Abstract
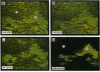
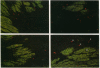
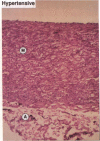
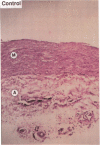
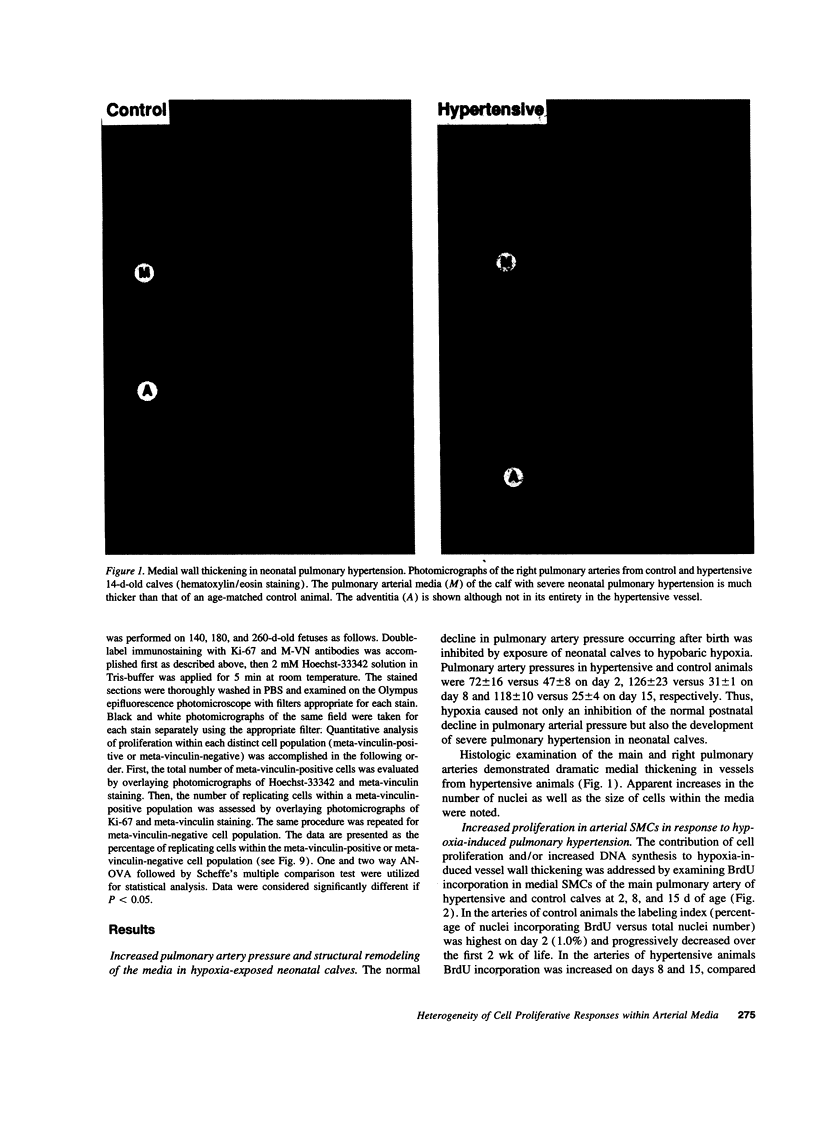
Medial thickening of the pulmonary arterial wall, secondary to smooth muscle cell (SMC) hyperplasia, is commonly observed in neonatal hypoxic pulmonary hypertension. Because recent studies have demonstrated the existence of multiple phenotypically distinct SMC populations within the arterial media, we hypothesized that these SMC subpopulations would differ in their proliferative responses to hypoxic pulmonary hypertension and thus contribute in selective ways to the vascular remodeling process. Expression of meta-vinculin, a muscle-specific cytoskeletal protein, has been shown to reliably distinguish two unique SMC subpopulations within the bovine pulmonary arterial media. Therefore, to assess the proliferative responses of phenotypically distinct SMC subpopulations in the setting of neonatal pulmonary hypertension, we performed double immunofluorescence staining on pulmonary artery cryosections from control and hypertensive calves with antibodies against meta-vinculin and the proliferation-associated nuclear antigen, Ki-67. We found that, although neonatal pulmonary hypertension caused significant increases in overall cell replication, proliferation occurred almost exclusively in one, the meta-vinculin-negative SMC population, but not the other SMC population expressing meta-vinculin. We also examined fetal pulmonary arteries, where proliferative rates were high and meta-vinculin expression again reliably distinguished two SMC subpopulations. In contrast to the hypertensive neonate, we found in the fetus that the relative proliferative rates of both SMC subpopulations were equal, thus suggesting the existence of different mechanisms controlling proliferation and expression of cytoskeletal proteins in the fetus and neonate. We conclude that phenotypically distinct SMC populations in the bovine arterial media exhibit specific and selective proliferative responses to neonatal pulmonary hypertension. Distinct SMC subpopulations may, thus, contribute in unique ways to vascular homeostasis under both normal and pathologic conditions.
