Abstract
The present study examined c-Fos expression in selected brain areas consequent to administration of lithium chloride, the typical illness-inducing agent used in laboratory studies of conditioned taste aversion. The results replicated previous findings of significant c-Fos expression in the parabrachial nucleus, the central nucleus of the amygdala and the basolateral amygdala. New findings indicate significant lithium-induced c-Fos in the gustatory region of the thalamus and the bed nucleus of the stria terminalis but not in the insular cortex. The results are discussed with respect to the neural substrates of conditioned taste aversion.
Keywords: C-Fos, Lithium chloride, Viscerosensory processing, Conditioned taste aversion, Rat
1. Introduction
Conditioned taste aversions (CTAs) develop when consumption of a novel taste is followed by transient gastrointestinal malaise (unconditioned stimulus, US). On subsequent encounters, the taste (now termed a conditioned stimulus, CS) is avoided because of the acquired association with the aversive US (e.g., Garcia & Ervin, 1968; Garcia, Kimeldorf, & Koelling, 1955; Revusky & Garcia, 1970). A large literature has examined the behavioral and biological mechanisms involved in this fundamental learning process that protects against the repeated self-administration of poisonous foods (e.g., Barker, Best & Domjan, 1977; Braveman & Bronstein, 1985; Bures, Bermudez-Rattoni & Yamamoto, 1998; Milgram, Krames & Alloway, 1977). Although the neural substrates of CTA have been investigated for over 30 years, to date no consensus has emerged regarding the component structures of the central CTA system. The general approach to this issue has been to examine CTA acquisition following placement of lesions in a selected brain structure. Unsurprisingly, the choice of brain structures has tended to be nonsystematic. A complementary approach involves the imaging of c-Fos expression to determine the areas involved in (1) processing of the visceral illness that constitutes the US, (2) processing of the novel gustatory stimulus that will become a CS, and (3) the processing of the CS (i.e., a taste cue that has previously been paired with the US). The present paper, which examined c-Fos expression consequent to an injection of a lithium chloride (LiCl)-induced US, represents our first step in this 3-stage enterprise.
Although traditional tract tracing methods have been used to assess the components of the ascending viscerosensory system, the picture, particularly with regard to structures higher in the brain, is far from clear. More recently, imaging c-Fos expression has been employed. The c-Fos protein represents an immediate early gene lasting only a few hours after transcription (Dragunow & Faull, 1989; Fenelton, Poulain, & Theodosis, 1993; Sagar, Sharp, & Curan, 1988). Thus, neuronal activation can be quantified and correlated to an activity, behavioral or physiological, of interest occurring within a constrained timeframe using this technique. It should be acknowledged, however, that c-Fos expression is related to neuronal excitation not inhibition (Hughes & Dragunow, 1995; Sheng & Greenberg, 1990). Thus, while c-Fos positive cells provide definitive evidence that the neuron/nucleus is, in some way, involved in the target activity, the absence of c-Fos expression provides no information relevant to the functional involvement of a neuron or nucleus. A clear demonstration of the importance of this latter issue can be seen with regard to the neural substrates of successive negative contrast, the exaggerated underresponding to a low value reward in a situation where a high value reward was expected (Flaherty, 1996). While Pecoraro and Dallman (2005) found no evidence of c-Fos activation in the gustatory thalamus (GT; parvicellular region of the ventral posteromedial nucleus) during the reward downshift trials, neurotoxic lesions of the GT completely prevent the occurrence of successive negative contrast (Reilly & Trifunovic, 1999; Reilly & Trifunovic, 2003).
Because CTA is a taste-guided behavior, our selection of target structures to examine for LiCl-induced c-Fos expression was based on the components of the central gustatory system (for a review see Lundy & Norgren, 2004). In particular, we were interested in the following structures: the parabrachial nucleus (PBN), the central nucleus of the amygdala (CNA), the basolateral nucleus of the amygdala (BLA), the GT, the bed nucleus of the stria terminalis (BNST) and the insular cortex (IC).
