Abstract
Considerable evidence supports novel functions for lysyl oxidase (LOX) beyond its traditional role in initiating crosslinkages in collagen and elastin within the extracellular matrix. These novel roles are particularly relevant during the transition of malignant epithelial cells towards a migratory and invasive phenotype. However, knowledge on cellular and matrix functions of LOX has been generated almost exclusively in mesenchymal cell types. But it is becoming increasingly evident that these cell types are not adequate to address these novel and highly significant roles for LOX in epithelial tissues. In this initial report, we demonstrate that active LOX is expressed by polarized MDCK II kidney and MCF-10A breast epithelial cells. Furthermore, we show evidence for the presence of mature LOX in the cytoplasm and establish these cell lines as models for epithelial LOX studies.
Keywords: Lysyl oxidase (LOX), epithelia, MDCK II cells, MCF-10A cells
Introduction
Lysyl oxidase (LOX) is essential for crosslinking of elastin fibers and collagen fibrils in extracellular matrices, thereby providing tissue integrity (reviewed in Kagan and Li, 2003; Fong et al., 2006). LOX function has been traditionally investigated using mesenchymal cell types as in vitro model systems, partially because those cells are believed to contribute most of the extracellular matrix components to the tissue stroma. Although epithelia face the extracellular matrix with their basal surfaces, there is poor knowledge about a potential role of LOX in epithelial cell types. A few reports however did provide evidence for LOX expression in some epithelial tissues based on immunohistochemistry data and one study detected LOX mRNA expression in cultured rabbit pigment epithelium cells (Omori et al., 2002, Hayashi et al., 2004, Noblesse et al., 2004; Fogelgren et al., 2005). Additionally, recent findings indicate that LOX is a critical factor when breast epithelial tumor cells acquire a migratory and invasive phenotype in vitro (Kirschmann et al., 1999; Kirschmann et al., 2002; Payne et al., 2005). A recent study further demonstrated that LOX is essential for hypoxia-induced metastasis in a breast cancer model in vivo (Erler et al., 2006).
Altogether, these observations raise the fundamental question for a role of LOX in epithelial cells and furthermore how this function might be altered during the course of malignant progression in cancer. Using two well-characterized epithelial cell lines, MDCK II and MCF-10A cells, we show for the first time that active LOX is expressed by normal polarized epithelial cells in culture. We additionally provide evidence that LOX is not only secreted into the extracellular space where it is thought to be subsequently processed, but that processed LOX is also present in the cytoplasm of these cells. We suggest these cell lines might be appropriate to serve as valuable model systems for future in vitro studies that aim to understand the unexplored role of LOX in epithelia.
Results and discussion
LOX expression in polarized epithelial cells
Over the years, extensive studies have demonstrated that MDCK II and MCF-10A cells exhibit crucial features of polarized epithelia in vivo, most importantly apico-basolateral polarity (Yeaman et al., 1999; Debnath et al., 2003). Both cell lines display the characteristic “cobblestone” morphology of epithelial cells in culture (Fig. 1A).
Figure 1.
