Abstract
Purpose
To explore the role of the complement system and complement regulatory proteins in an immune-privileged organ, the eye.
Methods
Eyes of normal Lewis rats were analyzed for the expression of complement regulatory proteins, membrane cofactor protein (MCP), decay-acceleration factor (DAF), membrane inhibitor of reactive lysis (MIRL, CD59), and cell surface regulator of complement (Crry), using immunohistochemistry, Western blot analysis, and reverse transcription–polymerase chain reaction (RT-PCR). Zymosan, a known activator of the alternative pathway of complement system was injected into the anterior chamber of the eye of Lewis rats. Animals were also injected intracamerally with 5 μl (25 μg) of neutralizing monoclonal antibody (mAb) against rat Crry (5I2) or CD59 (6D1) in an attempt to develop antibody induced anterior uveitis; control animals received 5 μl of sterile phosphate-buffered saline (PBS), OX-18 (25 μg), G-16-510E3 (25 μg), or MOPC-21 (25 μg). The role of complement system in antibody-induced uveitis was explored by intraperitoneal injection of 35 U cobra venom factor (CVF), 24 hours before antibody injection. Immunohistochemical staining and sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) with Western blot analysis were used to detect the presence of membrane attack complex (MAC) and C3 activation products, respectively, in normal and antibody-injected rat eyes.
Results
Complement activation product MAC was present in the normal rat eye, and intraocular injection of zymosan induced severe anterior uveitis. The complement regulatory proteins, MCP, DAF, CD59, and Crry, were identified in the normal rat eye. Soluble forms of Crry and CD59 were also detected in normal rat aqueous humor. Severe anterior uveitis developed in Lewis rats injected with a neutralizing mAb against Crry, with increased formation of C3 split products. Systemic complement depletion by CVF prevented the induction of anterior uveitis by anti-Crry mAb. Intracameral injection of anti-rat CD59 (6D1), anti-rat MHC class I antigen (OX-18), anti-rat Ig (G-16-510E3), or MOPC-21 caused no inflammatory reaction.
Conclusions
The results suggest that the complement system is continuously active at a low level in the normal eye and is tightly regulated by intraocular complement regulatory proteins.
The complement system is a major component of innate immunity and acts in parallel or in concert with the immune system. Complement activation provides an effective host defense mechanism against foreign organisms by generating effector molecules, which are involved in cell death and in immune and inflammatory responses.1 Although complement activation is a valuable first-line defense against potential pathogens, complement activation products have been reported to be spontaneously and continuously deposited on self-tissue in small amounts under normal conditions and in larger quantities during inflammatory reactions.2 Normally, damage to autologous tissue by complement is limited by several widely distributed membrane-associated glycoproteins that act on discrete steps of complement activation by interfering with C3 and C5 convertases and membrane attack complex (MAC) activity.3–5 We and others have noted the differential expression of membrane-bound complement regulatory proteins—namely, decay-acceleration factor (DAF, CD55), membrane cofactor protein (MCP, CD46) and membrane inhibitor of reactive lysis (MIRL, CD59)—in the normal human eye.6,7 Using polyclonal antibodies to human MCP and DAF, we have also shown by immunohistology that these (or similar) molecules are differentially expressed in the eye of the normal Lewis rat.8 Recently, the cell surface regulator of complement, Crry (5I2 antigen), and CD59 were identified in the normal rat eye and adnexal tissues.9,10
In man, soluble forms of various complement regulatory factors, such as C1 inhibitor, C3b inactivator, MCP, and DAF have been reported in normal intraocular fluid and tears.11–14 In 1998, Goslings et al.15 reported the presence of a 1.3-kDa complement inhibitory factor in normal rabbit aqueous humor. However, there are no reports in the literature describing the presence of soluble complement regulatory proteins in the aqueous and/or vitreous of normal rats.
The eye is an immune-privileged site that contains a number of immunosuppressive factors that presumably protect it from potentially dangerous immune and inflammatory reactions.16 In the past, several investigators have focused on the role of these factors in the protection of this important organ; however, the contribution of the complement system has been largely ignored. The present study was designed to investigate the immune relevance of the complement system and complement regulatory protein in the eye.
MATERIALS AND METHODS
Animals
Pathogen-free, male Lewis rats (5–6 weeks old) obtained from Harlan Sprague–Dawley (Indianapolis, IN) were used in this study. All animals were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Antibodies and Reagents
Monoclonal antibodies (mAbs), anti-rat Crry/p65 (clone 5I2, mouse IgG1) anti-rat MHC antigen RT 1A (clone OX-18, mouse IgG1), and anti-rat immunoglobulin (clone G-16-510E3, mouse IgG1) were purchased from PharMingen (San Diego, CA). IgG fraction of goat antiserum to rat C3 was from ICN Biochemicals (Aurora, OH). Rat monoclonal anti-CD59 (6D1, mouse IgG1) antibody was kindly provided by Bryan P. Morgan, Department of Medical Biochemistry, University of Wales College of Medicine (Cardiff, UK), and anti-rat CD59 (clone TH9, mouse IgG1) was obtained from Research Diagnostics (Flanders, NJ). A mouse mAb against rat C5b-9 complex (MAC), 2A1 (mouse IgG1) was a generous gift from William G. Couser (University of Washington, Seattle). MOPC-21 (mouse IgG1) was purchased from Sigma (St. Louis, MO). Purified cobra venom factor (CVF) was from Quidel (San Diego, CA).
Zymosan Injection
Stock suspension (10 mg/ml) of zymosan (Sigma) was prepared and diluted in sterile phosphate-buffered saline (PBS; pH 7.4) and was thoroughly mixed just before use. Rats were divided into six groups, each containing 12 animals. Rats in groups 1 and 2 were injected intracamerally with 5 and 50 μg zymosan suspension (5 μl), respectively, with a 30-gauge needle fitted to a Hamilton syringe. Control eyes (group 3) were injected with 5 μl of sterile PBS. Animals in groups 4 and 5 received purified CVF 24 hours before receiving 5 and 50 μg zymosan, respectively. Polystyrene latex beads (Sigma) were used as the control particles and were prepared as a 10-mg/ml suspension in sterile PBS. Twelve animals (group 6) were injected intracamerally with 5 μl of this suspension, as described. Development and severity of intraocular inflammation were monitored by clinical and histopathologic examination (in masked fashion), as previously described.17,18 The experiment was repeated twice.
In one experiment, the final dilution (1 μg/μl) of zymosan suspension was prepared in normal rat aqueous humor, and 5 μl of this suspension was injected intracamerally into normal Lewis rats (n = 24), as described earlier.
Antibody Injection and Histopathology
Animals were divided into 11 groups, each containing 16 animals. Lewis rats in groups 1 and 2 were injected intracamerally with 25 μg (5 μl) of neutralizing antibodies against rat CD59 (6D1) or Crry (5I2), respectively, by means of a 10-μl Hamilton syringe and 30-gauge needle. The control animals (group 3) received 5 μl of saline, and additional control treatments (groups 4, 5, and 6) consisted of intracameral injection of 25 μg OX-18, anti-rat immunoglobulin, or MOPC-21, respectively. To deplete complement in vivo, Lewis rats (groups 7 and 8) were injected with 35 U purified CVF intraperitoneally, 24 hours before anti-CD59 or anti-Crry injection, respectively. Groups 9, 10, and 11 consisted of control animals (as described) and received similar treatment with CVF. Animals were examined by slit lamp at 6, 12, 18, 24, 36, 48, 60, 72, 84, and 96 hours after antibody injection for clinical signs of uveitis, and anterior uveitis was graded, using the criteria previously described.17 18 Eyes were also harvested for histologic analysis to assess the development and severity of inflammation. Freshly enucleated eyes were fixed immediately for 24 hours in 2.5% buffered glutaraldehyde and transferred into 10% neutral formalin. Fixed and dehydrated tissues were then embedded in paraffin. Six-micrometer sections were stained with hematoxylin and eosin and examined by light microscopy. The histologically determined intensity of uveitis was scored on an arbitrary scale of 0 to 4.17,18 The experiment was repeated twice. Clinical and histologic examinations were performed in masked fashion.
CH50 Assay
Anticoagulant-free blood was collected from the tail vein of normal and CVF-injected Lewis rats, and serum was isolated. A CH50 assay was performed on these serum samples according to a method described in the literature.19
Immunohistochemical Studies
Frozen sections were stained with anti-Crry and anti-MAC antibodies, and paraffin sections were stained with anti-CD59. All antibodies were used at 1:200 dilution for immunohistochemical staining. For frozen sections, eyes were placed in optimal cutting temperature (OCT) compound (Miles, Indianapolis, IN), snap frozen, and stored in sealed vials at −80°C. Eyes were sectioned by cryostat at a thickness of 6 μm, air dried overnight (18 hours), fixed in cold acetone for 10 minutes, and rehydrated in PBS (pH 7.2). For paraffin sections, eyes were removed, fixed in formalin, and processed for paraffin sectioning. Sections were then deparaffinized two times for 5 minutes in xylene, two times for 5 minutes in absolute ethanol, 3 minutes in 95% ethanol, 3 minutes in 70% ethanol, and 5 minutes in PBS. Fixed sections were immersed in a 0.3% hydrogen peroxide-methanol solution for 20 minutes to inactivate endogenous peroxidase and then rinsed well in PBS. Immunohistochemical staining was performed with an anti-mouse IgG immunoperoxidase staining kit (Vector, Burlingame, CA) according to the manufacturer’s directions. The sections were treated with 3,3′-diaminobenzidine (DAB) for 10 minutes, counterstained with Mayer’s hematoxylin for 10 minutes, washed thoroughly in cold tap water, and coverslipped with an mounting media for viewing by light microscopy. Control stains were performed with nonrelevalant antibodies of the same immunoglobulin subclass at concentrations similar to those of the primary antibodies. Additional controls consisted of staining by omission of the primary antibody or secondary biotinylated antibodies.