Prior studies have found increased c-Fos activity within the PBN after LiCl administration (Lamprecht & Dudai, 1995; Swank & Bernstein, 1994), with some researchers noting increased activity primarily within the lateral subnuclei (Yamamoto et. al, 1992). These findings are consistent with the conclusions derived from lesion studies that examined the role of the PBN in CTA (e.g., Reilly, 1999). Although the roles of the CNA and BLA in CTA are not fully understood (Reilly & Bornovalova, 2005), these structures show elevated c-Fos activity after a LiCl injection (Lamprecht & Dudai, 1995; Gu et al., 1993; Spencer & Houpt, 2001; Yamamoto et. al, 1992; Yamamoto et. al, 1997). The remaining structures innervated by the PBN (GT, BNST, IC) have been examined to a much lesser degree typically in experiments in which lithium was administered as a control condition (e.g., LiCl-only) and therefore was not the focus of the investigation. These studies suggest (1) little involvement of the IC (Billig, Yates, & Rinaman, 2001; Ferreira, Ferry, Meurisse, & Lévy, 2006) and (2) that the medial BNST may express c-Fos following lithium treatment in the ferret (Billig et al., 2001); to our knowledge the GT has not been investigated. The purpose of this study is to confirm these prior findings and to explore additional structures implicated in LiCl-induced viscerosensory information processing.
2. Results
Due to histological as well as immunohistochemical techniques not all nuclei of interest were obtained from each rat. Missing data was estimated using SPSS Missing Value Analysis standard regression method with residual correction, a method that is both robust and reliable (Little & Rubin, 1987). A 2 (Condition: saline, LiCl) X 6 (Area: PBN, CNA, BLA, GT, BNST, IC) mixed design of variance on total c-Fos counts was conducted, with condition treated as a between subjects factor and area as a within subjects factor.
Statistical analysis revealed a significant main effect of condition, F (1, 10) = 50.97, p < .001, ω2 = .81, with LiCl inducing more c-Fos expression than saline. A significant main effect of area was also found, F (5, 50) = 21.37, p < .001, ω2 = .51. Tukey's HSD post hoc comparisons revealed the CNA to have significantly more LiCl-induced c-Fos expression than all other areas (see Figs. 1 - 4). No other areas differed significantly. Finally, a significant interaction was found, F (5, 50) = 21.37, p < .01, ω2 = .51.
Fig. 1.
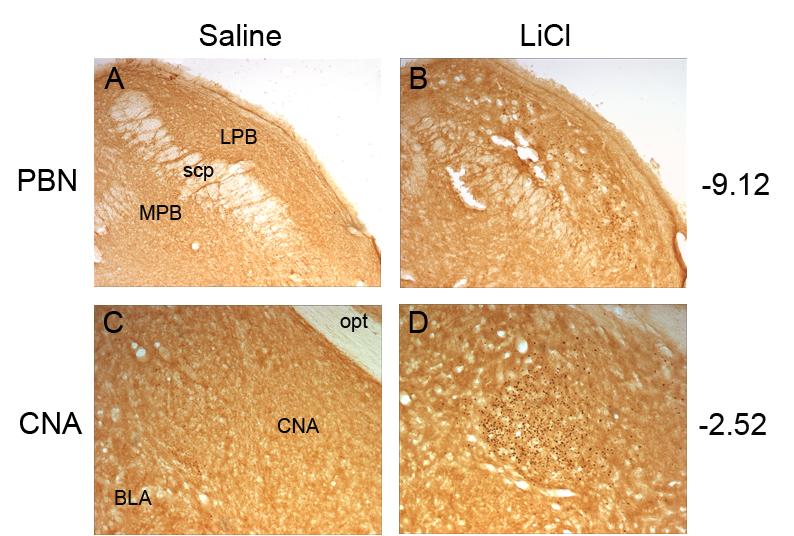
Representative photomicrographs of c-Fos activity within the parabrachial nucleus (PBN) after saline (A) or lithium chloride (LiCl) injection (B) and the central nucleus of the amygdala (CNA) after a saline (C) or lithium chloride (D) injection. All nuclei in the LiCl condition showed significant elevations in c-fos expression compared to the Saline control condition. Abbreviations: basolateral nucleus of the amygdala, BLA; central nucleus of the amygdala, CNA; lateral parabrachial nucleus, LPB; medial parabrachial nucleus, MPB; optic tract, opt; superior cerebellar peduncle, scp
Fig. 4.
