Abstract
Objective
To assess the interactions among three types of pathology (ie, cerebrovascular disease, hippocampal sclerosis [HS], and Alzheimer’s disease [AD]), cognitive status, and apolipoprotein E genotype.
Methods
We report clinicopathological correlations from 79 autopsy cases derived from a prospective longitudinal study of subcortical ischemic vascular disease and AD.
Results
Thirty percent of the cases had significant cerebrovascular parenchymal pathology scores (CVDPS), 54% had significant AD pathology, and 18% had HS. In an ordinal logistic regression analysis that included interaction terms to assess the effects of each pathological variable when the other variables are interpolated to zero, each of the three pathology variables contributed independently to cognitive status: Braak and Braak stage odds ratio (OR) = 2.84 (95% confidence interval, 1.81–4.45), HS score OR = 2.43 (95% confidence interval, 1.01–5.85), and CVDPS OR = 1.02 (95% confidence interval, 1.00–1.04). Only Braak and Braak stage contributed to a global neuropsychological measure of cognitive impairment. Apolipoprotein E4 genotype was associated with Braak and Braak stage (OR, 1.31 [95% confidence interval, 1.03–1.68]), but not CVDPS or HS scores.
Interpretation
In this convenience sample enriched for subcortical ischemic vascular disease, HS was a common unsuspected neuropathological finding. Apolipoprotein E4 genotype was associated with cerebral amyloid angiopathy, but not HS or arteriosclerosis. When Braak and Braak stage was interpolated to zero, both CVDPS and HS contributed to cognitive impairment. However, advancing stages of AD pathology overwhelmed the effects of CVDPS and HS, to become the major determinant of dementia.
Cerebrovascular disease (CVD) is an important, but heterogeneous, cause of cognitive impairment and dementia. Several subtypes of ischemic vascular dementia (IVD) have been proposed, including multiinfarct dementia, strategic infarct dementia, Binswanger’s syndrome, and subcortical ischemic vascular dementia (SIVD). Recently, SIVD has been defined by the presence of dementia often with prominent dysexecutive rather than amnestic features, combined with hyperintensities in subcortical gray and white matter as visualized by proton density and T2-weighted magnetic resonance imaging (MRI).1,2 (In this article, CVD and sCVD refer to pathological findings, whereas IVD and SIVD refer to clinical diagnoses.)
Several mechanisms have been postulated whereby subcortical cerebrovascular disease (sCVD) may cause or contribute to progressive cognitive impairment. According to the lacunar hypothesis, infarcts that are strategically located in frontal-subcortical loops may lead to abrupt changes in cognition and behavior.3 In Binswanger’s syndrome, hypoperfusion and demyelination of the deep white matter are postulated to cause slowly progressive cognitive impairment, gait disturbance, and urinary incontinence.4 In most cases of slowly progressive dementia, however, many investigators posit occult Alzheimer’s disease (AD), rather than lacunes, as the predominant cause of dementia.5 Mixed or combined contributions have also been proposed, with CVD and AD pathological changes contributing independently to dementia.6,7 Further elucidation of the cognitive impact of combined AD and CVD pathology depends on autopsy studies, because histological examination remains the best method for ascertaining the extent and severity of microscopic AD and CVD pathological alterations within the brain.
A major goal of the Ischemic Vascular Dementia (IVD) program project (PO1-AG12435) is to elucidate how CVD leads to cognitive impairment, either alone or in combination with AD. In this research project, individuals with cognitive impairment attributed to SIVD or AD, as well as cognitively normal (CN) elderly subjects, are followed longitudinally to autopsy with repeat neuropsychological testing and structural MRI studies.
Previous analyses from this project have shown that MR-assessed volumes of the hippocampus and cortical gray matter are stronger predictors of cognitive impairment and cognitive decline than are MR-assessed volumes of white matter hyperintensities and lacunes.8–10 The spectrum of pathological changes observed in the first 20 autopsy cases (eg, including frequent cortical microinfarcts and hippocampal sclerosis [HS])11 and the neuropsychological correlates for the first 46 autopsy cases12 have been published previously. We now report data from the first 79 consecutive autopsies regarding (1) correlations between clinical diagnosis and pathological findings, and (2) the relative contributions of CVD, AD pathology, and HS to cognitive impairment.
Subjects and Methods
Sample
The sample reported here comprises 79 autopsy cases (included in the September 2004 neuropathology database), drawn from a longitudinal study of subjects with SIVD, subjects with AD, and CN elderly subjects. Among the first 83 subjects coming to autopsy, 2 cases had dementia with Lewy bodies and 2 cases had frontotemporal lobe dementia; these 4 cases were excluded from this report. The autopsy cases were drawn from a total sample of 627 subjects, of whom 128 were deceased (autopsy rate, 64%).
Subjects with cognitive impairment and dementia were recruited mainly from university-affiliated memory clinics, whereas CN subjects were recruited from the community. The research project was approved by the institutional review boards at the University of Southern California, University of California Davis, University of California San Francisco, and Rancho Los Amigos National Rehabilitation Center. Written informed consent was obtained from all subjects or surrogate decision makers after institutional review board–approved protocols at each institution.
Initial evaluation included medical history, activities of daily living, physical examination, and neurological examination. Cognitive function was assessed using the Mini-Mental State Examination,13 Clinical Dementia Rating Scale (CDR),14 and neuropsychological examination. Laboratory studies included serum chemistry, blood count, vitamin B12, syphilis serology, thyroid function tests, and apolipoprotein E (ApoE) genotype. The entire brain was imaged with a 1.5-Tesla MR system (Siemen’s Vision [Siemens Medical Systems, South Iselin, NJ] or GE Signa [GE Healthcare, Chalfont St. Giles, United Kingdom,]) using a head coil with quadrature detection. The brain imaging protocol involved: (1) a sequence yielding proton density and T2-weighted spin-echo axial images, and (2) a sequence yielding T1-weighted coronal images with contiguous 1.4mm-thick slices. Lacunes were defined as discrete lesions larger than 2mm in diameter that were hyperintense relative to cerebrospinal fluid on proton density images, that is, slightly smaller than but otherwise similar to the lesions identified by the Cardiovascular Health Study criteria.15
Inclusion criteria included: age > 55 years, English speaking, CN, or cognitively impaired (CI; CDR ≤ 2) due to either SIVD or AD. Exclusion criteria included: severe dementia (CDR > 2), history of alcohol or substance abuse, head trauma with loss of consciousness of more than 15 minutes, severe medical illness, neurological or psychiatric disorders, or currently taking medications likely to affect cognitive function. Subjects were excluded if the initial clinical MRI showed evidence of cortical infarcts, hemorrhage, or structural brain disease other than atrophy, lacunes, or white matter lesions. They were retained in the study if cortical infarcts or hemorrhages developed subsequently.
Cognitive Status
Using clinical information obtained at the clinic visit closest to the time of death, we categorized subjects using the CDR into three categories: cognitively normal (CN), cognitively impaired (CI), or demented (D). The CDR was scored after interviewing a collateral informant regarding the subject’s function; the subject’s own performance on the Mini-Mental State Examination was also used in scoring the CDR. The CN group was defined as CDR = 0, CI group as CDR = 0.5, and D group as CDR ≥ 1.