Intraocular Content
Anesthetized animals were perfused through the heart with 200 ml sterile pyrogen-free saline. Eyes were then immediately enucleated. Conjunctiva and extraocular muscles were carefully removed, and the intraocular tissue from each eye was collected by using a previously described method.20 The intraocular tissues, which consisted of uvea, retina, lens, aqueous humor, and vitreous, were used for total RNA extraction and Western blot analysis.
RT-PCR Analysis
Equal amounts of the total RNA were used to detect the mRNA levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), MCP, DAF, CD59, and Crry by reverse transcription polymerase chain reaction (RT-PCR; RNA-PCR kit; Perkin–Elmer, Norwalk, CT). Total RNA was extracted from pooled intraocular content isolated from normal Lewis rats (20 eyes; RNeasy Mini Kit; Qiagen, Valencia, CA), according to the kit manufacturer’s specifications. The sense and anti-sense oligonucleotide primers for rat GAPDH, MCP, DAF, CD59, and Crry were synthesized at Gibco (Grand Island, NY). The primer sequences and the predicted sizes of am-plified cDNA are presented in Table 1. The cDNA was synthesized using an antisense primer corresponding to a particular rat complement regulatory protein, murine leukemia virus (MuLV) reverse transcriptase, and 4 μg of total RNA in a total volume of 20 μl (pH 8.3). RT reaction was first performed at 24°C for 10 minutes, then at 42°C for 15 minutes. The reaction mixture was heated at 99°C for 5 minutes, and the RT product was mixed with DNA polymerase (AmpliTaq; Perkin–Elmer) and sense primer in a buffer containing 20 mM Tris-HCl, 50 mM KCl, 2.0 mM MgCl2 (pH 8.3), and 50 mM of each dNTP in a 100-μl volume. The mixture was then amplified by PCR using 25 cycles. The thermal cycle profile used in this study was as follows: an initial denaturing at 94°C for 5 minutes and then 30 seconds in each cycle, annealing the primer with DNA at 60°C for 1 minute, and extending the primer at 72°C for 90 seconds. All reactions were normalized for GAPDH expression. The negative controls consisted of omission of RNA template or reverse transcriptase from the reaction mixture. PCR products were analyzed on 2% agarose gel. PCR-amplified DNA fragments were subcloned into PCR 2.1-TOPO cloning vector (TOPO TA cloning kit; Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Clones were sequenced using an automated DNA sequencer (ABI 310; Perkin–Elmer Applied Biosystems, Foster City, CA, with the ABI Prism dRhodamine terminator cycle kit with AmpliTaq DNA polymerase FS). Sequencing primers included M13 forward and reverse primers.
TABLE 1.
Primer Sequences used in RT-PCR
| Primer | Sequence | Amplified cDNA (bp) | |
|---|---|---|---|
| GAPDH | Sense
Antisense |
TGA-AGG-TCG-GTG-TCA-ACG-GAT-TTG-GC
CAT-GTA-GGC-CAT-GAG-GTC-CAC-CAC |
983 |
| MCP | Sense
Antisense |
CCA-TTT-GAA-GCT-ATG-GAA
ACA-TTT-TAC-CAC-TTT-ACA-CTC |
570 |
| DAF | Sense
Antisense |
AGA-GCA-TGC-CCT-AAT-CC
GGC-TTG-TGA-GAC-GTT-GG |
539 |
| Crry | Sense
Antisense |
GTT-CTT-GAT-GGA-GTG-GAA-AGC
CTG-AGG-CTG-AAC-ACA-GCT-GTC |
413 & 599 |
| CD59 | Sense
Antisense |
CTG-CTT-CTG-GCT-GTC-CTC-TG
ACG-CTG-TCT-TCC-CCA-ATA-GG |
302 |
The sequences of all oligonucleotides are shown in the 5′ to 3′ direction.
Western Blot Analysis
Intraocular content prepared as described was homogenized and solubilized in ice cold PBS (2.0 ml/eye) containing protease inhibitors, phenylmethylsulfonyl fluoride (1 μg/ml), aprotinin (1 μg/ml), and EDTA (1mM). The homogenate was centrifuged at 18,000g at 4°C for 30 minutes. The protein content of the supernatants was determined by the Bradford method.21 After sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on 10% linear slab gel, under reducing conditions, separated proteins were transferred to a polyvinylidene fluoride (PVDF) membrane using a semidry electrophoretic transfer cell (Trans-Blot; Bio-Rad, Richmond, CA). Blots were stained at room temperature with a 1:10,000 dilution of IgG fraction of goat anti-rat C3 for 2 hours or overnight at 4°C. Control blots were treated with the same dilution of normal goat serum. After washing and incubation with horseradish peroxidase–conjugated secondary antibody (1:10,000 dilution), blots were developed using the enhanced chemiluminescence Western blot analysis detection system (ECL Plus; Amersham Pharmacia Biotech, Arlington Heights, IL). Quantification of C3 split products was accomplished by densitometry (Alpha Imager 2200; Alpha Innotech; San Leandro, CA).
Western blot analysis was also performed on normal rat aqueous humor, essentially as just described. Pooled aqueous humor was analyzed on 10% SDS-PAGE under nonreducing conditions, and separated proteins were transferred to a PVDF membrane. The resultant blots were probed with 1:1000 dilution of mAb to rat CD59 (clone TH9) or anti-rat Crry mAb (1:1000). Control blots were reacted with the equivalent concentration of MOPC-21. Rat splenocytes purified by Ficoll density centrifugation were solubilized at a concentration of 5 × 107 cells/ml in PBS containing 1% NP-40 (Sigma) and the described protease inhibitors. The splenocyte membrane fraction separated from insoluble material by centrifugation at 33,000g for 20 minutes was used as the positive control in the Western blot analysis.
Statistical Analysis
Differences between groups were evaluated by Mann–Whitney test. P < 0.05 was considered significant.
Results
Complement Activation Products in the Normal Rat Eye
Frozen sections of the normal rat eye were stained for the complement activation product MAC. Staining was observed on the iris and ciliary body when using anti-MAC antibody (Fig. 1A). Control sections treated with MOPC-21, an irrelevant mAb (Fig. 1B), showed no staining. Staining was also observed on the corneal epithelium and stroma (Fig. 1A) and choroid (not shown). MAC was not identified on the retina (not shown).
Figure 1.
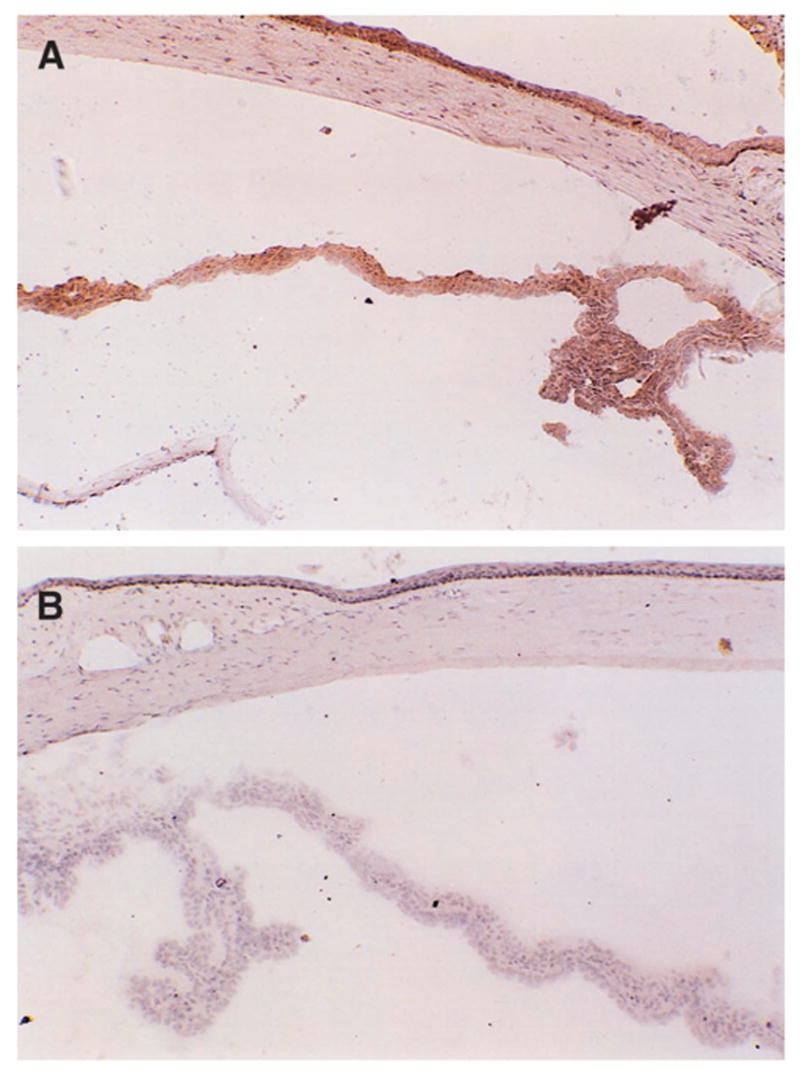
MAC in the rat eye (frozen sections). Immunohistochemical staining of the normal rat eye: anti-MAC (A) and MOPC-21 (B). Original magnification, (A) × 200; (B) × 250.
