sex of the infant, prematurity status, and parenting stress group on infant and maternal characteristics were performed using multivariate analysis of variance (MANOVA). All measures are presented in Table 1, as mean ± standard deviation (SD).
To address our hypotheses, we used hierarchical regression analyses to examine whether neonatal-pain related stress (number of skin-breaking procedures from birth to term, adjusted for initial illness severity and cumulative i.v. morphine exposure from birth to term: entered in block 1) and maternal behaviors score (entered in block 2) predicted the quality of focused attention (dependent variable), in PRETERM-LOWand PRETERM-HIGH parenting stress groups separately. In addition, we performed another set of analyses to investigate whether basal cortisol (covaried for time of testing and time since waking) was associated with the quality of focused attention in the PRETERM-LOW and PRETERM-HIGH parenting stress groups separately using linear regression analyses. For all analyses, statistical significance was set at p <.05.
Only a small number of mother–infant dyads received either antenatal (n = 8) or postnatal glucocorticoids (n = 6). None received glucocorticoids both ante- and postnatally. Due to the small sample size, rather than adding their user status as a grouping variable, we compared statistics obtained with and without including these mother–infant dyads in the analyses.
RESULTS
Demographic Characteristics
Infant Measures
As expected, MANOVA showed that birthweight and gestational age were lower in preterm than in full-term infants [F(1,120) = 525.24; p <.001 and F(1,120) = 553.54, p <.001, respectively]. Regardless of prematurity status, boys had greater birthweight than girls, F(1,120) = 3.99, p = .048. There was no main effect of parenting stress group, or interaction effects of sex of the infant, prematurity status, or parenting stress group on birthweight or gestational age. Importantly, neonatal illness severity and i.v. morphine exposure were significantly greater in the PRETERM-HIGH parenting stress compared to the PRETERM-LOW parenting stress group [F(1,79) = 6.44, p = .013 and F(1,79) = 7.01, p = .010, respectively]. No other main or interaction effects involving sex of the infant or parenting stress were observed for neonatal illness severity, morphine exposure. Infant characteristics are presented in Table 1.
Repeated measures ANOVAs performed across all four toys used in toy exploration showed that overall, there was a trend in lower quality of focused attention in preterm infants compared to full-term infants [F(1,173) = 3.60, p = .060; Preterm: 2.25±.08, 2.82 ±.10, 2.49 ±.09, and 2.58 ±.10 and Full-term: 2.36 ±.11, 3.13 ±.13, 3.01 ±.12, and 2.84 ±.13 for Toys 1, 2, 3, and 4, respectively]. No other main or interaction effects involving sex of the infant, prematurity status parenting, and stress group were observed.
We did not find any significant main or interaction effects of sex of the infant, prematurity status or parenting stress group on basal cortisol concentrations.
Maternal Measures
Main effect of prematurity status on years of maternal education, F(1,141) = 13.32; p <.001. There was no main effect of prematurity status on maternal age, however, among mothers of preterm infants, there was an overall effect of parenting stress groups on maternal age [F(1,129) = 6.13, p = .015]. Mothers of the PRETERM-HIGH parenting stress group were younger (by about 3 years) than mothers of the PRETERM-LOW parenting stress group (p = .022) and of the full-term groups (p = .004). Similarly, years of maternal education also differed among the groups [F(1,129) = 8.23, p = .005], with the lowest education in the PRETERM-HIGH parenting stress group (p <.001) compared to both the PRETERM-LOW and FULL-TERM parenting stress groups. There were significant differences in maternal age and years of education due to sex of the infant.
Principal component factor analysis (PCA) was used to reduce the four subscales of interactive maternal behaviors (Gratification, Affect, Sensitivity, and Organization) to one score. The contribution of these components was .857, .817, .623, and .615, respectively. Neither individual interactive maternal behavior components (Gratification, Affect, Sensitivity, or Organization) nor the overall score generated by PCA differed due to sex of the infant, parenting stress group, or prematurity status.
Mothers of preterm infants had higher scores on the Total PSI [F(1,120) = 13.13, p <.001], the Child Domain [F(1,120) = 7.60, p = .007], and the Parent Domain [F(1,120) = 6.30, p = .013], than mothers of full-term infants. However, within each group parenting stress, that is, lower median and upper median, scores in mothers of preterm infants did not differ from scores in mothers of full-term infants. No main or interaction effects of sex of the infant were noted for scores on the Child Domain, Parent Domain, and Total PSI. Maternal characteristics are presented in Table 1.
Because only birthweight was affected by sex of the infant, in the following set of analyses, boys and girls will be pooled together to be considered as one group.
Maternal Factors and Parenting Stress
Given the lower maternal age and education observed in PRETERM-HIGH compared to PRETERM-LOW parenting stress groups, analyses were conducted to examine potential associations between these demographic variables and PSI scores. Pearson correlations revealed no statistically significant association between maternal demographic characteristics and the Child Domain, Parent Domain, or Total PSI score.
Neonatal Pain-Related Stress and Basal Cortisol Concentrations
Neonatal pain-related stress, indexed by the number of skin-breaking procedures, did not correlate with basal cortisol concentrations in either the PRETERM-LOW or PRETERM-HIGH parenting stress groups.
Neonatal Pain-Related Stress and Interactive Maternal Behavior in Relation to Infant Focused Attention
In the PRETERM-LOW parenting stress group, after controlling for illness severity and i.v. morphine exposure, greater neonatal pain-related stress was associated with poorer focused attention (β= −.39, p = .047, Fig. 1), and greater interactive maternal behavior was associated with better focused attention (β = .35, p = .017, see Fig. 2). This model explained 19% of the variance observed. In the PRETERM-HIGH parenting stress group, though neonatal stress was also associated with poorer focused attention (β = −.35, p = .024, Fig. 3), there was no buffering effect of maternal behavior on infant focused attention. This model explained 15% of the variance observed.
FIGURE 1.
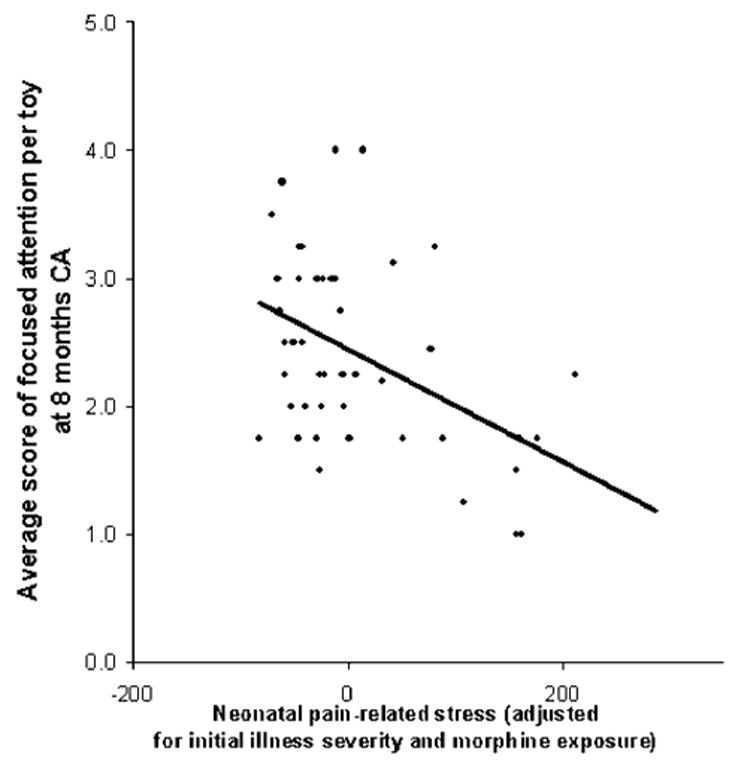
Relationship between the average score of focused attention per toy and cumulative neonatal pain-related stress (adjusted for initial illness severity and morphine exposure) in 8-month-old CA preterm infants exposed to low maternal parenting stress.
