Abstract
BACKGROUND
Chemotherapy improves survival for patients with stage III colon cancer, but some older patients with lymph node-positive colon cancer do not see a medical oncologist and, thus, do not receive adjuvant chemotherapy.
METHODS
To evaluate the role of the surgeon in determining referrals to medical oncology among patients with stage III colon cancer, the authors conducted a retrospective cohort study of 6158 patients aged ≥66 years who were diagnosed with stage III colon cancer from 1992 through 1999 by using the Surveillance, Epidemiology, and End Results-Medicare linked database. Multilevel analysis was used to simultaneously model variations in patients’ seeing a medical oncologist at the patient and surgeon levels.
RESULTS
Twenty-one percent of the total variance in seeing a medical oncologist was attributable to the surgeon after adjusting for available patient, tumor, and surgeon characteristics. The individual surgeon characteristics that significantly predicted whether the patient saw a medical oncologist were year since graduation (≤10 years vs >20 years; hazard ratio [HR], 1.60; 95% confidence interval [95% CI], 1.19–2.16), practicing in a teaching hospital (yes vs. no: HR; 1.30; 95% CI, 1.07–1.58), and volume of patients with colon cancer (<30 patients vs ≥121 patients; HR, 0.66; 95% CI, 0.46–0.94). Surgeon sex, race, board certification, and type of practice were not independent predictors of medical oncology referral.
CONCLUSIONS
Surgeons accounted for approximately 20% of the variation in patients seeing a medical oncologist. Interventions at the level of the surgeon may be appropriate to improve the care of patients with colon cancer.
Keywords: colon cancer, adjuvant chemotherapy, medical oncology, referral and consultation, oncology service, teaching hospitals, Surveillance, Epidemiology, End Results Program
The outcomes of patients with stage III colon cancer can be improved dramatically by the use of adjuvant chemotherapy.1–6 However, a substantial percentage of patients with colon cancer do not receive adjuvant chemotherapy7–9 and are at risk for poorer outcomes.10 Patients who are older and who are black reportedly are less likely to receive adjuvant chemotherapy,9–12 although it has not been demonstrated that the benefits of chemotherapy differ by age or ethnicity.2,10
Figure 1 shows a conceptual model for the factors that affect the receipt of adjuvant chemotherapy. First, a patient who is diagnosed with colon cancer must be referred to a medical oncologist, because, with rare exceptions, medical oncologists are the only physicians who prescribe and supervise chemotherapy administration. Second, after consultation with a medical oncologist, a decision must be made to proceed with chemotherapy.
FIGURE 1.
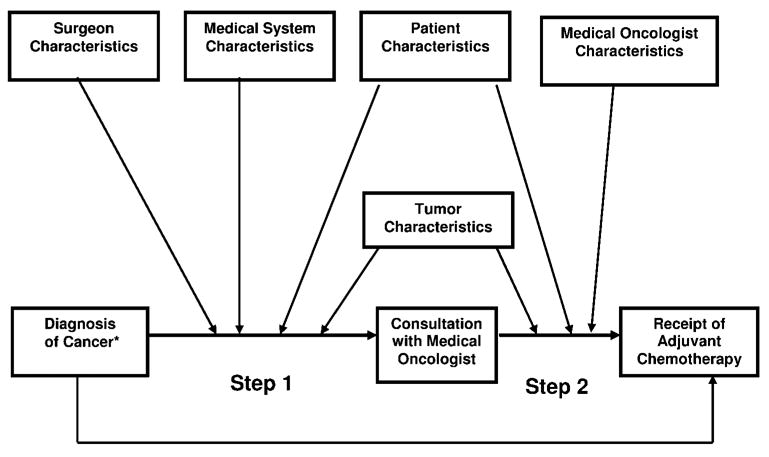
Conceptual model of the factors that influence the trajectory of stage III colon cancer patients resulting in receipt of chemotherapy. This figure illustrates a 2-step model for receipt of chemotherapy: The first is being referred to a medical oncologist, and the second step is the actual receipt of chemotherapy. In this model, both steps will be influenced by patient and tumor characteristics, whereas referral to an oncologist (Step 1) also will be influenced by characteristics of the surgeon who performs the primary surgery. The conceptual model may be used for reference in oncology care and cancer-related studies.