The presence of C3 cleavage fragments within the eye was documented by Western blot analysis. The soluble fraction of the homogenized intraocular content (uveal tract, retina, lens, and aqueous and vitreous humor) obtained from normal rat eye was probed using polyclonal antibodies directed at C3 determinants. Protein bands corresponding to C3b and iC3b were identified under reduced conditions (Fig. 2). The αchain of reduced C3b is present at 110 kDa (observed in Fig. 2); however, the βchain at 76 kDa was not seen. The 76-kDa βchain of C3b and iC3b did not undergo cleavage during complement activation and was only visualized after overnight incubation with the primary antibody (not shown). The 68-kDa and 43-kDa αchains of iC3b were also identified on Western blot (Fig. 2A). A similar banding pattern was observed with normal rat serum (the positive control; not shown). The blot incubated with normal goat serum did not show any immunoreactivity (Fig. 2B).
Figure 2.
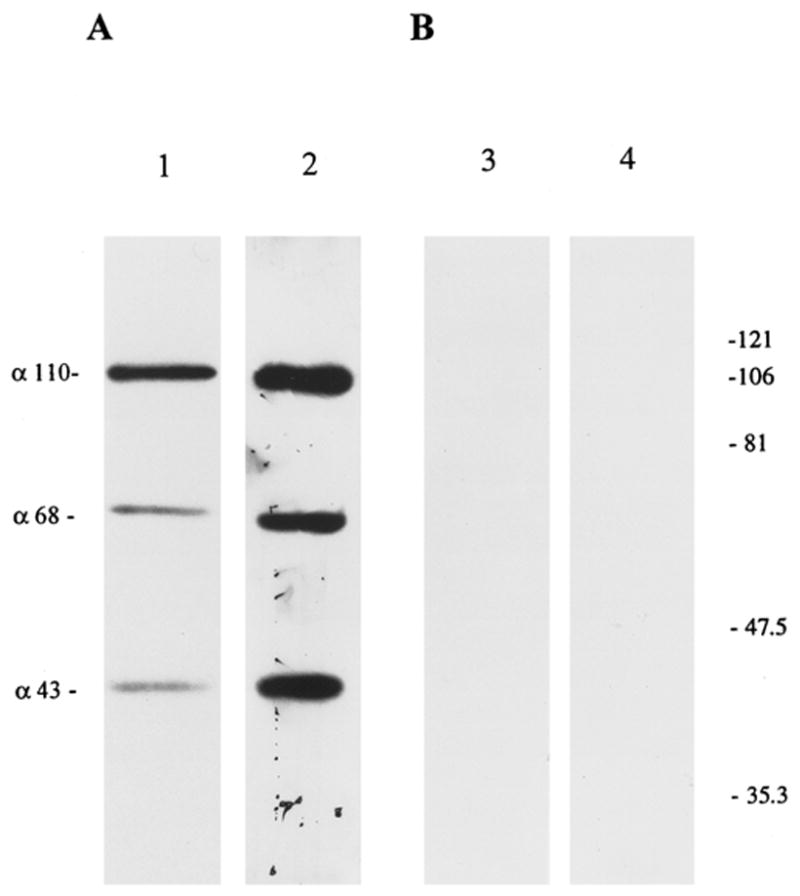
Immunoblot of ocular extracts analyzed on a 10% polyacrylamide gel under reducing conditions. Protein extracts from normal eyes (lanes 1 and 3) and from eyes with Crry-mAb–induced anterior uveitis (lanes 2 and 4). Equal amounts of total protein (20 μg) were loaded in each lane. Blot A was stained with anti-C3 polyclonal antibodies, and blot B was stained with equivalent dilution of normal goat serum. α-Polypeptide chains corresponding to C3b and iC3b (α-110, α-68, and α-43) are visualized in normal rat eye (lane 1), with an increase (P < 0.05) in anti-Crry–injected eyes (lane 2). The 76-kDa β chain of C3b and iC3b (not shown) was seen only after overnight incubation with the primary antibody. Molecular weight standards (in kilodaltons), right.
Zymosan-Induced Anterior Uveitis
Although complement activation products are present within the normal eye, intraocular inflammation is not present. However, the injection of zymosan (5 and 50 μg), an activator of complement, into the anterior chamber of the eye induced severe anterior uveitis in all (n = 24) Lewis rats (Fig. 3). Although iridocyclitis developed within 3 to 4 hours after injection of 5 or 50 μg zymosan, the clinical pattern of zymosan-induced uveitis was dose dependent. With 5 μg zymosan, maximum inflammation was observed between days 1 and 2, and the disease resolved by day 9 (Fig. 3A). However, with 50 μg zymosan, severe inflammation persisted until day 10; with complete resolution by day 19 (Fig. 3B). Histologically, within 3 to 4 hours after injection of zymosan (5 or 50 μg), polymorphonuclear cells (PMNs) infiltrated the iris and ciliary body, with prominent swelling of the iris and ciliary body within 2 days. PMNs appeared in the anterior chamber, with spillover into the anterior vitreous; the cornea, retina, and choroid were not inflamed (not shown). Additionally, the clinical and histopathologic features of anterior uveitis observed in Lewis rats (n = 24) injected intracamerally with 5 μg zymosan resuspended in rat aqueous humor were similar to those induced by 5 μg zymosan resuspended in PBS (Fig. 3A).
Figure 3.
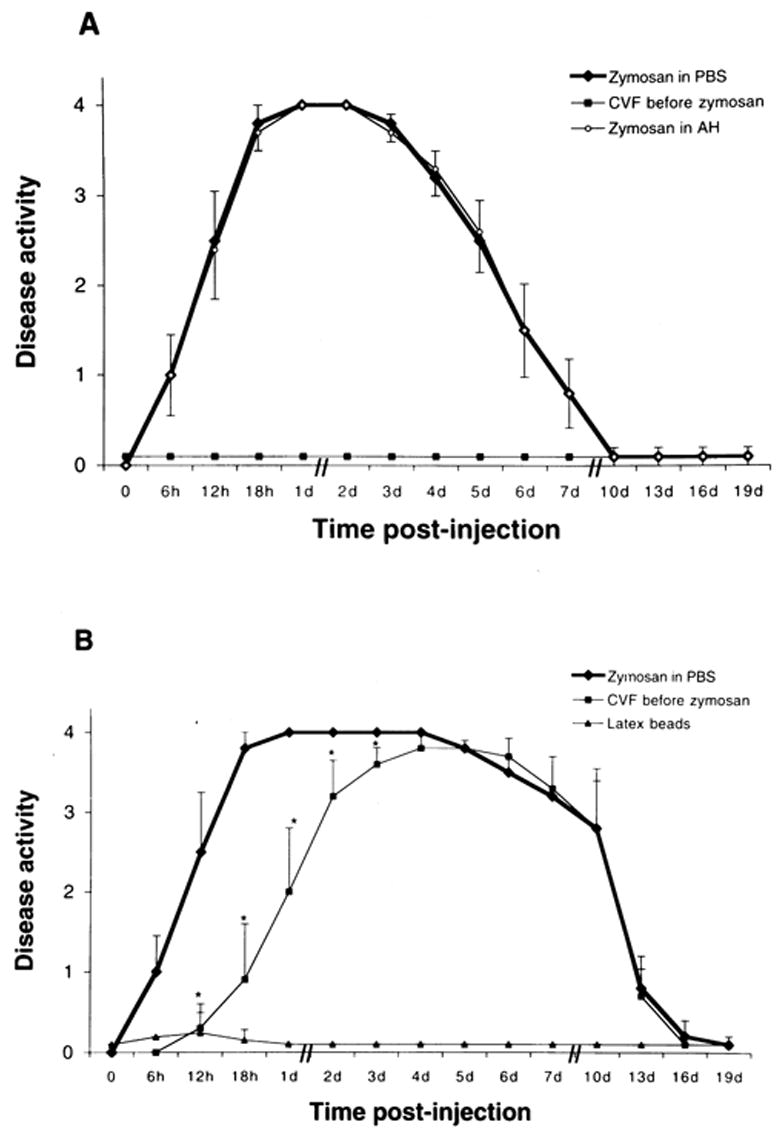
Kinetics of inflammatory response (anterior uveitis) induced by intracameral injection of zymosan, and the effect of a single dose of CVF. (A) Lewis rats were injected with 5 μg zymosan resuspended in 5 μl of PBS or 5 μl of aqueous humor. (B) Lewis rats were injected with 50 μg zymosan in PBS or polystyrene latex beads resuspended in PBS. The values represent the mean ± SD for two experiments (n = 24 rats/group). Comparisons were made between rats treated with zymosan with and without CVF (*P < 0.05).