FIGURE 2.
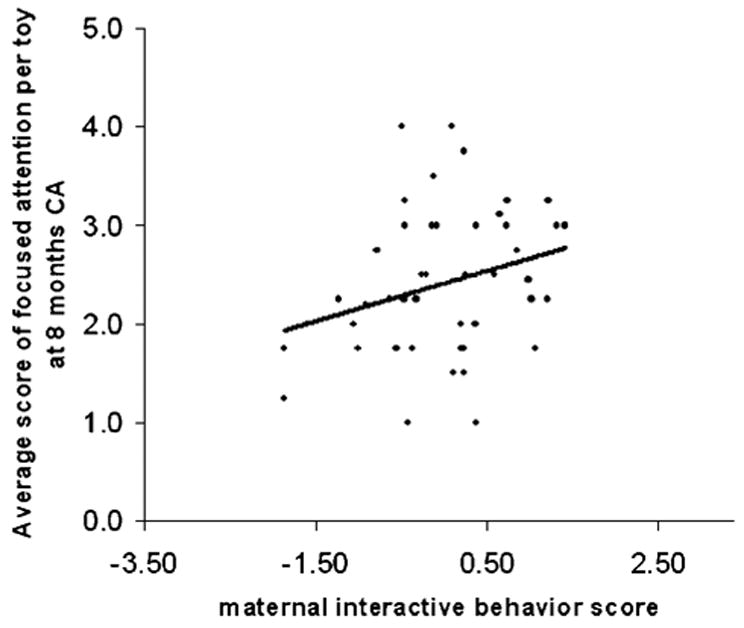
Relationship between the average score of focused attention per toy and interactive maternal behavior score in 8-month-old CA preterm infants exposed to low maternal parenting stress.
FIGURE 3.
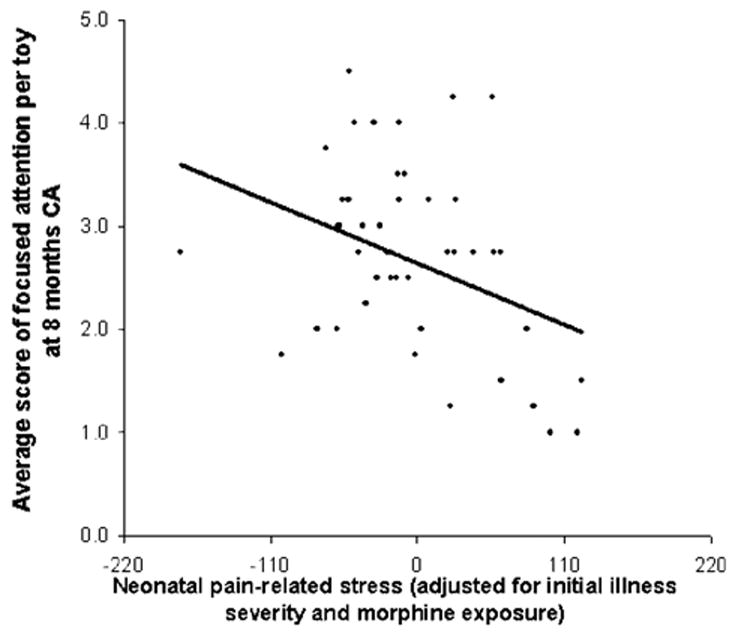
Relationship between the average score of focused attention per toy and cumulative neonatal pain-related stress (adjusted for initial illness severity and morphine exposure) in 8-month-old CA preterm infants exposed to high maternal parenting stress.
Infant Cortisol in Relation to Quality of Focused Attention
Linear regression analyses showed no significant association between infant basal cortisol (adjusted for time of testing and time since waking) and quality of focused attention in either the PRETERM-LOW or PRETERM-HIGH parenting stress groups. However, when the participants were subsequently divided into low and high interactive maternal behaviors (using median split), infants from the PRETERM-HIGH parenting stress group whose mothers displayed high interactive maternal behavior showed a negative association between basal cortisol and the quality of focused attention (β = −.34, p = .04, 23% variance explained, Fig. 4). High infant basal cortisol was associated with poorer attention in this subgroup. No significant correlations were seen for full-term infants.
FIGURE 4.
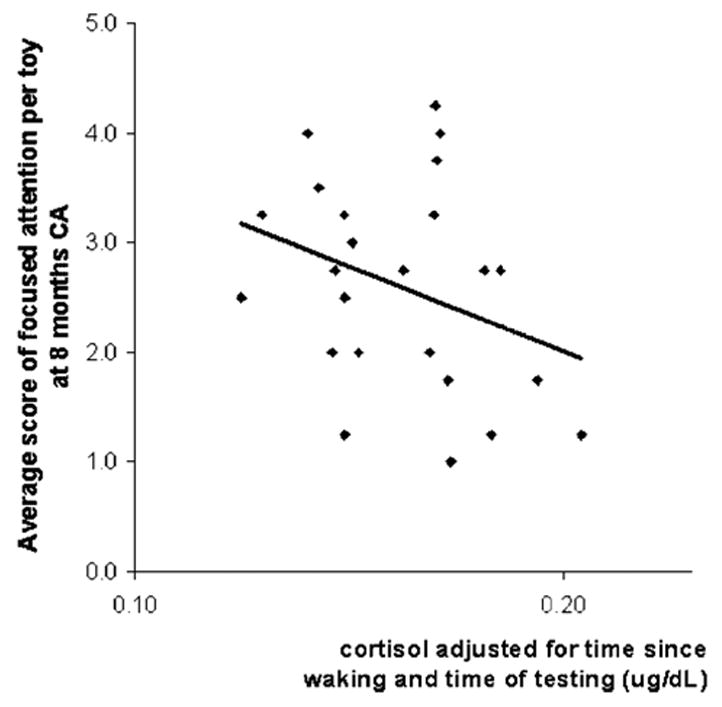
Relationship between basal cortisol (adjusted for time since waking and time of testing) and the average score of focused attention per toy in 8-month-old CA preterm infants of mothers who reported high parenting stress and overwhelming interactive behaviors.
Use of Antenatal and Postnatal Glucocorticoids
Analyses performed on a sample of mother–infant dyads unexposed to antenatal or postnatal glucocorticoids did not reveal any differences in the direction and significance of findings presented above. For both PRETERM-LOW and PRETERM-HIGH parenting stress group, larger number of painful procedures remained associated with poorer quality of focused attention and higher maternal interactive behavior score was associated with better attention in the PRETERM-LOW parenting stress group only (PRETERM-LOW: number of painful procedures, β = −.39, p = .05, maternal interactive behavior β =.33, p = .027, 18% variance explained; PRETERM-HIGH: number of painful procedures β = −.58, p = .017, 21% variance explained). In PRETERM-HIGH parenting stress group exposed to overwhelming maternal interactive behavior, higher basal cortisol concentrations were associated with poor quality of focused attention, β = −.34, p = .045, 23% variance explained.