Clinical Diagnosis
Patients with dementia were diagnosed as follows: (1) probable or possible AD, using National Institute of Neurological and Communication Disorders-Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria,16 and (2) probable or possible SIVD, using Alzheimer Disease Diagnostic and Treatment Centers criteria17 (except in this study, cortical infarcts were excluded). There were three clinical diagnostic categories for dementia: (1) AD, (2) SIVD, and (3) mixed AD/SIVD. Mixed dementia was diagnosed when clinicians believed that both AD and SIVD contributed significantly to the cognitive loss. If there was a history of slowly progressive memory loss, a diagnosis of probable AD, possible AD, or mixed AD/SIVD was made. CI cases were divided into two groups, those with lacunes on MRI (CI-CVD) and those without (CI-AD).
Global Cognition Score
Several specific tests were used to derive a measure of global cognitive ability (Global Cognition)18 that had desirable psychometric properties for evaluation of longitudinal change in this study. Donor tests were: (1) total recall on trials 1 and 2 on the Word List Learning Test of the Memory Assessment Scales, (2) Digit Span forward and backward from the Wechsler Memory Scale–Revised, (3) letter fluency (the letter “A” from the “FAS” test), and (4) animal category fluency. These tests were selected because they broadly assess cognitive domains relevant to AD and SIVD, have a broad range of measurement without appreciable floor or ceiling effects, can be administered quickly, and can be used even for patients with relatively severe dementia, as reported in previous publications.10,18
Neuropathological Evaluation
After fixation in 10% neutral buffered formalin for at least 2 weeks, each cerebral hemisphere was sectioned coronally, at 5mm thickness, using a rotary slicer. All macroscopic infarcts were measured, photographed, blocked for microscopic examination, and summarized as part of the CVD pathology score (described later). Tissue was obtained from 12 standardized regions in 1 cerebral hemisphere according to the “IVD blocking protocol.” The IVD protocol includes sections from the anterior and posterior deep white matter, in addition to the combined sections recommended by the Consortium to Establish a Registry for Alzheimer Disease (CERAD),19 the National Institute on Aging and Reagan Institute Working Group,20 and the Consensus Conference on Dementia with Lewy bodies.21
Tissue blocks were dehydrated through graded alcohols, embedded in paraffin, sectioned at 10μm thickness, and stained with hematoxylin and eosin, cresyl violet, Congo red, and Bielschowsky silver stain. At the pathologist’s discretion, certain cases were also immunolabeled using antibodies against α-synuclein, ubiquitin, glial fibrillary acidic protein, phosphorylated tau, and β-amyloid. Each case was reviewed at Consensus Neuropathology Conference, which included two Board-certified neuropathologists (H.V.V., W.G.E.) who were blinded to the clinical diagnoses and ApoE genotype. For each case, Braak and Braak stage,22 CERAD-neuritic plaque score,19 and Lewy body score21 were assigned at the Consensus Conference. Severity, grade, or extent of cerebral amyloid angiopathy,23 atherosclerosis, and arteriolosclerosis were each rated on a four-point scale (zero to three points). The atherosclerosis and arteriolosclerosis ratings were combined as an arteriosclerosis score (zero to six points). The severity of cerebrovascular ischemic brain injury was rated using a new CVD pathology scoring system developed within this project. Acute infarcts or hemorrhages near the time of death were noted, but were not included in the CVD pathology rating score.
The CVD pathology rating sheet (Fig 1) includes measures for (1) HS; (2) numbers and location of cystic infarcts, lacunar infarcts, microinfarcts in gray and white matter regions; and (3) white matter demyelination (not used in these analyses). Focal neuronal loss with gliosis in the CA1 sector of hippocampus, without evidence of adjacent neurofibrillary tangles, was called hippocampal injury and was rated in each available hemisphere on a scale from 0 to 3 (total possible score = 6). If neuronal loss extended over more than one hippocampal segment (eg, CA1 and subiculum) or over several coronal levels, it was termed “hippocampal sclerosis” (HS score ≥ 2). To avoid differential weighting, we created normalized subscores based on infarct size. Subscores for cystic infarcts, lacunar infarcts, and microinfarcts were created by summing the individual scores across all brain regions and normalizing to a scale of 0 to 100 (Fig 2). The three sub-scores were then summed to give a total cerebrovascular parenchymal pathology scores (CVDPS; 0–300).
Fig 1.
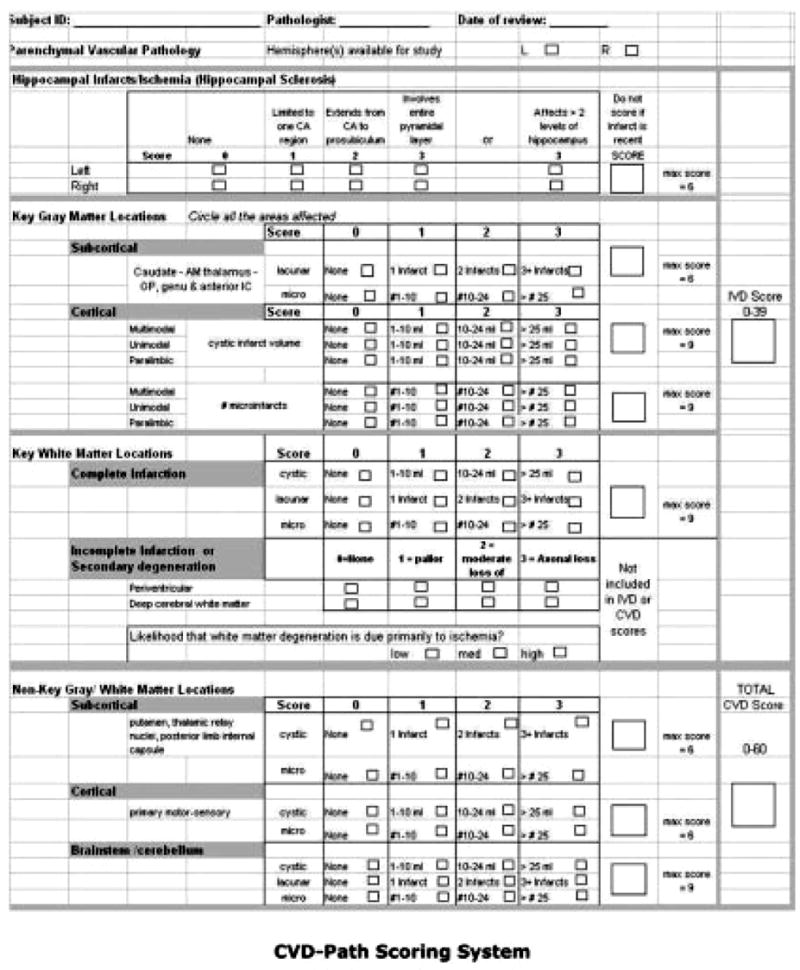
Cerebrovascular disease pathology scoring system.
Fig 2.