Pretreatment of Lewis rats with intraperitoneal CVF completely suppressed the anterior uveitis in all animals injected intracamerally with 5 μg zymosan (Fig. 3A). In contrast, CVF pretreatment did not result in complete blocking of anterior uveitis induced by 50 μg zymosan (Fig. 3B). In these animals the onset of zymosan-induced anterior uveitis was delayed, and the duration of severe inflammation was reduced (Fig. 3B). In addition, the severity of disease in CVF-treated animals was significantly reduced (P < 0.05) during the early phase (i.e., between 6 hours and 3 days) of the inflammation (Fig. 3B). Serum complement activity (determined by the CH50 level) was undetectable 24 hours after CVF administration and lasted for almost 5 days (Sohn et al., unpublished results, 1999). Polystyrene latex beads, which do not activate complement, were used as control particles. Intracameral injection of latex beads produced only a mild transient inflammatory reaction, which was completely resolved by 24 hours after latex injection (Fig. 3B). Thus, zymosan-induced uveitis differed significantly from latex-induced inflammation. Anterior uveitis did not develop in any of the animals injected with PBS in the anterior chamber (not shown).
These results demonstrate that components of the complement cascade are present within the normal eye and can be activated by the anterior chamber injection of zymosan to produce severe uveitis.
Presence of Complement Regulatory Proteins in the Normal Rat Eye
Because complement and complement activation products are present within the normal rat eye but intraocular inflammation is not unless the complement cascade is activated by zymosan, we hypothesized an important role for complement regulatory proteins in the normal eye. Consequently, normal rat eyes were analyzed for the presence of Crry, CD59, MCP, and DAF using immunohistochemistry, Western blot analysis, and RT-PCR.
Immunohistochemical Analysis
mAbs against rat CD59 and Crry were used to stain normal rat eyes. We do not have polyclonal antibodies to rat CD59 and Crry, and antibodies against rat MCP and DAF are not commercially available. Intensity of staining was graded using an arbitrary scale previously described by us.6 Controls in which equivalent concentrations of irrelevant mAb were substituted for the primary antibodies were negative. Our results are summarized in Table 2. The corneal epithelium, iris, ciliary body, and retina were moderately stained for CD59; weak staining was observed in the corneal stroma and choroid. A similar staining pattern was observed with anti-Crry, except that there was no staining in the retina.
TABLE 2.
Expression of CD59 and Crry in the Normal Rat Eye
| CD59 | Crry | |
|---|---|---|
| Cornea | ||
| Epithelium | ++ | ++ |
| Stroma | + | + |
| Ciliary Body | ++ | ++ |
| Iris | ++ | ++ |
| Choroid | + | ++ |
| Retina | ++ | 0 |
Comparative staining intensities were graded from extremely intense (++++) to negative (0).
Immunoblot Analysis
Immunoblot analysis studies were performed to detect the presence of complement regulatory proteins in the intraocular fluid. Pooled aqueous humor obtained from normal Lewis rats and mAbs against rat CD59 and Crry were used. We do not have polyclonal antibodies to rat CD59 and Crry, and antibodies against rat MCP and DAF are not commercially available. Representative immunoblot analysis profiles of Crry and CD59 in rat aqueous humor are shown in Figure 4. mAb to rat Crry specifically identified a single protein band with an apparent molecular weight of 65 kDa under nonreducing conditions in pooled rat aqueous humor (Fig. 4A, lane 1), whereas rat splenocyte Crry displayed two discrete protein bands of 65 and 75 kDa (4A, lane 2). CD59 was identified in rat aqueous humor as a protein of 20 to 22 kDa (Fig. 4B, lane 3) that comigrated with splenocyte CD59 (Fig. 4B, lane 4). A control blot stained with MOPC-21 did not show any reactivity (Fig. 4C).
Figure 4.
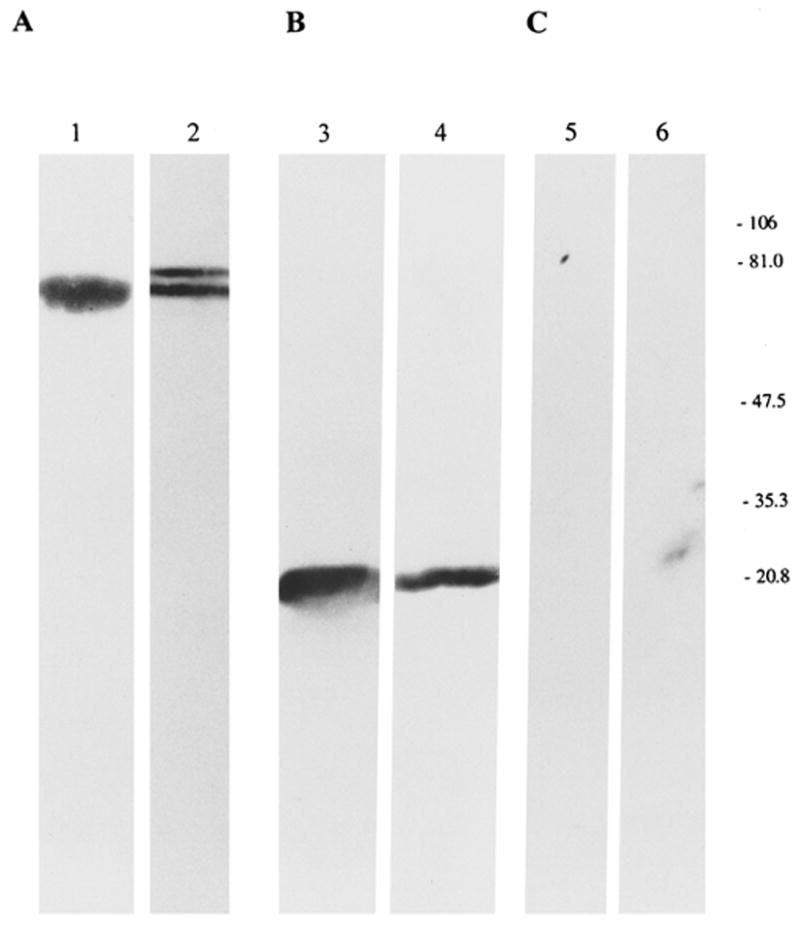
Immunoblot analysis of Crry and CD59 in normal rat aqueous humor. Aqueous humor (lanes 1, 3, and 5) was subjected to SDS-PAGE under nonreducing conditions. Solubilized preparation of normal rat splenocytes (lanes 2, 4, and 6) was used as the positive control. Blots were probed with mAb to rat Crry (A), rat CD59 (B), and MOPC-21 (C). Molecular masses, right.
mRNA Analysis
RT-PCR was used to detect mRNA expression for MCP, DAF, CD59, and Crry in the intraocular content obtained from normal rat eye. Sense and anti-sense primers were designed using published nucleotide sequences for rat proteins (Table 1). A single band of the appropriate molecular weight for Crry (599 and 413 bp), MCP (570 bp), DAF (539 bp), and CD59 (302 bp) was observed on an ethidium bromide–stained agarose gel after UV exposure (Fig. 5). No band was seen in the control without reverse transcriptase (Fig. 5A, lane 2) or RNA template (not shown). The authenticity of the PCR products was verified by nucleotide sequencing. Figure 6 shows the nucleotide sequences of the 570-bp RT-PCR product of MCP, amplified from rat eye. The entire nucleotide sequence of the PCR-amplified MCP band was identical with the published rat MCP sequences.
Figure 5.
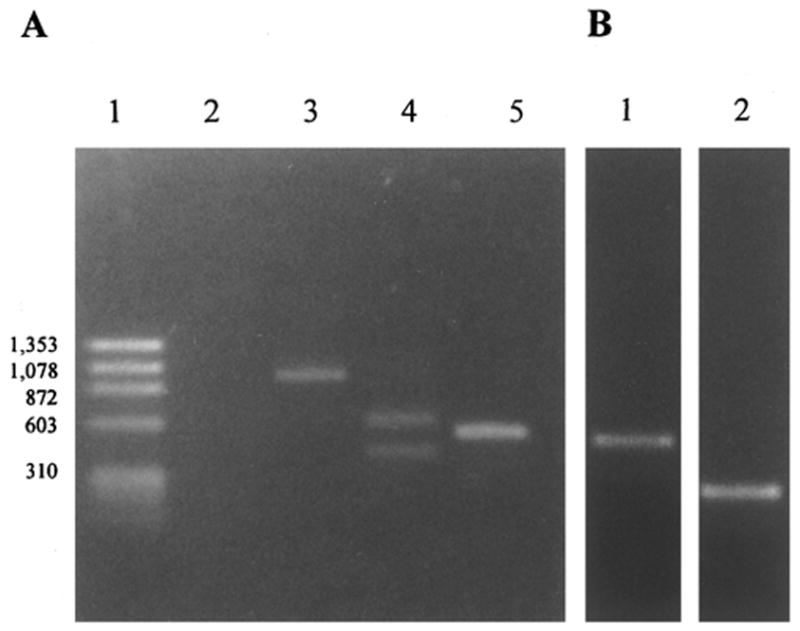
RT-PCR products of complement regulatory proteins in the eye of normal Lewis rats. (A) Ethidium bromide–stained gel showing reverse transcription-PCR products for GAPDH (lane 3), Crry (lane 4), and MCP (lane 5). The size markers were loaded in lanes 1 and 2 represents the negative control (without reverse transcriptase). (B) RT-PCR products for DAF (lane 1) and CD59 (lane 2).