DISCUSSION
To our knowledge, this is the first study to examine the contribution of maternal factors on the relationship between neonatal pain-related stress, cognitive indices, and basal cortisol in the preterm infant population. Simultaneously, we investigated the influence of maternal factors such as parenting stress and interactive behavior in relation to cognition and cortisol. We found that greater neonatal pain-related stress was associated with poorer quality of focused attention at 8 months CA and that maternal behavior can buffer this relationship in mothers reporting low parenting stress. Moreover, in infants whose mothers reported high parenting stress and displayed high interactive maternal behavior, higher basal cortisol was associated with poorer quality of focused attention in infants.
Children and adolescents born prematurely have a range of problems related to cognition, attention learning, and behavior (e.g., Grunau et al., 2004b). Little is known, however, about the etiology of these difficulties. Furthermore, although prolonged neonatal pain-related stress, at a time of rapid brain development, has been proposed as one possible contributing factor that may affect cognition and behavior (Anand, 2000; Grunau, 2002), this theory has received little direct empirical investigation. In this study, we used the quality of infant focused attention as a measure of cognition since it predicts later cognitive outcome (Lawson & Ruff, 2004). In agreement with previous work, we found that the quality of focused attention was poorer in preterm compared to full-term infants (Ruff, 1986, 1988; Ruff et al., 1990). Importantly, we show for the first time that neonatal pain-related stress, indexed by the total number of skin-breaking procedures from birth to term, is associated with poorer quality of focused attention in preterm infants. Low tactile threshold and immaturity of descending modulatory systems (Fitzgerald, 2005) underlie greater sensitivity to pain in VLGA preterm infants. Due to immaturity of the biological systems, sensitization occurs such that even inherently noninvasive routine nursing procedures induce stress responses (Holsti, Grunau, Oberlander, & Whitfield, 2005). Thus, the number of skin-breaking procedures is a marker of overall neonatal stress associated with tactile interventions. Other sources of stress, such as lighting and noise, are well-controlled in current NICU practice with the implementation of covered incubators and periods of no intervention as part of developmentally oriented care (Als et al., 1994). However, pain-related stress is highly correlated with number of days on mechanical ventilation, a situation that is inherently stressful. Further, since morphine treatment to reduce stress is associated with respiratory depression and lengthens time on ventilation (Bhandari, Berqvist, Kronsberg, Barton, & Anand, 2005), pain and stress inherent to NICU care is difficult to manage or eliminate. Therefore, we cannot evaluate a direct causal relationship between early pain and poorer cognition; rather we propose that pain-related stress, as a marker of neonatal stress, is likely a contributing factor. This is consistent with the animal literature on the adverse impact of early stress, especially in the case of prolonged maternal separation. Animal studies of neonatal pain have focused on long-term effects on pain systems, and to our knowledge, none has examined later cognition. Experimental animal models addressing whether neonatal pain affects later cognition are needed.
Importantly, we found that maternal parenting stress overrode the buffering effect of interactive maternal behaviors on the relationship between the high number of skin-breaking procedures in the NICU and poor quality of focused attention in preterm infants. Thus, VLGA infants exposed to neonatal pain-related stress appeared to benefit from positive interactive maternal behavior, but only if their mothers self-reported low concurrent stress. These findings support and extend previous reports that mother–infant interactions can mediate the trajectories of mental development in the at-risk infant population (Poehlmann & Fiese, 2001). Maternal parenting stress may act indirectly through interactive maternal behavior or may affect infant cognition directly. In infants born close to term (below 38 weeks gestation), maternal stress did not affect quality of interactive maternal behavior during a free play session (Crnic, Greenberg, Robinson, & Ragozin, 1984). However, in preterm infants born below 34 weeks gestation, interaction at 6-months was affected more by maternal traumatic stress since birth than perinatal risk factors (Muller-Nix et al., 2004). Maternal worries at the time of delivery also predict maternal stress during infancy (Combs-Orme, Cain, & Wilson, 2004). Consistent with these studies, maternal stress appears to have greater effects in mothers of infants born very preterm, such as in our study.
Our finding that the consequences of neonatal pain-related stress on cognitive outcomes might be partially compensated for by sensitive and balanced quality of interactive maternal behaviors, is congruent with the animal literature. Such literature has demonstrated that adverse effects of early stress can be partially ameliorated through enriched rearing environments (Bredy et al., 2003; Francis et al., 2002), and suggests possibilities for remedial intervention. Recent preliminary evidence in macaques suggests that maternal perinatal stress and maternal behavior are associated with infant behavior during development (Bardi & Huffman, 2006). We speculate that in the current study, maternal parenting stress interfered with interactive maternal behavior, resulting in poorer quality of caregiver ‘‘support’’ in promoting cognitive development, expressed as quality of focused infant attention. Most studies addressing the contribution of interactive maternal behavior to infant cognitive outcomes use ‘‘sensitivity to infant cues’’ as the key component of maternal behavior. Interestingly, in reducing the four components of interactive maternal behavior, that is, Gratification, Affect, Sensitivity, and Organization derived from Crnic et al. (1983), to a single factor, we found that Gratification and Affect might play a role that is at least as important as Sensitivity in the quality of interactive maternal behavior. Previously, maternal stress had been suggested to impede maternal sensitivity to infant cues (Ragozin, Basham, Crnic, Greenberg, & Robinson, 1982). Here, we report that other components of interactive maternal behavior, such as Affect, might interact with high maternal stress and possibly overwhelm the infant (Field, 1994). Given that communication styles between a mother and her preterm infant can change significantly over the first 2 years of life (Muller-Nix et al., 2004), it is important to extend this question beyond age 8 months. Additional work is needed to test this mediating role of maternal stress further.
Maternal behavior has long been known to be a strong regulator of an infant’s biological vulnerability (e.g., Gunnar, 1998; Hofer, 1994; Spangler, Schieche, Ilg, Maier, & Ackermann, 1994; Thompson et al., 1994) and has been suggested to mediate the developmental trajectories in at-risk populations (Magill-Evans & Harrison, 1999; Poehlmann & Fiese, 2001). Moreover, associations linking maternal stress and depressive symptoms, with elevated child cortisol levels and cognitive and behavioral outcomes have been reported in recent years (Essex et al., 2002; Lupien et al., 2000, 2001). Interestingly, Walker et al. (2003) suggested that increased maternal behavior buffered HPA responsiveness to stress in two week-old rat pups exposed to repeated neonatal pain. We extend these findings by suggesting that in infants born very preterm, maternal factors might mediate the vulnerability of preterm infants to early pain-related stress. It is important to keep in mind that in rodents, maternal care is essential for basic functions such as thermoregulation, feeding, and excretion. Conversely, in humans, the role of maternal behavior as a regulator of infant biological and cognitive development appears to act at a much higher and likely more complex cortical level. Protective factors play a greater role in individuals who are at higher risk of poor self-regulation. Further work is needed to elucidate the contribution of various aspects of maternal behavior on HPA buffering in the preterm and full-term infant population.