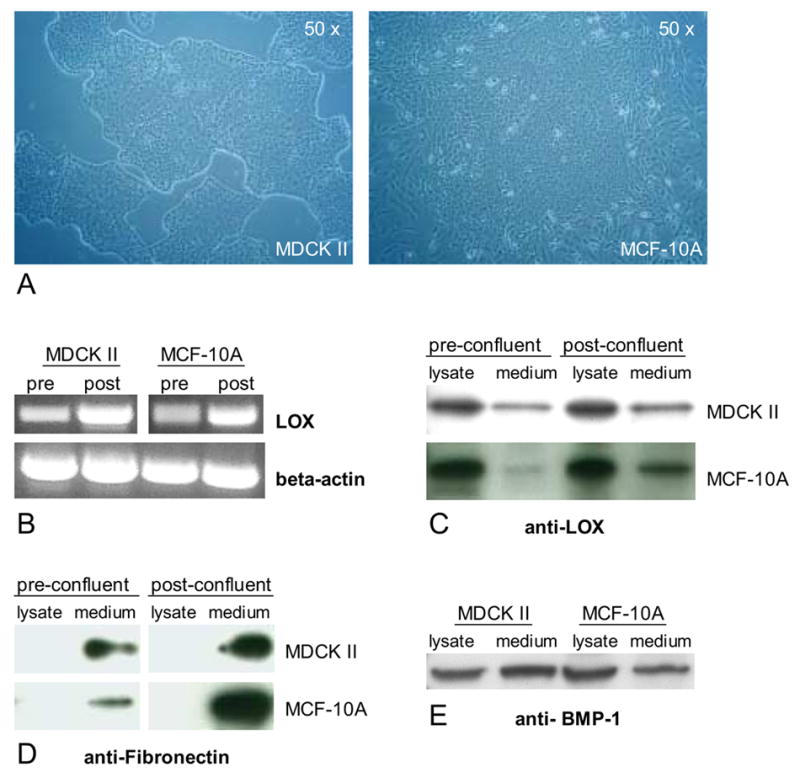
Expression of lysyl oxidase (LOX) in polarized epithelial cells. (A) MDCK II cells and MCF-10A cells display the characteristic “cobblestone” morphology of polarized epithelial cells in culture. (B) Detection of LOX mRNA transcripts in MDCK II cells and MCF-10A cells by RT-PCR. Western Blot analysis detected (C) mature (30 kD) LOX protein in cell lysates and medium fractions, (D) fibronectin only in medium fractions and (E) mature (70 kD) BMP-1 in cell lysates and medium fractions of MDCK II cells and MCF-10A cells.
It has been established that cultured epithelial cells in a pre-confluent stage reflect many properties of undifferentiated epithelia in vivo whereas post-confluent monolayers possess key features of differentiated epithelia in vivo (Simons and Fuller, 1985). We first analysed LOX expression in pre-confluent and post-confluent cultures of MDCK II and MCF-10A cells. Initially, we examined LOX expression at the mRNA level and detected LOX transcripts by semiquantitative RT-PCR in each cell line (Fig. 1B). LOX mRNA levels seemed to be slightly elevated in post-confluent cells compared to those of pre-confluent cultures. To determine LOX protein expression, we subjected cell lysates as well as extracellular medium fractions to Western blot analysis. LOX is known to be synthesized as a glycosylated 50 kD inactive pro-enzyme that is secreted into the extracellular space and subsequently processed by Bone Morphogenic Protein 1 (BMP-1) into the 30 kD active form (Smith-Mungo and Kagan, 1998). We detected mature 30 kD LOX in conditioned cell medium especially from post-confluent cultures in both cell lines (Fig. 1C), suggesting that cells in differentiated epithelial tissues might contribute to LOX-catalyzed crosslinking of ECM fibrils at their underlying basal surfaces and basement membranes. Surprisingly, we detected even higher amounts of 30 kD LOX in cell lysates that were cleared from nuclei (Fig. 1C), indicating the presence of mature LOX in the cytoplasm of these cells. Interestingly, the amount of 30 kD LOX in cell lysates is approximately equal within pre-and post-confluent cells, implying that intracellular LOX expression may be independent of the differentiation state in epithelial cells. Potential extracellular contamination in cell lysates seemed to be unlikely as fibronectin was exclusively detected in medium fractions (Fig. 1D). Consistent with these results is the presence of BMP-1 in both, cell lysates and medium fractions of MDCK II and MCF-10A cells (Fig. 1E). Using immunofluorescence we also observed intense cytoplasmic but not nuclear LOX staining in MDCK II cells, reinforcing our previous observations made by Western blot analysis (Fig. 2C, arrowheads indicate nuclei that were also counterstained with DAPI, not shown).
Figure 2.