Full text
PDF

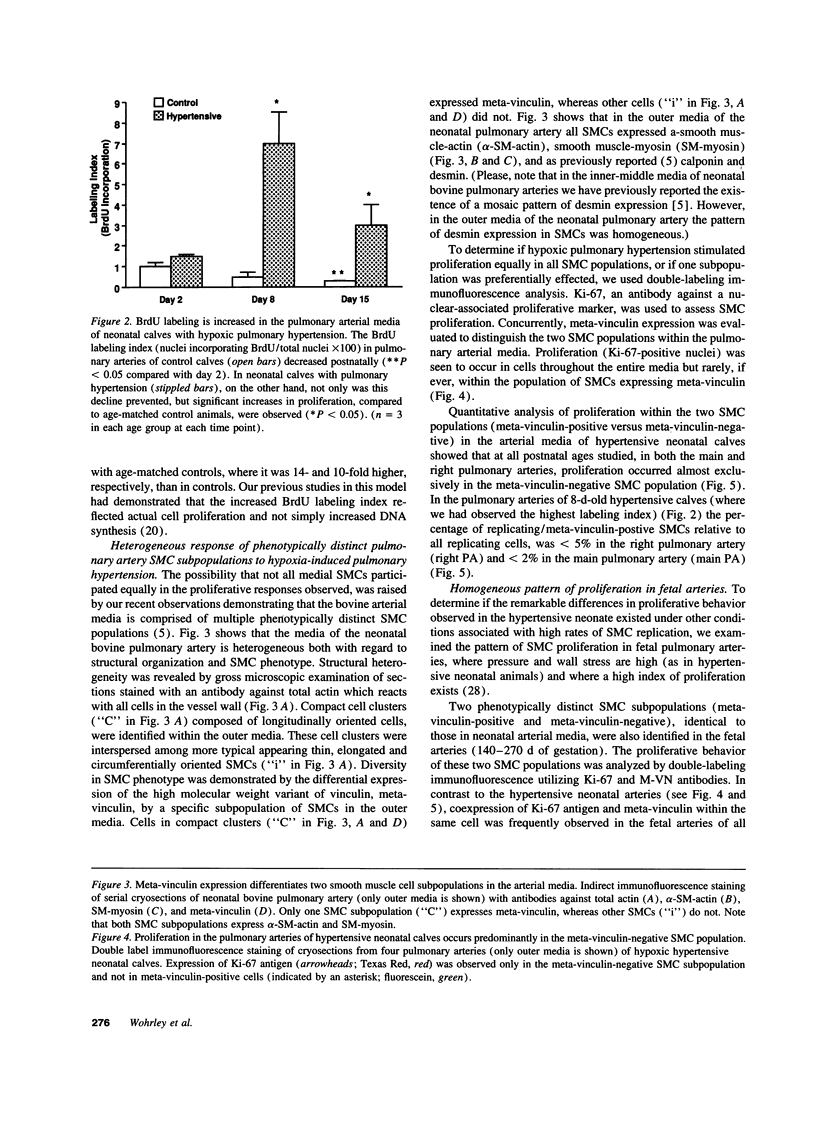

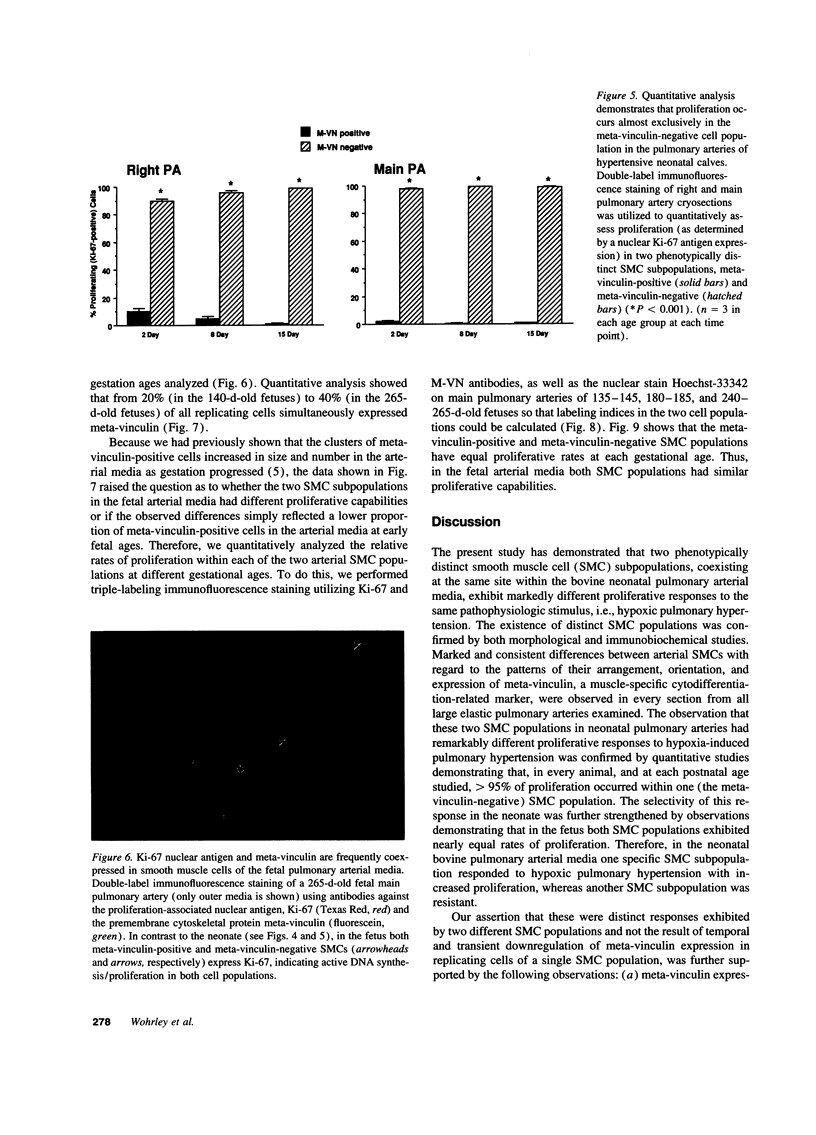
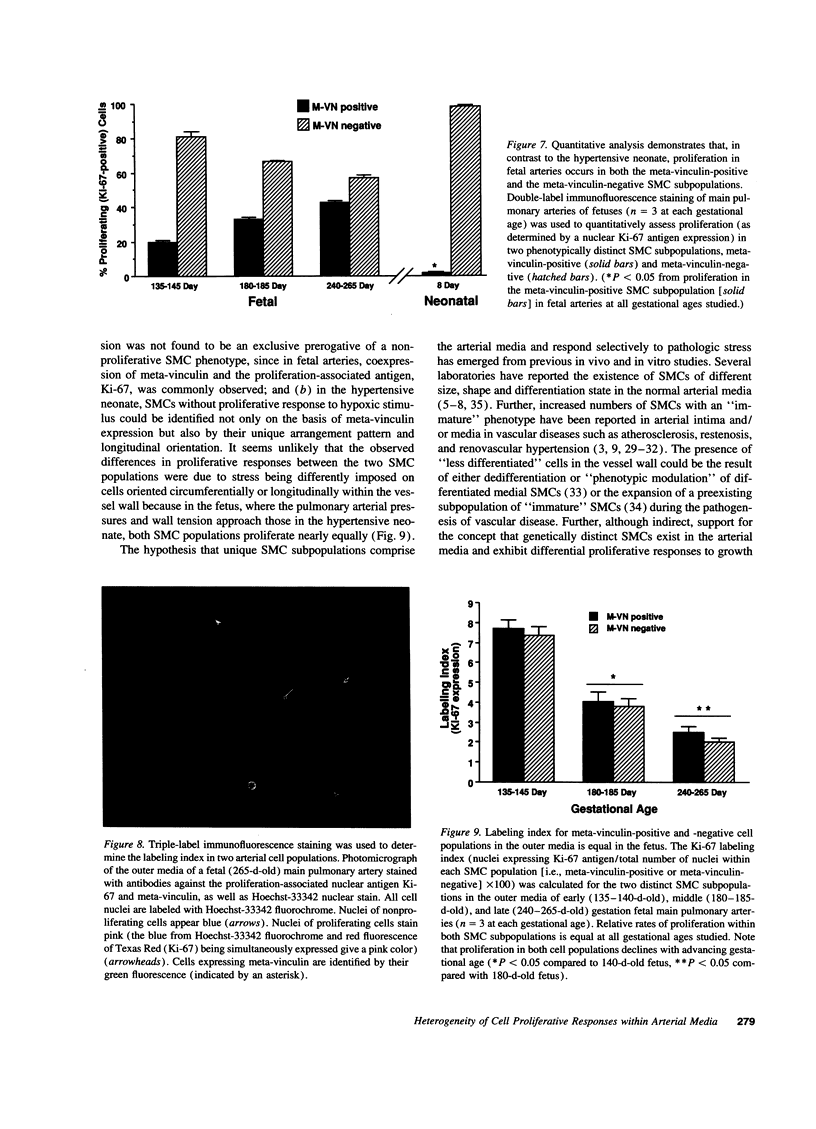


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown D. C., Gatter K. C. Monoclonal antibody Ki-67: its use in histopathology. Histopathology. 1990 Dec;17(6):489–503. doi: 10.1111/j.1365-2559.1990.tb00788.x. [DOI] [PubMed] [Google Scholar]
- Bârzu T., Herbert J. M., Desmoulière A., Carayon P., Pascal M. Characterization of rat aortic smooth muscle cells resistant to the antiproliferative activity of heparin following long-term heparin treatment. J Cell Physiol. 1994 Aug;160(2):239–248. doi: 10.1002/jcp.1041600205. [DOI] [PubMed] [Google Scholar]
- Campbell J. H., Kocher O., Skalli O., Gabbiani G., Campbell G. R. Cytodifferentiation and expression of alpha-smooth muscle actin mRNA and protein during primary culture of aortic smooth muscle cells. Correlation with cell density and proliferative state. Arteriosclerosis. 1989 Sep-Oct;9(5):633–643. doi: 10.1161/01.atv.9.5.633. [DOI] [PubMed] [Google Scholar]
- Cesarone C. F., Bolognesi C., Santi L. Improved microfluorometric DNA determination in biological material using 33258 Hoechst. Anal Biochem. 1979 Nov 15;100(1):188–197. doi: 10.1016/0003-2697(79)90131-3. [DOI] [PubMed] [Google Scholar]
- Chamley-Campbell J. H., Campbell G. R. What controls smooth muscle phenotype? Atherosclerosis. 1981 Nov-Dec;40(3-4):347–357. doi: 10.1016/0021-9150(81)90145-3. [DOI] [PubMed] [Google Scholar]
- Coltrera M. D., Gown A. M. PCNA/cyclin expression and BrdU uptake define different subpopulations in different cell lines. J Histochem Cytochem. 1991 Jan;39(1):23–30. doi: 10.1177/39.1.1670579. [DOI] [PubMed] [Google Scholar]
- Cook C. L., Weiser M. C., Schwartz P. E., Jones C. L., Majack R. A. Developmentally timed expression of an embryonic growth phenotype in vascular smooth muscle cells. Circ Res. 1994 Feb;74(2):189–196. doi: 10.1161/01.res.74.2.189. [DOI] [PubMed] [Google Scholar]