Seeing a medical oncologist is very important for several reasons. First, as noted above, medical oncologists are the specialists who administer chemotherapy. It is uncommon for surgeons or primary care physicians to administer chemotherapy. Second, medical oncologists are cancer specialists, whereas the vast majority of cancer resections are done by general surgeons.13,14 Third, oncologists are more likely than general surgeons to perform recommended oncology care.15 Finally, cancer patients who are treated by oncologists experience better outcomes than patients who are treated by physicians without oncology training.15–17
We hypothesized that, in addition to patient, tumor, and medical system characteristics, surgeon characteristics would be important in determining which patients would see a medical oncologist. Previous reports indicated that physician characteristics play an important role in referral patterns. Physicians who have more practice experience,18 and female physicians and internists19 tend to have higher referral rates. It also has been demonstrated that physician specialty,15–17,20 years in practice,21 practice setting,15,21,22 and patient volume23 all influence practice patterns. Therefore, in the current study, we investigated the role of surgeon characteristics in predicting which patients with stage III colon cancer are seen by a medical oncologist.
MATERIALS AND METHODS
Data Sources
Data for this study were obtained from several databases that were linked through collaborative projects and agreements involving the National Cancer Institute, the Centers for Medicare and Medicaid Services (CMS), and the American Medical Association (AMA).8,24,25 These databases contain 1) population-based tumor registry data obtained from the Surveillance, Epidemiology, and End Results (SEER) Program, 2) Medicare enrollment information and claims submitted to CMS for services provided to Medicare beneficiaries, and 3) information collected by the AMA on all physicians in the United States (AMA Master file). The SEER-Medicare database also contains the Hospital file, which includes information on hospital characteristics, such as academic affiliation, and is derived from the Provider of Services survey, which is submitted by hospitals to Medicare.26
Patients
Eligible patients for this study were Medicare beneficiaries who were diagnosed with stage III colon cancer from 1992 through 1999 and who were aged ≥66 at the time of their diagnosis (n = 14,217). We excluded patients who had a cancer diagnosis at ≥1 site (n = 2049), patients who died within 6 months of diagnosis (n = 1508), patients who were not covered by both Medicare Parts A and B or who were members of a health maintenance organization for any period from 12 months before to 6 months after diagnosis (n = 3085), patients who did not have a diagnosis month listed in SEER (n = 13), patients who had no information on surgeon or hospital (n = 675), and patients who were treated by a surgeon who saw only 1 patient with stage III colon cancer from 1992 through 1999 (n = 742). This resulted in a final sample size of 6158 patients who were diagnosed with stage III colon cancer, and those patients saw 1307 surgeons at 377 hospitals.
Identification of Patients Who Saw a Specialist
Patients who saw a medical oncologist were identified through information on their Medicare physician claims (Carrier Claims File). Physician claims have a 2-digit CMS provider specialty code that represents the specialty reported to the carrier who processed the claim.25 Physician claims also contain an encrypted Unique Physician Identification Number (UPIN) for the physician who provided the service. Through linkage with the AMA Master file, the primary and secondary specialty of a specific physician can be ascertained from residency training information and self-designated specialty.25 If a patient had a physician claim within the 6 months after the month of diagnosis and the physician specialty (primary or secondary) was medical oncology or hematology based on either the AMA or CMS data, then we defined the patient as having seen a medical oncologist.
Measures
Surgeon characteristics were identified from the AMA Master file and included sex, ethnicity, year of graduation, patient volume, board certification, affiliation with a teaching hospital, and primary employment setting. Surgeon graduation year was classified into 3 groups: <10 years since graduation, from 10 years to 20 years since graduation, and >20 years since graduation. The orientation for surgeon graduating year was 1995, which was the middle point of our data period (1992–1999). The estimated volume for each surgeon was the total number of all his or her patients with colon cancer who had claims in the SEER-Medicare database over the period from 1991 to 2001. It was calculated by using the surgery UPIN on the physician claims. Patient volume was classified as <31 patients, from 31 to 70 patients, from 71 to 120 patients, and ≥121 patients with colon cancer over the entire period from 1991 to 2001. Specialty certification categories included not board certified, board certified in general surgery, and board certified in colorectal surgery. Teaching hospital (yes/no) and primary employment (solo or owner/other) were dichotomous variables.
We used the SEER data to assign patients to different sociodemographic categories at the time of diagnosis according to race/ethnicity, marital status, and age. From the Medicare file, we obtained information regarding whether the individual was enrolled in state buy-in, low-income subsidy programs (Qualified Medicare Beneficiary) as an indicator of low income at the individual level. In addition, we selected socioeconomic characteristics by zip code of the patient’s Census tract in 1990, including median household income and percentage of individuals aged >25 years with <12 years of education.