Previous studies have shown that the tiniest preterm infants born at extremely low gestational age (ELGA, <29 weeks GA) had greater cortisol levels at 8 months CA, compared to preterm infants born with more maturity and full-term infants (Grunau et al., 2004a). In this study, at a similar age, we did not find higher basal cortisol concentrations in preterm infants born with greater gestational age (<32 weeks gestation), when compared to full-term infants. It is possible that in the current study, the inclusion of preterm infants born with greater maturity at birth might have prevented us from findings such group differences in basal cortisol concentrations. For similar reasons, we failed to detect any significant correlations between the number of skin-breaking procedures and basal cortisol concentrations, even though this association has already been reported previously in ELGA infants by our own research group (Grunau et al., 2004a).
In this study, we found that high cortisol was negatively associated with quality of focused attention only in preterm infants exposed to both high maternal parenting stress and high maternal interactive behavior. At first sight, this finding could seem unexpected given that a positive relationship between basal cortisol and cognition in preterm infants at 3 months CA has been recently described (Haley et al., 2006). However, at 3 months CA, VLGA infants are in a state of downregulation, when low cortisol levels are predominant (Haley et al., 2006). In contrast, at 8 months CA, ELGA infants, are in a state of upregulation, that is, levels are higher compared to full-term infants (Grunau et al., 2004a; Grunau et al., under review). When comparing our cortisol values collected at 8 months CA, to those presented in Haley et al. (2006), we found that in full-term infants, from 3 months CA to 8 months CA, basal cortisol levels decreased (mean .32 μg/dL vs. .25 μg/dL, respectively), while they remained similar for VLGA infants (.2 μg/dL at both time points). This shift from downregulation to upregulation state could explain the opposite direction of association we are reporting in this study.
It is encouraging that at 8 months CA, only a small proportion of preterm infants, that is, those exposed to both high parenting stress and high interactive behavior, presented poor cognition in association with higher basal cortisol concentrations. Further studies are needed to determine the exact reasons for this finding. Some stress-related events experienced by mothers have been linked to higher basal cortisol levels and poorer cognition in children (Essex et al., 2002; Lupien et al., 2000, 2001). Because we have reported that high maternal parenting stress may override the beneficial contribution of maternal interactive behavior on cognition in preterm infants exposed to neonatal pain-related stress, it is possible that both maternal parenting stress and interactive behavior could mediate the association between infant cognition and basal cortisol levels. In our search to understand these findings, we contemplate the hypothesis of ‘‘overwhelming’’ maternal influences on the preterm infant. We speculate that this maternal-induced ‘‘overwhelming’’ of the preterm infant could lead to a more rapid emergence from the HPA hyporesponsive state normally observed during the first year of life. This emergence to a responsive state would enable the HPA axis to present negative correlations with the quality of focused attention. This is in accordance with the hypothesis that cortisol levels during the early human development are regulated by social factors derived from the parents during the first years of life, and from interacting with the peers at the daycare and beyond (Gunnar & Donzella, 2002). Importantly, in our study, full-term infants did not readily show any association between cortisol and infant attention. Thus, it appears that based on our maternal measures, this maternal-induced ‘‘overwhelming’’ might be more easily detected in mothers of infants displaying poor self-regulation. Additional work is needed to investigate this matter further.
Cortisol levels collected in laboratory settings have been consistently reported to be lower than those measured in the same infants, in a home setting, at comparable times of day (Gunnar, Mangelsdorf, Larson, & Hertsgaard, 1989; Larson, Gunnar, & Hertsgaard, 1991). Although this could be an obstacle to the generalization of our findings to daily life situations, high correlations between baseline cortisol levels collected prior to laboratory-induced stressors and basal levels sampled at similar times at home suggest that observations made in the laboratory reflect the infant’s phenotype, without the need to collect home samples as reference levels (Goldberg et al., 2003). Therefore, not only would our findings regarding cortisol correlates represent a realistic phenotype of daily life in preterm infants, but also it is possible that the relationship might be even more pronounced in daily life situations.
Our findings were not affected by excluding mother–infant dyads exposed to antenatal or postnatal glucocorticoids from our analyses. However, because of the small sample size of infants exposed, we cannot entirely rule out the possibility that glucocorticoids exposure, whether ante- or postnatally, did not affect our findings. In contrast to the animal literature, only a limited number of human studies have investigated the long-term consequences of antenatal and postnatal exposure to glucocorticoids on the HPA in neonates (for a review, see Matthews, 2001; Purdy & Wiley, 2004). Findings suggest that despite showing greater behavior problems during childhood, infants exposed to antenatal and postnatal glucocorticoids might not show any detectable side effects impeding neurodevelopment until later in life (Purdy & Wiley, 2004). With animal work hypothesizing the presence of sex-specific long-term consequences of antenatal glucocorticoids exposure favoring females (Matthews, 2001), more work in the human population would be needed to better understand these interactions.
While examining the demographic characteristics of mother–infant dyads, we found that compared to the FULL-TERM group, mothers of the PRETERM-LOW parenting stress group had similar ages, years of education and Total, Child, and Parent Domains of the PSI. Conversely, PRETERM-HIGH parenting stress mothers were younger and had fewer years of education. Lower education has been linked to reduced coping and protective resources in the face of stressful events (for a review, see Kristenson, Eriksen, Sluiter, Starke, & Ursin, 2004). However, we did not find any significant association between maternal parenting stress and interactive behavior, and maternal education or age. Mothers in the PRETERM-HIGH parenting stress group were younger in age and had fewer years of education (average age 31.0 years and 14.2 years of education) compared to mothers in the PRETERM-LOW parenting stress group and mothers of full-term infants (average age 34.0 and 34.4 years, 15.8 and 17.2 years of education, respectively). However, given that maternal age and education were relatively high in all our groups, it is unlikely that these variables played a significant role in our findings. Importantly, although infants in the PRETERM-HIGH parenting stress group had higher illness severity on the first postnatal day, they did not differ from the PRE-TERM-LOW parenting stress group in birthweight, gestational age, days on mechanical ventilation, or amount of neonatal pain-related stress. Furthermore, we found that greater illness severity on Day 1 had a lasting impression on the parents only in the PRETERM-LOW parenting stress group. During early infancy, greater neonatal physiological vulnerability is known to be associated with greater maternal distress during infancy (Combs-Orme et al., 2004; Singer et al., 1999) and poorer developmental outcome (Miceli et al., 2000). However, this association appears to be more apparent during early infancy since over time, the impact of the early social environment appears to exceed the influence of neonatal factors as the infant reaches preschool age (Miceli et al., 2000).
Sex differences in developmental outcomes of preterm born children have been favoring girls over boys. Preterm male neonates seem to require more neonatal care than female preterm neonates (Elsmen, Hansen Pupp, & Hellstrom-Westas, 2004). Despite medical advances in recent years, boys are yet to show less severe neonatal brain injury and illness, and lower infant and perinatal mortality (Bhaumik et al., 2004; Nunez & McCarthy, 2003; Vatten & Skjarven, 2004). Despite this evidence of sex dichotomy in preterm outcome, we did not find any significant sex differences in the outcomes that we have measured in this study. Unlike child temperament and indices of developmental psychopathology, where sex differences are apparent at a relatively early age, very few have reported clear sex differences for cognitive abilities in preterm infants (Hindmarsh et al., 2000). It is possible that sex differences in the areas investigated in our study, that is, maternal stress, maternal interactive behavior, infant cortisol, and infant focused attention may require some time to unfold. Importantly, due to bias related to participation to research studies like ours, it is also possible that some of the boys enrolled in our study might be among those who fared better after hospital discharge.