- Durmowicz A. G., Orton E. C., Stenmark K. R. Progressive loss of vasodilator responsive component of pulmonary hypertension in neonatal calves exposed to 4,570 m. Am J Physiol. 1993 Dec;265(6 Pt 2):H2175–H2183. doi: 10.1152/ajpheart.1993.265.6.H2175. [DOI] [PubMed] [Google Scholar]
- Evans H. E., Sack W. O. Prenatal development of domestic and laboratory mammals: growth curves, external features and selected references. Zentralbl Veterinarmed C. 1973 Mar;2(1):11–45. doi: 10.1111/j.1439-0264.1973.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Fager G., Hansson G. K., Gown A. M., Larson D. M., Skalli O., Bondjers G. Human arterial smooth muscle cells in culture: inverse relationship between proliferation and expression of contractile proteins. In Vitro Cell Dev Biol. 1989 Jun;25(6):511–520. doi: 10.1007/BF02623563. [DOI] [PubMed] [Google Scholar]
- Frid M. G., Moiseeva E. P., Stenmark K. R. Multiple phenotypically distinct smooth muscle cell populations exist in the adult and developing bovine pulmonary arterial media in vivo. Circ Res. 1994 Oct;75(4):669–681. doi: 10.1161/01.res.75.4.669. [DOI] [PubMed] [Google Scholar]
- Frid M. G., Shekhonin B. V., Koteliansky V. E., Glukhova M. A. Phenotypic changes of human smooth muscle cells during development: late expression of heavy caldesmon and calponin. Dev Biol. 1992 Oct;153(2):185–193. doi: 10.1016/0012-1606(92)90104-o. [DOI] [PubMed] [Google Scholar]
- Fujita H., Shimokado K., Yutani C., Takaichi S., Masuda J., Ogata J. Human neonatal and adult vascular smooth muscle cells in culture. Exp Mol Pathol. 1993 Feb;58(1):25–39. doi: 10.1006/exmp.1993.1003. [DOI] [PubMed] [Google Scholar]