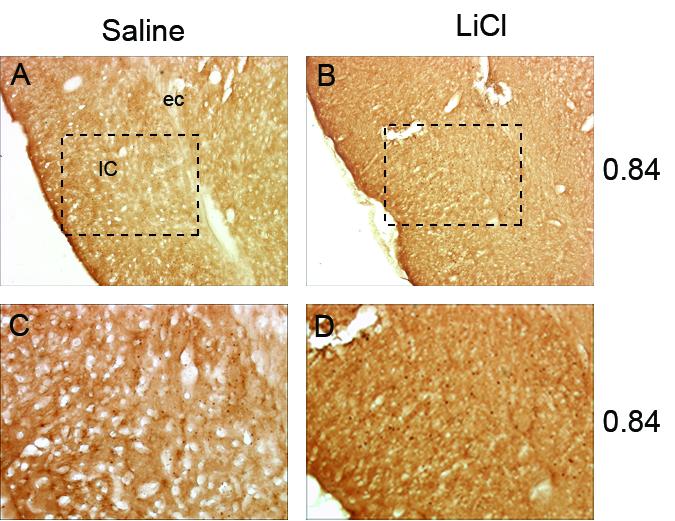
Representative photomicrographs of c-Fos activity within the insular cortex (IC) shown at low magnification after saline (A) or lithium chloride (LiCl) injection (B). Figures C and D are magnifications of images A and B with approximate site of magnification indicated by a dashed box. No differences were found between LiCl and Saline conditions. Abbreviations: external capsule, ec.
Planned comparisons of the simple main effects were conducted to follow-up the significant interaction. As shown in Table 1, significantly more c-Fos expression was found for the LiCl condition than the saline condition in the PBN, F (1,50) = 46.46, p < .01, CNA, F (1,50) = 199.67, p < .01, BLA, F (1,50) = 7.53, p < .01, GT, F (1,50) = 22.84, p < .01, BNST, F (1,50) = 27.87, p < .01; however, significant differences were not found in the IC (F < 1).
Table 1.
Mean number (±SD) of c-Fos positive nuclei, and effect size (ω2), in target brain areas following intraperitoneal injections of LiCl or saline.
| Saline |
LiCl |
||||
|---|---|---|---|---|---|
| Area | Mean | SD | Mean | SD | ω2 |
| PBN* | 13.86 | 7.94 | 101.33 | 36.65 | .415 |
| CNA* | 27.87 | 23.00 | 553.00 | 151.4 | .457 |
| BLA* | 14.35 | 6.37 | 42.61 | 16.51 | .344 |
| GT* | 21.98 | 7.44 | 79.44 | 49.33 | .347 |
| BNST* | 20.33 | 14.32 | 142.50 | 58.68 | .392 |
| IC | 75.67 | 56.05 | 141.00 | 59.03 | .153 |
Significant (p < .01) difference between Saline and LiCl conditions.
Abbreviations: BLA, basolateral region of the amygdala; BNST, bed nucleus of the stria terminalis; CNA, central nucleus of the amygdala; GT, gustatory thalamus; IC, insular cortex; LiCl, lithium chloride; PBN, parabrachial nucleus.
3. Discussion
The present experiment confirmed that administration of LiCl, the quintessential illness-inducing agent in CTA research, caused c-Fos expression in the PBN, BLA and CNA. We also established that LiCl-induced c-Fos occurred in the GT and BNST. There was, however, no significant difference between the number of c-Fos positive nuclei in the IC following IP administration of saline or LiCl.
The expression of c-Fos in the lateral subnuclei of the PBN is consistent with results obtained from lesion-behavior studies that indicate that the lateral PBN has a significant role in the processing of ascending viscerosensory information. For example, a number of reports show that lateral PBN lesions prevent the acquisition of CTAs (Mungarndee, Lundy, & Norgren, 2006; Navarro & Cubero, 2003; Reilly & Trifunovic, 2000a, 2001; Trifunovic & Reilly, 2002). Similarly, lateral PBN lesions using ibotenic acid prevent the acquisition of calorie-based conditioned flavor preferences (Reilly & Trifunovic, 2000a) and disrupt the concentration-dependent intake of sucrose (Reilly & Trifunovic, 2000b). Collectively, these neurobehavioral data suggest that the lateral subnuclei of the PBN are involved in the processing of aversive and appetitive ascending viscerosensory information.
In terms of absolute numbers the BLA had the lowest count of c-Fos positive nuclei (42.61) and the CNA the highest (553.00). Although these numbers suggest that the CNA is much more important for processing lithium-related information than the BLA, lesion-behavior studies have yielded results that do not seem to afford important roles to either area in taste aversion learning. In a recent review of the influence of permanent lesions of the amygdala on CTA, Reilly and Bornovalova (2005) found little evidence that the CNA has any role in taste aversion learning. Although these numbers suggest that the CNA is much more important for processing lithium-related information than the BLA, axon-sparing lesion-behavior studies have yielded results that provide little evidence that either structure serves such a function in taste aversion learning (St. Andre & Reilly, 2007).