Although mothers of preterm infants had greater parenting stress scores than mothers of full-term infants, within each of the LOW and HIGH maternal parenting stress groups, there was no difference in the Total PSI scores due to prematurity status. The higher Total PSI score found in mothers of a preterm infant (higher by 16 points) might be driven by slightly higher scores in the PRETERM-HIGH group, than in the FULL-TERM HIGH parenting stress group (mean of 242.6 vs. 229.3, respectively). Previous studies have shown that maternal factors contribute to a larger variance in developmental outcomes in the preterm population compared to the full-term infant population (e.g., Greenberg & Crnic, 1988; Hofer, 1994; Poehlmann & Fiese, 2001). Indeed, our analyses showed that at 8 months, full-term infants might not be as influenced by maternal factors as the preterm population. Protective factors such as maternal behavior come into greater play when the infant is at risk for poorer self-regulation. Thus, compared to an infant born very prematurely, a healthy full-term infant, with lower risk for poor self-regulation, might not rely on the maternal protective influence as much. It is important to remind our readers that our findings by no means imply that full-term infants will not be affected by poor quality of maternal interactive behavior. Our measures might simply not be sensitive enough to detect the contribution of maternal factors in full-term infants. Furthermore, our median of 220 on the Total PSI observed in mothers of preterm infants should be interpreted with caution. The PSI is a self-reported inventory that has limited clinical significance when used in a context of research such as the current study. The 50th percentile norms for parents of healthy infant at age 12 months are listed at approximately 200, and a suggestive cut-off score for clinical referral for excessive parenting stress suggested at 260 (Abidin, 1995), which none of our participants has reached, our findings indicate that mothers were only slightly into the upper range of normative scores. Therefore, in our study, PSI scores in mothers of preterm infants did not need to reach the excessive stress level that could interfere with infant outcomes. Finally, it is important to note that we have excluded mothers who portrayed a ‘‘defensive’’ profile, self-reporting abnormally low scores. These abnormally low scores have been believed to under-report underlying higher parenting stress profile. However, given the relevance of stressful events during the neonatal and infancy period, for parents of very preterm infants, they may self-report less stress once their infant’s health has stabilized after NICU discharge. Thus, excluding mothers with defensive profile from the analyses prevented us from including under-reporting in our analyses; it is also likely that we have excluded mothers who truly reported low parenting stress.
In our study, high scores in both the Child and Parent Domains contributed to the high total PSI score. The fact that high stress mothers reported higher scores on Domains indicates that interventions targeted to alleviate parenting stress attributed to parental characteristics might only partially reduce the level of parenting stress. These findings are in agreement with previous observations that maternal concerns during delivery might predict the extent of maternal stress during infancy (Combs-Orme et al., 2004). Moreover, the PSI is a self-report scale and we acknowledge that further studies using a more objective marker of stress, such as cortisol, would provide valuable information in conjunction with PSI scores, especially since anticipation of adverse events is known to be among the psychological determinants of cortisol secretion (e.g., Gaab, Rohleder, Nater, & Ehlert, 2005; Mason, 1968; Smyth et al., 1998).
Prenatal maternal anxiety and stress are predictors of premature delivery, poor neurodevelopment, and more disrupted HPA function in the infant (Austin & Leader, 2000; DiPietro, Novak, Costigan, Atella, & Reusing, 2006; Glover, 1997; King & Laplante, 2005; Laplante et al., 2004; Wadhwa, Sandman, & Garite, 2001). In our study, recruitment took place after delivery. To account for both prenatal and postnatal risks factors of poorer neurodevelopment and stress-regulation, a much larger sample of mother–infant dyads than in the current study would be needed. Therefore, it is not possible to verify whether presence of antenatal maternal stress and anxiety would contribute independently to poorer infant outcomes, in combination with high postnatal maternal stress.
Infants born very prematurely are vulnerable because they enter the world with immature physiological and central nervous systems. During this critical period, as part of routine neonatal care, they are exposed to considerable pain and stress, which may contribute to ‘‘resetting’’ basal cortisol levels (Grunau et al., 2004a; Grunau et al., under review). Importantly, little is known about maternal distress after hospital discharge, even though maternal worries during the perinatal period can predict maternal distress later during infancy (Combs-Orme et al., 2004). In this study, we have identified maternal factors that may ameliorate adverse effects of neonatal pain exposure on infant cognition and affect the vulnerability and resilience of psychobiological function of at-risk infants. Our findings will contribute to current knowledge regarding postnatal influences after hospital discharge, and suggest the importance of developing targeted interventions to alleviate stress in the mothers of preterm infants.
Footnotes
Contract grant sponsor: National Institute for Child Health and Human Development
Contract grant number: HD39783
Contract grant sponsor: Canadian Institutes for Health Research (CIHR)
Contract grant numbers: MOP42469, MOP91869
Contract grant sponsor: Human Early Learning Partnership
Contract grant numbers: 03-3112, 02-2403, 02-2410
Contract grant sponsor: Fond de la Recherche en Sante du Quebec
Contract grant sponsor: Michael Smith Foundation for Health Research
This study was supported by grant HD39783 (R.E.G.) from the National Institute for Child Health and Human Development, grant MOP42469 (R.E.G.) from the Canadian Institutes for Health Research (CIHR), and grants 03-3112 (R.E.G.), 02-2403 (R.E.G.) and 02-2410 (J.W.) from the Human Early Learning Partnership. Mai Thanh Tu is supported by the Pain in Child Health Strategic Training grant MOP91869 from CIHR and the Fond de la Recherche en Sante du Quebec # 10308, and Ruth Grunau by a Senior Scholar Award from the Michael Smith Foundation for Health Research. We would like to thank the pediatricians at BC Children’s and Women’s Health Centre who provided access to their patients, and research staff, especially lab manager Adi Amir, research nurse Gisela Gosse, and study coordinator Colleen Jantzen.