- Glukhova M. A., Kabakov A. E., Frid M. G., Ornatsky O. I., Belkin A. M., Mukhin D. N., Orekhov A. N., Koteliansky V. E., Smirnov V. N. Modulation of human aorta smooth muscle cell phenotype: a study of muscle-specific variants of vinculin, caldesmon, and actin expression. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9542–9546. doi: 10.1073/pnas.85.24.9542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadrava V., Tremblay J., Hamet P. Abnormalities in growth characteristics of aortic smooth muscle cells in spontaneously hypertensive rats. Hypertension. 1989 Jun;13(6 Pt 1):589–597. doi: 10.1161/01.hyp.13.6.589. [DOI] [PubMed] [Google Scholar]
- Holycross B. J., Blank R. S., Thompson M. M., Peach M. J., Owens G. K. Platelet-derived growth factor-BB-induced suppression of smooth muscle cell differentiation. Circ Res. 1992 Dec;71(6):1525–1532. doi: 10.1161/01.res.71.6.1525. [DOI] [PubMed] [Google Scholar]
- Johnson R. J., Iida H., Alpers C. E., Majesky M. W., Schwartz S. M., Pritzi P., Gordon K., Gown A. M. Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest. 1991 Mar;87(3):847–858. doi: 10.1172/JCI115089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koteliansky V. E., Ogryzko E. P., Zhidkova N. I., Weller P. A., Critchley D. R., Vancompernolle K., Vandekerckhove J., Strasser P., Way M., Gimona M. An additional exon in the human vinculin gene specifically encodes meta-vinculin-specific difference peptide. Cross-species comparison reveals variable and conserved motifs in the meta-vinculin insert. Eur J Biochem. 1992 Mar 1;204(2):767–772. doi: 10.1111/j.1432-1033.1992.tb16692.x. [DOI] [PubMed] [Google Scholar]
- Larson D. M., Fujiwara K., Alexander R. W., Gimbrone M. A., Jr Heterogeneity of myosin antigenic expression in vascular smooth muscle in vivo. Lab Invest. 1984 Apr;50(4):401–407. [PubMed] [Google Scholar]
- Lemire J. M., Covin C. W., White S., Giachelli C. M., Schwartz S. M. Characterization of cloned aortic smooth muscle cells from young rats. Am J Pathol. 1994 May;144(5):1068–1081. [PMC free article] [PubMed] [Google Scholar]