The novel finding of LiCl-induced c-Fos expression in the GT is an intriguing result that is not easy to interpret. Early research favored a role for the GT in CTA acquisition (e.g., Lasiter, 1985; Loullis, Wayner, & Jolicoeur, 1978; Yamamoto, 1993; Yamamoto & Fujimoto, 1991; Yamamoto et al. 1995). However, more recent research, using electrolytic lesions that caused minimal damage beyond the boundaries of the GT, finds no evidence that discrete GT lesions disrupt first-order CTA (e.g., Flynn, Grill, Schulkin, & Norgren, 1991; Grigson, Lyuboslavsky, & Tanase, 2000; Reilly & Pritchard, 1996). Although ibotenic acid GT lesions do disrupt CTA acquisition when multiple CSs are involved (Reilly, Bornovalova, Dengler, & Trifunovic, 2003), the deficit cannot be interpreted as a disruption in the processing of US-related information.
The BNST is a forebrain recipient of ascending gustatory information from the PBN (Alden, Besson, & Bernard, 1994; Saper & Loewy, 1980) and receives additional projections from the CNA, a component of the extended amygdala (Alheid, 2003; Alheid & Heimer, 1988). Two aspects of the BNST data are noteworthy, First, our findings differs from those reported by Billig et al. (2001) which found activation within the medial portion of the BNST work on ferrets whereas we found none. Second, in the present study the BNST, particularly the anterior part of the lateral region, was implicated in the processing of ascending LiCl-induced viscerosensory information. This finding encourages the view that the nucleus may have a role in the associative integration of the CS and US that underlies CTA acquisition. A definitive test of this hypothesis would be afforded by examining the effects of BNST lesions on CTA acquisition.
A surprising aspect of the IC data concerns the high number of c-Fos positive cells in the saline condition which was 3-5 times greater (with a corresponding high degree of variance) than the baseline in the other target areas. Thus, despite the fact that the c-Fos count in the IC was the third highest in any brain area (after the PBN and BNST) there was no significant treatment effect in the IC. It would appear, then, that the IC is not involved in visceral processing induced by ip administration of LiCl. This interpretation is strengthened by comparably low omega squared value (0.153), which was half the size of the next smallest value (0.344 for the BLA).
Future research will continue to examine the neural underpinnings of CTA formation using c-Fos imaging. The response to taste stimulus only and to a CS (a taste previously paired with lithium toxicosis) will be studied. Prior studies have examined c-Fos activity in some of the structures currently under examination consequent to ingestion of novel or familiar taste solutions. Elevations of activity were found within the CNA and IC (but not the PBN, BLA or NST) when novel salt and saccharin solutions were consumed relative to familiar solutions (Koh, Wilkins & Bernstein, 2003; Barot & Bernstein, 2005). These additional studies will help identify a taste aversion neural network based upon: US only condition, taste only condition, and CS condition.
Most drugs have multiple influences (i.e., side effects) beyond their selected target effect. For example, lithium, which is also used in the treatment of bipolar disorder (Fountoulakis, Vieta, Sanchez-Moreno, Kaprinis, Goikolea, & Kaprinis, 2005), inhibits glycogen synthase kinase-3 (Lin, Wang, Klein, & Lazar, 2006), which, in turn, regulates the circadian rhythms of many functions including body temperature, metabolism, and sleep. The many systems that are influenced by lithium likely have different, or at least overlapping, neural substrates relative to CTA. It is, then, hardly surprising that some brain structures that express LiCl-induced c-Fos (i.e., BLA, CNA, and GT) are not, as determined by lesion-behavior studies, involved in taste aversion learning. This is a useful reminder that the identification of functional neural systems (in the present case, the central CTA system) benefits from an approach that emphasizes converging lines of evidence from a number of complementary methods.
4. Experimental procedures
4.1. Subjects
A total of 12 Sprague-Dawley CD IGS rats (Charles River Laboratories, Wilmington, MA) served as subjects. Animals were individually housed in stainless steel hanging cages and were maintained on a 12 h: 12 h light-dark cycle (lights on at 0700). Food and water were provided ad libitum. Animals weighed between 257-267 g on the experimental day.
4.2 Procedure
On the day of the experiment animals were weighed and food and water removed. Two hours later rats received ip injections of either 0.4 M LiCl or physiological saline at 1.0 ml/100 g. The pattern and magnitude of c-Fos expression has been found to be the same following administration of 0.15 M or 0.4 M NaCl (Ferreira, Ferry, Meurisse, & Lévy, 2006). Therefore, 0.15 M saline was used in the control condition of the present study.