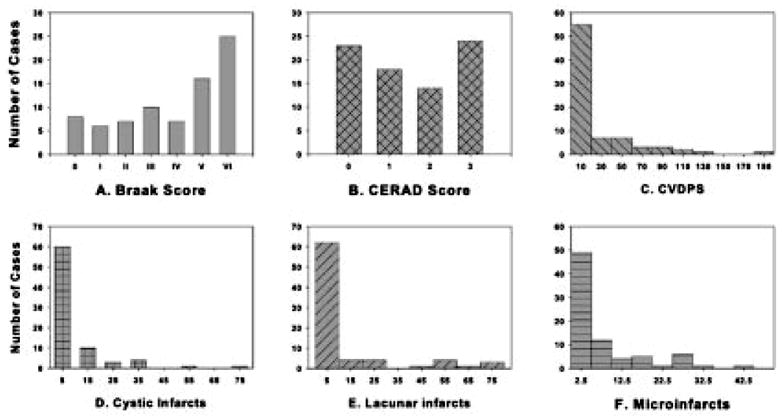
Distribution of pathology scores among 79 autopsy cases: (A) Braak and Braak stage (0–VI), (B) Consortium to Establish a Registry for Alzheimer Disease (CERAD) neuritic plaque score (0–3), (C) cerebrovascular parenchymal pathology scores (CVDPS; 0–300), (D) cystic infarct score (0–100), (E) lacunar infarct score (0–100), and (F) microinfarct score (0–100).
Pathological Diagnosis
For categoric analyses, cutoff scores were selected for Braak and Braak stage and CVDPS score to operationally define pathological subgroups (see Table 2). We considered a Braak stage ≥ IV, where AD is considered to be moderately likely by National Institute on Aging-Reagan criteria,20 to indicate AD. We used a CVDPS score ≥ 20, which divides the sample approximately into tertiles, as a cutoff score for CVD. (A CVDPS score ≥ 20 corresponds to a raw CVD pathology score of ≥ 4 [see Fig 1].) Because the maximum score for any single CVD pathology variable is 3 (see Fig 1), this cut point conceptually requires positive scores for at least two CVD pathology variables. Because both AD and CVD pathology reflect a continuous process, cutoff scores are admittedly arbitrary. Therefore, this article does not rely on categorical analyses for its major conclusions.
Table 2.
Distribution of Cerebrovascular Disease Pathology Scores among 79 Autopsy Cases by Four Pathological Diagnostic Categories
| CVDPS Scores | CVD: CVDPS ≥ 20, B&B < 4 | AD: CVDPS < 20, B&B ≥ 4 | AD/CVD: CVDPS ≥ 20, B&B ≥ 4 | NSP: CVDPS < 20, B&B < 4 |
|---|---|---|---|---|
| Median CVDPS (IQR) | 75 (41.7–88.9) | 4.2 (0–8.3) | 41.7 (37.5–44.5) | 0 (0–8.3) |
| Median cystic infarcts (IQR) | 16.7 (5.6–33.3) | 0 (0–0) | 11.1 (0–16.7) | 0 (0–0) |
| Median microinfarcts (IQR) | 12.5 (8.3–25) | 0 (0–4.2) | 16.7 (16.7–20.8) | 0 (0–8.3) |
| Median lacunar infarcts (IQR) | 41.7 (16.7, 50) | 0 (0, 0) | 0 (0, 25) | 0 (0, 0) |
| Hippocampal sclerosis ≥ 2, n (%) | 4 (27) | 4 (12) | 2 (22) | 4 (19) |
CVD = cerebrovascular disease; CVDPS = cerebrovascular disease parenchymal pathology scores; B&B = Braak and Braak stage; AD = Alzheimer’s disease; NSP = no significant pathologic abnormality; IQR = interquartile range.
Statistical Analyses
Sensitivity, specificity, and positive likelihood ratios were calculated in the subset of 53 cases with dementia, using the pathological diagnoses described earlier. (Positive likelihood ratios were calculated as sensitivity divided by 1 minus the specificity.) In the sample as a whole, associations between the three pathology scores (Braak and Braak stage [0–VI], HS score [0–6], and CVDPS score [0–300]; independent variables) and global cognitive score (0–100; dependent variable) were assessed by multiple linear regression analysis. Associations among the three pathology scores and cognitive status evaluated at the closest time to death (CN, CI, or D) or ApoE genotype (presence or absence of an e4 allele) were assessed using ordinal and dichotomous logistic regression analyses, respectively. Models were adjusted for age, sex, ethnicity, and education for each of the regression analyses. Because the infarct score distributions show a significant right skew, secondary analyses were performed dividing the CVDPS into tertiles (eg, no infarct [n = 28], 0 < CVDPS < 20 [n = 27], and CVDPS ≥ 20 [n = 24]). Also, secondary analyses were performed analyzing each type of infarct separately (cystic infarct, lacune infarct, and microinfarct scores). All statistical testing was performed at a 5% level of significance using SAS version 8.0 (SAS Institute, Cary, NC).
Results
Demographics
The 79 cases comprised 45 male and 34 female cases (mean age at death, 82.8 years; standard deviation [SD], 7.0 years) of mixed ethnicity (68 white, 4 black, 5 Asian, and 2 Latino cases) (Table 1). Clinical syndromes at the time of the final clinic visit were CN (n = 13), CI (n = 13), and D (n = 53). Among the CI cases, there were 8 with MRI lacunes (CI-CVD) and 5 without lacunes (CI-AD). Among the dementia cases, the diagnoses at last clinic visit were AD (n = 20: 18 probable AD and 2 possible AD), IVD (n = 11: 10 probable SIVD and 1 possible SIVD), and mixed AD/SIVD (n = 22). The mean interval between the last clinic visit and death was 10.7 months (SD, 9.4 months). A history of symptomatic stroke was obtained in approximately half of the CI-CVD or SIVD cases (ie, approximately half of these cases had asymptomatic or “silent” lacunes on MRI). In comparisons between deceased cases with and without autopsy, subjects with a clinical diagnosis of SIVD were less likely to be autopsied than those clinically diagnosed with AD. Similarly, autopsy rates were lower among black compared with white cases. Autopsy cases were also older than nonautopsied cases. No differences in autopsy rates were found based on sex or education.
Table 1.