- Mosse P. R., Campbell G. R., Wang Z. L., Campbell J. H. Smooth muscle phenotypic expression in human carotid arteries. I. Comparison of cells from diffuse intimal thickenings adjacent to atheromatous plaques with those of the media. Lab Invest. 1985 Nov;53(5):556–562. [PubMed] [Google Scholar]
- Neylon C. B., Avdonin P. V., Dilley R. J., Larsen M. A., Tkachuk V. A., Bobik A. Different electrical responses to vasoactive agonists in morphologically distinct smooth muscle cell types. Circ Res. 1994 Oct;75(4):733–741. doi: 10.1161/01.res.75.4.733. [DOI] [PubMed] [Google Scholar]
- Okamoto E., Imataka K., Fujii J., Kuro-o M., Nakahara K., Nishimura H., Yazaki Y., Nagai R. Heterogeneity in smooth muscle cell population accumulating in the neointimas and the media of poststenotic dilatation of the rabbit carotid artery. Biochem Biophys Res Commun. 1992 May 29;185(1):459–464. doi: 10.1016/s0006-291x(05)81007-1. [DOI] [PubMed] [Google Scholar]
- Orton E. C., LaRue S. M., Ensley B., Stenmark K. Bromodeoxyuridine labeling and DNA content of pulmonary arterial medial cells from hypoxia-exposed and nonexposed healthy calves. Am J Vet Res. 1992 Oct;53(10):1925–1930. [PubMed] [Google Scholar]
- Osborn M., Caselitz J., Weber K. Heterogeneity of intermediate filament expression in vascular smooth muscle: a gradient in desmin positive cells from the rat aortic arch to the level of the arteria iliaca communis. Differentiation. 1981;20(3):196–202. doi: 10.1111/j.1432-0436.1981.tb01176.x. [DOI] [PubMed] [Google Scholar]
- Owens G. K., Loeb A., Gordon D., Thompson M. M. Expression of smooth muscle-specific alpha-isoactin in cultured vascular smooth muscle cells: relationship between growth and cytodifferentiation. J Cell Biol. 1986 Feb;102(2):343–352. doi: 10.1083/jcb.102.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panettieri R. A., Yadvish P. A., Kelly A. M., Rubinstein N. A., Kotlikoff M. I. Histamine stimulates proliferation of airway smooth muscle and induces c-fos expression. Am J Physiol. 1990 Dec;259(6 Pt 1):L365–L371. doi: 10.1152/ajplung.1990.259.6.L365. [DOI] [PubMed] [Google Scholar]