Ninety min after injections animals were deeply anesthetized using sodium pentobarbital and transcardially perfused with phosphate buffered saline (PBS) followed by 37% paraformaldehyde in 0.1 M phosphate buffer. Brains were removed and postfixed for two days followed by two days in PBS containing 20% sucrose.
4.3. c-Fos Immunohistochemistry
Coronal sections were sliced at 60 μm using a cryostat and placed in PBS. Tissue was first treated for 20 min in 0.3% H2O2 PBS to block endogenous peroxidase activity, rinsed, and incubated for 30 min in 3% normal goat serum, and 0.5% Triton X-100 in PBS. Without rinsing, sections were transferred to c-Fos primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) containing c-Fos antibody (1:10,000), 1% normal goat serum, and 0.5% Triton X-100 in PBS. Slices were refrigerated in primary antibody solution for 48 h, rinsed, and placed in a secondary antibody containing biotinylated goat anti-rabbit IgG (1:1,000), 1% normal goat serum, and 0.5% Triton X-100 in PBS and refrigerated for an additional 2 h. Sections were rinsed, processed using the ABC method (Vector Laboratories, Burlingame, CA) and rinsed again. Reaction was visualized using a 3,3′-diaminobenzidine (DAB) kit (Vector Laboratories, Burlingame, CA), rinsed, mounted on subbed slides, rehydrated, and cover-slipped. All rinses were done three times at five minutes per rinse in PBS.
4.4 Data Analysis
Representative digital photomicrographs were taken at the same A/P coordinate for each nucleus of interest using a Zeiss Axioskop 40 microscope equipped with a Q-Imaging Camera running Q-Capture software (Quantitative Imaging Corporation, Burnaby, BC). Photomicrographs were scored by four blind raters who recorded total number of c-Fos reactive nuclei. Raters' scores by area were significantly positively correlated, r > .95, p < .01. Scores were averaged after results indicated no significant differences between raters.
Fig. 2.
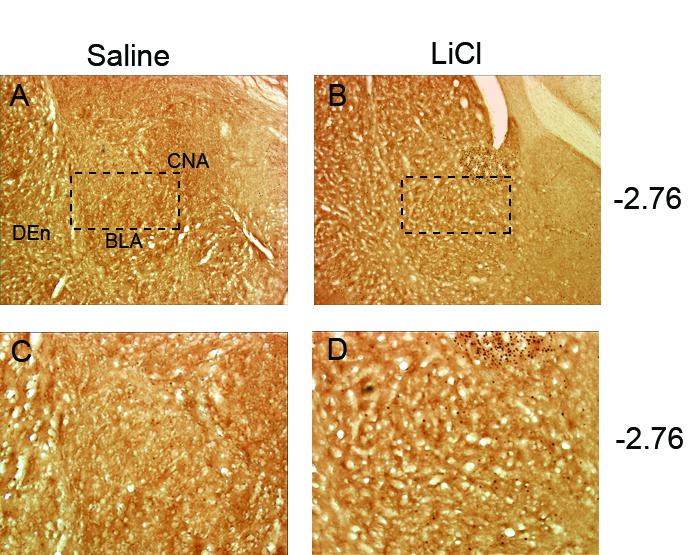
Representative photomicrographs of c-Fos activity within the basolateral amygdala (BLA) shown at low magnification after saline (A) or lithium chloride (LiCl) injection (B). Figures C and D are magnifications of images A and B with approximate site of magnification indicated by a dashed box. LiCl treated rats showed increased c-Fos expression compared to the Saline condition. Abbreviations: central nucleus of the amygdala, CNA; dorsal endopiriform nucleus, Den.
Fig. 3.