Each patient’s comorbidity score was determined from claims data by using the adaptation by Klabunde and colleagues of the Charlson Comorbidity Index.26,27 The claims data also were used to define receipt of chemotherapy based on Medicare codes, as described in previous publications.28 Patients were classified as having received chemotherapy if they had ≥1 of these codes on any claim within 6 months after their colon cancer diagnosis.
Statistical Analysis
Differences in the percentage of patients who saw a medical oncologist across strata were evaluated using chi-square statistics. Hierarchical generalized linear models (HGLM)29 were used to fit multilevel data in which individuals were nested within surgeons. From HGLM, the intraclass correlation coefficient (ICC) was estimated by using the threshold method.30 The ICC provides an estimate of the percent variance in patients seeing a medical oncologist that is attributable to the surgeon. A null model, which did not include any patient or surgeon characteristics, and 2 adjusted models (1 that included patient characteristics only and another that included both patient and surgeon characteristics) were constructed. From the adjusted models, the residual ICC was calculated, representing the percentage of variance attributable to the surgeon after adjustments. The odds ratios for seeing a medical oncologist for patient and surgeon characteristics from adjusted models were calculated along with 95% confidence intervals. All analyses were performed with Statistical Analysis Software (SAS) version 9.1. Models were fitted using the SAS GLIMMIX procedure.
RESULTS
In total, 6158 older patients with stage III colon cancer met eligibility criteria for this study. The characteristics of this patient population are shown in Table 1. The mean patient age was 77.4 years (range, 66–101 years). Overall, 59.2% of patients were female, 84.5% of patients were non-Hispanic whites, 51.5% of patients were married, 67.1% of patients had no identifiable comorbidities, 68.7% of patients had ≤3 positive lymph nodes, 60.2% of patients received chemotherapy, and 82.3% of patients saw a medical oncologist.
TABLE 1.
Patient Characteristics
| Patient characteristics | No. of patients (N = 6158) | Percentage of patients who saw an oncologist (N = 5066) | P | Patient characteristics | No. of patients (N = 6158) | Percentage of patients who saw an oncologist (N = 5066) | P |
|---|---|---|---|---|---|---|---|
| Age at diagnosis, y | Residence | ||||||
| 66–69 | 994 | 95.3 | Big metropolitan | 3519 | 83.7 | ||
| 70–74 | 1520 | 91.5 | Metropolitan | 1442 | 80.0 | ||
| 75–79 | 1524 | 86.2 | Urban | 1085 | 80.7 | ||
| 80–84 | 1172 | 76.6 | Rural | 112 | 83.0 | .0087 | |
| ≥85 | 948 | 54.6 | <.0001 | No. of positive lymph nodes | |||
| Sex | ≤3 | 4233 | 81.5 | ||||
| Men | 2514 | 84.6 | 4–6 | 1149 | 83.4 | ||
| Women | 3644 | 80.7 | <.0001 | ≥7 | 593 | 86.7 | |
| Race | Unknown | 183 | 79.2 | .0080 | |||
| Non-Hispanic white | 5203 | 82.6 | Received chemotherapy | ||||
| Black | 422 | 83.4 | No | 2454 | 58.0 | ||
| Hispanic | 190 | 80.0 | Yes | 3704 | 98.4 | <.0001 | |
| Other | 343 | 77.8 | .1149 | SEER site | |||
| Married | Atlanta | 313 | 85.6 | ||||
| Yes | 3171 | 87.3 | Connecticut | 989 | 82.1 | ||
| No | 2987 | 76.9 | <.0001 | Detroit | 973 | 88.4 | |
| Ever had state buy-in | Hawaii | 162 | 74.1 | ||||
| No | 5187 | 83.9 | Iowa | 1120 | 80.9 | ||
| Yes | 971 | 73.3 | <.0001 | New Mexico | 220 | 75.0 | |
| Zip household median income (1990), $ | San Francisco | 466 | 75.1 | ||||
| >35,000 | 2777 | 83.2 | San Jose | 256 | 82.4 | ||
| ≤35,000 | 3099 | 81.5 | Seattle | 591 | 81.9 | ||
| Missing | 282 | 81.2 | .2059 | Utah | 232 | 81.5 | |
| Zip Pct nonhigh school (1990), % | Los Angeles | 836 | 83.9 | <.0001 | |||
| <16 | 2190 | 83.0 | Year of diagnosis | ||||
| 16–25 | 1979 | 82.5 | 1992 | 743 | 76.5 | ||
| ≥25 | 1707 | 81.3 | 1993 | 758 | 79.8 | ||
| Missing | 282 | 81.2 | .5238 | 1994 | 825 | 81.6 | |
| Comorbidity score | 1995 | 848 | 82.9 | ||||
| 0 | 4133 | 83.6 | 1996 | 746 | 84.2 | ||
| 1 | 1314 | 81.9 | 1997 | 812 | 84.5 | ||
| 2 | 444 | 78.8 | 1998 | 787 | 83.4 | ||
| ≥3 | 267 | 69.3 | <.0001 | 1999 | 639 | 85.6 | <.0001 |
Pct indicates precinct; SEER, Surveillance, Epidemiology, and End Results.