- Pauletto P., Chiavegato A., Giuriato L., Scatena M., Faggin E., Grisenti A., Sarzani R., Paci M. V., Fulgeri P. D., Rappelli A. Hyperplastic growth of aortic smooth muscle cells in renovascular hypertensive rabbits is characterized by the expansion of an immature cell phenotype. Circ Res. 1994 May;74(5):774–788. doi: 10.1161/01.res.74.5.774. [DOI] [PubMed] [Google Scholar]
- Pauletto P., Sartore S., Giuriato L., Scatena M., Guidolin D., Scannapieco G., Pessina A. C. Computer-driven assessment of 'immature'-type smooth muscle cells in rabbit aorta. J Hypertens Suppl. 1991 Dec;9(6):S180–S181. [PubMed] [Google Scholar]
- San Antonio J. D., Karnovsky M. J., Ottlinger M. E., Schillig R., Pukac L. A. Isolation of heparin-insensitive aortic smooth muscle cells. Growth and differentiation. Arterioscler Thromb. 1993 May;13(5):748–757. doi: 10.1161/01.atv.13.5.748. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Campbell G. R., Campbell J. H. Replication of smooth muscle cells in vascular disease. Circ Res. 1986 Apr;58(4):427–444. doi: 10.1161/01.res.58.4.427. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Foy L., Bowen-Pope D. F., Ross R. Derivation and properties of platelet-derived growth factor-independent rat smooth muscle cells. Am J Pathol. 1990 Jun;136(6):1417–1428. [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. M., Heimark R. L., Majesky M. W. Developmental mechanisms underlying pathology of arteries. Physiol Rev. 1990 Oct;70(4):1177–1209. doi: 10.1152/physrev.1990.70.4.1177. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Reidy M. R., Clowes A. Kinetics of atherosclerosis: a stem cell model. Ann N Y Acad Sci. 1985;454:292–304. doi: 10.1111/j.1749-6632.1985.tb11869.x. [DOI] [PubMed] [Google Scholar]