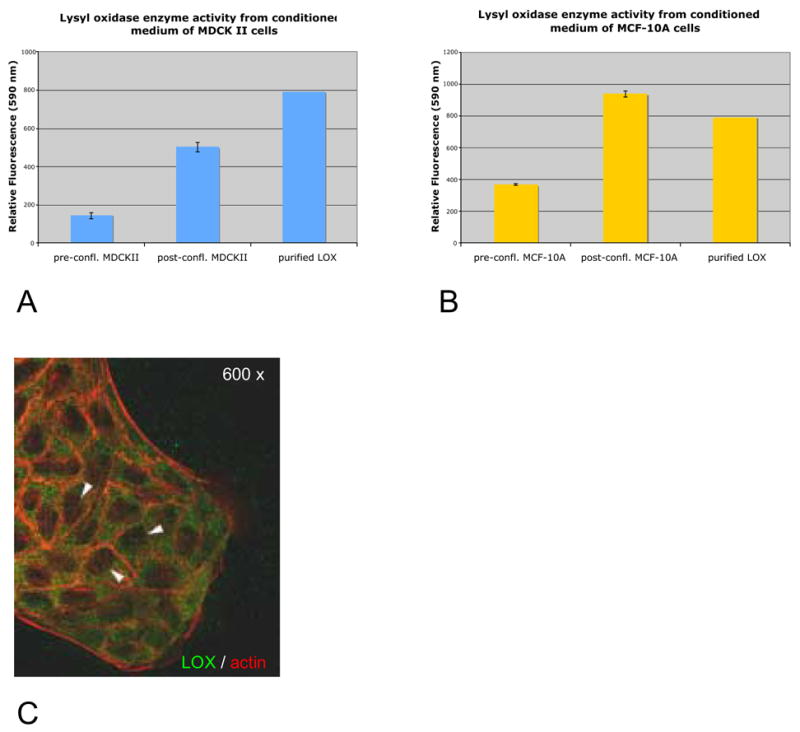
(A, B), BAPN-inhibitable lysyl oxidase enzyme activity in concentrated medium fractions of MDCK II cells and MCF-10A cells. (C) Immunofluorescence staining of LOX in the cytoplasm of MDCK II epithelial cells; arrowheads indicate nuclei (green = LOX, red = Phalloidin-TRITC).
We further investigated if mature LOX secreted by MDCK II and MCF-10A cells exhibits enzyme activity. We detected significant activity especially in medium fractions from post-confluent cultures of both cell lines (Fig. 2A, B). Notably, although on Western blots we observed the highest amounts of mature LOX in cytoplasmic fractions, we have not been able to detect lysyl oxidase enzyme activity from cell lysates yet (data not shown). It might be possible that different assay conditions are required for the detection of intracellular LOX activity. But these results could also indicate the possibility for a novel function of LOX within epithelial cells that is independent of its amine oxidase activity.
It has been recently shown in vitro and in vivo that LOX is essential for breast epithelial tumor cells to acquire a mesenchymal and therefore invasive phenotype (Payne et al., 2005, Erler et al., 2006). In this respect it is of special interest that the epithelial phenotype of MDCK II cells changes towards a fibroblast-like morphology after stable overexpression of LOX (M. Jansen and K. Csiszar, unpublished results). Furthermore, after induction of scattering in MDCK II cells, a process that reflects key features of epithelial-mesenchymal transition (EMT), the expression of intracellular 30 kD LOX is significantly upregulated (M. Jansen and K. Csiszar, unpublished results). Future studies will aim to decipher precisely the role(s) of LOX in epithelial cells and thereby provide important insights into potentially novel functions of LOX that have significant implications for diseases of epithelial tissues.
Experimental Procedures
Cell culture
MDCK II cells (dog kidney epithelial cells) were grown in DMEM low glucose (Invitrogen) supplemented with 10 % FBS (Mediatech) and 1 × Antibiotic-Antimycotic (Invitrogen). MCF-10A cells (human mammary epithelial cells) were grown in DMEM/F12 supplemented with 5% horse serum (Invitrogen), 20 ng/ml EGF (Peprotech), 500 ng/ml hydrocortisone (Sigma), 100 ng/ml Cholera toxin (Sigma), 10 μg/ml Insulin (Sigma) and 1 × Antibiotic-Antimycotic (Invitrogen).