Figure 6.
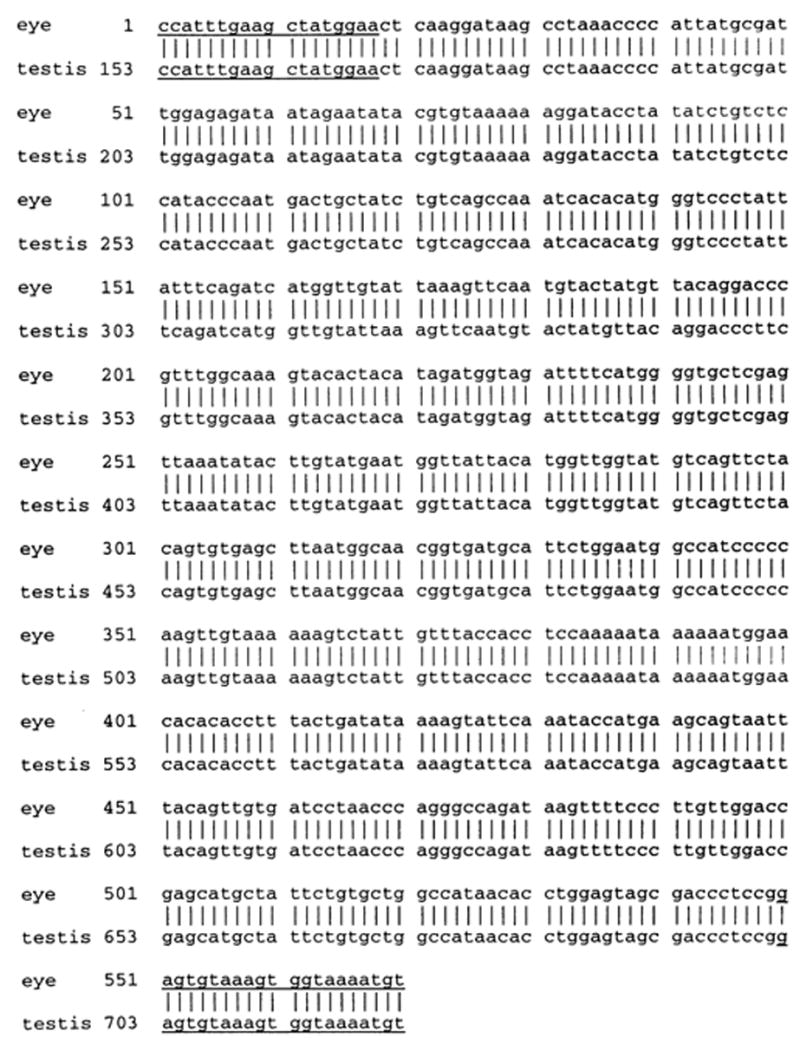
Nucleotide sequences of 570-bp RT-PCR product of MCP amplified from rat eye and its comparison with published rat MCP sequences. The underlined region represents the oligonucleotide primers used in the RT-PCR reaction and the vertical lines indicate the homologous nucleotides. Nucleotides are numbered at the left.
Thus, each of these four complement regulatory proteins appears to be expressed within the normal rat eye.
Antibody-Induced Uveitis
If complement regulatory proteins are important in normally preventing complement-induced anterior uveitis, neutralization of one or more of these proteins should induce inflammation. Anterior uveitis developed in all Lewis rats (n = 32) injected intracamerally with a single dose (25 μg) of neutralizing mAb to Crry (clone 5I2). Clinically, the inflammatory reaction was first noted 4 to 6 hours after antibody injection, peaked between 18 to 24 hours, and resolved by day 4 (Fig. 7A). Histologic examination revealed PMN infiltration of the iris and ciliary body with spillover into aqueous humor (Fig. 7B).
Figure 7.
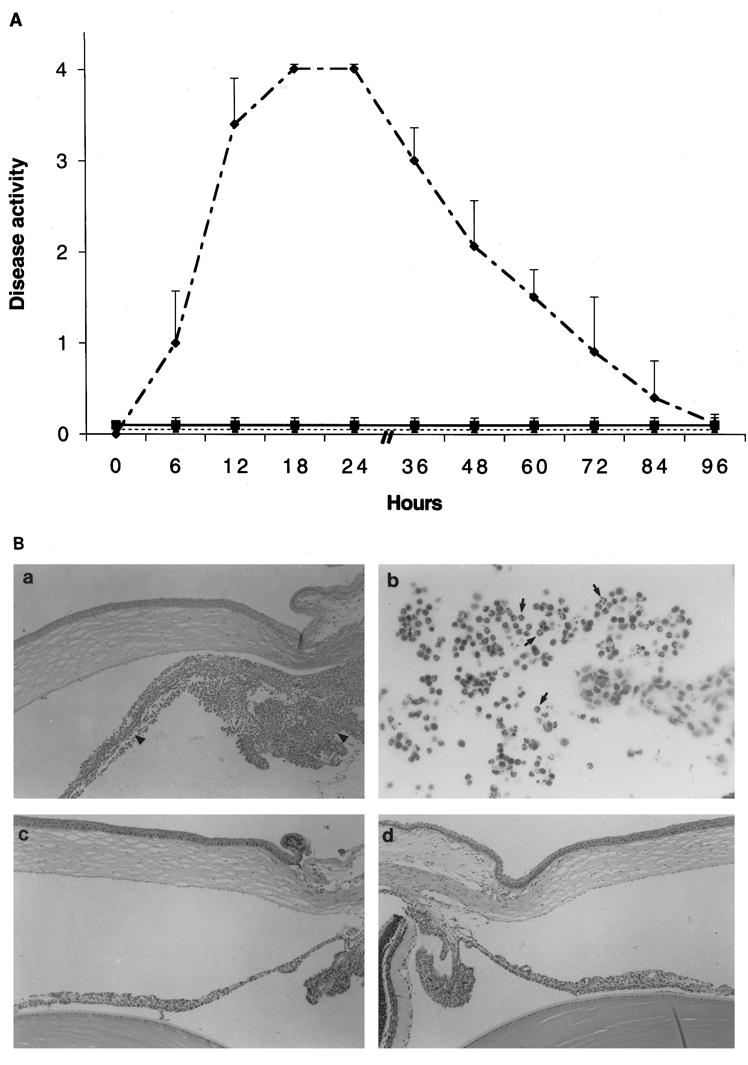
(A) Clinical course of acute anterior uveitis after the intracameral injection of an anti-Crry mAb (dashed curve); rats treated with 35 U of CVF 24 hours before antibody injection (solid line). Dashed straight line represents animals injected with irrelevant mAb, MOPC-21. Results show the mean ± SD from two experiments (n = 32 rats/group). (B) Histopathologic changes in the eye of Lewis rats after anti-Crry injection (a and b). At the peak of inflammation, iris and ciliary body (a) were severely inflamed (arrowheads). The anterior chamber (b) was also infiltrated by many cells, which were mainly PMNs (arrows). The cornea and the posterior segment of the eye were not involved. Intraocular inflammation did not develop in animals injected with CVF before antibody injection (c) and animals injected with anti-rat RT1A (d). Paraffin-embedded sections were stained with hematoxylin and eosin. Original magnification, (Ba, Bc, and Bd) × 100; (Bb) × 250.
The presence of C3 activation products after intracameral (i.e., anterior chamber) injection of anti-Crry antibody was investigated by using Western blot analysis. Intraocular content (uveal tract, retina, lens, and aqueous and vitreous humor) was isolated and used in the Western blot analysis. C3 activation products, C3b and iC3b, increased markedly in anti-Crry antibody–injected rat eyes compared with eyes of age-matched normal control animals (Fig. 2). Densitometric analysis of these polypeptide chains indicated a dramatic increase in the eyes of Crry-injected rats compared with controls rats that was statistically significant (P < 0.05). When complement was depleted by the intraperitoneal injection of 35 U CVF before the intra-cameral injection of 5I2, Crry antibody–induced anterior uveitis was completely suppressed (Figs. 7A, 7B). C3 split products were not detected (by Western blot analysis) in the aqueous humor of Lewis rats injected intraperitoneally with 35 U CVF (Sohn et al., unpublished results, 1999).