Patient characteristics that were associated strongly with seeing a medical oncologist were younger age, male gender, being married, not having state buy-in insurance, having a lower comorbidity score, having a larger number of involved lymph nodes, and living in certain SEER areas, such as Atlanta and Detroit. In addition, Table 1 illustrates an increase over time in the percent of patients with stage III colon cancer who saw a medical oncologist, from 76.5% in 1992 to 85.6% in 1999. The receipt of chemotherapy, as expected, also was linked closely to seeing a medical oncologist.
Table 2 presents the characteristics of 1307 surgeons who treated the patients in this study. Ninety-six percent of surgeons were male, and 53.6% of surgeons had graduated from medical school >20 years earlier. Only 12% of surgeons operated on <30 patients with colon cancer per year. Approximately 80% were board certified general surgeons, 10% had specialty training in colorectal surgery, and 11% were not board certified. One-third of surgeons operated at teaching hospitals (32.8%). Surgeons who referred patients to see a medical oncologist were significantly more likely to have graduated within the last 10 years, to be board certified as a colorectal surgeon, and to work in a teaching hospital.
TABLE 2.
Surgeon Characteristics
| Surgeon characteristics | No. of surgeons (N = 1307) (%) | Percentage of patients who saw an oncologist (Total No = 5066) | P |
|---|---|---|---|
| Years since graduation | |||
| ≤10 | 182 (13.9) | 85.9 | |
| 11–20 | 424 (32.4) | 83.0 | |
| >20 | 701 (53.6) | 81.0 | .0048 |
| Sex | |||
| Women | 58 (4.4) | 81.4 | |
| Men | 1249 (95.6) | 82.3 | .7329 |
| Race | |||
| White | 784 (59.9) | 82.0 | |
| Black | 14 (1.1) | 85.5 | |
| Hispanic | 27 (2.1) | 87.4 | |
| Asian | 126 (9.6) | 83.5 | |
| Unknown | 356 (27.2) | 82 | .5794 |
| Patient volume* | |||
| ≤30 | 156 (12.0) | 78.4 | |
| 31–70 | 501 (38.3) | 82.7 | |
| 71–120 | 452 (34.6) | 82.6 | |
| ≥121 | 198 (15.2) | 82.1 | .2501 |
| Board-certified specialty | |||
| Not board certified | 139 (10.6) | 84.3 | |
| Colorectal surgeon | 127 (9.7) | 85.5 | |
| General surgeon | 1041 (79.7) | 81.5 | .0076 |
| Teaching hospital | |||
| Yes | 428 (32.8) | 84.7 | |
| No | 879 (67.2) | 81.0 | .0003 |
| Primary employment | |||
| Solo or owner | 726 (55.6) | 81.9 | |
| Other | 581 (44.4) | 82.7 | .4089 |
The total number of colon cancer patients (all stages) seen by the surgeon from 1991 to 2001, as identified with physician claims in the Surveillance, Epidemiology, and End Results-Medicare database. Note that this may not reflect a surgeon’s overall patient volume, because it excludes younger patients and older patients who are members of a health maintenance organization and/or who are without Medicare Part B coverage.
Next, we used multilevel analyses to investigate characteristics that were associated with patients seeing a medical oncologist. The results are shown in Table 3. The ICC, which is shown in the first row of Table 3, provides an estimate of the percent variance in seeing a medical oncologist that is attributable to the surgeon. In Model 1, no predictors were included, ie, it is assumed that probability does not vary by individual patient or surgeon characteristics. The Model 1 ICC is estimated at 0.15, indicating that 15% of the variance in seeing a medical oncologist is attributable to the surgeon before adjusting for individual patient and surgeon characteristics.
TABLE 3.