- Shanahan C. M., Weissberg P. L., Metcalfe J. C. Isolation of gene markers of differentiated and proliferating vascular smooth muscle cells. Circ Res. 1993 Jul;73(1):193–204. doi: 10.1161/01.res.73.1.193. [DOI] [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark K. R., Aldashev A. A., Orton E. C., Durmowicz A. G., Badesch D. B., Parks W. C., Mecham R. P., Voelkel N. F., Reeves J. T. Cellular adaptation during chronic neonatal hypoxic pulmonary hypertension. Am J Physiol. 1991 Oct;261(4 Suppl):97–104. doi: 10.1152/ajplung.1991.261.4.L97. [DOI] [PubMed] [Google Scholar]
- Stenmark K. R., Durmowicz A. G., Roby J. D., Mecham R. P., Parks W. C. Persistence of the fetal pattern of tropoelastin gene expression in severe neonatal bovine pulmonary hypertension. J Clin Invest. 1994 Mar;93(3):1234–1242. doi: 10.1172/JCI117077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark K. R., Fasules J., Hyde D. M., Voelkel N. F., Henson J., Tucker A., Wilson H., Reeves J. T. Severe pulmonary hypertension and arterial adventitial changes in newborn calves at 4,300 m. J Appl Physiol (1985) 1987 Feb;62(2):821–830. doi: 10.1152/jappl.1987.62.2.821. [DOI] [PubMed] [Google Scholar]
- Sugihara H., Hattori T., Fukuda M. Immunohistochemical detection of bromodeoxyuridine in formalin-fixed tissues. Histochemistry. 1986;85(3):193–195. doi: 10.1007/BF00494803. [DOI] [PubMed] [Google Scholar]
- Thyberg J., Hedin U., Sjölund M., Palmberg L., Bottger B. A. Regulation of differentiated properties and proliferation of arterial smooth muscle cells. Arteriosclerosis. 1990 Nov-Dec;10(6):966–990. doi: 10.1161/01.atv.10.6.966. [DOI] [PubMed] [Google Scholar]
- Zanellato A. M., Borrione A. C., Giuriato L., Tonello M., Scannapieco G., Pauletto P., Sartore S. Myosin isoforms and cell heterogeneity in vascular smooth muscle. I. Developing and adult bovine aorta. Dev Biol. 1990 Oct;141(2):431–446. doi: 10.1016/0012-1606(90)90398-3. [DOI] [PubMed] [Google Scholar]