References
- Abidin R. Parenting stress index: Professional manual. 3. Lutz, FL: Psychological Assessment Resources, Inc; 1995. [Google Scholar]
- Als HA, Lawhon G, Duffy FH, McAnulty GB, Gibes-Grossman R, Blickman JG. Individualized developmental care for the very low-birth-weight preterm infant. Journal of the American Medical Association. 1994;272:853–858. [PubMed] [Google Scholar]
- Anand KJ, Coskun V, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiology and Behavior. 1999;66:627–637. doi: 10.1016/s0031-9384(98)00338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand KJS. Effects of perinatal pain and stress. Progress in Brain Research. 2000;122:117–129. doi: 10.1016/s0079-6123(08)62134-2. [DOI] [PubMed] [Google Scholar]
- Anderson PJ, Doyle LW the Victorian Infant Collaborative Study Group. Executive functioning in school-aged children who were born very preterm or with extremely low birth weight in the 1990s. Pediatrics. 2004;114:50–57. doi: 10.1542/peds.114.1.50. [DOI] [PubMed] [Google Scholar]
- Antonini SRR, Jorge SM, Moreira AC. The emergence of salivary cortisol circadian rhythm and its relationship to sleep activity in preterm infants. Clinical Endocrinology. 2000;52:423–426. [PubMed] [Google Scholar]
- Ashwood PJ, Crowther CA, Willson KJ, Haslam RR, Kennaway DJ, Hiller JE, Robinson JS. Neonatal adrenal function after repeat dose prenatal corticosteroids: A randomized controlled trial. Am J Obstet Gynecol. 2006;194:861–867. doi: 10.1016/j.ajog.2005.08.063. [DOI] [PubMed] [Google Scholar]
- Austin MP, Leader L. Maternal stress and obstetric and infant outcomes: Epidemiological findings and neuroendocrine mechanisms. The Australian & New Zealand Journal of Obstetrics & Gynaecology. 2000;40:331–337. doi: 10.1111/j.1479-828x.2000.tb03344.x. [DOI] [PubMed] [Google Scholar]
- Bardi M, Huffman MA. Maternal behavior and maternal stress are associated with infant behavioral development in macaques. Developmental Psychobiology. 2006;48:1–9. doi: 10.1002/dev.20111. [DOI] [PubMed] [Google Scholar]
- Bhandari V, Berqvist LL, Kronsberg SS, Barton B, Anand KJS. Morphine administration and short-term pulmonary outcomes among ventilated preterm infants. Pediatrics. 2005;116:352–359. doi: 10.1542/peds.2004-2123. [DOI] [PubMed] [Google Scholar]
- Bhaumik U, Aitken I, Kawachi I, Ringer S, Orav J, Lieberman E. Narrowing of sex differences in infant mortality in Massachusetts. Journal of Perinatology. 2004;24:94–99. doi: 10.1038/sj.jp.7211021. [DOI] [PubMed] [Google Scholar]
- Bhutta AT, Cleves MA, Casey PH, Craddock MM, Anand KJS. Cognitive and behavioral outcomes of school-aged children who were born preterm. A meta-analysis. Journal of American Medical Association. 2002;288:728–737. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Humpartzoomian RA, Cain DP, Meaney MJ. Partial reversal of the effect of maternal care on cognitive function through environmental enrichment. Neuroscience. 2003;118:571–576. doi: 10.1016/s0306-4522(02)00918-1. [DOI] [PubMed] [Google Scholar]
- Burlet G, Fernette B, Blanchard S, Angel E, Tankosic P, Maccari S, Burlet A. Antenatal glucocorticoids blunt the functioning of the hypothalamic-pituitary-adrenal axis of neonates and disturb some behaviors in juveniles. Neuroscience. 2005;133:221–230. doi: 10.1016/j.neuroscience.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Combs-Orme T, Cain DS, Wilson EE. Do maternal concerns at delivery predict parenting stress during infancy? Child Abuse & Neglect. 2004;28:377–392. doi: 10.1016/j.chiabu.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Crnic KA, Ragozin AS, Greenberg MT, Robinson NM, Basham RB. Social interaction and developmental competence of preterm and full-term infants during the first year of life. Child Development. 1983;54:1199–1210. [PubMed] [Google Scholar]
- Crnic KA, Greenberg MT, Robinson NM, Ragozin AS. Maternal stress and social support: Effect on the mother-infant relationship from birth to eighteen months. American Journal Orthopsychiatric. 1984;54:221–235. doi: 10.1111/j.1939-0025.1984.tb01490.x. [DOI] [PubMed] [Google Scholar]
- de Weerth C, Zijl RH, Buitelaar JK. Development of cortisol circadian rhythm in infancy. Early Human Development. 2003;73:39–52. doi: 10.1016/s0378-3782(03)00074-4. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Novak MF, Costigan KA, Atella LD, Reusing SP. Maternal psychological distress during pregnancy in relation to child development at age two. Child Development. 2006;77:573–587. doi: 10.1111/j.1467-8624.2006.00891.x. [DOI] [PubMed] [Google Scholar]
- Elsmen E, Hansen Pupp I, Hellstrom-Westas L. Preterm male infants need more initial respiratory and circulatory support than female infants. Acta Paediatrica. 2004;93:529–533. doi: 10.1080/08035250410024998. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: Effects on cortisol and behavior. Biological Psychiatry. 2002;52:776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- Field T. The effects of mother’s physical and emotional unavailability on emotion regulation. Monographs of the Society for Research in Child Development. 1994;59:208–227. [PubMed] [Google Scholar]
- Fitzgerald M. The development of nociceptive circuits. Nature Reviews Neuroscience. 2005;6:507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. Journal of Neuroscience. 2002;22:7840–7843. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaab J, Rohleder N, Nater UM, Ehlert U. Psychological determinants of the cortisol stress response: The role of anticipatory cognitive appraisal. Psychoneuroendocrinology. 2005;30:599–610. doi: 10.1016/j.psyneuen.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Goldberg S, Levitan R, Leung E, Masellis M, Basile VS, Nemeroff CB, Atkinson L. Cortisol concentrations in 12- to 18-month-old infants: Stability over time, location, and stressor. Biological Psychiatry. 2003;54:719–726. doi: 10.1016/s0006-3223(03)00010-6. [DOI] [PubMed] [Google Scholar]
- Glover V. Maternal stress or anxiety in pregnancy and emotional development of the child. British Journal of Psychiatry. 1997;171:105–106. doi: 10.1192/bjp.171.2.105. [DOI] [PubMed] [Google Scholar]
- Glover V, Miles R, Matta S, Modi N, Stevenson J. Glucocorticoid exposure in preterm babies predicts saliva cortisol response to immunization at 4 months. Pediatric Research. 2005;58:1233–1237. doi: 10.1203/01.pdr.0000185132.38209.73. [DOI] [PubMed] [Google Scholar]
- Greenberg MT, Crnic KA. Longitudinal predictors of developmental status and social interaction in premature and full-term infants at age two. Child Development. 1988;59:554–570. doi: 10.1111/j.1467-8624.1988.tb03216.x. [DOI] [PubMed] [Google Scholar]
- Grunau R. Early pain in preterm infants. A model of long-term effects. Clinics in Perinatology. 2002;29:373–394. doi: 10.1016/s0095-5108(02)00012-x. [DOI] [PubMed] [Google Scholar]
- Grunau RE. Self-regulation and behavior in preterm children: Effects of early pain. In: McGrath PJ, Finley GA, editors. Pediatric pain: Biological and social context, progress in pain research and management. Vol. 26. Seattle, WA: IASP Press; 2003. pp. 23–55. [Google Scholar]