Multilevel Analysis of Characteristics Associated With Seeing a Medical Oncologist Within 6 Months of a Diagnosis of Stage III Colon Cancer
| OR (95% CI)
|
OR (95% CI)
|
||||||
|---|---|---|---|---|---|---|---|
| Variable | Model 1 | Model 2 | Model 3 | Variable | Model 1 | Model 2 | Model 3 |
| Intraclass correlation coefficient | 0.150 | 0.221 | 0.213 | 70–74 | 9.39 (7.62–11.58) | 9.43 (7.63–11.65) | |
| Surgeon | 75–79 | 5.35 (4.44–6.44) | 5.68 (4.45–6.48) | ||||
| Years since graduation | 80–84 | 2.68 (2.24–3.20) | 2.68 (2.23–3.21) | ||||
| ≤10 | 1.60 (1.19–2.16) | ≥85 | 1 | 1 | |||
| 11–20 | 1.15 (0.95–1.40) | Sex | |||||
| ≥20 | 1 | Men | 0.88 (0.76–1.02) | 0.88 (0.75, 1.02) | |||
| Sex | Women | 1 | 1 | ||||
| Men | 1.47 (0.94–2.30) | Race | |||||
| Women | 1 | Black | 1.00 (0.74–1.34) | 0.94 (0.70–1.27) | |||
| Race | Hispanic | 0.82 (0.56–1.19) | 0.84 (0.57–1.24) | ||||
| Nonwhite | 1.09 (0.91–1.31) | Other | 0.70 (0.52–0.96) | 0.70 (0.51–0.96) | |||
| White | 1 | White | 1 | 1 | |||
| Patient volume | Married | ||||||
| ≤30 | 0.66 (0.46–0.94) | No | 0.77 (0.66–0.89) | 0.76 (0.65–0.88) | |||
| 31–70 | 1.05 (0.82–1.36) | Yes | 1 | 1 | |||
| 71–120 | 1.03 (0.81–1.31) | State buy-in | |||||
| ≥121 | 1 | No | 1.55 (1.30–1.85) | 1.54 (1.29–1.94) | |||
| Board-certified specialist | Yes | 1 | 1 | ||||
| Not certified | 1.10 (0.80–1.49) | Zip code median income, $ | |||||
| Colorectal surgeon | 1.02 (0.77–1.36) | ≤35,000 | 1 (0.86–1.16) | 1.02 (0.87–1.19) | |||
| General surgeon | 1 | >35,000 | 1 | 1 | |||
| Major teaching hospital | Comorbidity score | ||||||
| Yes | 1.30 (1.07–1.58) | 0 | 2.10 (1.59–2.78) | 2.11 (1.59–2.80) | |||
| No | 1 | 1 | 2.20 (1.63–2.97) | 2.21 (1.64–3.00) | |||
| Practice setting | 2 | 1.90 (1.34–2.69) | 1.89 (1.33–2.68) | ||||
| Other | 0.98 (0.81–1.17) | ≥3 | 1 | 1 | |||
| Solo | 1 | No. of positive lymph nodes | |||||
| Patient | ≤3 | 0.65 (0.51–0.83) | 0.64 (0.50–0.82) | ||||
| Age, y | 4–6 | 0.75 (0.56–0.98) | 0.74 (0.56–0.98) | ||||
| 66–69 | 16.92 (12.64–22.66) | 17.05 (12.69–22.90) | ≥7 | 1 | 1 | ||
OR indicates odds ratio; 95% CI, 95% confidence interval. OR and 95% CI calculations: eparameter (eparameter−1.96*standard error, eparameter+1.96*standard error).
In Model 2, patient characteristics are added to the model. After adjustment for patient-level variables, the percent variance attributable to the surgeon increases to 22.1%. In Model 3, adjustments are made for both patient and surgeon characteristics. It is noteworthy that, adjusting for individual surgeon characteristics has little effect on the overall variance attributable to the surgeon: It decreases from 22% to 21%. In the full model (Model 3), patient characteristics that significantly predict whether the patient was seen by a medical oncologist include younger age, being married, not having state buy-in (ie, not indigent), lower comorbidity score, and increasing number of involved lymph nodes. After adjustment for patient characteristics, surgeons with more years in practice, surgeons with a low volume of colon cancer patients, and surgeons who do not work in a teaching hospital were significantly less likely to have patients who were seen by a medical oncologist.