Demographic Data by Clinical Diagnosis Closest to Death (N = 79)
| Characteristics | CN (n = 13) | CI-CVD (n = 8) | CI-AD (n = 5) | SIVD (n = 11)a | AD (n = 20)b | Mixed AD/SIVD (n = 22) | pc |
|---|---|---|---|---|---|---|---|
| Mean age at death (SD), yr | 82.7 (5.6) | 79.4 (5.3) | 83.7 (6.7) | 84.6 (8.6) | 81.6 (8.5) | 84.2 (6.2) | 0.54 |
| Mean education (SD), yr | 13.7 (3.1) | 15.5 (2.7) | 15.8 (3.9) | 13.3 (2.8) | 15.0 (3.6) | 12.7 (3.6) | 0.14 |
| Mean duration of illness (SD), yr | — | 4.1 (4.5) | 3.5 (1.3) | 8.9 (3.9) | 9.2 (3.0) | 8.9 (5.2) | 0.03 |
| Total lacunar volume (%ICV) | 0.008 (0.013) | 0.099 (0.083) | 0.009 (0.019) | 0.076 (0.092) | 0.005 (0.014) | 0.059 (0.130) | 0.03 |
| WMSH (%ICV) | 1.1 (1.0) | 2.7 (1.8) | 1.9 (2.7) | 3.4 (2.9) | 0.9 (0.9) | 3.1 (2.3) | 0.002 |
| Mean time from last MRI to death (SD), yr | 2.8 (1.8) | 1.3 (1.0) | 1.0 (0.4) | 2.7 (2.2) | 3.1 (2.1) | 2.0 (1.4) | 0.06 |
| Mean global cognitive score (SD) | 94.7 (16.3) | 93.3 (15.1) | 88.6 (21.4) | 53.3 (20.5) | 46.7 (17.6) | 52.1 (18.1) | <0.0001 |
| Mean time from last neuropsychological testing to death (SD), yr | 1.2 (1.0) | 0.8 (0.9) | 0.8 (0.4) | 2.0 (2.0) | 2.1 (1.7) | 1.6 (0.9) | 0.12 |
| Mean time from last clinic visit to death (SD), yr | 1.4 (0.9) | 0.7 (1.0) | 1.4 (2.5) | 1.6 (1.4) | 1.0 (0.6) | 0.9 (0.6) | 0.28 |
| Sex, n (%) | 0.81 | ||||||
| Male | 6 (46.2) | 6 (75) | 3 (60) | 5 (45.5) | 12 (60) | 13 (59.1) | |
| Female | 7 (53.8) | 2 (25) | 2 (40) | 6 (54.5) | 8 (40) | 9 (40.9) | |
| Race, n (%) | 0.40 | ||||||
| White | 12 (92.3) | 7 (87.5) | 5 (100) | 8 (72.7) | 20 (100) | 16 (72.7) | |
| Hispanic | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (9.1) | |
| Black | 0 (0) | 1 (12.5) | 0 (0) | 1 (9.1) | 0 (0) | 2 (9.1) | |
| Asian | 1 (7.7) | 0 (0) | 0 (0) | 2 (18.2) | 0 (0) | 2 (9.1) | |
| ApoE E4 allele, n (%) | 0.10 | ||||||
| No | 10 (83.3) | 4 (50) | 3 (60) | 6 (66.7) | 5 (29.4) | 13 (59.1) | |
| Yes | 2 (16.7) | 4 (50) | 2 (40) | 3 (33.3) | 12 (70.6) | 9 (40.9) | |
| History of stroke, n (%) | 0.001 | ||||||
| No | 13 (100) | 4 (50) | 4 (80) | 5 (45.5) | 19 (95) | 15 (68.2) | |
| Yes | 0 (0) | 4 (50) | 1 (20) | 6 (54.5) | 1 (5) | 7 (31.8) |
SIVD includes probable (n = 10) and possible SIVD (n = 1).
AD subjects are composed of probable (n = 18) and possible AD (n = 2).
p value from analysis of variance for continuous variables and from Fisher’s exact test for categorical variables.
CN = cognitively normal; CI-CVD = cognitively impaired cases with lacunes on MRI; CI-AD = cognitively impaired cases without lacunes on MRI; SIVD = subcortical ischemic vascular dementia; AD = Alzheimer’s disease; SD = standard deviation; WMSH = white matter signal hyperintensities; MRI = magnetic resonance imaging; ApoE = apolipoprotein E; %ICV = % of intracranial volume.
Neuropathological Observations
Neurofibrillary tangles, neuritic plaques, ischemic lesions, and HS were common overlapping pathological findings. The distribution of Braak and Braak stage, CERAD scores, CVDPS, as well as the cystic infarct, lacunar infarct, and microinfarct subscores are shown for the entire sample in Figure 2. The percentage of subjects with infarcts in various brain regions were as follows: 11% had brainstem infarcts, 37% had subcortical gray matter infarcts, 24% had white matter infarcts, and 52% had cortical infarcts (including 44% with microinfarcts and 28% with cystic infarcts). Using cutoff scores to define “significant” pathology: 54% of subjects had significant AD pathology (Braak Stage ≥ IV), 30% of cases had CVDPS ≥ 20, and 18% had HS score ≥ 2. The highest CVDPS scores were found among the subjects with CI-CVD. The distribution of CVDPS, cystic infarct, lacunar infarct, and microinfarct subscores are shown by clinical diagnosis in Figure 3, and their median (interquartile range) are summarized by pathological diagnosis in Table 2. Figure 3 shows that among the three subtypes of infarcts, microinfarcts are the most likely to be found at autopsy among subjects diagnosed clinically as AD.
Fig 3.
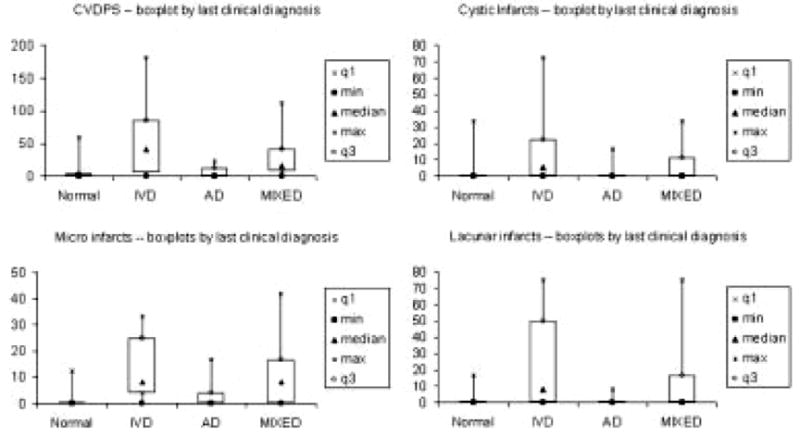
Distribution of cerebrovascular parenchymal pathology scores (CVDPS) and infarct subscores by last clinical diagnosis: minimum, first quartile q1, median, third quartile q3, maximum. AD = Alzheimer’s disease; IVD = ischemic vascular dementia.
Braak and Braak stage and CERAD scores were significantly correlated with each other (Spearman’s r = 0.85; p < 0.0001). Therefore, only the analyses using Braak and Braak stage are reported in this study. Severity of atherosclerosis and arteriolosclerosis correlated with CVDPS (atherosclerosis: Spearman’s r = 0.62, p < 0.0001; arteriolosclerosis: r = 0.53, p < 0.0001), whereas cerebral amyloid angiopathy correlated with Braak and Braak stage (Spearman’s r = 0.45, p < 0.0001). Braak and Braak stage and CVDPS were not correlated. The infarct subscores were intercorrelated: cystic-infarct and microinfarct Spearman’s r = 0.43 ( p < 0.0001); cystic- and lacunar-infarct Spearman’s r = 0.40 ( p < 0.0002); microinfarct and lacunar-infarct Spearman’s r = 0.53 ( p < 0.0001).
Clinicopathological Correlations
We used Braak stage ≥ IV and CVDPS ≥ 20 to define four pathological subgroups: 15 CVD, 34 AD, 9 mixed AD/CVD, and 21 no significant AD or CVD pathology (see Table 3). For the subset of 53 dementia cases, we calculated sensitivities, specificities, and positive likelihood ratios. For the clinical diagnosis of AD (n = 20), sensitivity was 58.1%, specificity was 90.9%, and positive likelihood ratio was 6.4. For a clinical diagnosis of SIVD (n = 11), sensitivity was 57.1%, specificity was 84.7%, and positive likelihood ratio was 3.7. For a clinical diagnosis of mixed AD/SIVD (n = 22), sensitivity was 77.8%, specificity was 65.9%, and the positive likelihood ratio was 2.3. Clinical diagnoses for the 14 cases with HS ≥ 2 were: 1 CN, 4 IVD (2 CI-CVD + 2 SIVD), 3 AD (1 CI-AD + 2 AD), and 6 mixed AD/SIVD. Figure 4 shows that HS can be found at autopsy among subjects from all clinical diagnostic categories.