- Grunau RE, Weinberg J, Whitfield MF. Neonatal procedural pain and preterm infant cortisol response to novelty at 8 months. Pediatrics. 2004a;114:e77–e84. doi: 10.1542/peds.114.1.e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau RE, Whitfield MF, Davis C. Pattern of learning disabilities in children with extremely low birth weight and broadly average intelligence. Archives of Pediatrics & Adolescent Medicine. 2002;156:615–620. doi: 10.1001/archpedi.156.6.615. [DOI] [PubMed] [Google Scholar]
- Grunau RE, Whitfield MF, Fay TB. Psychosocial and academic characteristics of ELBW (≤800 g) survivors in late adolescence compared to term-born controls. Pediatrics. 2004b;114:e725–e732. doi: 10.1542/peds.2004-0932. [DOI] [PubMed] [Google Scholar]
- Grunau RE, Haley D, Whitfield MF, Weinberg J. Altered basal cortisol levels at 3, 6, 8 and 18 months in preterm infants born extremely low gestational age. J Pediatrics (in press) doi: 10.1016/j.jpeds.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau RE, Holsti L, Haley DW, Oberlander TF, Weinberg J, Solimano A, Whitfield MF, Fitzgerald C, Yu W. Neonatal procedural pain exposure predicts lower cortisol and behavioral reactivity in preterm infants in the NICU. Pain. 2005;113:293–300. doi: 10.1016/j.pain.2004.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR. Reactivity of the hypothalamic-pituitary-adrenocortical system to stressors in normal infants and children. Pediatrics. 1992;90:491–497. [PubMed] [Google Scholar]
- Gunnar MR. Quality of early care and buffering of neuroendocrine stress reactions: Potential effects on the developing human brain. American Journal of Preventive Medicine. 1998;27:208–211. doi: 10.1006/pmed.1998.0276. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Mangelsdorf S, Larson M, Hertsgaard L. Attachment, temperament, and adrenocortical activity in infancy: A study of psychoendocrine regulation. Developmental Psychology. 1989;25:355–363. [Google Scholar]
- Hack M, Taylor HG, Klein N, Eiben R, Schatschneider C, Mercuri-Minich N. School age outcomes in children with birth weights under 750 g. New England Journal of Medicine. 1994;331:753–759. doi: 10.1056/NEJM199409223311201. [DOI] [PubMed] [Google Scholar]
- Haley DW, Grunau RE, Weinberg J, Whitfield MF. Parenting stress and infant cortisol responses in preterm and full-term infants at 3 months. Pediatric Research. 2004;55(4):80A. [Google Scholar]
- Haley DW, Weinberg J, Grunau RE. Cortisol, contingency learning, and memory in preterm and full-term infants. Psychoneuroendocrinology. 2006;31:108–117. doi: 10.1016/j.psyneuen.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindmarsh GJ, O’Callaghan MJ, Mohay HA, Rogers YM. Gender differences in cognitive abilities at 2 years in ELBW infants. Extremely low birth weight. Early Human Development. 2000;60:115–122. doi: 10.1016/s0378-3782(00)00105-5. [DOI] [PubMed] [Google Scholar]
- Hingre RV, Gross SJ, Hingre KS, Mayes DM, Richman RA. Adrenal steroidogenesis in very low birth weight preterm infants. The Journal of Clinical Endocrinology and Metabolism. 1994;78:266–270. doi: 10.1210/jcem.78.2.8106610. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Early relationships as regulators of infant physiology and behavior. Acta Paediatrica Supplementum. 1994;397:9–18. doi: 10.1111/j.1651-2227.1994.tb13260.x. [DOI] [PubMed] [Google Scholar]
- Holsti L, Grunau RE, Oberlander TF, Whitfield MF. Prior pain induces heightened motor responses during clustered care in preterm infants in the NICU. Early Human Development. 2005;81:293–302. doi: 10.1016/j.earlhumdev.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Huysman MW, Hokken-Koelega AC, De Ridder MA, Sauer PJ. Adrenal function in sick very preterm infants. Pediatric Research. 2000;48:629–633. doi: 10.1203/00006450-200011000-00013. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Raivio T, Janne OA, Hovi P, Dunkel L, Andersson S. Circulating glucocorticoid bioactivity in the preterm newborn after antenatal betamethasone treatment. The Journal of Clinical Endocrinology and Metabolism. 2004;89:3999–4003. doi: 10.1210/jc.2004-0013. [DOI] [PubMed] [Google Scholar]
- Kivlighan KT, Granger DA, Schwartz EB, Nelson V, Curran M. Quantifying blood leakage into the oral mucosa and its effects on the measurement of cortisol, dehydroepiandrosterone, and testosterone in saliva. Hormones and Behavior. 2004;46:39–46. doi: 10.1016/j.yhbeh.2004.01.006. [DOI] [PubMed] [Google Scholar]
- King S, Laplante DP. The effects of prenatal maternal stress on children’s cognitive development: Project Ice Storm. Stress. 2005;8:35–45. doi: 10.1080/10253890500108391. [DOI] [PubMed] [Google Scholar]
- Kristenson M, Eriksen HR, Sluiter JK, Starke D, Ursin H. Psychobiological mechanisms of socioeconomic differences in health. Social Science and Medicine. 2004;58:1511–1522. doi: 10.1016/S0277-9536(03)00353-8. [DOI] [PubMed] [Google Scholar]
- Laplante DP, Barr RG, Brunet A, Galbaud du Fort G, Meaney ML, Saucier JF, Zelazo PR, King S. Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatric Research. 2004;56:400–410. doi: 10.1203/01.PDR.0000136281.34035.44. [DOI] [PubMed] [Google Scholar]
- Larson MC, Gunnar MR, Hertsgaard L. The effects of morning naps, car trips, and maternal separation on adrenocortical activity in human infants. Child Development. 1991;62:362–372. [PubMed] [Google Scholar]
- Larson MC, White BP, Cochran A, Donzella B, Gunnar M. Dampening of the cortisol response to handling at 3 months in human infants and its relation to sleep, circadian cortisol activity, and behavioral distress. Developmental Psychobiology. 1998;33:327–337. [PubMed] [Google Scholar]
- Lawson KR, Ruff HA. Focused attention: Assessing a fundamental cognitive process in infancy. In: Singer LT, Zeskind PS, editors. Biobehavioral assessment of the infant. New York: Guilford Press; 2001. [Google Scholar]
- Lawson KR, Ruff HA. Early focused attention predicts outcome for children born prematurely. Journal of Developmental and Behaviorial Pediatrics. 2004;25:399–406. doi: 10.1097/00004703-200412000-00003. [DOI] [PubMed] [Google Scholar]
- Lee MM, Rajagopalan L, Berg GJ, Moshang T., Jr Serum adrenal steroid concentrations in premature infants. The Journal of Clinical Endocrinology and Metabolism. 1989;69:1133–1136. doi: 10.1210/jcem-69-6-1133. [DOI] [PubMed] [Google Scholar]
- Levy-Shiff R, Einat G, Mogilner MB, Lerman M, Krikler R. Biological and environmental correlates of developmental outcome of prematurely born infants in early adolescence. Journal of Pediatric Psychology. 1994;19:63–78. doi: 10.1093/jpepsy/19.1.63. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Child’s stress hormone levels correlate with mother’s socioeconomic status and depressive state. Biological Psychiatry. 2000;48:976–980. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Development and Psychopathology. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- Magill-Evans J, Harrison MJ. Parent-child interactions and development of toddlers born preterm. Western Journal of Nursing Research. 1999;21:292–312. doi: 10.1177/01939459922043893. [DOI] [PubMed] [Google Scholar]