Intracameral injection of 6D1 (25 μg), an mAb that suppresses the function of rat CD59 caused no inflammatory reaction (not shown). Additionally, similar intracameral injection of anti-rat RT1A (Fig. 7B), anti-rat immunoglobulin (data not shown), MOPC-21 (Fig. 7A), or PBS alone (not shown) did not cause intraocular inflammation. Low levels of complement activation products were detected in the eyes of these animals (not shown), similar to normal control animals. Thus, the neutralization of only Crry, a complement regulatory protein, resulted in complement-mediated anterior uveitis, which was abolished by the depletion of complement with CVF.
Discussion
We investigated the role of the complement system and complement regulatory proteins in an immune privileged organ, the eye. In the past, several in vitro studies have suggested the presence of functionally active components of the classic and alternative pathways in human aqueous humor, tears, and cornea.11,22–24 We observed in immunohistologic staining of the normal rat eye that the complement activation product MAC was present in the anterior segment and choroid, even though there was no intraocular inflammation. To the best of our knowledge this is the first report describing the presence of MAC in normal rat ocular tissue.
The presence of complement activation products within the normal eye was confirmed by Western blot analysis. Specifically, we identified C3b and iC3b, which are cleavage products of C3, in the buffer extracts of intraocular content obtained from the normal rat eye. Activation of the complement cascade through the classic or the alternative pathway results in the conversion of C3 to C3b; the latter, consists of two peptide chains, α and β, with a molecular weight of 110 and 76 kDa, respectively.25,26 C3b has a short half-life and is rapidly inactivated to iC3b by the enzyme factor I and a cofactor MCP. Factor I has been reported in normal eyes,11 and we have shown in the present study that MCP is present in the normal rat eye. With SDS-PAGE, iC3b shows three peptides that have apparent molecular weight of 76, 68, and 43 kDa under reducing conditions.25,26 Activation of the complement system is necessary to generation of complement split products; thus, their presence is indisputable evidence of complement activation in the normal eye. Cleavage of C3 protein is essential, not only for activation of either complement pathway, but also for the generation of MAC (C5b-9). In humans the presence of MAC has been described in the normal cornea.27 Our immunohistologic demonstration of MAC in tissues of the anterior segment of rat eye is consistent with these observations and is further evidence that complement activation occurs at low levels under normal conditions in the eye.
C3 and complement activation products, including MAC and C3 fragments, have been reported in various human inflammatory diseases, such as systemic lupus erythematosus,28 rheumatoid arthritis,29,30 and anterior uveitis.31 However, the presence of complement activation products in normal tissue has been the subject of some controversy. Using immunoelectron microscopy, immunofluorescence and enzyme-linked immunosorbent assay (ELISA) several groups have noted the presence of complement activation product, at low levels in normal human skin,32 kidney,33 and serum.34 Using RT-PCR, Yasojima et al.35 showed that mRNAs for C3 and other complement proteins are present in normal human brain, liver, spleen, kidney, and heart. These investigators also reported the presence of C3 activation products in extracts of the normal human hippocampus; however, MAC was not detected.35 Similarly, using an immunoperoxidase technique Pham et al.36 did not observe staining for MAC on normal human liver cells. Although C3 was not identified in the heart of normal Wistar rats37 and MAC formation was not detected in the synovium of Lewis rats,38 C3 and MAC deposits have been identified in the kidney of normal Lewis rats.39 These conflicting reports in various normal tissues may represent organ-specific complement activation or the varying sensitivities of the reagents and techniques used to identify complement components.
If a functional complement system is present and active at a low level in the normal eye then agents known to activate complement by the alternative pathway, such as zymosan,40 may be able to elicit severe inflammation (i.e., anterior uveitis). Zymosan, an insoluble polysaccharide extracted from yeast wall, has been reported to induce severe, complement-mediated inflammation at various sites. Intratracheal administration of zymosan has been shown to induce a marked inflammatory response similar to Farmer’s lung41 and pleurisy.42 Keystone et al.43 showed in 1977 that chronic proliferative arthritis could be induced in mice by single intra-articular injection of zymosan. In the present study, a single intracameral (i.e., anterior chamber) injection of zymosan (5 or 50 μg) produced severe anterior uveitis in Lewis rats. However, the clinical course of the disease revealed dose dependence. With the higher dose, there was prolonged inflammation and severity.
We explored the role of complement in zymosan-induced uveitis by using CVF, a C3bBb enzyme of cobra, which depletes complement by unrestricted activation of complement through the alternative pathway.44 We observed that a single intraperitoneal injection of 35 units of CVF resulted in almost total loss of serum complement activity (determined by CH50 level) 24 hours after injection, which lasted for 5 days (Sohn et al., unpublished results, 1999). Our results demonstrated that anterior uveitis induced with 5 μg zymosan was completely abolished by decomplementation using CVF. In contrast, pre-treatment of rats with CVF did not have a dramatic effect on disease expression when 50 μg zymosan was used, although, the intensity of inflammation was significantly reduced in these CVF-treated rats during the initial phase (i.e., 6 hours and 3 day) of the disease.
Collectively, these results suggest that the administration of a low dose of zymosan resulted in anterior uveitis caused by activation of the alternative pathway of complement. However, with a high challenge dose there was activation of complement during the initial phase of the disease, followed by stimulation of other proinflammatory pathways by high dose of zymosan, which prolonged the intraocular inflammation. A high dose of zymosan has been reported to have multiple proinflammatory activities.45 These results are in accord with observations of Imai et al.,42 who reported that pretreatment of rats with CVF reduced the exudate formation during the early phase (at 0.5 and 5 h) of rat pleurisy induced with a high dose of zymosan. Thus, our results confirmed for the first time that a functionally active complement system is present in the normal rat eye.
Because the normal eye has a functional complement system and there is chronic, low-level complement activation, what prevents persistent complement-mediated intraocular inflammation? Using immunohistochemistry, we demonstrated a differential expression within the eye of the cell surface regulators of complement, CD59, and Crry. CD59 was observed in the corneal epithelium, ciliary body, and retina; whereas Crry was not demonstrated in the retina. Additionally, our immunoblot studies demonstrated the presence of soluble forms of Crry and CD59 in normal rat aqueous humor for the first time. There are no reports in the literature describing the presence of soluble Crry in rat body fluids; however, a previously reported study has identified CD59 in normal rat urine.46
In 1994, Funabashi et al.9 demonstrated that anti-CD59 and anti-Crry antibodies stained the cornea of female Wistar rats. Bardenstein et al.10 recently confirmed that Crry and CD59 are expressed in the eye and also observed staining of the adnexal tissues in these animals. Thus, our present immunohistochemical studies demonstrating the presence of CD59 and Crry in the normal eye are consistent with previous observations of others.9,10
We further investigated the expression of complement regulatory proteins, CD59, Crry, MCP, and DAF, in the normal eye using rat-specific sense and antisense oligonucleotide primers and RT-PCR. During the past 2 years, rat analogues of human MCP and DAF have been identified,47,48 and cDNAs for rat CD59, Crry, MCP, and DAF have been isolated and cloned.47–50 Our studies demonstrated for the first time the expression of all four complement regulatory proteins within the eye. These findings also confirmed our previous observations. In 1994, we reported the presence of MCP and DAF (or similar molecules) in the normal rat eye, by using polyclonal anti-human antibodies.8
The presence of membrane-bound and soluble complement regulatory proteins in the normal eye suggests an important in vivo regulatory function. Could these molecules be regulating the complement system in the normal eye so that frank intraocular inflammation was not observed in the face of chronic, low-level complement activation? We neutralized the effect of these molecules with the intracameral injection of blocking mAbs to rat CD59 and Crry (antibodies to rat MCP and DAF are not commercially available). A single injection of 25 μg of anti-Crry induced severe acute anterior uveitis. Histopathology revealed an inflamed iris and ciliary body with a heavy infiltration of mainly PMNs. Protein immunoblot analysis demonstrated that C3 activation products, C3b and iC3b, increased markedly in the eye of Crry antibody–injected animals. Finally, anti-Crry–induced uveitis was completely abrogated by complement depletion with CVF, suggesting that the intraocular inflammation was complement dependent. To our knowledge, this is the first in vivo demonstration that uveitis can be elicited by blocking a complement regulatory protein, Crry.
In contrast, intracameral injection of anti-rat CD59, anti-rat RT1A, and anti-rat immunoglobulin did not induce intraocular inflammation. We used purified IgG1 fractions of these mAbs in our experiments and not the F(ab)2 fragments. The IgG1 fractions of these antibodies or MOPC-21 did not induce inflammation when injected into the rat eye. Furthermore, antibodies that are not directed toward complement regulatory proteins (such as anti-rat RT1A and anti-rat immunoglobulin) did not provoke any inflammatory response when injected intracamerally. Thus, on the basis of these results we do not think that anti-Crry–induced uveitis is due to complement activation triggered by the Fc portion of IgG1 or by immune complexes formed within the eye.