Table 3.
Concordance between Clinical and Pathologic Diagnoses in 79 Autopsy Cases: Four Pathological Diagnostic Categories
| Pathological/Clinical Diagnoses | CVD: CVDPS ≥ 20, B&B < 4 | AD: CVDPS < 20, B&B ≥ 4 | Mixed AD/CVD: CVDPS ≥ 20, B&B ≥ 4 | NSP: CVDPS < 20, B&B < 4 |
|---|---|---|---|---|
| Normal (n = 13) | 1 | 2 | 0 | 10 |
| CI-CVD (n = 8) | 6 | 0 | 0 | 2 |
| CI-AD (n = 5) | 1 | 1 | 0 | 3 |
| SIVD (n = 11) | 4 | 5 | 1 | 1 |
| AD (n = 20) | 0 | 18 | 1 | 1 |
| Mixed AD/SIVD (n = 22) | 3 | 8 | 7 | 4 |
| Total (n = 79) | 15 | 34 | 9 | 21 |
CVD = cerebrovascular disease; CVDPS = cerebrovascular disease parenchymal pathology scores; B&B = Braak and Braak stage; AD = Alzheimer’s disease; CI = cognitive impairment not meeting criteria for dementia; SIVD = subcortical ischemic vascular dementia; mixed AD/SIVD = clinical diagnosis of mixed cases; NSP = no significant pathologic abnormality, clinical diagnosis of AD = 19 probable AD + 2 possible AD = possible AD, clinical diagnosis of SIVD = 10 probable SIVD + 1 possible SIVD.
Fig 4.
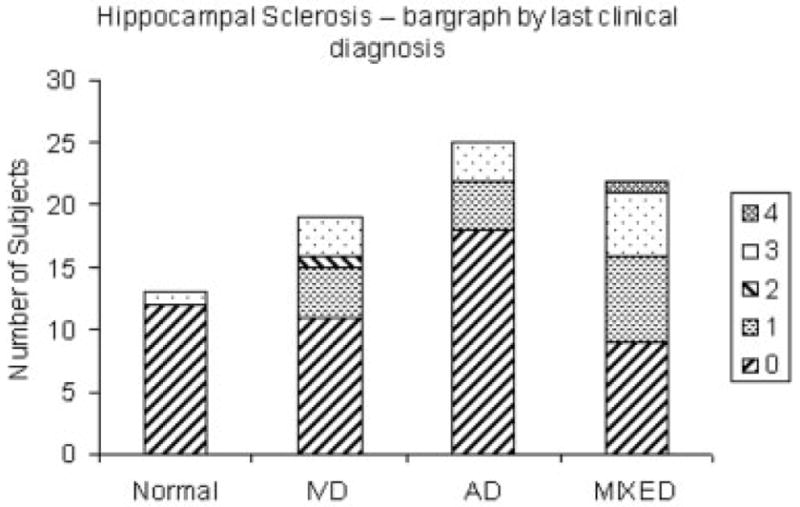
Distribution of hippocampal sclerosis scores by diagnosis at last clinic visit. (Ischemic vascular dementia [IVD] includes cognitively impaired cerebrovascular disease [CI-CVD] and subcortical ischemic vascular dementia [SIVD]; Alzheimer’s disease [AD] includes CI-AD and AD.)
Correlations between Pathology Scores and Global Cognition
The mean interval between the last neuropsychological assessment to death was 1.6 (SD, 1.4) years. In a linear multiple regression analysis, only Braak and Braak stage (not CVDPS or HS) was a significant correlate of the global cognitive score. This remained the case in secondary analyses using CVDPS categorized by tertiles and using infarct subtype scores (Table 4).
Table 4.
Linear Regression Evaluating the Association between Neuropathological Variables and Global Cognitive Score at Visit Closest to Death (n = 79)
| Independent variables | β | Standard Error | p |
|---|---|---|---|
| Intercept | 92.22 | 6.17 | <0.0001 |
| CVDPS | −0.04 | 0.08 | 0.64 |
| Hippocampal sclerosis score | 2.04 | 2.38 | 0.39 |
| Braak and Braak stage | −7.55 | 1.34 | <0.0001 |
Mean (standard deviation) of time from last neuropsychological test to death was 1.6 (1.4) years. Model was adjusted for age, education, sex, and race.
CVDPS = cerebrovascular disease parenchymal pathology scores.
Correlations between Pathology and Cognitive Status
The mean interval from the last clinic visit to death was 1.1 (SD, ±1.0) years. Ordinal logistic regression analyses were performed with cognitive syndrome (ie, CN, CI, and D) at the time of the last clinic visit as the dependent variable. Each of the three pathology scores (Braak and Braak stage, CVDPS, and HS) were simultaneously modeled as independent variables. In the main-effects model (Table 5, top), only Braak and Braak stage was significantly associated with cognitive syndrome (odds ratio [OR], 2.03 [95% confidence interval, 1.51–2.73]), with higher scores associated with higher likelihood of CI and D.
Table 5.
Ordinal Logistic Regression Evaluating Association between Neuropathological Findings and Cognitive Status Closest to Death (N = 79)
| Independent variables | OR (95% CI) | p |
|---|---|---|
| Main-effects model | ||
| CVDPS score | 1.01 (1.00–1.03) | 0.16 |
| HS score | 1.29 (0.77–2.15) | 0.33 |
| Braak and Braak stage | 2.03 (1.51–2.73) | <0.0001 |
| Interaction model | ||
| CVDPS score | 1.02 (1.00–1.04) | 0.07 |
| HS score | 2.43 (1.01–5.85) | 0.048 |
| Braak and Braak stage | 2.84 (1.81–4.45) | <0.0001 |
| CVDPSa Braak and Braak stage | 0.99 (0.98–1.00) | 0.13 |
| HS scorea Braak and Braak stage | 0.83 (0.65–1.05) | 0.13 |
| Age at death | 0.97 (0.88–1.06) | 0.49 |
OR = odds ratio; CI = confidence interval; CVDPS = cerebrovascular disease parenchymal pathology scores; HS = hippocampal sclerosis.
We then added pathology interaction terms (CVDPS × Braak and Braak stage, HS × Braak and Braak stage) to the model to test whether associations of CVDPS or HS with cognitive syndrome varied by Braak and Braak stage. Though not statistically significant, trends were noted in the pathology interaction terms (CVDPS score × Braak and Braak stage, p = 0.13; HS score × Braak and Braak Stage, p = 0.13). Because the sample size was small, we included the interaction terms in the logistic regression model (see Table 5, bottom). The main effect for Braak and Braak stage (OR, 2.84 [95% confidence interval, 1.81–4.45] per unit Braak and Braak stage) is the association between Braak and Braak stage and cognition among subjects with CVDPS score = 0 and HS score = 0.
In the interaction model, the main-effect term for CVDPS (OR, 1.02 [95% confidence interval, 1.00–1.04] per unit of CVDPS is the OR associated with CVDPS among subjects with Braak and Braak stage = 0. The estimate for the interaction term was essentially equal to 1 (CVDPS × Braak and Braak stage OR = 0.99), suggesting that effects of CVDPS and AD pathology are additive, rather than synergistic. Similarly, the main-effect OR of 2.43 (95% confidence interval, 1.01–5.85) per unit of the HS score represents the association between HS score and cognition among subjects with Braak and Braak stage = 0. The interaction between HS score and Braak and Braak stage was less than 1 (OR = 0.83), indicating that the association between HS and cognition is reduced as AD pathology increases.