- Mason JW. A review of psychoendocrine research on the sympathetic-adrenal medullary system. Psychosomatic Medicine. 1968;30:631–653. doi: 10.1097/00006842-196809000-00022. [DOI] [PubMed] [Google Scholar]
- Matthews SG. Antenatal glucocorticoids and the developing brain: Mechanisms of action. Seminars in Neonatology. 2001;6:309–317. doi: 10.1053/siny.2001.0066. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neurosciences. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Miceli PJ, Goeke-Morey MC, Whitman TL, Kolberg KS, Miller-Loncar C, White RD. Brief report: Birth status, medical complications, and social environment—Individual differences in development of preterm, very low birth weight infants. Journal of Pediatric Psychology. 2000;25:353–358. doi: 10.1093/jpepsy/25.5.353. [DOI] [PubMed] [Google Scholar]
- Mirsky AF. Disorders of attention: A neuropsychological perspective. In: Lyon GR, Krasnegor NA, editors. Attention, memory, and executive function. Baltimore, MD: Brooks; 1996. pp. 71–96. [Google Scholar]
- Muller-Nix C, Forcada-Guex M, Pierrehumbert B, Jaunin L, Borghini A, Ansermet F. Prematurity, maternal stress and mother-child interactions. Early Human Development. 2004;79:145–158. doi: 10.1016/j.earlhumdev.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Ng PC, Lee CH, Lam CW, Ma KC, Fok TF, Chan IH, Wong E. Transient adrenocortical insufficiency of prematurity and systemic hypotension in very low birthweight infants. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2004;89:F119–F126. doi: 10.1136/adc.2002.021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, McCarthy MM. Sex differences and hormonal effects in a model of preterm infant brain injury. Annals of New York Academy of Sciences. 2003;1008:281–284. doi: 10.1196/annals.1301.032. [DOI] [PubMed] [Google Scholar]
- Poehlmann J, Fiese BH. Parent-infant interaction as a mediator of the relation between neonatal risk status and 12-month cognitive development. Infant Behavior and Development. 2001;24:171–188. [Google Scholar]
- Posner MI, Petersen S. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner MI, Raichle ME. Images of mind. New York: Scientific American Books; 1994. [Google Scholar]
- Purdy IB, Wiley DJ. Perinatal corticosteroids: A review of research. Part I: Antenatal administration. Neonatal Network. 2004;23:15–30. doi: 10.1891/0730-0832.23.2.15. [DOI] [PubMed] [Google Scholar]
- Ragozin AS, Basham RB, Crnic KA, Greenberg MT, Robinson NM. Effects of maternal age on parenting. Developmental Psychology. 1982;18:627–634. [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Attention and recognition memory in the first year of life: A longitudinal study of preterms and full-terms. Developmental Psychology. 2001;37:135–151. [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Processing speed in the first year of life: A longitudinal study of preterms and full-terms. Developmental Psychology. 2002;38:895–902. doi: 10.1037//0012-1649.38.6.895. [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ, van Rossem R. Pathways from prematurity and infant abilities to later cognition. Child Development. 2005;76:1172–1184. doi: 10.1111/j.1467-8624.2005.00843.x. [DOI] [PubMed] [Google Scholar]
- Ruff HA. Components of attention during infants’ manipulative exploration. Child Development. 1986;57:105–114. doi: 10.1111/j.1467-8624.1986.tb00011.x. [DOI] [PubMed] [Google Scholar]
- Ruff HA. The measurement of attention in high risk infants. In: Viestz PM, Vaughan HG, editors. Early identification of infants with developmental disabilities. Philadelphia, PA: Grune & Stratton; 1988. pp. 282–296. [Google Scholar]
- Ruff HA, Lawson KR, Parrinello R, Weissberg R. Long-term stability of individual differences in sustained attentionin the early years. Child Development. 1990;61:60–75. [PubMed] [Google Scholar]
- Ruff HA, McCarton C, Kurtzberg D, Vaughan HG., Jr Preterm infants’ manipulative exploration of objects. Child Development. 1984;55:1166–1173. [PubMed] [Google Scholar]
- Ruff HA, Rothbart MK. Attention in early development: Themes and viarations. New York: Oxford University Press; 1996. [Google Scholar]
- Saigal S, Hoult LA, Streiner DL, Stoskopf BL, Rosenbaum PL. School difficulties at adolescence in a regional cohort of children who were extremely low birth weight. Pediatrics. 2000;105:325–331. doi: 10.1542/peds.105.2.325. [DOI] [PubMed] [Google Scholar]
- Schwartz EB, Granger DA, Susman EJ, Gunnar MR, Laird B. Assessing salivary cortisol in studies of child development. Child Development. 1998;69:1503–1513. [PubMed] [Google Scholar]
- Singer LT, Salvator A, Guo S, Collin M, Lilien L, Baley J. Maternal psychological distress and parenting stress after the birth of a very low-birth-weight infant. Journal of American Medical Association. 1999;281:799–805. doi: 10.1001/jama.281.9.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth J, Ockenfels MC, Porter L, Kirschbaum C, Hellhammer DH, Stone AA. Stressors and mood measured on a momentary basis are associated with salivary cortisol secretion. Psychoneuroendocrinology. 1998;23:353–370. doi: 10.1016/s0306-4530(98)00008-0. [DOI] [PubMed] [Google Scholar]
- Spangler G, Schieche M, Ilg U, Maier U, Ackermann C. Maternal sensitivity as an external organizer for biobehavioral regulation in infancy. Developmental Psychobiology. 1994;27:425–437. doi: 10.1002/dev.420270702. [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Goldstein RF, Oehler JM, Gustafson KE, Catlett AT, Brazy JE. Developmental outcome of very low birth weight infants as a function of biological risk and social risk. Developmental and Behavioral Pediatrics. 1994;12:232–238. [PubMed] [Google Scholar]
- Vatten LJ, Skjaerven R. Offspring sex and pregnancy outcome by length of gestation. Early Human Development. 2004;76:47–54. doi: 10.1016/j.earlhumdev.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Neurology of the newborn. 4. Philadelphia: WB Saunders; 2001. [Google Scholar]
- Wadhwa PD, Sandman CA, Garite TJ. The neurobiology of stress in human pregnancy: Implications for prematurity and development of the fetal central nervous system. Progress in Brain Research. 2001;133:131–142. doi: 10.1016/s0079-6123(01)33010-8. [DOI] [PubMed] [Google Scholar]
- Walker CD, Kudreikis K, Sherrard A, Johnston CC. Repeated neonatal pain influences maternal behavior, but not stress responsiveness in rat offspring. Brain Research: Developmental Brain Research. 2003;140:253–261. doi: 10.1016/s0165-3806(02)00611-9. [DOI] [PubMed] [Google Scholar]
- Watamura SE, Sebanc AM, Gunnar MR. Rising cortisol at childcare: Relations with nap, rest, and temperament. Developmental Psychobiology. 2002;40:33–42. doi: 10.1002/dev.10011. [DOI] [PubMed] [Google Scholar]
- Watterberg KL, Gerdes JS, Cook KL. Impaired glucocorticoid synthesis in premature infants developing chronic lung disease. Pediatric Research. 2001;50:190–195. doi: 10.1203/00006450-200108000-00005. [DOI] [PubMed] [Google Scholar]
- Whitfield MF, Grunau RV, Holsti L. Extremely premature (<or = 800 g) schoolchildren: Multiple areas of hidden disability. Archives of disease in childhood. Fetal and neonatal edition. 1997;77:F85–F90. doi: 10.1136/fn.77.2.f85. [DOI] [PMC free article] [PubMed] [Google Scholar]