The reason that suppression of Crry, and not CD59, induced severe anterior uveitis may be that these two molecules restrict different steps in the complement cascade. Crry blocks complement activation at an earlier point (C3-convertase level),51 whereas CD59 acts on the terminal step of complement activation.52 Thus, blockade of regulation at an earlier point in the pathway may lead to enhanced complement activation and intraocular inflammation. It should be noted that rat eyes have MCP and DAF (our present results), which, similar to Crry, also control complement activation at the critical step of C3 convertase formation. However, our results, showing that blocking of Crry by mAb (5I2) induced very strong complement activation and intraocular inflammation, suggest that Crry may have a more important regulatory role for C3 convertase than either MCP or DAF in the rat eye. Additionally, rat MCP and DAF have only recently been identified, and their in vivo roles have yet to be clarified.
In light of these observations, we conclude that intraocular complement regulatory proteins tightly control spontaneous complement activation within the normal rat eye. However, under pathologic conditions (analogous to zymosan injection in the anterior chamber) the increased complement activation overcomes the restriction by local complement regulatory proteins, thus causing intraocular inflammation. It is conceivable that by injecting 5 μl of zymosan suspension, we depleted the anterior chamber of soluble complement regulatory proteins. Therefore, the final dilution (1 μg/μl) of zymosan was prepared in normal rat aqueous humor, and 5 μl of this suspension was used for intracameral injection. This concentration was chosen, because our results demonstrated that intraocular inflammation induced with 5 μg zymosan was mediated by the complement activation. Our results show that 5 μg zymosan induced severe anterior uveitis, even if soluble complement regulatory proteins were supplemented by resuspending zymosan in 5 μl of rat aqueous humor instead of in 5 μl of PBS. These results further suggest that zymosan induces increased complement activation that overcomes the restriction by local complement regulatory proteins, but they do not suggest that zymosan-induced anterior uveitis is due to the acute depletion soluble complement inhibitory proteins in the anterior chamber.
Functional suppression of CD59 and Crry has been reported to cause local inflammation.38,53,54 Mizuno et al.38 reported that the intra-articular injection of 6D1 (an mAb to rat CD59) caused joint swelling, thickening of synovial tissues, and infiltration of inflammatory cells. In 1996, Nishikawa et al.53 studied the effect of blocking antibodies to Crry and CD59 after intracutaneous injection in rats. Similar to our observations, these groups also reported that anti-Crry antibodies induce a severe local inflammatory reaction, whereas functional suppression of CD59 has no effect. Matsuo et al.54 studied the in vivo effects of monoclonal and blocking antibodies to CD59 (6D1) and Crry (5I2) after intravenous injection in male Wistar rats. They concluded that mAb 5I2 induces a biphasic change in mean arterial pressure that is complement dependent; suppression of CD59 has no effect.
In conclusion, our studies revealed a novel and unique role for complement in the eye. The complement system is continuously activated (through an alternative pathway) at low levels in normal eye, and intraocular complement regulatory proteins present in the intraocular fluid as well as on the cell membrane tightly regulate this spontaneous complement activation. Control of complement activation at the level of C3 convertase is sufficient to prevent complement-mediated intraocular inflammation. We postulate that a chronic low level of complement activation is necessary as a primary defense mechanism in the eye against endogenous pathogenic infection. However, such a surveillance function of complement must be closely regulated to allow destruction of the putative pathogen without severe inflammation, which would be deleterious to the intraocular structures that subserve vision. Thus, the view we propose is that the endogenous complement inhibitory activity of the eye is not feeble, but rather is finely regulated to allow protection of the eye and that it has the potential to be markedly enhanced if maintenance of vision is threatened. We are currently exploring the role of intraocular complement in the protection against pathogenic invasion and in immune privilege in our laboratory.
Footnotes
Supported by National Institutes of Health grants EY09730, EY10543, and EY13094 and Core Grant EY02687.
References
- 1.Muller–Eberhard HJ. Molecular organization and function of the complement system. Annu Rev Biochem. 1988;57:321–347. doi: 10.1146/annurev.bi.57.070188.001541. [DOI] [PubMed] [Google Scholar]
- 2.Liszewski MK, Farries TC, Lublin DM, Rooney IA, Atkinson JP. Control of the complement system. Adv Immunol. 1996;61:201–283. doi: 10.1016/s0065-2776(08)60868-8. [DOI] [PubMed] [Google Scholar]
- 3.Nicholson–Weller A. Decay accelerating factor (CD55) Curr Topics Microbiol Immunol. 1992;178:7–30. doi: 10.1007/978-3-642-77014-2_2. [DOI] [PubMed] [Google Scholar]
- 4.Liszewski MK, Post TW, Atkinson JP. Membrane cofactor protein (MCP or CD46): Newest member of the regulators of complement activation gene cluster. Ann Rev Immunol. 1991;9:431–455. doi: 10.1146/annurev.iy.09.040191.002243. [DOI] [PubMed] [Google Scholar]
- 5.Holguin MH, Parker CJ. Membrane inhibitor of reactive lysis. Curr Top Microbiol Imunol. 1992;178:61–85. doi: 10.1007/978-3-642-77014-2_5. [DOI] [PubMed] [Google Scholar]
- 6.Bora NS, Gobleman CL, Atkinson JP, Pepose JS, Kaplan HJ. Differential expression of the complement regulatory proteins in the human eye. Invest Ophthalmol Vis Sci. 1993;34:3579–3584. [PubMed] [Google Scholar]
- 7.Bardenstein DS, Dietz Y, Lass JH, Medof ME. Localization of the complement membrane attack complex inhibitor (CD59) in human conjunctiva and lacrimal gland. Curr Eye Res. 1994;13:851–855. doi: 10.3109/02713689409015085. [DOI] [PubMed] [Google Scholar]
- 8.Bora NS, Kabeer NH, Kim MC, Paryjas S, Atkinson JP, Kaplan HJ. Expression of the complement regulatory proteins in the normal and diseased (EAI) rat eye. Adv Ocul Immunol. 1994:83–86. [Google Scholar]
- 9.Funabashi K, Okada N, Matsuo S, Yamamoto T, Morgan BP, Okada H. Tissue distribution of complement regulatory membrane proteins in rats. Immunology. 1994;81:444–451. [PMC free article] [PubMed] [Google Scholar]
- 10.Bardenstein DS, Cheyer C, Okada N, Morgan BP, Medof ME. Cell surface regulators of complement, 5I2 antigen, and CD59, in the rat eye and adnexal tissues. Invest Ophthalmol Vis Sci. 1999;40:519–524. [PubMed] [Google Scholar]
- 11.Mondino BL, Sumner H. Complement inhibitors in normal cornea and aqueous humor. Invest Ophthalmol Vis Sci. 1984;25:483–486. [PubMed] [Google Scholar]
- 12.Hara T, Kuriyama S, Kiyohara H, Nagase Y, Matsumoto M, Seya T. Soluble forms of membrane cofactor protein (CD46, MCP) are present in plasma, tears, and seminal fluid in normal subjects. Clinic Exp Immunol. 1992;89:490–494. doi: 10.1111/j.1365-2249.1992.tb06986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medof ME, Walter EI, Rutgers JL, Knowles DM, Nussenzweig V. Identification of the complement decay-accelerating factor (DAF) on epithelium and glandular cells and in body fluid. J Exp Med. 1987;165:848–864. doi: 10.1084/jem.165.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willcox MDP, Morris CA, Thakur A, Sack RA, Wickson J, Boey W. Complement and complement regulatory proteins in human tears. Invest Ophthalmol Vis Sci. 1997;38:1–8. [PubMed] [Google Scholar]
- 15.Goslings WRO, Prodeus AP, Streilein JW, Carroll MC, Jager MJ, Taylor AW. A small molecular weight factor in aqueous humor acts on C1q to prevent antibody-dependent complement activation. Invest Ophthalmol Vis Sci. 1998;39:989–995. [PubMed] [Google Scholar]
- 16.Streilein JW. Immunologic privilege of the eye. Semin Immunopathol. 1999;21:95–111. doi: 10.1007/BF00810243. [DOI] [PubMed] [Google Scholar]
- 17.Broekhuyse RM, Kuhlman ED, Winkens HJ, Van Vugt AHM. Experimental autoimmune anterior uveitis (EAAU), a new form of experimental uveitis, I. induction by a detergent-insoluble, intrinsic protein fraction of the retina pigment epithelium. Exp Eye Res. 1991;52:465–474. doi: 10.1016/0014-4835(91)90044-f. [DOI] [PubMed] [Google Scholar]