Because the infarct score distributions (see Fig 2) show a significant right skew, secondary analyses were performed by dividing CVDPS into three categories by approximate tertiles (no infarcts, <20, and ≥ 20), keeping Braak and Braak stage and HS scores as continuous variables. In the main-effects model, the highest CVDPS group (CVDPS ≥ 20) was significantly associated with poorer cognitive status (OR, 5.91 [95% confidence interval, 1.38–25.30]; p < 0.02), as was Braak and Braak stage (OR = 2.13 [95% confidence interval, 1.56–2.90]). In the interaction model, increasing severity of CVD pathology was again associated with cognitive impairment in the absence of AD pathology. Secondary analyses were also performed using the infarct subtype scores. In the main-effects model, none of these subscores contributed significantly to cognitive status. In the interaction model, the main-effects terms for microinfarcts and lacunar infarcts, but not cystic infarcts, became significant when Braak and Braak stage was interpolated to zero (data not shown). Thus, the secondary analyses were consistent with and strengthened the primary findings.
Apolipoprotein E
ApoE genotype was available for 73 subjects. The e4 allele was more frequent among subjects with AD than SIVD (see Table 1). Specifically, ApoE e4 was positively associated with Braak and Braak stage (univariate regression: OR, 1.35 [95% confidence interval, 1.07–1.72]; p = 0.01) and cerebral amyloid angiopathy (univariate regression: OR, 2.3 [95% confidence interval, 1.4–3.77]; p < 0.001). In multivariate regression analyses (Table 6), ApoE e4 was associated with only cerebral amyloid angiopathy (OR, 2.16 [95% confidence interval, 1.21–3.85]; p < 0.01), and no longer with Braak stage. ApoE genotype was not associated with arteriosclerosis, CVDPS, or HS. Secondary analyses using CVDPS tertiles and infarct subscores did not change the findings.
Table 6.
Logistic Regression Evaluating the Association between Neuropathological Findings and Apolipoprotein E4 Allele (N = 73)
| Independent Variables | OR (95% CI) | p |
|---|---|---|
| Cerebral amyloid angiopathy | 2.16 (1.21–3.85) | 0.009 |
| Arteriosclerosis | 0.94 (0.85–1.04) | 0.21 |
| Braak and Braak stage | 1.20 (0.89–1.62) | 0.23 |
| CVDPS | 1.0 (0.98–1.01) | 0.61 |
| HS score | 1.14 (0.60–1.9) | 0.61 |
Model was adjusted for age, education, sex, and race.
OR = odds ratio; CI = confidence interval; CVDPS = cerebrovascular disease parenchymal pathology scores; HS = hippocampal sclerosis.
Discussion
This clinicopathological study focuses on the relationship among three types of pathology (ie, AD, CVD, and HS), cognitive impairment, and ApoE genotype. We used a novel CVDPS score to summarize the severity of CVD-related brain injury. SIVD was clinically defined by the presence of discrete, but often clinically silent, subcortical hyperintensities on proton density MRI. In community-based studies, asymptomatic hyperintensities are found in 21 to 28% of elderly subjects and are established risk factors for stroke.24,25 Despite their common prevalence, the contribution of MRI-identified hyperintensities to cognitive impairment remains controversial,4–7 and the presence of confounding AD remains undetermined until autopsy.
Using cutoff scores to define “significant” levels of CVD, AD, and HS pathology (see Table 3), we found that 30% of cases had significant CVD pathology (CVDPS ≥ 20), 54% had significant AD pathology (Braak and Braak stage ≥ IV), and 18% had HS (HS score ≥ 2). There were 15 cases (19%) with “pure” CVD pathology and another 9 cases (11%) with mixed AD/CVD pathology. Although we excluded cases with evidence of cortical infarcts on MRI at the outset, 52% of the cases had cortical infarcts (including 44% with microinfarcts and 28% with cystic infarcts) at the time of autopsy. Thus, pure sCVD cases were relatively rare, and there was often an admixture of large- as well as small-vessel disease, cortical as well as subcortical infarcts, AD, and HS.
Among the subset of 53 dementia cases, sensitivity, specificity, and positive likelihood ratios were calculated. For the clinical diagnosis of AD by NINCDS-ADRDA criteria,16 sensitivity was 58.1%, specificity was 90.9%, and positive likelihood ratio was 6.4. For a clinical diagnosis of SIVD by Alzheimer Disease Diagnostic and Treatment Centers criteria17 (applied after the exclusion of cases with cortical infarcts), sensitivity was 57.1%, specificity was 84.7%, and positive likelihood ratio was 3.7. For a clinical diagnosis of mixed AD/SIVD, sensitivity was 77.8%, specificity was 65.9%, and the positive likelihood ratio was 2.3. These values and rank order are comparable with the positive likelihood ratios between 2 and 5 reported in the dementia literature,26,27 which may produce small, but sometimes important, changes in pretest to posttest probability.28 None of the eight cases clinically diagnosed as CI-CVD cases (not included formally in the sensitivity/specificity analyses) showed significant AD pathology. Few of the AD dementia cases showed significant CVD pathology. However, many of the dementia cases clinically diagnosed as SIVD or mixed AD/SIVD showed “pure” AD pathology. Thus, the dementia but not the CI subsample was relatively “Alzheimerized.”
In AD research centers, where “typical” AD cases tend to be enrolled, the clinical diagnosis of AD is more sensitive (93%) than specific (55%) (positive likelihood ratio = 2.07).29 In this study, the opposite was seen; that is, the clinical diagnosis of AD was less sensitive (58.1%) but more specific (90.9%) (positive likelihood ratio = 6.4). The NINCDS-ADRDA criteria for probable AD16 do not offer specific guidelines on how to handle MRI findings consistent with SIVD (eg, silent hyperintensities and confluent white matter changes), which may lead to differences in the “operational” definitions of AD, CVD, and mixed AD/CVD. Thus, application of current diagnostic criteria for AD varies and, in the context where the possibilities of other pathologies are not minimized, may be undersensitive.
Fourteen cases (18%) in this series showed HS at autopsy. None of these cases had been recognized as having HS premortem. The clinical diagnoses varied, with six cases receiving a diagnosed as mixed AD/ SIVD. Leverenz and colleagues30 observed HS in 12% of an elderly community-based dementia autopsy series. It is possible that the high prevalence of 18% noted in our sample may be related to enrichment of the sample for SIVD. The pathogenesis of HS is unknown; both ischemic31 and neurodegenerative origins32,33 have been proposed. In this study, HS was found less frequently in pure AD (11.8%), compared with mixed AD/CVD (22.2%), CVD (26.7%), or no significant AD or CVD pathology (19.1%), but these differences were not significant (Fisher’s exact test, p = 0.53). No association was found between ApoE genotype and HS (see Table 6), suggesting that HS occurs independently of AD. Two cases of FTD in our autopsy series were excluded; thus, we are unable to comment on possible associations between HS and FTD. This study emphasizes that regardless of the pathogenic mechanism, HS is an important, prevalent, and clinically underrecognized cause of memory impairment in late life.