RT-PCR
Total RNA was extracted using RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. For each sample 2 μg of RNA were processed for cDNA synthesis with first strand cDNA Kit (Invitrogen). PCR amplifications on MDCK II templates were performed with primers against hLOX amplifying a fragment of 529 bp (Peinado et al., 2005) and β-actin primers (Agudo et al., 2004) for 35 cycles at 62°C annealing temperature. PCR amplification on MCF-10A templates was performed with primers against hLOX amplifying a fragment of 324 bp (Kirschmann et al., 2002) and β-actin primers (Agudo et al., 2004) for 35 cycles at 59°C annealing temperature.
Western blot analysis
Pre-confluent (70–80 % confluency) or post-confluent (48 h after 100 % confluency) monolayers of each cell line were cultured for 48 h in serum-free medium prior to protein harvest. Conditioned cell medium was prepared as previously described (Fogelgren et al., 2005). Cells were lysed in M-PER Mammalian Protein Extraction Reagent (Pierce). Nuclei and cell fragments were pelleted for 10 min. at 1000 RCF. The supernatant (crude cytoplasmic fraction) was collected for Western blot analysis. Briefly, protein samples (20 μg each) were separated by SDS-PAGE on 4–12% NuPAGE Bis-Tris gradient gels (Invitrogen) and transferred onto PVDF membranes in a X-Cell Tank Blot unit (Invitrogen). Protein transfer was verified by Ponceau staining. Blot membranes were blocked in PBS-T containing 5 % non-fat dry milk prior to antibody incubations. Chemiluminescence detection was performed using ECL plus (Amersham).
Immunofluorescence staining
MDCK II cells were seeded overnight on glass cover slips in six-well plates resulting in an 80% confluent monolayer the next day. Cells were fixed in 2 % paraformaldehyde, permeabilized in 0.1 % Triton-X-100 and blocked in 3 % BSA prior to antibody incubation. Primary antibody incubation was applied for 1 h at RT followed by 45 min. incubation with the secondary antibody. Subsequently, the actin cytoskeleton was stained for 20 min. using Phalloidin-TRITC (Molecular Probes, Eugene). Finally, cells were mounted in anti-fading fluorescence mounting medium (Molecular Probes, Eugene). Slides were analysed with a Zeiss LSM Pascal confocal microscope.
Antibodies
LOX was detected using a rabbit polyclonal antibody against the N-terminus of the mature protein, seven amino acids down from the BMP-1 cleavage site (Li et al., 2004; Hayashi et al., 2004). Fibronectin expression was detected with a mouse monoclonal antibody (sc-18827, Santa Cruz Biotechnology) against the adhesive peptide FN CH/1 within the carboxy Hep II region. Expression of BMP-1 was detected with a rabbit polyclonal antibody against the CUB-2 Domain (Affinity Bioreagents).
Assay for lysyl oxidase enzyme activity
Activity assays for detection of BAPN-inhibitable lysyl oxidase enzyme activity were performed as previously described (Fogelgren et al., 2005). Briefly, conditioned cell medium was concentrated in sequential centrifugation steps using Amicon 10 kD cut-off filters (Millipore, Bedford). Aliquots equal to 100 μg of protein each were added to the final reaction mix (50 mM sodium borate pH 8.2, 1.2 M urea, 50 μM Amplex Red, 0.1 units/ml horseradish peroxidase, 10 mM 1,5-diaminopentane substrate) in the presence or absence of 500 μM BAPN (β-Aminoproprionitrile) and incubated at 37°C. The fluorescent product was excited at 560 nm and the emission was read at 590 nm every five minutes for three hours using a BMG Labtechnologies Inc. Polarstar Optima plate reader. Activity assays were repeated three times and samples for each experiment were performed at least in triplicate.