DISCUSSION
In the current study, we demonstrated that, after adjusting for patient and tumor characteristics, approximately 21% of the variability in whether a patient with lymph node-positive colon cancer sees a medical oncologist is attributable to the surgeon. Individual surgeon characteristics predicted which patients were seen by a medical oncologist. In particular, surgeons with fewer years since graduation and surgeons who practiced in a teaching hospital were more likely to have patients who were seen by a medical oncologist, whereas surgeons who had a lower volume of patients with colon cancer were less likely to send their patients to medical oncology. It is worth noting that surgeon sex, race, and type of practice were not significant predictors of medical oncology referral in either univariate or multilevel analyses. Although board certification was significant in the univariate analysis, it was not a significant predictor of medical oncology referral after adjusting for variables related to patient mix (analyses not shown).
We assumed that the primary reason why cancer patients would not see a medical oncologist was that they were not referred to one.15,31,32 However, there may be other reasons. For example, a patient may already have ruled out chemotherapy and refused to see an oncologist.33
The reasons for lower referral rates in some groups of surgeons are less clear. Surgeons who have finished their training more recently may be more aware that patients with stage III colon cancer should be referred to medical oncology for consideration of adjuvant chemotherapy.34 Conversely, surgeons who have more years in practice may be more comfortable in recognizing patients who are unlikely to receive chemotherapy and, thus, may not send such patients for a visit which is perceived as unnecessary. A surgeon who practices in a teaching hospital may be more likely to be aware of new information regarding cancer care, may be more likely to adopt new procedures, or may be more likely to be aware of the utility of new practice patterns than their counterparts who practice in rural or solo settings.21,22,34 Finally, it has been demonstrated previously that surgeons with higher patient volumes have better performance and may be more aware of practice guidelines than their less experienced counterparts.23,34
Patient and tumor characteristics also were important predictors of whether a patient saw a medical oncologist. Younger patient age, being married, not being indigent, having fewer than 3 comorbidities, and more than 7 positive lymph nodes all were associated with referral to medical oncology. These findings are consistent with clinical practice, in that younger, healthier patients and patients with higher risk tumors are likely to be the best candidates for chemotherapy.
Our reported relationships between patient characteristics and seeing a medical oncologist also suggest plausible mechanisms for observed disparities in cancer care. For example, we found that sex and race were not associated with seeing a medical oncologist. This suggests that the lower use of adjuvant chemotherapy by women and African Americans reported by Jessup et al35 may be attributed to factors that affect an oncologist’s decision to recommend chemotherapy as opposed to lower rates of referral to medical oncology. Conversely, the reported lower rates of chemotherapy use in older patients35 may be attributed to lower rates of referral to a medical oncologist with increasing patient age.
Our study has some limitations. Clinical information available from billing data is not as detailed as that available from chart review, and some variables, such as UPINs, may have missing values.7 The AMA Master file is missing data on ethnicity for approximately 33% of surgeons, which makes it difficult to evaluate the impact of the surgeon’s ethnicity on referral patterns. The patient-volume variable is calculated based on care related to colon cancer in older patients within the SEER-Medicare database and, thus, may not reflect surgeons’ overall patient volume.12 For instance, it is possible that a surgeon may have a high volume of younger patients and a limited number of Medicare patients, which would give the false impression of low operative volume. We also note that surgeon characteristics may be less accurate for physicians at teaching hospitals. The UPINs were those of the attending physicians rather than residents or fellows, and patients visiting a surgeon who is affiliated with a teaching hospital may be treated by residents or fellows instead of attending physicians.12 However, the attending physician is supervising the patient’s care. Finally, our study is limited to men and women aged ≥66 years, so our findings may not be generalizable to younger patients in the United States. However, nearly two-thirds of incident colon cancer occurs in older individuals (aged >65 years); thus, our findings would be relevant to the majority of patients.
The main findings from this study are that surgeons account for approximately 20% of the variation in patients seeing a medical oncologist and that fewer years since graduation, higher patient volume, and affiliation with a teaching hospital are major positive determinants in referral patterns. Therefore, interventions at the level of the surgeon may be appropriate to improve the care of patients with colon cancer. Surgeons may need more information about making appropriate referrals to oncologists, given the possibility that not all surgeons may be aware of optimal care available or practice guidelines for older patients with colon cancer. By enhancing surgeons’ knowledge of optimal oncology care and practice guidelines, we can increase the likelihood that older and vulnerable patients will see a medical oncologist, will be considered for chemotherapy, and will have optimal outcomes.