Given the great overlap in pathological findings, the arbitrariness of using cutoff scores, and the lack of consensus criteria for a pathological diagnosis of “vascular dementia,” we relied primarily on continuous regression and ordinal and dichotomous regression analyses to model the associations among three types of pathology, cognitive status, or ApoE genotype. In this study, only Braak and Braak stage correlated significantly with global cognitive score and cognitive status. However, independent contributions could be observed between CVDPS or HS and cognitive status in interaction models where Braak and Braak stage is interpolated to zero. The interaction terms, although not significant, suggest that as AD pathology (ie, Braak and Braak stage) increases the relatively small effects of CVDPS on cognition are hidden and the effects of HS diminish.
The Nun Study6 reported that cerebral infarction significantly increases the likelihood of dementia in cases with AD pathology. In the Honolulu Asia Aging study, AD, HS, Lewy bodies, and microinfarcts all contributed independently to dementia.34 In the Religious Orders Study, the likelihood of dementia increased with both AD pathology and the number of macroscopic infarcts.35 Similar to our findings, there was no evidence of significant interaction,7 indicating that the effects between AD and infarcts are additive rather than synergistic.
The association between ApoE4 and AD is robust, whereas its association with vascular dementia remains controversial. In clinically diagnosed cases, some investigators have reported increased frequency of ApoE4 in vascular dementia or AD with CVD,36 whereas others have not.37 Marin and colleagues38 reported elevations of ApoE4 with both vascular dementia (26%) and AD (22%), but not normal “control subjects” (7%) with atherosclerotic heart disease, hypertension, or stroke without dementia. In autopsy-diagnosed cases of pure CVD, Betard and coworkers39 found no increase in ApoE4 compared with mixed cases of AD/CVD. In a recent autopsy study of 215 subjects enrolled in the Religious Orders Study, ApoE e4 allele was independently associated with cerebral infarction, cerebral amyloid angiopathy, and AD pathology,40 although cases of pure CVD were not examined as a separate group.
In univariate analyses, we found correlations between ApoE e4 carrier status with cerebral amyloid angiopathy and Braak and Braak stage, but not with CVDPS, HS, or arteriosclerosis scores. In multiple logistic regression analyses (see Table 6), only the association between ApoE e4 and cerebral amyloid angiopathy remained. This is consistent with a neuropathological study of AD,41 where ApoE e4 was associated with amyloid angiopathy and deep microinfarcts, but not with basal atherosclerosis or macroscopic infarcts. Taken together, these findings suggest that (1) the apoE4 allele is associated with amyloid angiopathy but not arteriosclerotic-related ischemic brain injury, and (2) previous associations between ApoE4 genotype and vascular dementia may reflect the inclusion of cases with mixed AD/CVD or AD plus cerebral amyloid angiopathy.
This study has several strengths and limitations. All of the cases were followed longitudinally with common neuropsychological instruments, including a composite score of global cognitive status, which has desirable linear measurement properties. Similarly, all of the cases were evaluated under a common neuropathological protocol and reviewed at a consensus conference, blind to clinical information and ApoE genotype. A novel method (ie, the CVDPS score) was used to characterize the severity of cerebrovascular brain injury, but it has not been optimized as a predictor of cognitive impairment. In the sensitivity and specificity analyses, the threshold for setting levels of “significant pathology” was conservative and did not include a cutoff for HS. (The no significant AD or CVD pathology group included 10 CN, 5 CI, and 6 D cases, including 4 cases with HS). Recognizing that the choices of pathology cutoffs scores are arbitrary, we do not rely on them for our primary conclusions.
Our convenience sample drew heavily on memory clinics for subject enrollment, and subjects with a clinical diagnosis of AD were more likely to come to autopsy. Consequently, referral and autopsy bias might have contributed to the relative “Alzheimerization” of the subsample with dementia. The study focused by design on SIVD, defined by relatively mild MRI findings (ie, hyperintense lesions > 2mm), rather than symptomatic stroke, and excluded cortical infarcts at the time of initial enrollment. Therefore, this study is not representative of vascular dementia in general or of stroke-related dementia, but focuses instead on the milder end of SIVD.
Several important findings emerge from this convenience sample drawn from university-affiliated memory clinics, enriched for SIVD. HS was a common unexpected pathological finding. ApoE e4 carrier status was associated with cerebral amyloid angiopathy and AD, but not HS or arteriosclerosis. In the absence of AD pathology (ie, Braak and Braak stage = 0), CVDPS and HS contribute to mild cognitive impairment. However, advancing AD pathology overwhelms the effects of CVDPS and HS and becomes the major determinant of dementia in patients. The impact of symptomatic or large-vessel stroke, however, may make stronger contributions to the dementia syndrome, even in the presence of AD pathology, and deserves further investigation.
Footnotes
This study was supported by the National Institute on Aging, P01-AG12435, P50-AG05142, H.C.C.. W.J.M., L.Z.; P50-AG16570, H.V.V., P30-AG10129, C.C.D., W.G.E., D.M., B.R.R.; Chui was supported in part by the Raymond & Betty McCarron Chair of Neurology; Vinters was supported in part by the Dalijit S. & Elaine Sarkaria Chair in Diagnostic Medicine.