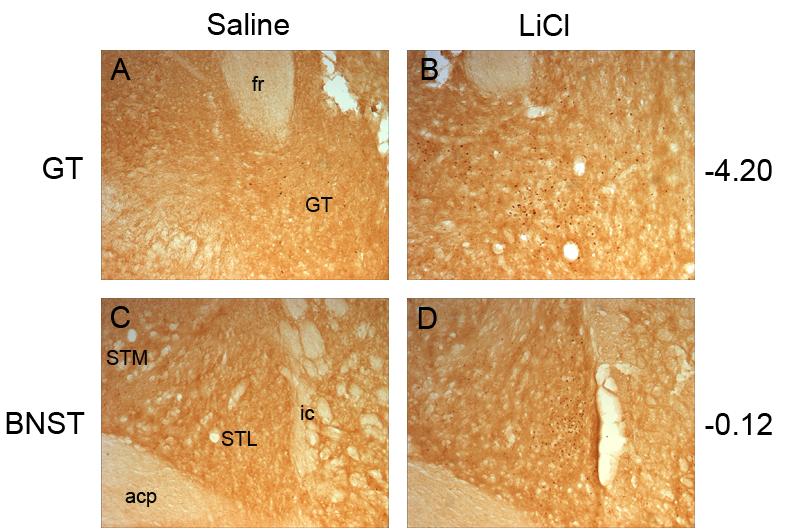
Representative photomicrographs of c-Fos activity within the gustatory thalamus (GT) after saline (A) or lithium chloride (LiCl) injection (B) and the bed nucleus of the stria terminalis (BNST) after a saline (C) or lithium chloride (D) injection. The GT and BNST showed significant elevations in c-fos expression in the LiCl condition compared to the Saline controls. Abbreviations: posterior portion of the anterior commissure, acp; fasciculus retroflexus, fr; gustatory thalamus, GT; internal capsule, ic; lateral division of the bed nucleus of the stria terminalis, STL; medial division of the bed nucleus of the stria terminalis STM.
Footnotes
This research was supported by Grant DC06456 from the National Institute of Deafness and Other Communication Disorders. Portions of the data reported in this article were originally presented at the 78th Annual Meeting of the Midwestern Psychological Association, Chicago, IL May 2006.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alden M, Besson JM, Bernard JF. Organization of the efferent projections from the pontine parabrachial area to the bed nucleus of the stria terminalis and neighboring regions: a PHA-L study in the rat. J. Comp. Neurol. 1994;341:289–314. doi: 10.1002/cne.903410302. [DOI] [PubMed] [Google Scholar]
- Alheid GF. Extended amygdala and basal forebrain. Ann. New York Acad. Sci. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: The striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neurosci. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Barker LM, Best MR, Domjan M, editors. Learning mechanisms in food selection. Baylor University Press; Waco, Texas: 1977. [Google Scholar]
- Barot SK, Bernstein IL. Polycose taste pre-exposure fails to influence behavioral and neural indices of taste novelty. Behav. Neurosci. 2005;119:1640–1647. doi: 10.1037/0735-7044.119.6.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billig I, Yates BJ, Rinaman L. Plasma hormone levels and central c-Fos expression in ferrets after systemic administration of cholocystokin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R1243–R1255. doi: 10.1152/ajpregu.2001.281.4.R1243. [DOI] [PubMed] [Google Scholar]
- Braveman NS, Bronstein P, editors. Ann. New York Acad. Sci. Vol. 443. 1985. Experimental assessments and clinical applications of conditioned food aversions; pp. 1–441. [PubMed] [Google Scholar]
- Bures J, Bermudez-Rattoni F, Yamamoto T. Conditioned taste aversion: Memory of a special kind. Oxford University Press; Oxford, UK: 1998. [Google Scholar]
- Dragunow M, Faull RLM. The use of c-fos as a metabolic marker in neuronal pathway tracing. J. Neurosci. Methods. 1989;29:261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- Fenelton VS, Poulain DA, Theodosis DT. Oxytocin neuron activation and Fos expression: a quantitative immunocytochemical analysis of the effect of lactation, parturition, osmotic and cardiovascular stimulation. Neurosci. 1993;53:777–789. doi: 10.1016/0306-4522(93)90286-o. [DOI] [PubMed] [Google Scholar]
- Ferreira G, Ferry B, Meurisse M, Lévy F. Forebrain structures specifically activated by conditioned taste aversion. Behav. Neurosci. 2006;120:952–62. doi: 10.1037/0735-7044.120.4.952. [DOI] [PubMed] [Google Scholar]
- Flaherty CF. Incentive relativity. Cambridge University Press; Cambridge, England: 1996. [Google Scholar]
- Flynn FW, Grill HJ, Schulkin J, Norgren R. Central gustatory lesions: II. Effects on sodium appetite, taste aversion learning, and feeding behavior. Behav. Neurosci. 1991;105:944–954. doi: 10.1037//0735-7044.105.6.944. [DOI] [PubMed] [Google Scholar]
- Fountoulakis KN, Vieta E, Sanchez-Moreno J, Kaprinis SG, Goikolea JM, Kaprinis GS. Treatment guidelines for bipolar disorder: A critical review. J. Affect. Disorders. 2005;86:1–10. doi: 10.1016/j.jad.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Garcia J, Ervin FR. Gustatory-visceral and telereceptor-cutaneous conditioning: Adaptation in internal and external milieus. Comm. Behav. Biol. 1968;1:389–415. [Google Scholar]