Footnotes
Supported by the University of Texas Medical Branch Center for Population Health and Health Disparities (P50 CA10563) and grants from the National Cancer Institute (CA 104949) and the Agency for Healthcare Research and Quality (R24 HS011618); Grant sponsor: We are indebted to the Applied Research Program, National Cancer Institute; to the Office of Research, Development, and Information, Centers for Medicare and Medicaid Services; to Information Management Services; and to the Surveillance, Epidemiology, and End Results (SEER) Program for the creation of the SEER-Medicare database. The interpretation and reporting of the data are the sole responsibility of the authors.
References
- 1.Weitz J, Koch M, Debus J, Hohler T, Galle PR, Buchler MW. Colorectal cancer. Lancet. 2005;365:153–165. doi: 10.1016/S0140-6736(05)17706-X. [DOI] [PubMed] [Google Scholar]
- 2.Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345:1091–1097. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 3.O’Connell MJ, Laurie JA, Kahn M, et al. Prospectively randomized trial of postoperative adjuvant chemotherapy in patients with high-risk colon cancer. J Clin Oncol. 1998;16:295–300. doi: 10.1200/JCO.1998.16.1.295. [DOI] [PubMed] [Google Scholar]
- 4.Francini G, Petrioli R, Lorenzini L, et al. Folinic acid and 5-flourouracil as adjuvant chemotherapy in colon cancer. Gastroenterology. 1994;106:899–906. doi: 10.1016/0016-5085(94)90748-x. [DOI] [PubMed] [Google Scholar]
- 5.International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) Investigators. Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. Lancet. 1995;345:939–944. [PubMed] [Google Scholar]
- 6.Mamounas E, Wieland S, Wolmark N, et al. Comparative efficacy of adjuvant chemotherapy in patients with Dukes’ B versus Dukes’ C colon cancer: results from four National Surgical Adjuvant Breast and Bowel Project adjuvant studies (C-01, C-02, C-03, and C-04) J Clin Oncol. 1999;17:1349–1355. doi: 10.1200/JCO.1999.17.5.1349. [DOI] [PubMed] [Google Scholar]
- 7.Schrag D, Cramer LD, Bach PB, Begg CB. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst. 2001;93:850–857. doi: 10.1093/jnci/93.11.850. [DOI] [PubMed] [Google Scholar]
- 8.Schrag D, Bach PB, Dahlman C, Warren JL. Identifying and measuring hospital characteristics using the SEER-Medicare data and other claims-based sources. Med Care. 2002;40(8 suppl):IV-96–IV-103. doi: 10.1097/00005650-200208001-00013. [DOI] [PubMed] [Google Scholar]
- 9.Sundararajan V, Grann VR, Jacobson JS, Ahsan H, Neugut AI. Variations in the use of adjuvant chemotherapy for node-positive colorectal cancer in the elderly: a population-based study. Cancer J. 2001;7:213–218. [PubMed] [Google Scholar]
- 10.Sundararajan V, Mitra N, Jacobson JS, Grann VR, Heitjan DF, Neugut AI. Survival associated with 5-fluorouracil-based adjuvant chemotherapy among elderly patients with node-positive colon cancer. Ann Intern Med. 2002;136:349–357. doi: 10.7326/0003-4819-136-5-200203050-00007. [DOI] [PubMed] [Google Scholar]
- 11.Goodwin JS, Hunt WC, Samet JM. Determinants of cancer therapy in elderly patients. Cancer. 1993;72:594–601. doi: 10.1002/1097-0142(19930715)72:2<594::aid-cncr2820720243>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Baldwin LM, Dobie SA, Billingsley K, et al. Explaining black-white differences in receipt of recommended colon cancer treatment. J Natl Cancer Inst. 2005;97:1211–1220. doi: 10.1093/jnci/dji241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jessup JM, McGinnis LS, Steele GD, Jr, Menck HR, Winchester DP. The National Cancer Data Base: report on colon cancer. Cancer. 1996;78:918–926. doi: 10.1002/(SICI)1097-0142(19960815)78:4<918::AID-CNCR32>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 14.Nattinger AB, Gottlieb MS, Hoffman RG, Walker AP, Goodwin JS. Minimal increase in use of breast conserving surgery from 1986 to 1990. Med Care. 1996;34:479–489. doi: 10.1097/00005650-199605000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Earle CC, Neumann PJ, Gelber RD, Weinstein MC, Weeks JC. Impact of referral patterns on the use of chemotherapy for lung cancer. J Clin Oncol. 2002;20:1786–1792. doi: 10.1200/JCO.2002.07.142. [DOI] [PubMed] [Google Scholar]