References
- 1.Erkinjuntti T, Inzitari D, Pantoni L, et al. Research criteria for subcortical vascular dementia in clinical trials. J Neural Transm Suppl. 2000;59:23–30. doi: 10.1007/978-3-7091-6781-6_4. [DOI] [PubMed] [Google Scholar]
- 2.Roman GC, Erkinjuntti T, Wallin A, et al. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;7:426–436. doi: 10.1016/s1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- 3.Tatemichi TK, Desmond DW, Prohovnik I, et al. Confusion and memory loss from capsular genu infarction: a thalamocortical disconnection syndrome? Neurology. 1992;10:1966–1979. doi: 10.1212/wnl.42.10.1966. [DOI] [PubMed] [Google Scholar]
- 4.Roman GC. Senile dementia of the Binswanger type. A vascular form of dementia in the elderly. JAMA. 1987;258:1782–1788. doi: 10.1001/jama.1987.03400130096040. [DOI] [PubMed] [Google Scholar]
- 5.Gorelick PB, Nyenhuis DL, Garron DC, Cochran E. Is vascular dementia really Alzheimer disease or mixed dementia? Neuroepidemiology. 1996;15:286–290. doi: 10.1159/000109918. [DOI] [PubMed] [Google Scholar]
- 6.Snowdon DA, Greiner LH, Mortimer JA, et al. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- 7.Schneider JA, Wilson RS, Bienias JL, et al. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62:1148–1155. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 8.Fein G, Di Sclafani V, Tanabe J, et al. Hippocampal and cortical atrophy predict dementia in subcortical ischemic vascular disease. Neurology. 2000;55:1626–1635. doi: 10.1212/wnl.55.11.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mungas D, Jagust WJ, Reed BR, et al. MRI predictors of cognition in subcortical ischemic vascular disease and Alzheimer’s disease. Neurology. 2001;57:2229–2235. doi: 10.1212/wnl.57.12.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mungas D, Reed BR, Jagust WJ, et al. Volumetric MRI predicts rate of cognitive decline related to AD and cerebrovascular disease. Neurology. 2002;59:867–873. doi: 10.1212/wnl.59.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vinters HV, Ellis WG, Zarow C, et al. Neuropathologic substrates of ischemic vascular dementia. J Neuropathol Exp Neurol. 2000;59:911–945. doi: 10.1093/jnen/59.11.931. [DOI] [PubMed] [Google Scholar]
- 12.Reed BR, Mungas DM, Kramer JH, et al. Clinical and neuropsychological features in autopsy-defined vascular dementia. Clin Psychol. 2004;18:63–74. doi: 10.1080/13854040490507163. [DOI] [PubMed] [Google Scholar]
- 13.Folstein M, Folstein S, McHugh PR. Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.Hughes CP, Berg L, Danziger W, et al. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 15.Longstreth WT, Jr, Bernick C, Manolio TA, et al. for the Cardiovascular Health Study Collaborative Research Group. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Arch Neurol. 1998;55:1217–1225. doi: 10.1001/archneur.55.9.1217. [DOI] [PubMed] [Google Scholar]
- 16.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 17.Chui HC, Victoroff JI, Margolin D, et al. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer Disease Diagnostic and Treatment Centers (ADDTC) Neurology. 1992;42:473–480. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- 18.Mungas D, Reed BR, Kramer JH. Psychometrically matched measures of global cognition, memory, and executive function for assessment of cognitive decline in older persons. Neuropsychology. 2003;17:380–392. doi: 10.1037/0894-4105.17.3.380. [DOI] [PubMed] [Google Scholar]
- 19.Mirra SS, Heyman A, McKeel D, et al. participating CERAD neuropathologists. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 20.NIA and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Consensus recommendations for the postmortem diagnosis of Alzheimer’s Disease. Neurobiol Aging. 1997;18:S1–S2. suppl 4. [PubMed] [Google Scholar]
- 21.McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathological diagnosis of dementia with Lewy bodies (DLB): Report of the Consortium on DLB International Workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 22.Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. Eur Neurol. 1993;33:403–408. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- 23.Vonsattel JP, Myers RH, Hedley-Whyte ET, et al. Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol. 1991;30:637–649. doi: 10.1002/ana.410300503. [DOI] [PubMed] [Google Scholar]
- 24.Vermeer SE, Hollander M, van Dijk EJ, et al. Silent brain infarcts and white matter lesions increase stroke risk in the general population. The Rotterdam Scan Study. Stroke. 2003;34:1126–1129. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- 25.Bernick CB, Kuller L, Dulberg C, et al. for the Cardiovascular Health Study Collaborative Research Group. Silent MRI infarcts and the risk of future stroke: the Cardiovascular Health Study. Neurology. 2001;57:1222–1229. doi: 10.1212/wnl.57.7.1222. [DOI] [PubMed] [Google Scholar]
- 26.Gold G, Bouras C, Canuta A, et al. Clinicopathological validation study of four sets of clinical criteria for vascular dementia. Am J Psychiatry. 2001;159:82–87. doi: 10.1176/appi.ajp.159.1.82. [DOI] [PubMed] [Google Scholar]
- 27.Chui HC. Evidence-based diagnosis of dementia. In: Qizilbash N, Schneider L, Chui H, et al., editors. Evidence-based dementia: a practical guide to diagnosis and management. Oxford, United Kingdom: Blackwell Science; 2002. [Google Scholar]
- 28.Jaeschke R, Guyatt GH, Sackett DL for the Evidence-Based Medicine Working Group. User’s guide to the medical literature. III. How to use an article about a diagnostic test. A. What are the results and will they help me in caring for my patients? JAMA. 1994;271:703–707. doi: 10.1001/jama.271.9.703. [DOI] [PubMed] [Google Scholar]
- 29.Mayeux R, Saunders AM, Shea S, et al. Utility of the apolipoprotein E genotype in the diagnosis of Alzheimer’s disease. Alzheimer’s Disease Centers Consortium on Apolipoprotein E and Alzheimer’s Disease. N Engl J Med. 1998;338:506–511. doi: 10.1056/NEJM199802193380804. [DOI] [PubMed] [Google Scholar]
- 30.Leverenz JB, Agustin CM, Tsuang D, et al. Clinical and neuropathological characteristics of hippocampal sclerosis: a community-based study. Arch Neurol. 2002;59:1099–1106. doi: 10.1001/archneur.59.7.1099. [DOI] [PubMed] [Google Scholar]
- 31.Dickson DW, Davie P, Bevona C, et al. Hippocampal sclerosis: a common pathological feature of dementia in the very old (greater than 80 years of age) humans. Acta Neuropathol. 1994;88:212–221. doi: 10.1007/BF00293396. [DOI] [PubMed] [Google Scholar]
- 32.Beach TG, Sue L, Scott S, et al. Hippocampal sclerosis dementia with tauopathy. Brain Pathol. 2003;13:263–278. doi: 10.1111/j.1750-3639.2003.tb00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hatanpaa KJ, Blass DM, Pletnikova O, et al. Most cases of dementia with hippocampal sclerosis may represent frontotemporal dementia. Neurology. 2004;63:538–542. doi: 10.1212/01.wnl.0000129543.46734.c0. [DOI] [PubMed] [Google Scholar]
- 34.White L, Petrovitch H, Hardman J, et al. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann N Y Acad Sci. 2002;977:9–23. doi: 10.1111/j.1749-6632.2002.tb04794.x. [DOI] [PubMed] [Google Scholar]
- 35.Schneider JA, Wilson RS, Cochran EJ, et al. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology. 2003;60:1082–1088. doi: 10.1212/01.wnl.0000055863.87435.b2. [DOI] [PubMed] [Google Scholar]
- 36.Slooter AJC, Tang M-X, van Duijn CM, et al. Apolipoprotein E4 and the risk of dementia with stroke: a population-based investigation. JAMA. 1997;277:818–821. doi: 10.1001/jama.277.10.818. [DOI] [PubMed] [Google Scholar]
- 37.Traykov L, Rigaud AS, Caputo L, et al. Apolipoprotein E phenotypes in demented and cognitively impaired patients with and without cerebrovascular disease. Eur J Neurol. 1999;6:415–421. doi: 10.1046/j.1468-1331.1999.640415.x. [DOI] [PubMed] [Google Scholar]
- 38.Marin DB, Breuer B, Marin ML, et al. The relationship between apolipoproteins E, dementia, and vascular illness. Atherosclerosis. 1998;140:173–180. doi: 10.1016/s0021-9150(98)00105-1. [DOI] [PubMed] [Google Scholar]
- 39.Betard C, Robitaille Y, Gee M, et al. Apo E allele frequencies in Alzheimer’s disease, Lewy body dementia, Alzheimer’s disease with cerebrovascular disease and vascular dementia. NeuroReport. 1994;5:1893–1896. doi: 10.1097/00001756-199410000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Schneider JA, Bienias JL, Wilson RS, et al. The apolipoprotein E ε4 allele increases the odds of chronic cerebral infarction detected at autopsy in older persons. Stroke. 2005;36:954–959. doi: 10.1161/01.STR.0000160747.27470.2a. [DOI] [PubMed] [Google Scholar]
- 41.Yip AG, McKee AC, Green RC, et al. APOE, vascular pathology and the AD brain. Neurology. 2005;65:259–265. doi: 10.1212/01.wnl.0000168863.49053.4d. [DOI] [PubMed] [Google Scholar]