- Garcia J, Kimeldorf DJ, Koelling R. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science. 1955;122:157–158. [PubMed] [Google Scholar]
- Gu Y, Gonzales MF, Chen D, Deutsch JA. Expression of c-fos in brain subcortical structures in response to nauseant lithium chloride and osmotic pressure in rats. Neurosci. Lett. 1993;157:49–52. doi: 10.1016/0304-3940(93)90640-7. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Lyuboslavsky P, Tanase D. Bilateral lesions of the gustatory thalamus disrupt morphine, but not LiCl-induced conditioned taste aversions in rats: Evidence for the reward comparison hypothesis. Brain Res. 2000;858:327–337. doi: 10.1016/s0006-8993(00)01939-9. [DOI] [PubMed] [Google Scholar]
- Hughes P, Dragunow M. Induction of immediate-early genes and the control of neurotransmitter-regulated gene expression within the nervous system. Pharmacol. Rev. 1995;47:133–78. [PubMed] [Google Scholar]
- Koh MT, Wilkins EE, Bernstein IL. Novel tastes elevate c-fos expression in the central amygdala and insular cortex: implication for taste aversion learning. Behav. Neurosci. 2003;117:1614–1422. doi: 10.1037/0735-7044.117.6.1416. [DOI] [PubMed] [Google Scholar]
- Lamprecht R, Dudai Y. Differential modulation of brain immediate early genes by intraperitoneal LiCl. NeuroReport. 1995;7:289–293. [PubMed] [Google Scholar]
- Lasiter PS. Thalamocortical relations in taste aversion learning: II. Involvement of the medial ventrobasal thalamic complex in taste aversion learning. Behav. Neurosci. 1985;99:477–495. doi: 10.1037//0735-7044.99.3.477. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical analysis with missing data. Wiley; New York: 1987. [Google Scholar]
- Loullis CC, Wayner MJ, Jolicoeur FB. Thalamic taste nucleus lesions and taste aversion. Physiol. Behav. 1978;20:653–655. doi: 10.1016/0031-9384(78)90259-7. [DOI] [PubMed] [Google Scholar]
- Lundy RF, Jr., Norgren R. Gustatory system. In: Paxinos G, editor. The rat nervous system. 3rd Edition Academic Press; San Diego: 2004. pp. 891–921. [Google Scholar]
- Milgram NW, Krames L, Alloway TM, editors. Food aversion learning. Plenum Press; New York: 1977. [Google Scholar]
- Mungarndee SS, Lundy RF, Jr., Norgren R. Central gustatory lesions and learned taste aversions: Unconditioned stimuli. Physiol. Behav. 2006;87:542–551. doi: 10.1016/j.physbeh.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Cubero I. Lateral parabrachial lesions impair lithium chloride-induced aversive responses but not saccharin-induced flavor preference. Brain Res. 2003;990:195–202. doi: 10.1016/s0006-8993(03)03530-3. [DOI] [PubMed] [Google Scholar]
- Pecoraro N, Dallman MF. c-Fos after incentive shifts: Expectancy, incredulity, and recovery. Behav. Neurosci. 2005;119:366–387. doi: 10.1037/0735-7044.119.2.366. [DOI] [PubMed] [Google Scholar]
- Reilly S. The parabrachial nucleus and conditioned taste aversion. Brain Res. Bull. 1999;48:239–254. doi: 10.1016/s0361-9230(98)00173-7. [DOI] [PubMed] [Google Scholar]
- Reilly S, Bornovalova M. Conditioned taste aversion and amygdala lesions in the rat: A critical review. Neurosci. Biobehav. Rev. 2005;29:1067–1088. doi: 10.1016/j.neubiorev.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Reilly S, Bornovalova M, Dengler C, Trifunovic R. Effects of excitotoxic lesions of the gustatory thalamus on latent inhibition and blocking of conditioned taste aversion in rats. Brain Res. Bull. 2003;62:117–128. doi: 10.1016/j.brainresbull.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Reilly S, Pritchard TC. Gustatory thalamus lesions in the rat: II. Aversive and appetitive taste conditioning. Behav. Neurosci. 1996;110:746–759. [PubMed] [Google Scholar]