- 16.Junor EJ, Hole DJ, Gillis CR. Management of ovarian cancer: referral to a multidisciplinary team matters. Br J Cancer. 1994;70:363–370. doi: 10.1038/bjc.1994.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skinner KA, Helsper JT, Deapen D, Ye W, Sposto R. Breast cancer: do specialists make a difference? Ann Surg Oncol. 2003;10:606–615. doi: 10.1245/aso.2003.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds GA, Chitnis JG, Roland MO. General practitioner outpatient referrals: do good doctors refer more patients to the hospital? BMJ. 1991;302:1250–52. doi: 10.1136/bmj.302.6787.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franks P, Clancy CM. Referrals of adult patients from primary care: demographic disparities and their relationship to HMO insurance. J Fam Pract. 1997;45:47–53. [PubMed] [Google Scholar]
- 20.Grilli R, Apolone G, Marsoni J, Nocolucci A, Zola P, Liberati A. The impact of patient management guidelines on the care of breast, colorectal and ovarian cancer patients in Italy. Med Care. 1991;29:50–63. doi: 10.1097/00005650-199101000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Freiman MP. The rate of adoption of new procedures among physicians: the impact of specialty and practice characteristics. Med Care. 1985;23:939–945. doi: 10.1097/00005650-198508000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Coleman JS, Katz E, Menzel H. Medical Innovation: A Diffusion Study. New York: Bobbs-Merrill; 1966. [Google Scholar]
- 23.Jollis JG, Peterson ED, DeLong ER, et al. The relation between the volume of coronary angioplasty procedures at hospitals treating Medicare beneficiaries and short-term mortality. N Engl J Med. 1994;331:1625–1629. doi: 10.1056/NEJM199412153312406. [DOI] [PubMed] [Google Scholar]
- 24.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Med Care. 8 suppl. Vol. 40. 2002. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population; pp. IV-3–IV-18. [DOI] [PubMed] [Google Scholar]
- 25.Baldwin LM, Adamache W, Klabunde CN, Kenward K, Dahlman C, Warren JL. Med Care. 8 suppl. Vol. 40. 2002. Linking physician characteristics and Medicare claims data: issues in data availability, quality, and measurement; pp. IV-82–IV-95. [DOI] [PubMed] [Google Scholar]
- 26.SEER-Medicare provider files. [Accessed August 1, 2006]; Available at: http://healthservices.cancer.gov/seermedicare/aboutdata/provider.html.
- 27.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 28.Luo RL, Giordano SH, Freeman JL, Zhang D, Goodwin JS. Referral to medical oncology: a crucial step in the treatment of older patients with stage III colon cancer. Oncologist. 2006:1025–1033. doi: 10.1634/theoncologist.11-9-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstein H. Multilevel Statistical Models. 2. New York: Halsted Press; 1995. [Google Scholar]
- 30.Snijders TAB, Bosker RJ. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. Thousand Oaks, Calif: Sage; 1999. [Google Scholar]
- 31.Neugut AI, Grann VR. Referral to medical oncologists: are there barriers at the gate? J Clin Oncol. 2002;20:1716–1718. doi: 10.1200/JCO.2002.20.7.1716. [DOI] [PubMed] [Google Scholar]
- 32.Oliveria SA, Yood MU, Campbell UB, Yood SM, Stang P. Treatment and referral patterns for colorectal cancer. Med Care. 2004;42:901–906. doi: 10.1097/01.mlr.0000135820.44720.89. [DOI] [PubMed] [Google Scholar]
- 33.Townsley CA, Naidoo K, Pond GR, Melnick W, Straus SE, Siu LL. Are older cancer patients being referred to oncologists? A mail questionnaire of Ontario primary care practitioners to evaluate their referral patterns. J Clin Oncol. 2003;21:4627–4635. doi: 10.1200/JCO.2003.06.073. [DOI] [PubMed] [Google Scholar]
- 34.McFall SL, Warnecke RB, Kaluzny AD, Ford L. Practice setting and physician influences on judgments of colon cancer treatment by community physicians. Health Serv Res. 1996;31:5–19. [PMC free article] [PubMed] [Google Scholar]
- 35.Jessup J, Stewart A, Greene FL, Minsky BD. Adjuvant chemotherapy for stage III colon cancer: implications of race/ethnicity, age and differentiation. JAMA. 2005;294:2703–2711. doi: 10.1001/jama.294.21.2703. [DOI] [PubMed] [Google Scholar]