- 18.Bora NS, Kim MC, Kabeer NH, Simpson SC, et al. Experimental autoimmune anterior uveitis (EAAU):Induction with melanin associated antigen from the iris and ciliary body. Invest Ophthalmol Vis Sci. 1995;36:1056–1066. [PubMed] [Google Scholar]
- 19.Kabat EA, Mayer MM. Experimental Immunochemistry. Spring-field, IL: Charles C. Thomas; 1961. pp. 133–239. [Google Scholar]
- 20.Okada AA, Sakai J, Usui M, Mizuguchi J. Intraocular cytokine quantification of experimental autoimmune uveoretinitis in rats. Ocular Immunmol Inflamm. 1998;6:111–120. doi: 10.1076/ocii.6.2.111.4046. [DOI] [PubMed] [Google Scholar]
- 21.Bradford MM. A refined and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 22.Chandler JW, Leder R, Kaufman HE, Caldwell JR. Quantitative determinations of complement components and immunoglobulins in tears and aqueous humor. Invest Ophthalmol Vis Sci. 1974;13:151–153. [PubMed] [Google Scholar]
- 23.Mondino BJ, Rao H. Complement levels in normal and inflamed aqueous humor. Invest Ophthalmol Vis Sci. 1983;24:380–384. [PubMed] [Google Scholar]
- 24.Mondino BJ, Ratajczak HV, Goldberg DB, Schanzlin DJ, Brown SI. Alternate and classical pathway components of complement in the normal cornea. Arch Ophthalmol. 1980;98:346–349. doi: 10.1001/archopht.1980.01020030342023. [DOI] [PubMed] [Google Scholar]
- 25.Daha MR, Stuffers–Heiman M, Kijlstra A, Vanes LA. Isolation and characterization of the third component of rat complement. Immunology. 1979;36:63–70. [PMC free article] [PubMed] [Google Scholar]
- 26.Davis AE, III, Harrison RA, Lachmann PJ. Physiologic inactivation of fluid phase C3b: isolation and structural analysis of C3c, C3d, g (alpha 2D), and C3g. J Immunol. 1984;132:1960–1966. [PubMed] [Google Scholar]
- 27.Mondino BJ, Chou HJ, Sumner HL. Generation of complement membrane attack complex in normal human corneas. Invest Ophthalmol Vis Sci. 1996;37:1576–1581. [PubMed] [Google Scholar]
- 28.Manzi S, Rairie JE, Carpenter AB, et al. Sensitivity and specificity of plasma and urine complement split products as indicators of lupus disease activity. Arthritis Rheum. 1996;39:1178–1188. doi: 10.1002/art.1780390716. [DOI] [PubMed] [Google Scholar]
- 29.Corvetta A, Pomponio G, Rinaldi N, Luchetti MM, Di Loreto C. Terminal complement complex in synovial tissue from patients affected by rheumatoid arthritis, osteoarthritis and acute joint trauma. Clin Exp Rheumatol. 1992;10:433–438. [PubMed] [Google Scholar]
- 30.Guc D, Gulati P, Lemercier C, Lappin D, Birnie GD, Whaley K. Expression of the components and regulatory proteins of the alternative complement pathway and the membrane attack complex in normal and diseased synovium. Rheumatol Int. 1993;13:139–146. doi: 10.1007/BF00301260. [DOI] [PubMed] [Google Scholar]
- 31.Mondino BJ, Glovsky MM, Chekiere L. Activated complement in inflamed aqueous humor. Invest Ophthalmol Vis Sci. 1984;25:871–873. [PubMed] [Google Scholar]
- 32.Dovezenski N, Billeta R, Gigli I. Expression and localization of proteins of the complement system in human skin. J Clin Invest. 1992;90:2000–2012. doi: 10.1172/JCI116080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinglais N, Kaztchkine MD, Bhakdi S, et al. Immunohistochemical study of the C5b-9 complex of complement in human kidneys. Kidney Int. 1986;30:399–410. doi: 10.1038/ki.1986.198. [DOI] [PubMed] [Google Scholar]
- 34.Hugo F, Kramer S, Bhakdi S. Sensitive ELISA for quantitating the terminal membrane C5b-9 and fluid-phase SC5b-9 complex of human complement. J Immunol Methods. 1987;20:243–251. doi: 10.1016/0022-1759(87)90134-7. [DOI] [PubMed] [Google Scholar]
- 35.Yasojima K, Schwab C, McGeer EG, McGeer PL. Up-regulated production and activation of the complement system in Alzheimer’s disease brain. Am J Physiol. 1999;154:927–936. doi: 10.1016/S0002-9440(10)65340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pham B-N, Mosnier J-F, Durand F, et al. Immunostaining for membrane attack complex of complement is related to cell necrosis in fulminant and acute hepatitis. Gastroenterology. 1995;108:495–504. doi: 10.1016/0016-5085(95)90079-9. [DOI] [PubMed] [Google Scholar]
- 37.Vakeva A, Morgan BP, Tikkanen I, Helin K, Laurila P, Meri S. Time course of complement activation and inhibitor expression after ischemic injury of rat myocardium. Am J Pathol. 1994;144:1357–1368. [PMC free article] [PubMed] [Google Scholar]
- 38.Mizuno M, Nishikawa K, Goodfellow RM, Piddlesden SJ, Morgan BP, Matsuo S. The effects of functional suppression of a membrane-bound complement regulatory protein, CD59, in the synovial tissue in rats. Arthritis Rheum. 1997;40:527–533. doi: 10.1002/art.1780400319. [DOI] [PubMed] [Google Scholar]
- 39.Koffler D, Biesecker G, Noble B, Andres GA, Martinez–Hernandez A. Localization of the membrane attack complex (MAC) in experimental immune complex glomerulonephritis. J Exp Med. 1983;157:1885–1905. doi: 10.1084/jem.157.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fearon DT, Austen KF. Activation of the alternative complement pathway due to resistance of the zymosan-bound amplification convertase to endogenous regulatory mechanism. Proc Natl Sci USA. 1977;74:1683–1687. doi: 10.1073/pnas.74.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edwards JH, Wagner JC, Seal RME. Pulmonary responses to particulate materials capable of activating the alternative pathway of complement. Clin Allergy. 1976;6:155–164. doi: 10.1111/j.1365-2222.1976.tb01893.x. [DOI] [PubMed] [Google Scholar]
- 42.Imai Y, Hayashi M, Oh-ishi S. Key role of complement activation and platelet-activating factor in exudate formation in zymosan-induced rat pleurisy. Jpn J Pharmacol. 1991;57:225–232. doi: 10.1254/jjp.57.225. [DOI] [PubMed] [Google Scholar]
- 43.Keystone EC, Schorlemmer HU, Rope C, Allison AC. Zymosan-induced arthritis: a model of chronic proliferative arthritis following activation of the alternative pathway of complement. Arthritis Rheum. 1977;20:1396–1401. doi: 10.1002/art.1780200714. [DOI] [PubMed] [Google Scholar]
- 44.Fritzinger DC, Bredehorst R, Vogel CW. Molecular cloning and derived primary structure of cobra venom factor. Proc Natl Acad Sci USA. 1994;91:12775–12779. doi: 10.1073/pnas.91.26.12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rao TS, Currie JL, Shaffer AF, Isakson PC. In vivo characterization of Zymosan-induced mouse peritoneal inflammation. J Pharmacol Exp Ther. 1994;269:917–925. [PubMed] [Google Scholar]
- 46.Lehto T, Morgan BP, Meri S. Binding of human and rat CD59 to the terminal complement complexes. Immunology. 1997;90:121–128. doi: 10.1046/j.1365-2567.1997.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsujimura A, Shida K, Kitamura M, et al. Molecular cloning of a murine homologue of membrane cofactor protein (CD46): preferential expression in testicular germ cells. Biochem J. 1998;330:163–168. doi: 10.1042/bj3300163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spicer AP, Seldin MF, Gendler SJ. Molecular cloning and chromosomal localization of the mouse decay-accelerating factor genes: duplicated genes encode glycosylphophatidylinositol-anchored and transmembrane forms. J Immunol. 1995;155:3079 –3091. [PubMed] [Google Scholar]
- 49.Rushmere NK, Harrison RA, Berg CWVD, Morgan BP. Molecular cloning of the rat analogue of human CD59 and identification of a putative active site. Biochem J. 1994;304:595–601. doi: 10.1042/bj3040595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakurada C, Seno H, Dohi N, et al. Molecular cloning of the rat complement regulatory protein, 5I2 antigen. Biochem Biophys Res Commun. 1994;198:819–826. doi: 10.1006/bbrc.1994.1117. [DOI] [PubMed] [Google Scholar]
- 51.Takizawa H, Okada N, Okada H. Complement inhibitor of rat cell membrane resembling mouse Crry/p65. J Immunol. 1994;152:3032–3038. [PubMed] [Google Scholar]
- 52.Hughes TR, Piddlesden SJ, Williams JD, Harrison RA, Morgan BP. Isolation and characterization of a membrane protein from rat erythrocytes which inhibits lysis by the membrane attack complex of rat complement. Biochem J. 1992;284:169–176. doi: 10.1042/bj2840169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishikawa K, Matsuo S, Okada N, Morgan BP, Okada H. Local inflammation caused by a monoclonal antibody that blocks the function of the rat membrane inhibitor of C3 convertase. J Immunol. 1996;156:1182–1188. [PubMed] [Google Scholar]
- 54.Matsuo S, Ichida S, Takizawa H, et al. In vivo effects of monoclonal antibodies that functionally inhibit complement regulatory proteins in rats. J Exp Med. 1994;180:1619–1627. doi: 10.1084/jem.180.5.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]