- Reilly S, Trifunovic R. Gustatory thalamus lesions eliminate successive negative contrast in the rat. Behav. Neurosci. 1999;113:1242–1248. [PubMed] [Google Scholar]
- Reilly S, Trifunovic R. Lateral parabrachial nucleus lesions in the rat: Aversive and appetitive gustatory conditioning. Brain Res. Bull. 2000a;52:269–278. doi: 10.1016/s0361-9230(00)00263-x. [DOI] [PubMed] [Google Scholar]
- Reilly S, Trifunovic R. Lateral parabrachial nucleus lesions in the rat: Long-and short-duration gustatory preference tests. Brain Res. Bull. 2000b;51:177–186. doi: 10.1016/s0361-9230(99)00218-x. [DOI] [PubMed] [Google Scholar]
- Reilly S, Trifunovic R. Lateral parabrachial nucleus lesions in the rat: Neophobia and conditioned taste aversion. Brain Res. Bull. 2001;55:359–366. doi: 10.1016/s0361-9230(01)00517-2. [DOI] [PubMed] [Google Scholar]
- Reilly S, Trifunovic R. Gustatory thalamus lesions disrupt successive negative contrast: Evidence against a memory deficit. Behav. Neurosci. 2003;117:606–615. doi: 10.1037/0735-7044.117.3.606. [DOI] [PubMed] [Google Scholar]
- Revusky S, Garcia J. Learned associations over long delays. In: Bower GH, Spence JT, editors. The psychology of learning and motivation: Advances in research and theory. Vol. 4. Academic Press; New York: 1970. pp. 1–84. [Google Scholar]
- Sagar SM, Sharp FR, Curan T. Expression of c-fos protein in brain: Metabolic mapping at the cellular level. Science. 1988;240:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- Saper CB, Loewy A. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 1980;197:291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–85. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Spencer CM, Houpt TA. Dynamics of c-fos and ICER mRNA expression in rat forebrain following lithium chloride injection. Molecular Brain Res. 2001;93:113–126. doi: 10.1016/s0169-328x(01)00173-5. [DOI] [PubMed] [Google Scholar]
- St. Andre J, Reilly S. Effects of central and basolateral amygdala lesions on conditioned taste aversion and latent inhibition. Behav. Neurosci. 2007;121 doi: 10.1037/0735-7044.121.1.90. 000-000. [DOI] [PubMed] [Google Scholar]
- Swank MW, Bernstein IL. c-Fos induction in response to a conditioned stimulus after single trial taste aversion learning. Brain Res. 1994;636:202–208. doi: 10.1016/0006-8993(94)91018-9. [DOI] [PubMed] [Google Scholar]
- Trifunovic R, Reilly S. Medial versus lateral parabrachial nucleus lesions in the rat: Effects on mercaptoacetate-induced feeding and conditioned taste aversion. Brain Res. Bull. 2002;58:107–113. doi: 10.1016/s0361-9230(02)00766-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto T. Neural mechanisms of taste aversion learning. Neurosci. Res. 1993;16:181–185. doi: 10.1016/0168-0102(93)90122-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Fujimoto Y. Brain mechanisms of taste aversion learning in the rat. Brain Res. Bull. 1991;27:403–406. doi: 10.1016/0361-9230(91)90133-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Fujimoto Y, Shimura T, Sakai N. Conditioned taste aversion in the rat with excitotoxic brain lesions. Neurosci. Res. 1995;22:31–49. doi: 10.1016/0168-0102(95)00875-t. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Sako N, Sakai N, Iwafune A. Gustatory and visceral inputs to the amygdale of the rat: conditioned taste aversion and induction of c-fos-like immunoreactivity. Neurosci. Lett. 1997;226:127–130. doi: 10.1016/s0304-3940(97)00265-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Shimura T, Sako N, Azuma S, Bai W.-Zh., Wakisaka S. C-fos expression in the rat brain after intraperitoneal injection of lithium chloride. NeuroReport. 1992;3:1049–1052. doi: 10.1097/00001756-199212000-00004. [DOI] [PubMed] [Google Scholar]
- Yin I, Wang J, Klein PS, Lazar MA. Nuclear receptor rev-erbα is a critical lithium-sensitive component of the circadian clock. Science. 2006;311:1002–1005. doi: 10.1126/science.1121613. [DOI] [PubMed] [Google Scholar]