Acknowledgments
This work was supported by NIH grants RR03061 and AR47662.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agudo D, Gomez-Esquer F, Martinez-Arribas F, Nunez-Villar MJ, Pollan M, Schneider J. Nup88 mRNA overexpression is associated with high aggressiveness of breast cancer. Int J Cancer. 2004;109:717–720. doi: 10.1002/ijc.20034. [DOI] [PubMed] [Google Scholar]
- Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- Erler JT, Bennewith KL, Nicolau M, Dornhoefer N, Kong C, Le QT, Ashley Chi JT, Jeffrey SS, Giaccia AJ. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- Fogelgren B, Polgar N, Molnarne Szauter K, Ujfaludi Z, Laczko R, Fong KS, Csiszar K. Cellular fibronectin binds to lysyl oxidase with high affinity and is critical for its proteolytic activation. J Biol Chem. 2005;280:24690–24697. doi: 10.1074/jbc.M412979200. [DOI] [PubMed] [Google Scholar]
- Fong KS, Fogelgren B, Fong SF, Csiszar K. Lysyl oxidase. AfCS-Nature Molecule pages. 2006 doi: 10.1038/mp.a002989.01. [Google Scholar]
- Hayashi K, Fong KS, Mercier F, Boyd CD, Csiszar K, Hayashi M. Comparative immunocytochemical localization of lysyl oxidase (LOX) and the lysyl oxidase-like (LOXL) proteins: changes in the expression of LOXL during development and growth of mouse tissues. J Mol Histol. 2004;35:845–55. doi: 10.1007/s10735-004-2340-1. [DOI] [PubMed] [Google Scholar]
- Kagan HM, Li W. Lysyl Oxidase: Proerties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88:660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- Kirschmann DA, Seftor EA, Nieva DRC, Mariano EA, Hendrix MJC. Differentially expressed genes associated with the metastatic phenotype in breast cancer. Breast Cancer Res Treat. 1999;55:127–136. doi: 10.1023/a:1006188129423. [DOI] [PubMed] [Google Scholar]
- Kirschmann DA, Seftor EA, Fong SFT, Nieva DRC, Sullivan CM, Edwards EM, Sommer P, Csiszar K, Hendrix MJC. A molecular role for Lysyl Oxidase in breast cancer invasion. Cancer Res. 2002;62:4478–4483. [PubMed] [Google Scholar]
- Li PA, He Q, Cao T, Yong G, Szauter KM, Fong KS, Karlsson J, Keep MF, Csiszar K. Up-regulation and altered distribution of lysyl oxidase in the central nervous system of mutant SOD1 transgenic mouse model of amyotrophic lateral sclerosis. Brain Res Mol Brain Res. 2004;120:115–22. doi: 10.1016/j.molbrainres.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Noblesse E, Cenizo V, Bouez C, Borel A, Gleyzal C, Peyrol S, Jacob MP, Sommer P, Damour O. Lysyl oxidase-like and lysyl oxidase are present in the dermis and epidermis of a skin equivalent and in human skin and are associated to elastic fibers. J Invest Dermatol. 2004;122:621–630. doi: 10.1111/j.0022-202X.2004.22330.x. [DOI] [PubMed] [Google Scholar]
- Omori K, Fujiseki Y, Omori K, Suzukawa J, Inagaki C. Regulation of the expression of lysyl oxidase mRNA in cultured rabbit retinal pigment epithelium cells. Matrix Biol. 2002;21:337–348. doi: 10.1016/s0945-053x(02)00013-6. [DOI] [PubMed] [Google Scholar]
- Payne SL, Fogelgren B, Hess AR, Seftor EA, Wiley EL, Fong SFT, Csiszar K, Hendrix MJC, Kirschmann DA. Lysyl oxidase regulates breast cancer cell migration and adhesion through a hydrogen peroxide-mediated mechanism. Cancer Res. 2005;65:11429–11436. doi: 10.1158/0008-5472.CAN-05-1274. [DOI] [PubMed] [Google Scholar]
- Peinado H, Iglesias-de la Cruz MC, Olmeda D, Csiszar K, Fong KSK, Vega S, Nieto MA, Cano A, Portillo F. A molecular role for Lysyl oxidase-like 2 enzyme in Snail regulation and tumor progression. EMBO J. 2005;24:3446–3458. doi: 10.1038/sj.emboj.7600781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Fuller SD. Cell surface polarity in epithelia. Ann Rev Cell Biol. 1985;1:243–288. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- Yeaman C, Grindstaff KK, Nelson WJ. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol Rev. 1999;79:73–98. doi: 10.1152/physrev.1999.79.1.73. [DOI] [PubMed] [Google Scholar]


